Breast reconstruction
Introduction
Surgery for breast cancer is not finished until the reconstruction has been completed in those patients who choose to have it. Mastectomy for breast cancer can lead to negative psychological effects on the patient and breast reconstruction, whether immediate or delayed, can provide significant psychosocial benefits.1–4 Even the most sophisticated breast reconstruction, however, will never fully replicate the breast that has been lost in terms of feel, movement, and erogenous sensation, although some spontaneous sensory recovery may occur.5
Women must be fully informed of all available options for breast reconstruction at the time of planning initial surgical treatment so that they can make informed decisions, even if it is their personal preference to have a delayed reconstruction or no reconstruction at all.6,7 The ultimate goal of breast reconstruction is to produce a ‘breast’ that satisfies the patient’s wishes and matches the contralateral breast, also improving the preoperative breast aesthetics if possible. Breast reconstruction may be either autologous, non-autologous, or a combination of the two, with the use of symmetrising mastopexy, reduction or augmentation surgery if necessary.8 The decision regarding the timing and technique of breast reconstruction should be made by the patient and a multidisciplinary breast cancer team, which should include reconstructive surgeons who are able to provide the full range of commonly used reconstructive procedures.
Timing
The principal aim of breast cancer surgery is to provide safe and successful oncological treatment. The decision for delayed or immediate breast reconstruction and the reconstruction offered may be affected by the anticipated need for adjuvant therapy.8
Immediate breast reconstruction
The main advantage of immediate breast reconstruction is that the patient does not have to spend any time without a breast mound. It allows preservation of the native breast skin envelope and inframammary fold and therefore the reconstruction usually assumes a more natural shape when the breast volume is restored. The mastectomy skin flaps are pliable and unaffected by soft-tissue contracture and scar, and have not suffered the effects of radiotherapy. Skin-sparing and subcutaneous mastectomy techniques can lead to better cosmetic results, with a reduced need for contralateral symmetrisation surgery.8–10
The disadvantages of immediate reconstruction are the limited time for decision-making by the patient due to the need to perform the oncological surgery, increased operating time, and the difficulties of coordinating two surgical teams where different surgeons are required to perform the mastectomy and desired reconstruction. Immediate breast reconstruction does not compromise adjuvant treatment, although there is a potential in individual patients for complications to result in a delay in starting adjuvant treatment.8,11,12
The current indications for post-mastectomy radiotherapy lead many patients to receive radiotherapy as part of their breast reconstruction algorithm. The possibility of radiotherapy should be anticipated before proceeding with immediate breast reconstruction. Radiotherapy can have detrimental effects on breast reconstructions, but these can be reduced by choosing autologous reconstruction over implant-based procedures. With current radiotherapy delivery regimens good cosmetic outcomes can be expected in the majority of cases.13,14 Delayed-immediate reconstruction is also an option in these circumstances. Whether the use of acellular dermal matrix confers any protective effect for implant reconstruction requiring radiotherapy is unclear at present.15
It is now well established that immediate breast reconstruction does not adversely affect breast cancer outcome.13,16–18 Breast reconstruction may be indicated even in advanced disease to control locoregional disease and improve quality of remaining life.8,19,20 There is also evidence to suggest that survival may be improved by removal of the primary tumour.21
Delayed breast reconstruction
Delayed breast reconstruction allows the patient time for decision-making, psychological adjustment following their breast cancer diagnosis and mastectomy, and allows the full pathology to be available prior to reconstructive surgery. It avoids any potential delay of adjuvant treatment and also avoids any detrimental effects of adjuvant therapy on the reconstruction. In addition, the mastectomy skin flaps can be allowed to heal if necessary and any skin damaged by radiotherapy can be excised. The main disadvantage is that skin-sparing mastectomy techniques cannot be used due to the poor aesthetic outcomes of a contracted skin envelope, and therefore a much larger skin paddle is required. In addition, a second operation and episode of hospitalisation is required and treatment costs are increased compared with immediate reconstruction (see Box 9.1).22
Delayed-immediate breast reconstruction
Delayed-immediate breast reconstruction provides some of the benefits of both immediate and delayed breast reconstruction. A skin-sparing mastectomy and immediate reconstruction with a tissue expander is performed. Once the final pathology is available, patients who do not require adjuvant radiotherapy proceed to immediate breast reconstruction. Those who require radiotherapy have their expander fully deflated prior to radiotherapy to allow optimal delivery of the radiotherapy, following which the expander is serially re-expanded within a few weeks of completion of radiotherapy to prevent contraction of the skin envelope whilst awaiting delayed reconstruction.23
Techniques
The aesthetics of autologous reconstruction do not deteriorate with time as with implant-based reconstruction, and are considered to be superior in terms of more natural appearance, feel and durability.24 Autologous tissue can also better withstand radiotherapy.25,26
Non-autologous reconstruction
Breast reconstruction by tissue expansion involves the serial expansion of chest-wall tissue to replace permanently the skin lost following mastectomy by repeated injections of saline into an inflatable silicone expander placed behind the pectoralis major muscle28 (Fig. 9.1). This may either be followed by replacement with a definitive implant once expansion is complete, or in the case of a permanent expandable breast implant that consists of a silicone outer lumen and an expandable saline inner lumen, only the filling port may need removal if it is not integrated into the device.
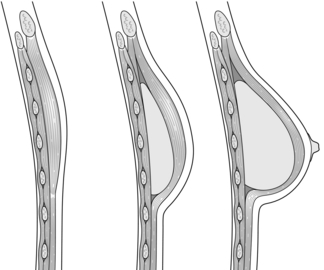
Figure 9.1 Breast reconstruction with submuscular tissue expander. Courtesy of Eva M. Weiler-Mithoff.
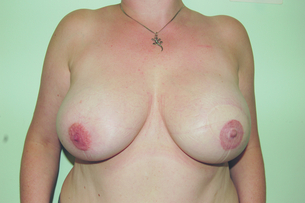
The outcomes of the technique are dependent on careful patient and implant selection. The technique appears simple and is generally good for restoring volume (Fig. 9.2), but it is difficult to create ptosis, and therefore good symmetry with the unaffected breast and true symmetry with implant-based reconstruction is best achieved by bilateral procedures (Fig. 9.3).
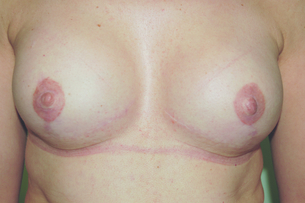
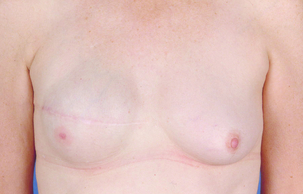
Figure 9.3 Bilateral mastectomy with breast reconstruction with implants and nipple reconstructions.
Indications
This technique is most suitable for patients with small non-ptotic breasts, when performing bilateral reconstruction, or for women who are happy to accept a mastopexy or augmentation procedure on the opposite breast. It is ideal for patients who want minimal scarring and are unwilling or unfit to undergo autologous tissue reconstruction.8,27
Contraindications
Patients are unsuitable for implant reconstruction if the chest-wall tissues are very thin, if the mastectomy skins flaps are of uncertain viability, or if the pectoralis major muscle is absent, either congenitally or following radical mastectomy. Radiotherapy significantly increases the risk of complications and diminishes the aesthetic result of implant/expander breast reconstruction.25,26 This may therefore not be the best method of reconstruction if adjuvant radiotherapy is planned or has already been given.
Surgical techniques
The inframammary fold is an important landmark for implant reconstruction that can be preserved safely during mastectomy and should be restored with sutures if it has been violated. Careful choice of the expander is important, and the size should take into account the base width, height and projection of the normal, intact breast.29
Tissue expanders are placed under the pectoralis major muscle and the inferolateral portion may be covered by serratus fascia, allo- or xenograft, or the serratus anterior and external oblique muscles in a submuscular plane to reduce palpability. There is growing popularity for using acellular dermal matrix, most commonly human (AlloDerm®) or porcine (Strattice®) skin derived, as well as bovine skin and pericardium, to cover the inferolateral portion of the implant (Fig. 9.4). This potentially allows a one-stage immediate implant reconstruction or shortens the time taken for expansion. This technique expands the indications for immediate implant-based reconstruction in women with large ptotic breasts; however, these advantages need to be offset against the costs of the product. In the setting of one- versus two-stage reconstruction, though, the initial increased costs may be offset overall. Acellular dermal matrices (ADM) do allow much better inframammary fold definition. They are not without complications. There is a higher rate of seroma, infection and reconstruction failure with the use of ADMs. The key is to try and place the mastectomy incision over muscle and not over the matrix when inserted and to ensure primary wound healing by refreshing the mastectomy wound. This is achieved by excising the traumatised skin edges at the end of the operation. A good alternative in immediate reconstruction of large ptotic breasts is to perform a skin-reducing mastectomy and use the deepithelialised lower skin flap sutured to the caudal edge of the pectoralis major muscle to cover the inferolateral portion of the prosthesis as a vascularised dermal flap, although a contralateral reduction procedure is usually necessary.
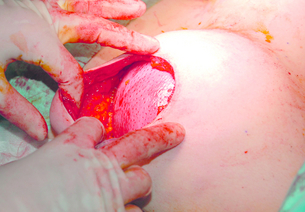
Figure 9.4 One-stage reconstruction after mastectomy with Strattice sling visible covering lower half of implant.
Tissue expansion can be used for immediate or delayed breast reconstruction (Fig. 9.5). The expander is only partially inflated at insertion to allow closure of overlying mastectomy skin flaps without tension. The actual expansion starts 2–4 weeks postoperatively following an interval for healing and is usually performed at weekly intervals. The volume of expansion at each occasion should be guided by patient comfort. Overexpansion was a technique that was used to create a degree of ptosis to produce a more natural-looking breast, but this is unnecessary when anatomical devices are used. Once expansion is completed, the expander is left in place for 1–3 months to allow the skin envelope to maintain its stretch permanently. The expander is then removed, a capsulectomy or capsulotomy is performed, and a definitive implant is inserted based on the width and height of the pocket and the desired projection. Reconstruction of the breast mound can therefore take up to 6 months using tissue expansion. A slightly larger definitive prosthesis is often used following expansion with an anatomical device to reduce the risk of rotation, which can be problematic. Revisional procedures are often required to optimise the aesthetic appearance of the reconstructed breast, and over one-third of patients require further surgery within the first 5 years after implant-based breast reconstruction.30 In addition mastopexy, reduction or augmentation of the contralateral breast and lipofilling are often required to improve symmetry. The long-term aesthetic results of implant-based reconstruction can be expected to decline with time, independent of the implant type or volume, due to gradual ptosis of the contralateral side and failure of the implanted side to undergo normal ptosis, leading to late asymmetry.31 This procedure requires approximately 1 hour of operating time, a short period of hospitalisation and 2–4 weeks of recovery time.
Complications
Early complications include haematoma, infection, mastectomy skin flap necrosis and wound dehiscence, and late complications include implant rupture/deflation, capsular contracture, implant malposition/rotation, implant rippling, extrusion and asymmetry. Even with the latest prosthetic materials and modern radiation delivery techniques, the complication rate for implant-based breast reconstruction in patients undergoing post-mastectomy radiation therapy may be as high as 40%, and the extrusion rate is 15%.32 The commonest and least predictable complication of implant reconstruction is capsular contracture, which may lead to firmness and visible distortion of the breast, as well as pain in advanced cases, and may warrant surgical revision. The risk of capsular contracture is significantly increased following radiotherapy.33 There is some evidence that textured implants may reduce the risk of capsular contracture.34 Lipofilling appears to improve capsular contracture and can help improve cosmetic outcomes. It is particularly valuable for implant rippling and achieving a greater degree of symmetry.
Autologous breast reconstruction
Latissimus dorsi (LD) flap reconstruction
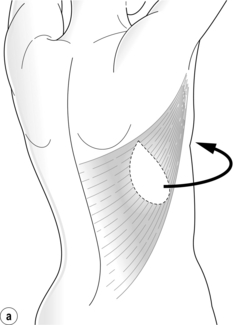
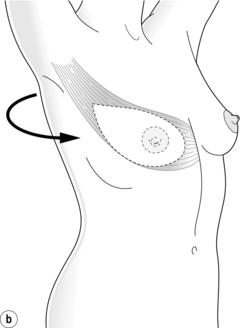
The LD flap may be used either as a muscle or musculocutaneous flap. With its excellent blood supply to the overlying skin it affords a variety of skin paddle designs that can be hidden within the bra strap lines (Fig. 9.7). It is usually combined with an implant and reduces clinically evident capsular contracture and rippling of the prosthesis (Fig. 9.8). The extended LD flap includes the subcutaneous fat overlying the muscle deep to the superficial fascia to increase volume and reduce the chance of needing an implant (Fig. 9.9). Where volume is still deficient with this method, later lipofilling can be used to provide the necessary volume without the need for an implant.
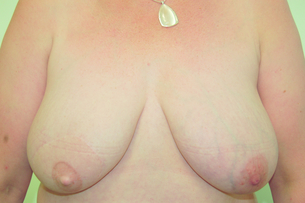
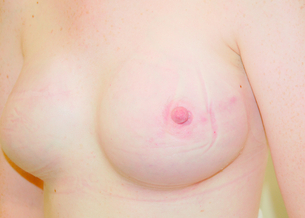
Figure 9.8 Immediate right breast reconstruction with extended LD flap, a very small implant and nipple reconstruction.
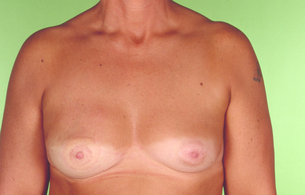
Figure 9.9 Immediate right breast reconstruction by autologous latissimus dorsi flap after skin-sparing mastectomy. Courtesy of Eva M. Weiler-Mithoff.
The pedicled LD flap has the lowest flap failure rate of the autologous reconstructions available and may be indicated in patients who are higher risk for autologous reconstruction. The best indication is in cases where the abdomen is unsuitable as a donor site either due to insufficient tissue volume or the presence of multiple scars, or where the deep inferior epigastric pedicle has been previously ligated. Disadvantages include a scar on the back, possible shoulder stiffness and impairment of upper limb function. The functional deficit of the upper limb has been investigated in multiple studies, and although its absence is well compensated for by the teres major muscle, it is necessary to counsel patients who have high demands of their upper limb, in particular for activities involving shoulder extension and adduction such as climbing and swimming, that this option may result in some functional deficit. Additional physiotherapy may also be required to restore full shoulder mobility.35 The tissue from the back is thicker than that of the native chest skin and the colour match may be different, and this needs to be taken into consideration. The procedure generally requires 3–4 hours operating time, with an extended LD usually taking longer than an LD and implant, a hospital stay of 5–7 days and a recovery time of 4–8 weeks.
Indications: Indications for this technique include the reconstruction of large ptotic breasts, if the chest wall tissues are unsuitable for tissue expansion, or if additional skin needs to be imported following mastectomy. Additional indications include chest-wall reconstruction in locally advanced breast cancer, partial breast reconstruction after breast conservation surgery, or for salvage following loss of an abdominal tissue flap.
Contraindications: The LD flap is contraindicated where it is suspected that previous surgery has damaged the flap pedicle, such as a thoracotomy or extensive and radical axillary surgery, congenital absence of the LD muscle, and significant patient comorbidity. Immediate LD breast reconstruction, even in the setting of postoperative radiotherapy, yields satisfactory results.36
Flap options: The LD flap is most commonly used as a musculocutaneous flap with either an oblique or horizontally orientated skin paddle. A muscle-only flap can be used where no additional skin is required, and where only skin is required, a muscle-sparing or thoracodorsal artery perforator flap may be used.37–40
Preoperative planning: It is necessary to confirm the presence of the LD muscle prior to surgery by asking patients to push down onto their hips and palpate the anterior axillary fold for muscle contraction. This is also particularly important following previous axillary surgery to indicate that the pedicle is likely to be intact, as the nerve lies in close proximity. Next it is important to decide how much skin needs to be replaced and to test the amount of skin that can be taken from the back whilst allowing closure of the donor site, taking into account skin-fold thickness. This is usually between 6 and 9 cm in width, with a lesser amount of skin taken in high risk patients such as smokers to reduce the risk of wound breakdown, and approximately 20 to 25 cm in length. In our experience using the extended LD flap, the total volume in a lean back can be expected to be approximately 200 cc, an average back 400–700 cc, and a variable amount more can be harvested in larger backs.
Surgical technique: The patient is positioned in the lateral decubitus position and secured with well-padded table attachments with the arm supported with attachments at 90°. Infiltration of the incision lines is performed using local anaesthetic with adrenaline to reduce postoperative pain, induce haemostasis and facilitate location of Scarpa’s fascia through tissue tumescence where an extended LD flap is planned. The plane of dissection in an extended flap is immediately deep to Scarpa’s fascia to preserve the blood supply to the back skin and it can be difficult to locate in some patients. In this situation it is easiest to start with the caudal flap, where it is usually better defined. Additional areas of subcutaneous fat harvest including the parascapular area, fat anterior to the anterior border of the muscle and supra-iliac fat deposits are included in the extended flap.
Complications: Early postoperative complications include haematoma, infection, breast skin necrosis, partial or complete flap failure, or wound breakdown. Late complications include seroma, implant rupture and capsular contracture. Seroma formation may be reduced by quilting sutures at the donor site, and once established may be reduced by the use of intracavity steroid injections.41,42
Breast reconstruction with lower abdominal tissue
The lower abdominal pannus is usually an excellent source of tissue for autologous breast reconstruction and leaves an acceptable donor scar as well as serving as a simultaneous aesthetic abdominoplasty (Fig. 9.10). This technique achieves aesthetically stable results that are stable with time24,51 (Fig. 9.2). It must be acknowledged, however, that there is a risk of donor-site bulge and hernia with any technique that transgresses the anterior rectus sheath.52,53
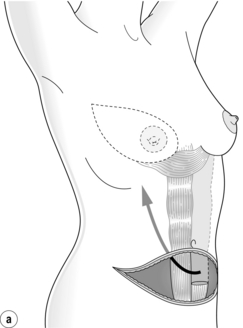
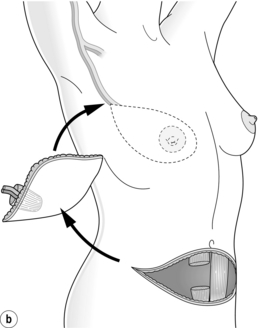
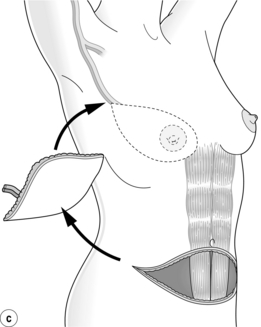
Figure 9.10 Techniques of breast reconstruction using abdominal tissue: (a) pedicled TRAM flap; (b) free TRAM flap; (c) free DIEP flap. See text for explanation of abbreviations. Courtesy of Eva M. Weiler-Mithoff.
Indications: Lower abdominal tissue can be used for immediate or delayed breast reconstruction in any patient with sufficient tissue. For a microvascular procedure patients need to be surgically fit. Due to the versatility, resemblance to a normal breast and excellent long-term outcome, lower abdominal flaps have become the first choice for breast reconstruction for many surgeons.
Contraindications: The only absolute contraindications are previous ligation of the flap pedicle or previous abdominoplasty. Multiple abdominal scars are a relative contraindication, as is previous abdominal liposuction, and imaging of the vascularity may be indicated in these cases. Midline abdominal scars may necessitate harvest of only a hemiflap, or use of a bipedicled flap in certain circumstances. The most predictable and safest outcomes after prior breast irradiation involve the use of autologous tissue, and where radiotherapy is anticipated to follow immediate breast reconstruction, autologous tissue is the current accepted standard due to its better tolerance of irradiation.14,26
Surgical techniques: The lower abdominal pannus receives its dominant blood supply from perforators of the deep inferior epigastric artery, a branch of the external iliac artery, through the rectus abdominis muscle. This vessel connects through reduced calibre vessels within the muscle with the deep superior epigastric artery, the terminal branch of the internal mammary artery, which is the blood supply to the pedicled flap, and this flap therefore necessitates inclusion of the muscle and the venous drainage is retrograde. The lower abdominal flap also receives a variable contribution from the superficial inferior epigastric artery (SIEA), which lies superficial to the anterior rectus sheath.
The triple blood supply to the lower abdominal tissue allows it to be used in a variety of techniques, including the pedicled TRAM flap, free TRAM flap, free deep inferior epigastric artery perforator (DIEP) flap and free SIEA flap.54–59 The free TRAM and DIEP flaps utilise the dominant blood supply and are associated with a reduced risk of flap complications compared with the pedicled TRAM flap. In addition, the potential to completely or partially preserve the rectus muscle and its intercostal motor nerves leads to reduced donor-site morbidity. It is important, therefore, to take a careful history of the activities and hobbies of the patient when considering the most appropriate reconstruction. With any technique there may always be persistent absence of sensory recovery in a triangle between the new umbilicus position and the abdominal scar.
Pedicled TRAM flap: The pedicled TRAM flap relies on blood flow through the deep superior epigastric vessels within the substance of the rectus abdominis muscle. The flap is transferred onto the chest wall through a large epigastric subcutaneous tunnel that may be either ipsilateral or contralateral. The contralateral pedicle may produce superior aesthetic results because it reduces the bulge in the epigastrium and may avoid disruption of the ipsilateral inframammary fold.56 The only absolute contraindication is previous ligation of the deep superior epigastric artery pedicle. The flap does not require microvascular skills; however, perfusion through the non-dominant blood supply leads to higher rates of complications than the free flap, including fat necrosis. For this reason some surgeons advocate flap ‘delay’ by ligation of the ipsilateral deep and superficial inferior epigastric arteries before transfer, to allow augmentation of the remaining blood supply, especially in those considered high risk for flap necrosis, such as smokers and obese persons.
As the full muscle width is required, the donor site needs to be reconstructed with prosthetic material and the donor-site morbidity is higher than with free flap options.56,60 Bilateral pedicled TRAM flaps may further increase donor-site morbidity.61
Free TRAM flap: In many centres free flaps from the lower abdominal wall are the first choice in breast reconstruction with autologous tissue (Fig. 9.11). The deep inferior epigastric vessels are the dominant blood supply for a free TRAM flap. The lower abdominal skin is transferred with a segment of rectus abdominis muscle and the deep inferior epigastric vessels, which are anastomosed to the recipient vessels of the subscapular axis or the internal mammary system. An ipsilateral pedicle will place the better vascularised tissue towards the midline. Muscle- and fascial-sparing techniques are now widely used to avoid the need for insertion of synthetic mesh at the donor site. Due to the improved blood supply the rate of fat necrosis is reduced and a larger flap can be safely transferred compared with the pedicled flap.62 Muscle-sparing free TRAM flap techniques have demonstrated reduced donor-site morbidity.63–65 Many large-volume centres are reporting total flap failure rates of around 1%.53
Deep inferior epigastric perforator (DIEP) flap: The DIEP flap spares the whole of the rectus abdominis muscle through meticulous dissection of deep inferior epigastric artery perforators within the rectus abdominis muscle and preservation of the intercostal motor nerves (Fig. 9.12). This reduces the donor-site morbidity when compared with the TRAM flap.59,66,67 No muscle and little or no fascia is harvested and mesh is not usually required for donor-site closure.
Superficial inferior epigastric artery (SIEA) flap: The SIEA flap is based on the superficial inferior epigastric artery and vein, which arise from the common femoral artery and saphenous bulb, respectively. Donor-site morbidity from SIEA flap harvest is minimal as the vessels are dissected at the level of Scarpa’s fascia and the rectus fascia is left intact.68 The main disadvantage of the SIEA flap is variability of the SIEA in presence, calibre and cutaneous territory. Vessels of at least 1 mm in diameter at the level of the inferior incision can be used safely for flap transfer. The vascular pedicle is short and therefore the internal mammary recipient vessels are preferred, and flap inset requires special consideration due to the peripheral location of the pedicle. Perfusion of the flap across the midline is unreliable, and thus its use is limited to where only a hemiflap is required and for bilateral procedures.68–71
Techniques: The flaps are harvested through standard abdominoplasty incisions extending laterally to the anterior superior iliac spines.59,66 Dissection is from lateral to medial, taking care to identify the superficial inferior epigastric pedicle. If the artery is of sufficient size, then a SIEA flap can be harvested, otherwise the superficial inferior epigastric vein is dissected for a short distance for use in case of venous compromise later. The perforators are inspected and if one dominant perforator or two or three smaller suitable perforators in the same intramuscular septum can be harvested then a DIEP flap is harvested with careful intramuscular dissection of the perforators to the pedicle, which is located on the underside of the muscle. Where suitable perforators for DIEP flap harvest are not present, muscle is included (as much as is necessary to incorporate the perforators) and the dissection continues until a pedicle of sufficient length and calibre is obtained. Sensory nerves to the flap typically run with the perforators and may be connected to the lateral branch of the fourth intercostal nerve, although spontaneous recovery of sensation often occurs.5
Complications: Early complications include thrombosis of the arterial or venous anastomosis, haematoma, partial or total flap loss, fat necrosis, wound breakdown, and infection of prosthetic mesh if used. Late complications include donor-site bulge or hernia and reduced abdominal strength (Table 9.1). Overweight (body mass index 25–29) and obese (body mass index ≥ 30) patients have a significantly increased rate of flap and donor-site complications.73 Smokers have a higher incidence of mastectomy flap necrosis and donor-site abdominal flap necrosis and hernia, although not thrombosis of the anastomosis or flap loss.74
Table 9.1
Pooled complication rates for DIEP and free TRAM flap patients (%)
| DIEP flap | Free TRAM flap | |
| Fat necrosis | 10.1 | 4.9 |
| Partial flap loss | 2.5 | 1.8 |
| Total flap loss | 2.0 | 1.0 |
| Abdominal bulge | 3.1 | 5.9 |
| Abdominal hernia | 0.8 | 3.9 |
DIEP, deep inferior epigastric perforator; TRAM, transverse rectus abdominis myocutaneous.
Modified from Man LX, Selber JC, Serletti JM. Abdominal wall following free TRAM or DIEP flap reconstruction: a meta-analysis and critical review. Plast Reconstr Surg 2009; 124(3):752–64.
Superior and inferior gluteal artery perforator flaps: A superior or inferior gluteal artery perforator (SGAP, IGAP) flap is indicated when the abdominal donor site is unavailable due to insufficient tissue or the presence of multiple abdominal scars or if the patient wants a more inconspicuous donor-site scar.75–77 The flaps are limited to small-volume reconstructions and tissue is firmer and less able to create ptosis than that from the abdomen. The donor site, however, particularly with the IGAP flap, can be excellent in well-selected patients and recovery is shorter than with abdominal flaps. The internal mammary vessels are preferred for the anastomosis to aid inset, due to the relatively short pedicle.
Transverse upper gracilis flap: The transverse upper gracilis (TUG) flap utilises a transverse skin ellipse from the upper thigh, which is usually discarded in a traditional aesthetic medial thigh lift, based on musculocutaneous perforators through the gracilis muscle from the medial circumflex iliac artery. As with the gluteal artery perforator flaps the disadvantages include small volume and firmer tissue than from the abdomen, but the donor-site scar is very well hidden and there is good patient satisfaction.78–80
Alternative free flap donor sites: Alternative options of autologous tissue breast reconstruction in selected patients without other suitable donor sites include the anterolateral thigh flap, omentum and Rubens flap based on the deep circumflex iliac artery. The free contralateral LD flap may be used in selected cases of ipsilateral congenital absence.
Finishing touches
Further surgery may be necessary to the reconstructed breast, the contralateral breast or the donor site of the autologous reconstruction. Complete breast reconstruction including nipple–areola reconstruction requires on average 3.3 separate procedures.81
Surgery to the reconstructed breast
The reconstructed breast may require adjustment in size or shape by liposuction, excision of fat necrosis, mastopexy or augmentation. Lipomodelling transfers fat cells that have been harvested by liposuction into autologous breast reconstructions such as the autologous LD flap or the DIEP. This technique is particularly useful for contour irregularities or generalised volume loss after adjuvant radiotherapy. Lipomodelling may be used for adding volume to the reconstructed breast and smoothing out irregularities, and also for preparing the irradiated bed prior to implant breast reconstruction.82–84 Further adjustments of the position of the breast on the chest wall, improvement of projection, adjustment of the inframammary fold or revisional surgery for capsular contracture may be necessary.
Surgery to the contralateral breast
Symmetrising surgery may be achieved by mastopexy, reduction or breast augmentation. Augmentation is particularly useful for gaining symmetry where implant breast reconstruction has been utilised. Some patients may want a contralateral risk-reducing mastectomy with reconstruction where they are deemed at high risk of contralateral breast cancer after a formal assessment of genetic risk.85
Nipple–areola reconstruction
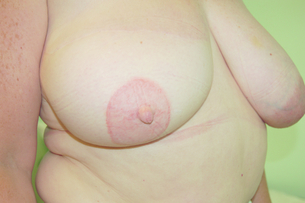
The breast reconstruction is not complete until the nipple–areola complex (NAC) has been reconstructed, although some patients may be happy with a customised prosthetic nipple alone.86 NAC reconstruction is usually the last step of the reconstruction as its position is difficult to alter. The aims of NAC reconstruction are to achieve symmetry with the contralateral NAC in terms of size, colour, texture, position and projection (see Figs 9.3, 9.8 and 9.11). The ideal NAC reconstruction technique has not yet been discovered, as evidenced by the number of techniques that have been described, mainly as a result of loss of projection with time.87
Areola reconstruction can be performed by full-thickness skin grafting or by tattooing. Grafts are usually obtained from the contralateral areola or the labia majora, with the aim to match the pigmentation and texture of the contralateral areolar as closely as possible. For this reason, where the contralateral areola is not suitable, tattooing is usually preferred. It is a quick and simple technique with minimal morbidity and very few complications apart from fading with time, and may be performed either before or after the nipple reconstruction.88
Complications of breast reconstruction
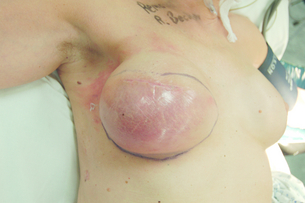
Mastectomy skin flap necrosis can be a common complication of immediate breast reconstruction. Management may be conservative with dressings if small, or the area can be excised and closed by advancement of the skin flap or autologous flap skin paddle, or by split skin grafting. Partial autologous flap failure usually requires debridement and management involves dressings, excision, and possibly skin grafting, but complete flap loss requires removal of the flap and either direct closure of the skin flaps, or placement of a tissue expander or implant, or immediate LD flap breast reconstruction, depending on the patient’s wishes. Implant infection can sometimes be salvaged by washout and reinsertion of the implant, but severe infection or extrusion usually requires removal of the prosthesis (Fig. 9.13) and replacement at a later date once the tissues are healed and free from infection. Contour defects following fat necrosis, muscle atrophy or following radiotherapy may be corrected either by prosthetic augmentation or by lipomodelling.
Local recurrence
Salvage surgery for chest-wall recurrence is best dealt with in a multidisciplinary setting. The aims of surgery are local control of disease, palliation of symptoms and enhancement of the quality of remaining life. Chest-wall reconstruction may be required, and importation of well-vascularised non-irradiated flap tissues may allow for further radiotherapy. Reconstruction of the resultant defect often requires extensive surgery utilising local flaps or abdominal advancement, regional flaps such as LD, pectoralis major and parascapular flaps, omental transposition, pedicled or free abdominal flaps, or a combination of these techniques.19,20,89,90
Summary
The aim of breast reconstruction is to recreate the natural breast as closely as possible following mastectomy. Decisions regarding breast reconstruction are best made by the fully informed patient within the setting of a multidisciplinary breast cancer team that can deliver the oncological surgery as well as the full range of commonly used breast reconstruction techniques. Breast reconstruction leads to a high degree of satisfaction but high levels of preoperative information and psychological support are necessary.91 Close collaboration between oncological and reconstructive surgeons or management by an oncoplastic surgeon and careful patient selection and counselling can achieve excellent outcomes for breast reconstruction in the majority of patients.
References
1. Harcourt, D.M., Rumsey, N.J., Ambler, N.R., et al, The psychological effect of mastectomy with or without breast reconstruction: a prospective, multicenter study. Plast Reconstr Surg 2003; 111:1060–1068. 12621175
2. Elder, E.E., Brandberg, Y., Bjorklund, T., et al, Quality of life and patient satisfaction in breast cancer patients after immediate breast reconstruction: a prospective study. Breast 2005; 14:201. 15927829
3. Atisha, D., Alderman, A.K., Lowery, J.C., et al, Prospective analysis of long-term psychosocial outcomes in breast reconstruction: two-year postoperative results from the Michigan Breast Reconstruction Outcomes Study. Ann Surg. 2008;247(6):1019–1028. 18520230
4. Fourth annual report of the National Mastectomy and Breast Reconstruction Audit. www.ic.nhs.uk/services/national-clinical-audit-support-programme-ncasp/audit-reports/mastectomy-and-breast-reconstruction, 2011. [[accessed 11.08.12]].
5. Shridharani, S.M., Magarakis, M., Stapleton, S.M., et al, Breast sensation after breast reconstruction: a systematic review. J Reconstr Microsurg. 2010;26(5):303–310. 20195965
6. Alderman, A.K., Hawley, S.T., Waljee, J., et al, Understanding the impact of breast reconstruction on the surgical decision-making process for breast cancer. Cancer. 2008;112(3):489–494. 18157830
7. Second annual report of the National Mastectomy and Breast Reconstruction Audit. www.ic.nhs.uk/services/national-clinical-audit-support-programme-ncasp/audit-reports/mastectomy-and-breast-reconstruction, 2009. [[accessed 11.08.12]].
8. Baildam, A., Bishop, H., Boland, G., et al, Oncoplastic breast surgery – A guide to good practice. Eur J Surg Oncol. 2007;33(Suppl. 1):S1–23. 17604938
9. Yi, M., Kronowitz, S.J., Meric-Bernstam, F., et al, Local, regional, and systemic recurrence rates in patients undergoing skin-sparing mastectomy compared with conventional mastectomy. Cancer. 2011;117(5):916–924. 20945319
10. Kronowitz, S.J., Immediate versus delayed reconstruction. Clin Plast Surg. 2007;34(1):39–50. 17307070
11. Alderman, A.K., Collins, E.D., Schott, A., et al, The impact of breast reconstruction on the delivery of chemotherapy. Cancer. 2010;116(7):1791–1800. 20143440
12. Wilson, C.R., Brown, I.M., Weiler-Mithoff, E.M., et al, Immediate breast reconstruction is not associated with a delay in the delivery of adjuvant chemotherapy. Eur J Surg Oncol 2004; 30:624–627. 15256235 No statistically significant difference was found in the time between surgery and first dose of adjuvant chemotherapy in 285 patients undergoing wide local excision, simple mastectomy or mastectomy and immediate breast reconstruction with a variety of techniques.
13. Veronesi, P., Ballardini, B., De Lorenzi, F., et al, Immediate breast reconstruction after mastectomy. Breast. 2011;20(Suppl. 3):S104–S107. 22015274
14. Barry, M., Kell, M.R., Radiotherapy and breast reconstruction: a meta-analysis. Breast Cancer Res Treat. 2011;127(1):15–22. 21336948
15. Spear, S.L., Parikh, P.M., Reisin, E., et al, Acellular dermis-assisted breast reconstruction. Aesthet Plast Surg 2008; 32:418–425. 18338102
16. Nedumpara, T., Jonker, L., Williams, M.R., Impact of immediate breast reconstruction on breast cancer recurrence and survival. Breast. 2011;20(5):437–443. 21601458
17. Eriksen, C., Frisell, J., Wickman, M., et al, Immediate reconstruction with implants in women with invasive breast cancer does not affect oncological safety in a matched cohort study. Breast Cancer Res Treat. 2011;127(2):439–446. 21409394
18. Petit, J.Y., Gentilini, O., Rotmensz, N., et al, Oncological results of immediate breast reconstruction: long term follow-up of a large series at a single institution. Breast Cancer Res Treat. 2008;112(3):545–549. 18210199
19. Crisera, C.A., Chang, E.I., Da Lio, A.L., et al, Immediate free flap reconstruction for advanced-stage breast cancer: is it safe? Plast Reconstr Surg. 2011;128(1):32–41. 21399562
20. Lim, W., Ko, B.S., Kim, H.J., et al, Oncological safety of skin sparing mastectomy followed by immediate reconstruction for locally advanced breast cancer. J Surg Oncol. 2010;102(1):39–42. 20578076
21. Ruiterkamp, J., Ernst, M.F., van de Poll-Franse, L.V., et al, Surgical resection of the primary tumour is associated with improved survival in patients with distant metastatic breast cancer at diagnosis. Eur J Surg Oncol 2009; 35:1146–1151. 19398188
22. Khoo, A., Kroll, S.S., Reece, G.P., et al, A comparison of resource costs of immediate and delayed breast reconstruction. Plast Reconstr Surg. 1998;101(4):964–968. 9514328
23. Kronowitz, S.J., Lam, C., Terefe, W., et al, A multidisciplinary protocol for planned skin-preserving delayed breast reconstruction for patients with locally advanced breast cancer requiring postmastectomy radiation therapy: 3-year follow-up. Plast Reconstr Surg. 2011;127(6):2154–2166. 21311392
24. Hu, E.S., Pusic, A.L., Waljee, J.F., et al, Patient-reported aesthetic satisfaction with breast reconstruction during the long-term survivorship period. Plast Reconstr Surg. 2009;124(1):1–8. 19568038
25. Chevray, P.M., Timing of breast reconstruction: immediate versus delayed. Cancer J. 2008;14(4):223–229. 18677129
26. Jugenburg, M., Disa, J.J., Pusic, A.L., et al, Impact of radiotherapy on breast reconstruction. Clin Plast Surg. 2007;34(1):29–37. 17307069
27. Bostwick, J., III. Plastic and reconstructive breast surgery. St Louis: Quality Medical Publishing; 1990.
28. Radovan, C., Breast reconstruction after mastectomy using the temporary expander. Plast Reconstr Surg 1982; 69:195–208. 7054790
29. Spear, S.L., Mesbahi, A.N., Implant-based reconstruction. Clin Plast Surg. 2007;34(1):63–73. 17307072
30. Gabriel, S.E., Woods, J.E., O’Fallon, W.M., et al, Complications leading to surgery after breast implantation. N Engl J Med 1997; 336:677–682. 9041097
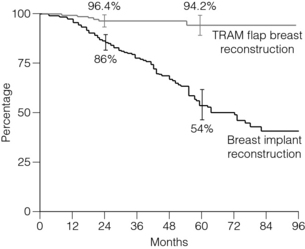
31. Clough, K.B., O’Donoghue, J.M., Fitoussi, A.D., et al, Prospective evaluation of late cosmetic results following breast reconstruction: implant reconstruction. Plast Reconstr Surg 2001; 107:1702–1709. 11391188 Prospective evaluation of morbidity and cosmesis of 334 patients with unilateral implant reconstructions showed a significant deterioration of long-term results in a linear fashion, from an initial acceptable result of 86% 2 years after patients completed their reconstruction to only 54% at 5 years, despite a 92.5% rate of symmetry surgery. This deterioration was unrelated to the type of implant used, the volume of the implant, the age of the patient or the type of mastectomy incision employed, but after a retrospective photographic review the authors are of the opinion that it was related to late asymmetry produced by the failure of both breasts to undergo symmetrical ptosis with ageing.
32. Kronowitz, S.J., Robb, G.L., Radiation therapy and breast reconstruction: a critical review of the literature. Plast Reconstr Surg. 2009;124(2):395–408. 19644254
33. Cordeiro, P.G., Pusic, A.L., Disa, J.J., et al, Irradiation after immediate tissue expander/implant breast reconstruction: outcomes, complications, aesthetic results, and satisfaction among 156 patients. Plast Reconstr Surg. 2004;113(3):877–881. 15108879
34. Wickman, M., Jurell, G., Low capsular contraction rate after primary and secondary breast reconstruction with a textured expander prosthesis. Plast Reconstr Surg. 1997;99(3):692–697. 9047188
35. Clough, K.B., Louis-Sylvestre, C., Fitoussi, A., et al, Donor site sequelae after autologous breast reconstruction with an extended latissimus dorsi flap. Plast Reconstr Surg 2002; 109:1904–1911. 11994592
36. McKeown, D.J., Hogg, F.J., Brown, I.M., et al, The timing of autologous latissimus dorsi breast reconstruction and effect of radiotherapy on outcome. J Plast Reconstr Aesthet Surg. 2009;62(4):488–493. 18262481
37. Delay, E., Gounot, N., Bouillot, A., et al, Autologous latissimus breast reconstruction: a 3 year clinical experience with 100 patients. Plast Reconstr Surg 1998; 102:1461–1478. 9774000
38. McCraw, J.B., Papp, C., Edwards, A., et al, The autogenous latissimus breast reconstruction. Clin Plast Surg 1994; 21:279–288. 8187421
39. Germann, G., Steinau, H.U., Breast reconstruction with the extended latissimus dorsi flap. Plast Reconstr Surg 1996; 97:519–526. 8596782
40. Saint-Cyr, M., Nagarkar, P., Schaverien, M., et al, The pedicled descending branch muscle-sparing latissimus dorsi flap for breast reconstruction. Plast Reconstr Surg. 2009;123(1):13–24. 19116510
41. Dancey, A.L., Cheema, M., Thomas, S.S., A prospective randomized trial of the efficacy of marginal quilting sutures and fibrin sealant in reducing the incidence of seromas in the extended latissimus dorsi donor site. Plast Reconstr Surg. 2010;125(5):1309–1317. 20440152 Prospective, double-blinded, clinical trial under a single surgeon involving 26 patients followed up for 6 months randomised to receive either quilting sutures only or a combination of fibrin sealant and marginal quilting sutures. The combination of fibrin sealant and marginal quilting sutures significantly reduced total drainage, hospital stay and seroma formation. The authors caution that potential, albeit small, risk of virus transmission and allergic reaction, however, needs to be taken into consideration with the use of fibrin sealant, as with any blood transfusion product.
42. Taghizadeh, R., Shoaib, T., Hart, A.M., et al, Triamcinolone reduces seroma re-accumulation in the extended latissimus dorsi donor site. J Plast Reconstr Aesthet Surg. 2008;61(6):636–642. 17499035 This prospective study involved 52 extended LD breast reconstructions in 49 patients, with patients exhibiting seromas at their first postoperative visit randomised to receive either intracavity triamcinolone 80 mg or saline following seroma aspiration. Triamcinolone significantly reduced the need for any further aspiration, total number of aspirations, total volume aspirated and total time to dryness. Steroid injections were well tolerated and there were no infective complications.
43. Kroll, S.S., Baldwin, B., A comparison of outcomes using three different methods of breast reconstruction. Plast Reconstr Surg 1992; 90:455–462. 1387483
44. Roy, M.K., Shrotia, S., Holcombe, C., et al, Complications of latissimus dorsi myocutaneous flap breast reconstruction. Eur J Surg Oncol 1998; 24:162–165. 9630851
45. Hardwicke, J.T., Prinsloo, D.J., An analysis of 277 consecutive latissimus dorsi breast reconstructions: a focus on capsular contracture. Plast Reconstr Surg. 2011;128(1):63–70. 21701322
46. Hammond, D.C., Latissimus dorsi flap breast reconstruction. Clin Plast Surg. 2007;34(1):75–82. 17307073
47. Gui, G.P., Tan, S.M., Faliakou, E.C., Immediate breast reconstruction using biodimensional anatomical permanent expander implants: a prospective analysis of outcome and patient satisfaction. Plast Reconstr Surg 2003; 111:125–138. 12496573
48. Tarantino, I., Banic, A., Fisher, T., Evaluation of late results in breast reconstruction by latissimus dorsi flap and prosthesis implantation. Plast Reconstr Surg 2006; 117:1387–1394. 16641703
49. Chang, D.W., Barnea, Y., Robb, G.L., Effects of an autologous flap combined with an implant for breast reconstruction: an evaluation of 1000 consecutive reconstructions of previously irradiated breasts. Plast Reconstr Surg. 2008;122(2):356–362. 18626350
50. Report of the Independent Review Group. Silicone gel breast implants. London: HMSO; 1998. The Independent Review Group considered immense amounts of complex evidence and reached a number of conclusions, including that there is no histopathological or conclusive immunological evidence for an abnormal immune response to silicone from breast implants in tissue, and that there is no epidemiological evidence for any link between silicone gel breast implants and any established connective tissue disease.
51. Clough, B.C., O’Donoghue, J.M., Fitoussi, A.D., et al, Prospective evaluation of late cosmetic results following breast reconstruction. II. TRAM flap reconstruction. Plast Reconstr Surg 2001; 107:1710–1716. 11391189 Prospective study of 171 TRAM flap patients for 8 years showed aesthetically pleasing long-term results in 94.2%.
52. Yueh, J.H., Slavin, S.A., Adesiyun, T., et al, Patient satisfaction in postmastectomy breast reconstruction: a comparative evaluation of DIEP, TRAM, latissimus flap, and implant techniques. Plast Reconstr Surg. 2010;125(6):1585–1595. 20517080
53. Man, L.X., Selber, J.C., Serletti, J.M., Abdominal wall following free TRAM or DIEP flap reconstruction: a meta-analysis and critical review. Plast Reconstr Surg. 2009;124(3):752–764. 19342994
54. Robbins, T.H., Rectus abdominis myocutaneous flap for breast reconstruction. Aust N Z J Surg 1979; 49:527–530. 159684
55. Hartrampf, C.R., Breast reconstruction with a transverse abdominal island flap. Plast Reconstr Surg 1982; 69:216–224. 6459602
56. Jones, G., The pedicled TRAM flap in breast reconstruction. Clin Plast Surg. 2007;34(1):83–104. 17307074
57. Holmstroem, H., The free abdominoplasty flap and its use in breast reconstruction. Scand J Plast Reconstr Surg 1979; 13:423–426. 396670
58. Koshima, I., Soeda, S., Inferior epigastric artery skin flaps without rectus abdominis muscle. Br J Plast Surg 1989; 42:645–648. 2605399
59. Allen, R.J., Treece, P., Deep inferior epigastric perforator flap for breast reconstruction. Ann Plast Surg 1994; 32:32–38. 8141534
60. Atisha, D., Alderman, A.K., A systematic review of abdominal wall function following abdominal flaps for postmastectomy breast reconstruction. Ann Plast Surg. 2009;63(2):222–230. 19593108
61. Chun, Y.S., Sinha, I., Turko, A., et al, Comparison of morbidity, functional outcome, and satisfaction following bilateral TRAM versus bilateral DIEP flap breast reconstruction. Plast Reconstr Surg. 2010;126(4):1133–1141. 20555301
62. Kroll, S.S., Gherardini, G., Martin, J.E., et al, Fat necrosis in free and pedicled TRAM flaps. Plast Reconstr Surg. 1998;102(5):1502–1507. 9774003
63. Nelson, J.A., Guo, Y., Sonnad, S.S., et al, A comparison between DIEP and muscle-sparing free TRAM flaps in breast reconstruction: a single surgeon’s recent experience. Plast Reconstr Surg. 2010;126(5):1428–1435. 21042098
64. Wu, L.C., Bajaj, A., Chang, D.W., et al, Comparison of donor-site morbidity of SIEA, DIEP, and muscle-sparing TRAM flaps for breast reconstruction. Plast Reconstr Surg. 2008;122(3):702–709. 18766032
65. Bajaj, A.K., Chevray, P.M., Chang, D.W., Comparison of donor-site complications and functional outcomes in free muscle-sparing TRAM flap and free DIEP flap breast reconstruction. Plast Reconstr Surg. 2006;117(3):737–746. 16525258
66. Granzow, J.W., Levine, J.L., Chiu, E.S., et al, Breast reconstruction using perforator flaps. J Surg Oncol. 2006;94(6):441–454. 17061279
67. Futter, C.M., Webster, M.H., Hagen, S., et al, A retrospective comparison of abdominal muscle strength following breast reconstruction with a free TRAM or DIEP flap. Br J Plast Surg 2000; 53:578–583. 11000074
68. Grotting, J., The free abdominoplasty flap for immediate breast reconstruction. Ann Plast Surg. 1991;27(4):351–354. 1663325
69. Chevray, P., Update on breast reconstruction using free TRAM, DIEP, and SIEA flaps. Semin Plast Surg. 2004;18(2):97–104. 20574488
70. Lipa, J., Breast reconstruction with free flaps from the abdominal donor site: TRAM, DIEAP, and SIEA flaps. Clin Plast Surg. 2007;34(1):105–121. 17307075
71. Selber, J., Samra, F., Bristol, M., et al, A head-to-head comparison between the muscle-sparing free TRAM and the SIEA flaps: is the rate of flap loss worth the gain in abdominal wall function? Plast Reconstr Surg. 2008;122(2):348–355. 18626349
72. Busic, V., Das-Gupta, R., Mesic, H., et al, The deep inferior epigastric perforator flap for breast reconstruction, the learning curve explored. J Plast Reconstr Aesthet Surg. 2006;59(6):580–584. 16817256
73. Chang, D., Wang, B., Robb, G., et al, Effect of obesity on flap and donor-site complications in free transverse rectus abdominis myocutaneous flap breast reconstruction. Plast Reconstr Surg. 2000;105(5):1640–1648. 10809092
74. Chang, D., Reece, G., Wang, B., et al, Effect of smoking on complications in patients undergoing free TRAM flap breast reconstruction. Plast Reconstr Surg. 2000;105(7):2374–2380. 10845289
75. Allen, R., Tucker, C., Jr., Superior gluteal artery perforator free flap for breast reconstruction. Plast Reconstr Surg. 1995;95(7):1207–1212. 7761507
76. Allen, R., Levine, J., Granzow, J., The in-the-crease inferior gluteal artery perforator flap for breast reconstruction. Plast Reconstr Surg. 2006;118(2):333–339. 16874198
77. LoTempio, M., Allen, R., Breast reconstruction with SGAP and IGAP flaps. Plast Reconstr Surg. 2010;126(2):393–401. 20679825
78. Arnez, Z., Pogorelec, D., Planinsek, F., et al, Breast reconstruction by the free transverse gracilis (TUG) flap. Br J Plast Surg. 2004;57(1):20–26. 14672674
79. Schoeller, T., Huemer, G., Wechselberger, G., The transverse musculocutaneous gracilis flap for breast reconstruction: guidelines for flap and patient selection. Plast Reconstr Surg. 2008;122(1):29–38. 18594364
80. Pülzl, P., Schoeller, T., Kleewein, K., et al, Donor-site morbidity of the transverse musculocutaneous gracilis flap in autologous breast reconstruction: short-term and long-term results. Plast Reconstr Surg. 2011;128(4):233e–242e. 21921734
81. Malyon, A., Husein, M., Weiler-Mithoff, E., How many procedures to make a breast? Br J Plast Surg 2001; 54:227–231. 11254415
82. Petit, J., Lohsiriwat, V., Clough, K., et al, The oncologic outcome and immediate surgical complications of lipofilling in breast cancer patients: a multicenter study – Milan–Paris–Lyon experience of 646 lipofilling procedures. Plast Reconstr Surg. 2011;128(2):341–346. 21502905
83. Sarfati, I., Ihrai, T., Kaufman, G., et al, Adipose-tissue grafting to the post-mastectomy irradiated chest wall: preparing the ground for implant reconstruction. J Plast Reconstr Aesthet Surg. 2011;64(9):1161–1166. 21514910
84. Delay, E., Garson, S., Tousson, G., et al, Fat injection to the breast: technique, results, and indications based on 880 procedures over 10 years. Aesthet Surg J. 2009;29(5):360–376. 19825464
85. Sauven, P., Guidelines for the management of women at increased familial risk of breast cancer. Eur J Cancer 2004; 40:653–665. 15010065
86. Wellisch, D., Schain, W., Noone, R., et al, The psychological contribution of nipple addition in breast reconstruction. Plast Reconstr Surg 1987; 80:699–704. 3671562
87. Henseler, H., Cheong, V., Weiler-Mithoff, E., et al, The use of Munsell colour charts in nipple areola tattooing. Br J Plast Surg 2001; 54:338–340. 11355995
88. Farhadi, J., Maksvytyte, G., Schaefer, D., et al, Reconstruction of the nipple–areola complex: an update. J Plast Reconstr Aesthet Surg. 2006;59(1):40–53. 16482789
89. Hathaway, C., Rand, R., Moe, R., et al, Salvage surgery for locally advanced and locally recurrent breast cancer. Arch Surg 1994; 129:582–587. 8204031
90. Munhoz, A., Montag, E., Arruda, E., et al, Immediate locally advanced breast cancer and chest wall reconstruction: surgical planning and reconstruction strategies with extended V-Y latissimus dorsi myocutaneous flap. Plast Reconstr Surg. 2011;127(6):2186–2197. 21617452
91. Damen, T., de Bekker-Grob, E., Mureau, M., et al, Patients’ preferences for breast reconstruction: a discrete choice experiment. J Plast Reconstr Aesthet Surg. 2011;64(1):75–83. 20570232