Chapter 10 Breast reshaping using autologous tissues after massive weight loss
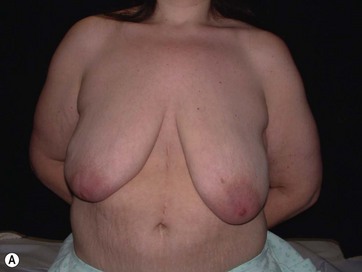
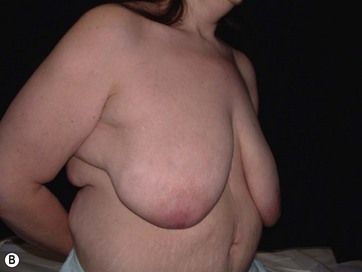
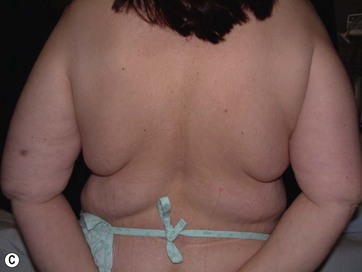
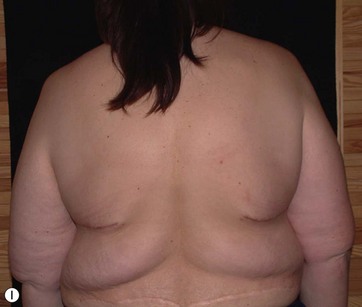
• Breast shape in patients after massive weight loss (MWL) often appears as a “pancake” deformity.
• Conventional techniques of mammaplasty usually fail to correct breast shape in this specific group of patients.
• Breast volume deficiency is better addressed using autologous tissue whenever available.
• “Spare-parts” and “recycled tissue” concepts are the fundamental basis of the surgical technique.
• Redundant tissue over the axillary region, back, abdomen, or thigh usually provides adequate tissue for flap transfer, which can be used for autologous breast augmentation.
Introduction
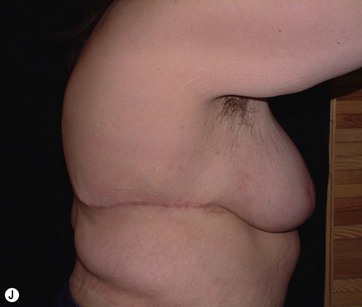
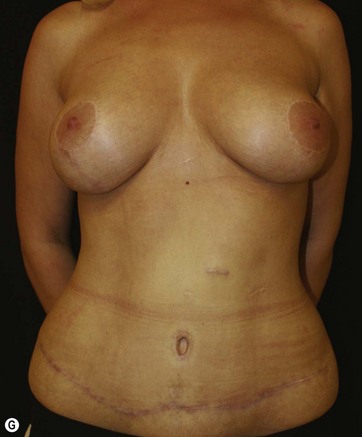
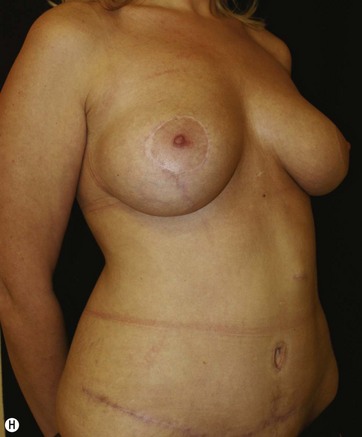
Breast deformities are the most challenging and variable part of the massive weight loss (MWL) patient; thus a thorough definition of the problem and an accordingly chosen technique determine the success of the surgical outcome.1–5 Breast deformities of the MWL patient differ from typically enlarged or ptotic breasts and therefore well-known techniques usually fail in this group. Significant breast volume depletion, and loss of upper pole and medial fullness resulting in the formation of flattened parenchyma against the chest wall, known as the “pancake” appearance, are the most common changes.4,5 The majority of patients present with grade III ptosis and the two breasts are usually asymmetric because of disproportional volume loss. The breasts are displaced laterally in continuity with the lateral chest rolls and the nipple–areola complex is displaced medially. The skin envelope shows significant laxity and is covered by stretch marks.
The patients can present with excessive, sufficient, or insufficient breast volume.1 Although MWL patients rarely have enlarged breasts, the deformity is treated by one of the reduction techniques. However, instead of standard reduction techniques, ligamentous system preservation combined with retightening procedures provides more satisfactory and long-lasting results. Therefore, we prefer a Würinger’s septum-based reduction technique.6 The second group includes patients who have droopy breasts with sufficient volume. Existing dermoglandular tissues are usually adequate to form a natural-looking breast and most of the patients benefit from one of the mastopexy techniques. Lastly, the majority of patients have saggy breasts with insufficient volume, so providing extra volume is mandatory to establish naturally projecting breasts. One option to correct this type of deformity is to combine mastopexy techniques with implant augmentation.1,7 However, there are certain challenges with implant augmentation–mastopexy in MWL patients. Due to excessive skin laxity and loss of dermoglandular suspension, an unnatural appearance and recurrent ptosis are the most common problems after implant augmentation.7
Clinical Approach
Techniques Based on Locoregional Tissue
In most MWL patients, breast deformity is accompanied by lateral skin redundancy or “side rolls” in the lateral thoracic area. This axillary extension of the breast can be used to provide autoaugmentation in patients with insufficient breast volume, using different surgical techniques.1–5,8,10 Rubin described a total parenchymal breast reshaping with dermal suspension mastopexy using the excess tissues around the breast mound.2,5 A modified Wise pattern with lateral extension to encompass any significant lateral rolls was used. The technique was based on de-epithelialization of the entire region, then lateral and medial dermoglandular flaps were elevated securing them together with the central pedicle to the second or third rib periosteum with permanent sutures. Hurwitz et al described the use of lateral side rolls as a spiral flap to augment and reshape the breast in MWL patients.8 In this technique they de-epithelialized fasciocutaneous flap extensions of the Wise pattern mastopexy.
We suggested the use of lateral redundant dermoglandular tissue based on lateral intercostal artery perforators (LICAP) with some personal modifications.3 The technique evolved from our initial experience with the LICAP flap for partial breast reconstruction within a clinical algorithm based on the location of the defect and the availability of these perforators.9 A case report of pedicled perforator flaps for breast augmentation was subsequently published.10 We also reported our clinical experience with ICAP flaps and addressed the use of pedicled LICAP flaps in MWL patients.3 In a recent study we described the anatomical details of the perforators and the flap design.11 As the side rolls extend more posteriorly to the back of the patient, perforators based on the thoracodorsal system can also be used as the main pedicle of the redundant tissue. The thoracodorsal artery perforator (TDAP) flap is another valuable tool that can be used for reshaping the breast via the autoaugmentation principle.10,12
Techniques Based on Distant Tissue
Other parts of the body that need to be addressed during body contouring of the MWL patient are the abdomen, thighs, and buttocks. Contouring of all three parts is well described, with several modifications. The main concept in body reshaping is based on removal of these excess tissues. However, these skin and subcutaneous tissues can be transferred as free flaps and used for augmenting the breast in a single session. Although usage of these tissues is popular and widely used in postmastectomy breast reconstruction, there is little experience of augmenting the breast with free flaps for cosmetic purposes. The whole abdomen can be raised as two individual deep inferior epigastric perforator (DIEAP) or muscle sparing TRAM flaps to be transferred to the breast. Also, during the inner thigh lifting procedure, transverse skin islands can be prepared as gracilis musculocutaneous flaps and used for bilateral breast augmentation. Schoeller et al proposed a medial thigh lift free flap for autologous breast augmentation and presented their experience with three MWL patients.13 Likewise, upper gluteal excess and saggy infragluteal parts can be prepared on superior gluteal or inferior gluteal artery perforator flaps, respectively, to be used for breast augmentation of the MWL patient.
Preoperative Preparation
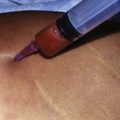
Mapping of the vascular anatomy of the chosen flap before the operation makes the surgery easier and shortens the operative time.14 Combining two procedures and an additional free flap transfer lengthens the operative time; hence any method used to facilitate the surgery is invaluable. Usually, perforators of the LICAP flap or TDAP flap are located with a unidirectional hand-held Doppler. Following the previously described anatomical outlines, it is relatively straightforward to find a reliable perforator on the back. However, the surface area of the abdomen is wide and the number and location of the paraumbilical perforators show great variability. Therefore more objective and reliable methods are needed for perforator mapping. Multidetector computed tomographic (MDCT) angiography and magnetic resonance angiography are extremely helpful tools to navigate the major perforators to be selected. Both techniques provide the exact coordinates of the points where the major perforator pierces the fascia referring to the umblicus.15,16
Surgical Technique
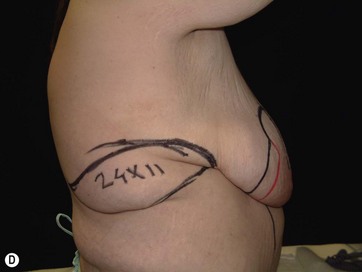
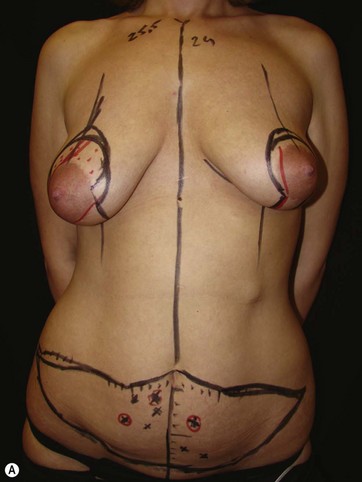
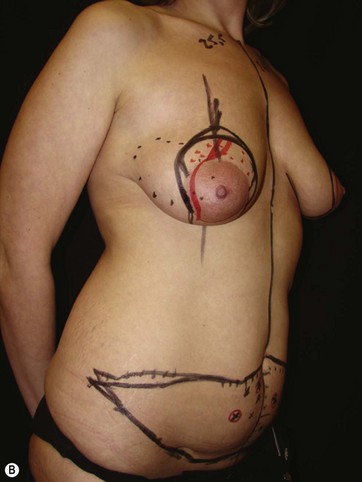
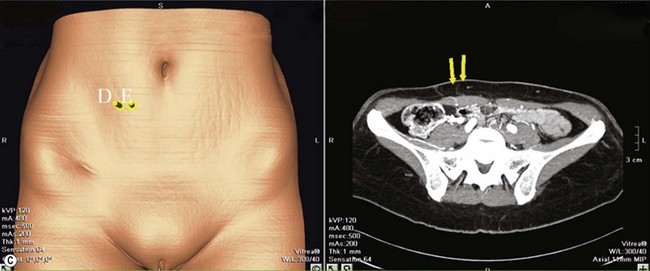
LICAP Flap (Fig. 10.1)
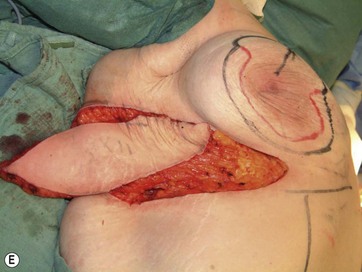
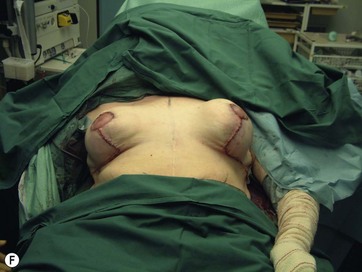
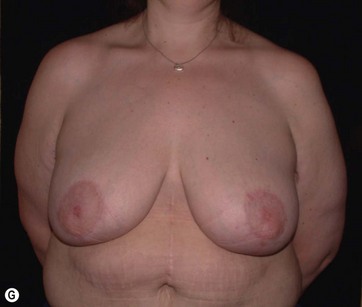
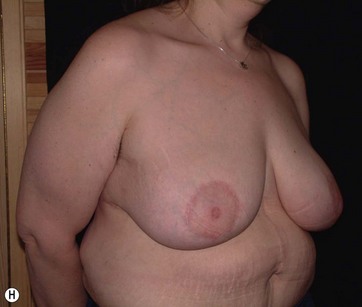
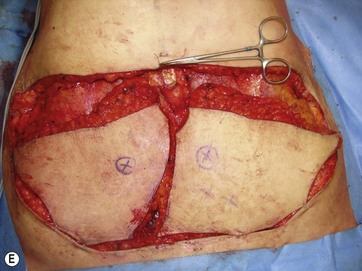
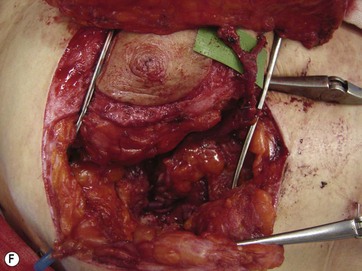
The largest or “dominant” perforators are found between the 5th and 7th intercostal spaces in 84% of cases. The mean distance of these dominant perforators from the anterior border of the latissimus dorsi (LD) muscle varies between 2.77 and 3.68 cm.11 The flap is designed lateral to the breast over the axilla and lateral thoracic area. The anterior border of the flap should include the junction of the inframammary fold (IMF) with the anterior axillary line to allow primary donor site closure. The width of the flap depends on skin redundancy and varies between 8 and 15 cm. The perforators are located with a Doppler and the closest and the most anterior perforator to the breast is chosen for adequate arc of rotation of the flap. The LD muscle is exposed after incision and the flap is elevated above the muscle fascia. Once the largest perforator closer to the pectoralis major muscle is found, it is freed off the surrounding tissue. The serratus anterior muscle is split and the perforator is dissected up to its exit above the rib. If a longer pedicle is required, dissection of the main pedicle can proceed within the costal groove. Once dissection of the perforator is complete, the rest of the flap is elevated easily above the muscle fascia. The inferior incision of the flap is then extended into the IMF. The breast gland is dissected and a retroglandular pocket is prepared for the flap. The mastopexy is first marked in a vertical scar mammaplasty pattern. The horizontal extent of the reduction pattern is determined during surgery, after harvesting and insetting the flap.
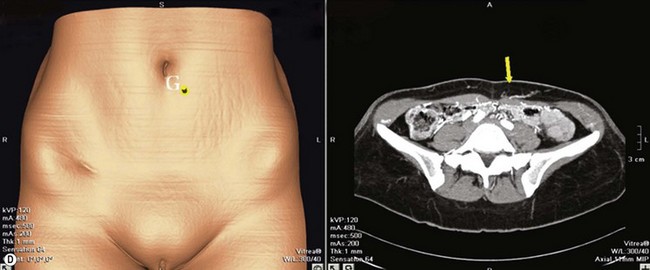
DIEAP Flap (Fig. 10.2)
The abdomen is considered the ideal donor site for autologous breast reconstruction after mastectomy. The morbidity associated with abdominal wall complications has limited the use of the TRAM flap and a search to minimize these complications led to the rise of the DIEAP flap.17,18 In the majority of the MWL patients an abdominoplasty procedure is needed. In carefully selected patients, the whole abdominal tissue can be raised on DIEAP flaps on each side of the abdomen to be transferred to the breast as a free flap. Preoperative mapping of the perforator anatomy with MDCT facilitates identification of the dominant perforator and the intramuscular course of the vessels.15 Abdominal scars from previous surgeries should also be considered before the operation. In general, most MWL patients present with different types of abdominal scars from bariatric surgery. The majority of these scars are midline scars and during two hemi-flap transfers they have no negative impact on flap circulation. Because the traditional belt lipectomy procedure is less effective in most of the MWL patients, a fleur-de-lis pattern excision, to achieve greater improvement above the umbilicus, and resulting in an extra midline scar, is safely performed by some surgeons.19,20 On the other hand, a retrospective study by Parrett et al demonstrated that donor site morbidities, including wound breakdown, abdominal laxity and bulging, were significantly higher compared to DIEAP flap patients with no previous scars.21
TMG Flap
The use of a TMG free flap for the reconstruction of various anatomical regions was described a long time ago, and Yousif et al demonstrated that this flap could be raised safely when the skin paddle is designed in a transverse fashion.22 Historically, the tissue of the upper medial thigh has received very little attention for the purpose of reconstructing an esthetically pleasing breast mound. During the last few years the use of free TMG flaps for breast reconstruction has been popularized, especially for women who have inadequate abdominal bulk or who had previous abdominal surgery.23,24
Excessive skin and soft tissue on the medial thigh after MWL is a common problem among this patient group and an appropriate lifting procedure is almost always needed.25 Schoeller et al reported medial thigh lifting combined with the use of excised tissues as bilateral free gracilis musculocutaneous flaps for breast augmentation in a single-stage procedure.13
Postoperative Care
Postoperative care can sometimes pose a challenge for this group as MWL patients usually have multiple accompanying comorbidities along with an increased risk for general postoperative complications.26,27 Especially in cases where multiple procedures have been carried out, patients should be observed under ICU conditions in the early postoperative phase (24 hours) or in a so-called semi-ICU where patients can stay overnight, mainly for free flap monitoring. Careful monitoring of vital signs, along with urinary output, is critical and fluid replacement must be administered accordingly. A control total blood count and biochemistry panel should be obtained to determine any critical blood loss or biochemical imbalances and the necessary measures must be taken according to the results.
Precautions must also be taken to avoid the risk of thromboembolus, especially in the first hours after surgery when the patient is immobilized.26 The use of compression socks or compression devices is advised for all patients. Pneumatic compression systems for the lower extremity are quite useful devices and serve well for the purpose. Patients may also be given low molecular weight heparin during the immobilized period. It is extremely important to mobilize patients as early as possible to avoid any complications related to prolonged immobilization.
Complications and Their Management
MWL patients are susceptible to various complications after body contouring procedures. In a previous study of 449 post-bariatric surgery patients, 42% of the subjects developed complications and the rates ranged from 8 to 66% in different series.28–30 Complications in the postoperative period include wound healing problems, seromas, thromboembolic complications, infection, hematoma, and necrosis.28–31 Patients’ body mass index, overall medical condition and comorbid factors play an important role in developing postoperative problems. Both seroma formation and wound healing problems are seen in the abdominal and thigh regions and the incidence of such complications is lower in the breast area.28,29 The most important risk factor for seroma was found to be the weight of skin excised at the time of surgery.29 In the thigh region, keeping the anterior border of the excision posterior to the femoral triangle is very important in order to prevent seroma, lymphatic collection and lymphedema. Another important determinant for postoperative complications is combining procedures or more aggressively applying total body lifting procedures.32 Additional application of two free flap transfers for breast autoaugmentation further lengthens the operative time and might increase the overall complication rates. However, there are no data regarding the complication rates after free flap transfer in MWL patients. On the other hand, perfusion complications can be expected at the flaps. Previous clinical data demonstrate that, as the body mass index gets higher, the incidence of venous congestion and fat necrosis increases.33–35 Fat necrosis or partial flap necrosis necessitates debridement, and if a significant amount of flap loss occurs, volume replacement can be achieved by fat grafting. In case of total flap loss the situation can be catastrophic both for the patient and the surgeon and the flap should be replaced by a silicone implant. Preoperative determination of the risk factors, careful patient selection for combining procedures, and appropriate postoperative care are the key steps to be followed for minimizing major and minor complications.
1 Colwell AS, Driscoll D, Breuing KH. Mastopexy techniques after massive weight loss: An algorithmic approach and review of the literature. Ann Plast Surg. 2009;63:28–33.
2 Rubin JP, Gusenoff JA, Coon D. Dermal suspension and parenchymal reshaping mastopexy after massive weight loss: statistical analysis with concomitant procedures from a prospective registry. Plast Reconstr Surg. 2009;123:782–789.
3 Hamdi M, Van Landuyt K, Blondeel P, et al. Autologous breast augmentation with the lateral intercostal artery perforator flap in massive weight loss patients. J Plast Reconstr Aesth Surg. 2009;62:65–70.
4 Kwei S, Borud LJ, Lee BT. Mastopexy with autologous augmentation after massive weight loss: the intercostal artery perforator (ICAP) flap. Ann Plast Surg. 2006;57:361–365.
5 Rubin JP, Agha-Mohammadi S, O’Toole JP. Breast reshaping after massive weight loss. In: Aly A, ed. Body Contouring after Massive Weight Loss. St. Louis: Quality Medical Publishing; 2006:361–378.
6 Hamdi M, Van Landuyt K, Tonnard P, et al. Septum-based mammaplasty: a surgical technique based on Würinger’s septum for breast reduction. Plast Reconstr Surg. 2009;123:443–454.
7 Spear SL, Boehmler JH, Clemens MW. Augmentation/mastopexy: a 3-year review of a single surgeon’s practice. Plast Reconstr Surg. 2006;118:136S–147S. discussion 148S–9S, 150S–1S
8 Hurwitz DJ, Agha-Mohammadi S. Postbariatric surgery breast reshaping: the spiral flap. Ann Plast Surg. 2006;56:481–486. discussion 486
9 Hamdi M, Van Landuyt K, Monstrey S, et al. Pedicled perforator flaps in breast reconstruction: a new concept. Br J Plast Surg. 2004;57:531–539.
10 Van Landuyt K, Hamdi M, Blondeel P, et al. Autologous breast augmentation by pedicled perforator flaps. Ann Plast Surg. 2004;53:322–327.
11 Hamdi M, Spano A, Van Landuyt K, et al. The lateral intercostal artery perforators: anatomical study and clinical application in breast surgery. Plast Reconstr Surg. 2008;121:389–396.
12 Hamdi M, Van Landuyt M, Hijjawi JB, et al. Surgical technique in pedicled thoracodorsal artery perforator flaps: a clinical experience with 99 patients. Plast Reconstr Surg. 2008;121:1632–1641.
13 Schoeller T, Meirer R, Otto-Schoeller A, et al. Medial thigh lift free flap for autologous breast augmentation after bariatric surgery. Obesity Surg. 2002;12:831–834.
14 Smit JM, Klein S, Werker PMN. An overview of methods for vascular mapping in the planning of free flaps. J Plast Reconstr Surg. 2010;63:e674–e682.
15 Gacto-Sanchez P, Sicilia-Castro D, Gomez-Cıa T, et al. Computed tomographic angiography with VirSSPA three-dimensional software for perforator navigation ımproves perioperative outcomes in DIEP flap breast reconstruction. Plast Reconstr Surg. 2010;125:24–31.
16 Alonso-Burgos A, Garcıa-Tutor E, Bastarrika G, et al. Preoperative planning of DIEP and SGAP flaps: Preliminary experience with magnetic resonance angiography using 3-tesla equipment and blood-pool contrast medium J Plast Reconstr. Aesth Surg. 2010;63:298–304.
17 Selber JC, Fosnot J, Nelson J, et al. A prospective study comparing the functional ımpact of SIEA, DIEP, and muscle-sparing free TRAM flaps on the abdominal wall: Part II. Bilateral reconstruction. Plast Reconstr Surg. 2010;126:1438–1453.
18 Nelson JA, Guo Y, Sonnad SS, et al. A Comparison between DIEP and muscle-sparing free TRAM flaps in breast reconstruction: a single surgeon’s recent experience. Plast Reconstr Surg. 2010;126:1428–1435.
19 Wallach SG. Abdominal contour surgery for the massive weight loss patient: the fleur-de-lis approach. Aesth Surg J. 2005;25:454–465.
20 Fernando da Costa L, Landecker A, Marinho Manta A. Optimizing body contour in massive weight loss patients: the modified vertical abdominoplasty. Plast Reconstr Surg. 2004;114:1917–1923.
21 Parrett BM, Caterson SA, Tobias AM, et al. DIEP flaps in women with abdominal scars: are complication rates affected? Plast Reconstr Surg. 2008;121:1527–1531.
22 Yousif NJ, Matloub HS, Kolachalam R, et al. The transverse gracilis musculocutaneous flap. Ann Plast Surg. 1992;29:482.
23 Wechselberger G, Schoeller T. The transverse myocutaneous gracilis free flap: a valuable tissue source in autologous breast reconstruction. Plast Reconstr Surg. 2004;114:69–73.
24 Arnez ZM, Pogorelec D, Planinsek F, et al. Breast reconstruction by the free transverse gracilis (TUG) flap. Br J Plast Surg. 2004;57:20–26.
25 Moreno CH, Neto HJG, Helene Junior A, et al. Thighplasty after bariatric surgery: evaluation of lymphatic drainage in lower extremities. Obes Surg. 2008;18:1160–1164.
26 Shermak MA, Chang DC, Heller J. Factors impacting thromboembolism after bariatric body contouring surgery. Plast Reconstr Surg. 2007;119:1590–1596.
27 Coon D, Michaels VJ, Gusenoff JA, et al. Multiple procedures and staging in the massive weight loss population. Plast Reconstr Surg. 2010;125:691–698.
28 Greco JA, 3rd., Castaldo ET, Nanney LB, et al. The effect of weight loss surgery and body mass index on wound complications after abdominal contouring operations. Ann Plast Surg. 2008;61:235–242.
29 Shermak MA, Rotellini-Coltvet LA, Chang D. Seroma development following body contouring surgery for massive weight loss: patient risk factors and treatment strategies. Plast Reconstr Surg. 2008;122:280–288.
30 Shermak MA, Chang DC, Heller J. Factors impacting thromboembolism after bariatric body contouring surgery. Plast Reconstr Surg. 2007;119:1590–1596. discussion 1597–8
31 Albino FP, Koltz PF, Gusenoff JA. A comparative analysis and systematic review of the wound-healing milieu: implications for body contouring after massive weight loss. Plast Reconstr Surg. 2009;124:1675–1682. discussion 1683–4
32 Coon D, Michaels VJ, Gusenoff JA, et al. Multiple procedures and staging in the massive weight loss population. Plast Reconstr Surg. 2010;125:691–698.
33 Seidenstuecker K, Munder B, Mahajan AL, et al. Morbidity of microsurgical breast reconstruction in patients with comorbid conditions. Plast Reconstr Surg. 2011;127:1086–1092.
34 Vyas RM, Dickinson BP, Fastekjian JH, et al. Risk factors for abdominal donor-site morbidity in free flap breast reconstruction. Plast Reconstr Surg. 2008;121:1519–1526.
35 Garvey PB, Buchel EW, Pockaj BA, et al. The deep ınferior epigastric perforator flap for breast reconstruction in overweight and obese patients. Plast Reconstr Surg. 2005;115:447–457.