Chapter 9 Breast Implants and the Reconstructed Breast
Implant Types
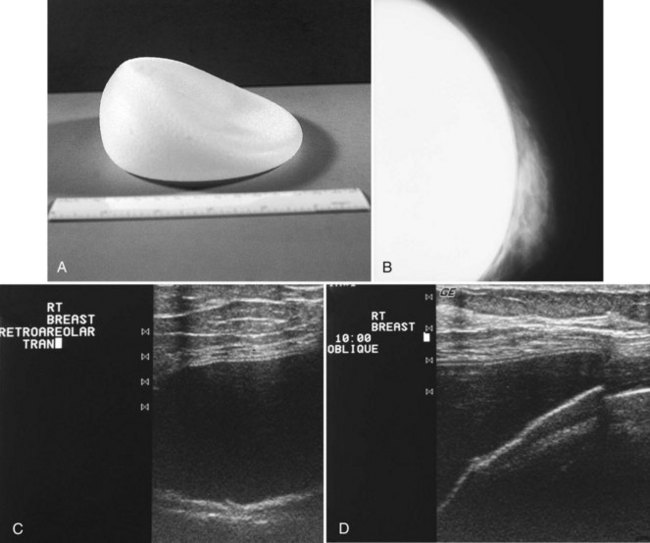
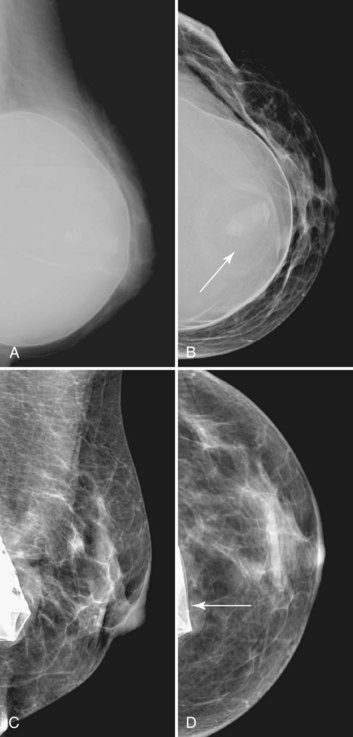
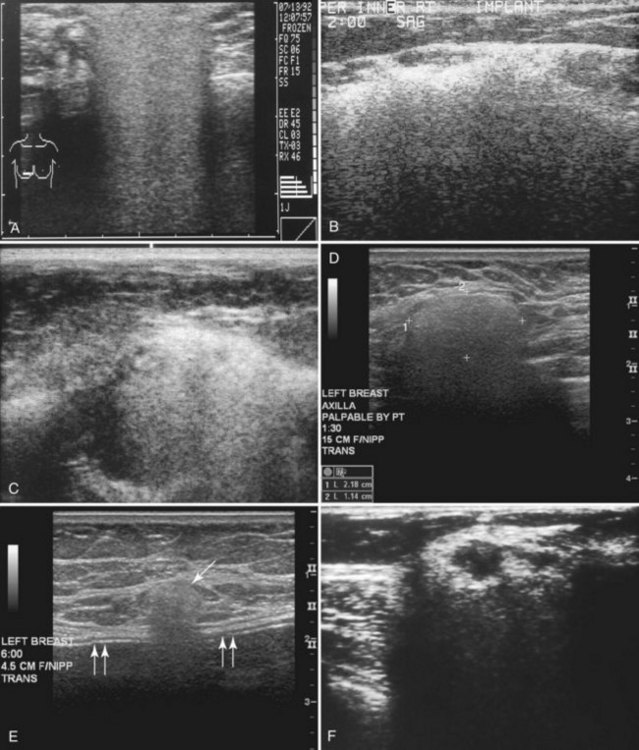
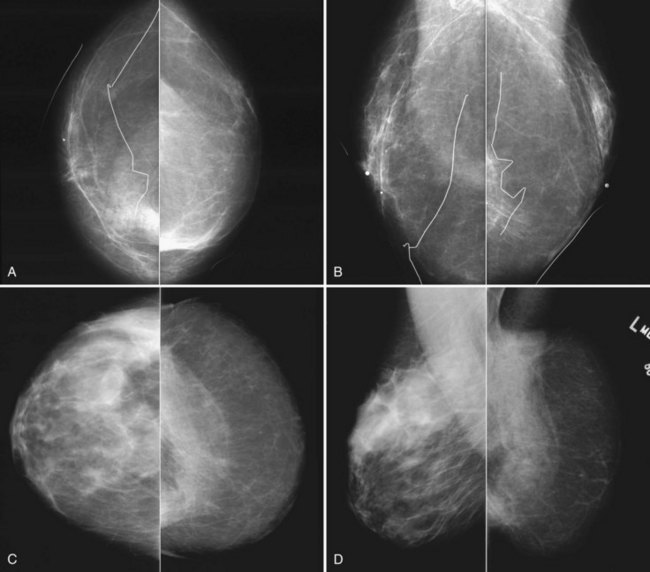
The most common breast implants are single-lumen and filled with either silicone or saline. Silicone implants are composed of a silicone elastomer shell filled with silicone made from a synthetic polymer of cross-linked chains of dimethylsiloxane that makes the implant soft and movable (Box 9-1 and Fig. 9-1). The outer envelope can be textured or smooth, polyurethane-coated or uncoated. The inner silicone can be a gel, a liquid, or a solid form. Saline implants are composed of an outer silicone shell and an inner envelope filled with saline (Fig. 9-2A to D). Of note, newer generation silicone implants have had a very small rupture rate in augmentation patients. Double- or triple-lumen implants have two or more envelopes inside one another, and each can contain saline or silicone gel. A common double-lumen implant is the saline outer, silicone inner implant. More recent common double-lumen implants have silicone outer and saline inner components. All implants are placed behind the breast tissue, and some implants are placed behind the pectoralis muscle.
Mammography of Normal Implants
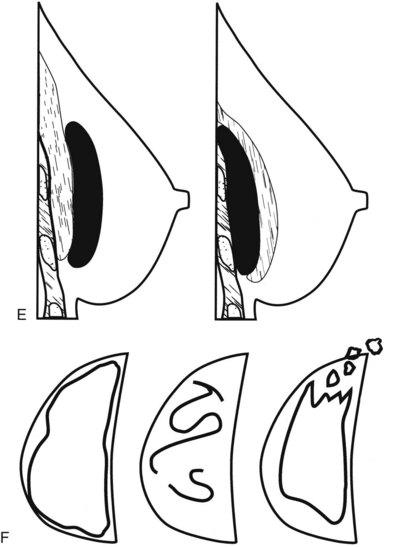
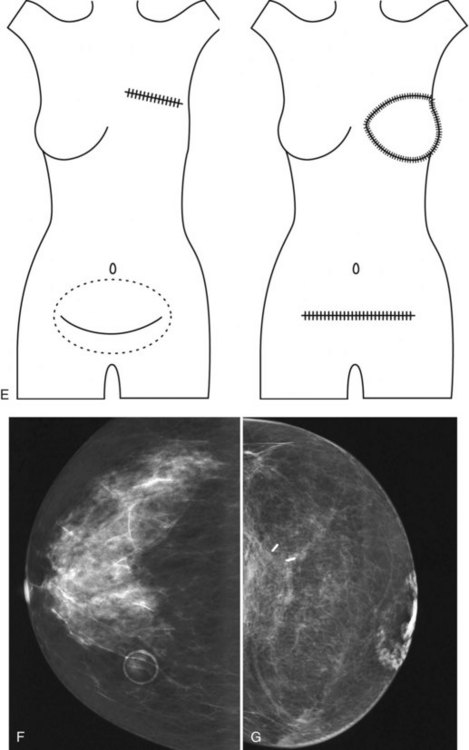
Surgeons place silicone gel-filled implants behind the breast tissue on the chest wall in the subglandular or subpectoral position (see Fig. 9-2E and F). In either case, the body generally forms a fibrous capsule around the implant. The fibrous capsule is usually soft, nonpalpable, and undetectable to physical examination, but with time, the capsule hardens or calcifies in some individuals. If a silicone implant ruptures, this fibrous capsule holds the silicone within as long as it, too, does not rupture.
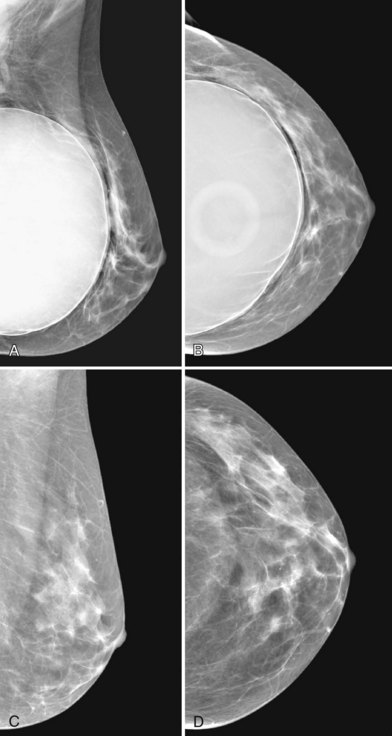
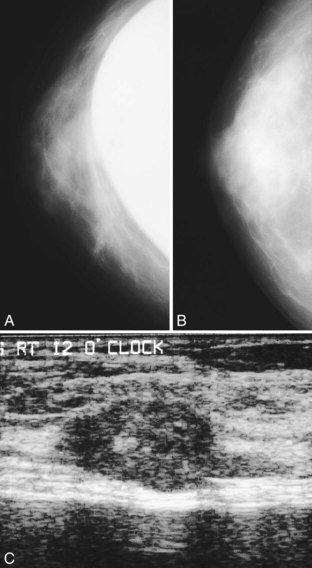
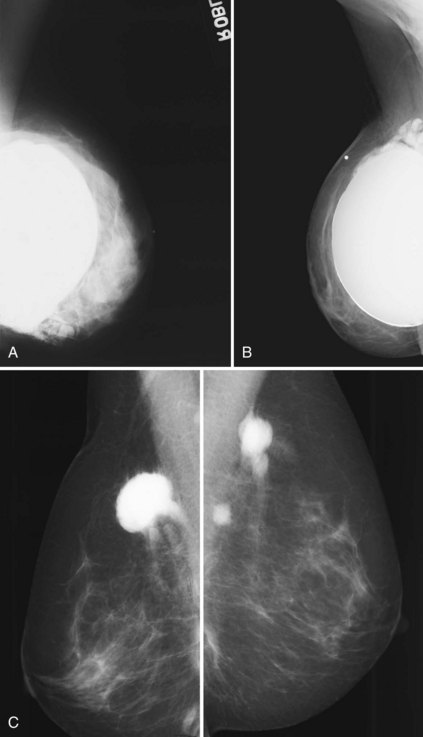
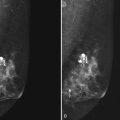
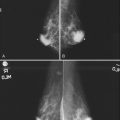
A normal silicone implant is quite dense and completely opaque, and obscures and displaces much of the surrounding breast tissue. On mammography the implant appears as a smooth white oval opacity near the chest wall (Fig. 9-3). The pectoralis muscle curves over the implant in subpectoral implants and lies underneath the implant in subglandular implants. A silicone gel-filled implant is not as compressible as breast tissue and can be ruptured if compressed too hard during mammography or closed capsulotomy. Because limited compression decreases visualization of the surrounding breast tissue for breast cancer screening, the Mammography Quality Standards Act (MQSA) recommends four views of each implanted breast. Two views are implant-displaced views, in which the technologist pinches the breast tissue in front of the implant to compress it, and two views include the implant but do not use much compression (Box 9-2). Breast tissue is evaluated on the implant-displaced views; implant integrity is evaluated on limited-compression mammograms in which the implant is surrounded by noncompressed breast tissue. Even with the implant-displaced views, the radiologist sees only about 80% of the breast tissue because it is hidden by the implant.
Box 9-2 Mammography of Implants
CC, craniocaudal; ML, mediolateral; MLO, mediolateral oblique.
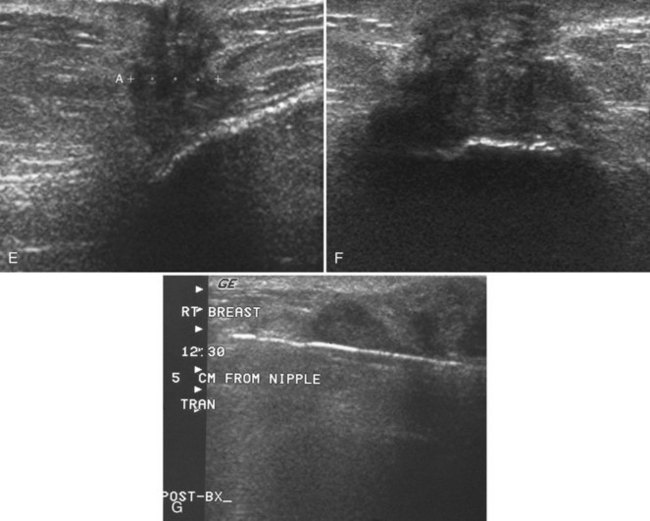
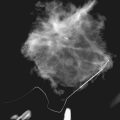
The implant-displaced technique does not completely resolve the problem of imaging small breast cancers, but it does optimize the amount of breast tissue displayed on the mammogram. Both physical examination and breast ultrasound as an adjunct to mammography are helpful in evaluating mammographically detected breast masses or palpable findings because mammography is limited with implants in place. Ultrasound can be especially helpful in determining whether a true mass exists, because ultrasound can evaluate the entire breadth of the breast tissue down to the implant. Ultrasound also distinguishes breast masses from the snowstorm appearance of silicone granulomas caused by ruptured implants (Fig. 9-4).
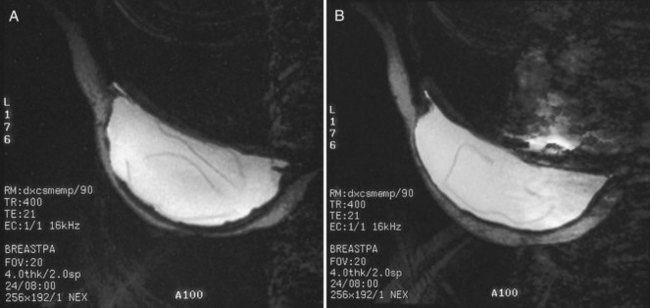
Unlike opaque silicone implants, saline implants contain radiolucent saline surrounded by a dense silicone outer envelope, in which small wrinkles may be seen. When a saline implant ruptures, the saline diffuses into the breast tissue and the envelope shrinks back against the chest wall (Fig. 9-5). In contradistinction, when a silicone implant ruptures, most of the silicone may be contained by the fibrous capsule and the implant retains much of its shape and volume. Saline outer, silicone inner double-lumen implants have an outer envelope containing saline surrounding a dense inner silicone implant filling.
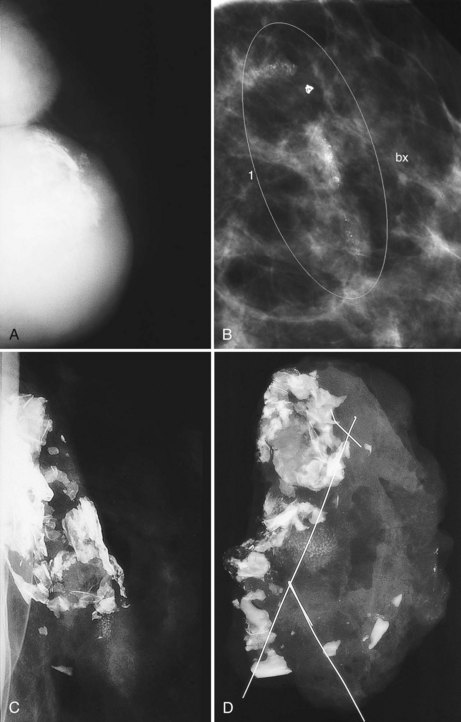
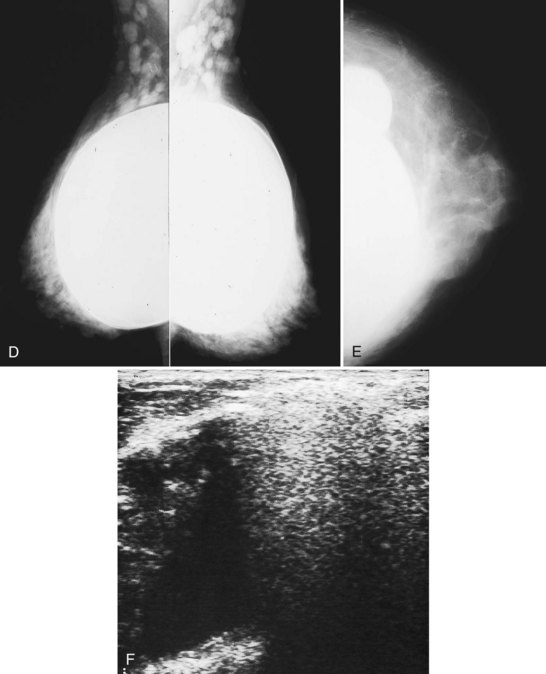
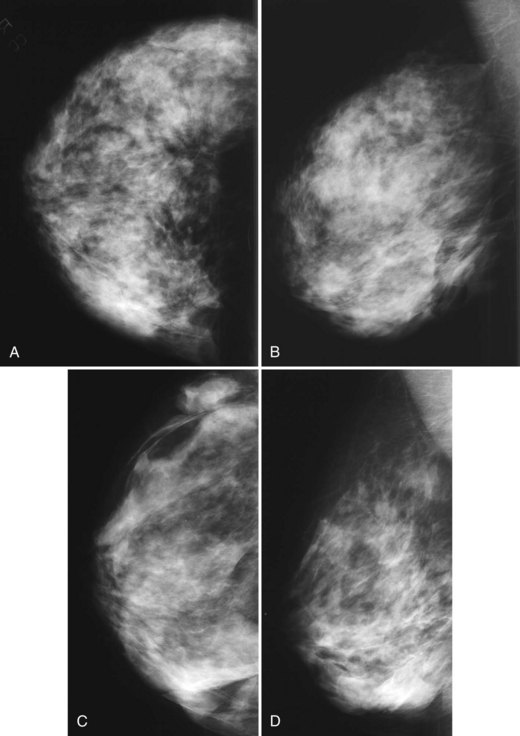
The fibrous capsule surrounding implants of any type is not usually visible unless it calcifies. A calcified fibrous capsule contains dystrophic sheetlike calcifications and appears bumpy on the nondisplaced-implant views (Fig. 9-6A). Implant-displaced views displace the capsular calcifications away from the implant for analysis if one is concerned that the calcifications are in breast parenchyma rather than in the implant capsule. Spot magnification mammograms also help the radiologist analyze intraparenchymal calcifications and distinguish them from capsular calcifications.
If an implant ruptures, the surgeon removes the ruptured implant but does not always remove the fibrous capsule. If the fibrous capsule has calcified, the mammogram shows dystrophic calcifications in a sheetlike curvilinear pattern because they reside in the retained fibrous capsule. These calcifications can be hard to distinguish from cancer and can prompt biopsy (see Fig. 9-6B). Another specific type of capsular calcification that can be mistaken for cancer and will sometimes prompt biopsy is from calcifying polyurethane-covered implants. These implants are covered with a spongelike material, and when they calcify produce a typical fine meshlike calcification that can mimic ductal carcinoma in situ (see Fig. 9-6C and D).
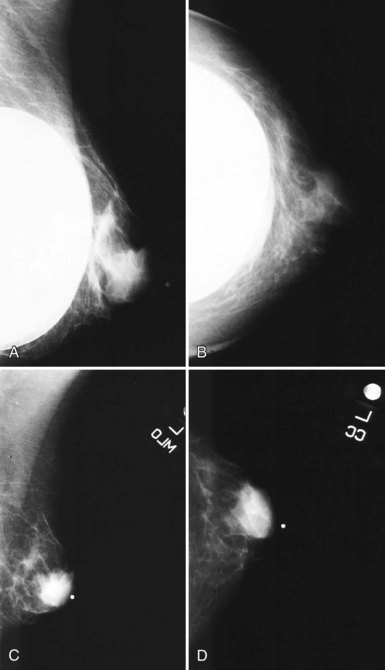
When evaluating breast tissue for cancer in women with implants, it is important to inspect both the implant-displaced views and the breast tissue adjacent to the implant on the nonimplant-displaced views. The standard views may display a mass near the implant or the fibrous capsule not evident on implant-displaced views. Masses on the fibrous capsule can be pushed away from the field of view with the implant-displaced views (Fig. 9-7). However, for masses or suspicious calcifications, spot compression or other fine-detail views can be used in women with implants, just as with any other woman. Needle localization, ultrasound-guided core biopsy, and stereotactic core biopsy all can be performed in women with implants as well. The radiologist just has to obtain informed consent from the woman, including implant rupture as a possible complication for percutaneous biopsies.
Implant Complications and Rupture
Untoward complications associated with silicone gel-filled breast implants include contracture of the fibrous capsule, calcification of the fibrous capsule, hematoma, infection, implant rupture, and the controversial silicone gel “bleed,” in which silicone gel leaks outside the implant through an intact envelope (Box 9-3). Capsular contracture is the most common complication, with a reported incidence of more than 70% in some older series and only about a 20% incidence in more recent series.
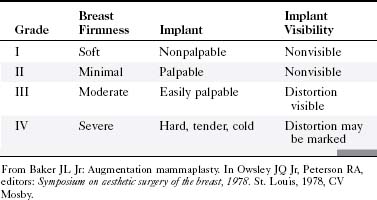
Implants can undergo capsular contraction, becoming hard and resistant, leading to a round, hard appearance and feel. The Baker classification of capsular formation on implants describes increasing levels of capsular contracture (Table 9-1). The incidence of capsular contracture may be diminished by the use of textured submuscular implants, although the use of such implants remains controversial. Open surgical capsulotomy, in which the hardened implant capsule is removed, was used to solve the problem of capsular contracture. Alternatively, surgeons would squeeze the implant to break the hardened fibrous capsule to allow the implant to become soft and pliable again, called closed capsulotomy. Unfortunately, closed capsulotomy could result in implant rupture.
Different types of fillers for implants were examined in trials, resulting in varying rates of fibrous capsule contracture. Munker and colleagues reported on Trilucent implants in 27 patients who elected to exchange their implants for a fourth-generation cohesive silicone implant. Of these 27 patients, 14 had a change in the volume of their implants but not all were aware of the change, and capsular contracture was not present (Baker grade II) (see Table 9-1); 55% of the implants had thickening or color changes caused by peroxidation of the triglyceride contents, and the implant capsule was adherent to breast tissues—in particular, the pectoralis muscle, which led to prolonged operative times. Rizkalla and colleagues reported similar results, with a reoperation rate of 20% (10/50) and an implant deflation rate of 10% (5/50). The Medical Devices Agency in the United Kingdom (which merged with the Medicines Control Agency in April 2003 to form the Medicines and Healthcare Products Regulatory Agency) withdrew the Trilucent implant from the market in March 1999, with a subsequent recommendation in June 2000 that the implants be removed from patients. A new type of alloplastic material for implants that contains carboxymethylcellulose, called Hydrogel, was introduced into the European market. Of 12 patients with 20 implants placed between 1996 and 1997, as reported by Cicchetti and colleagues, none showed immediate complications and had Baker grade I or II capsular contracture at 3.5 years of follow-up.
Implant Rupture
Implant integrity is classified as intact, intact with gel bleed, intracapsular rupture, or extracapsular rupture (Table 9-2). Extracapsular rupture is defined as implant rupture with silicone gel extruded outside a broken fibrous capsule. Intracapsular rupture is defined as implant rupture with silicone gel still contained within an intact fibrous capsule. Gel bleed is defined as silicone gel leakage through an intact implant envelope, although the existence of gel bleed versus small, undetected ruptures remains controversial.
| Rupture Types | Silicone Location | Envelope Status |
|---|---|---|
| Intracapsular rupture | Fibrous capsule contains silicone gel | Envelope ruptured |
| Extracapsular rupture | Silicone gel outside fibrous capsule | Envelope ruptured |
| Gel bleed (controversial) | Silicone outside envelope | Envelope intact |
Implant Imaging
Mammography
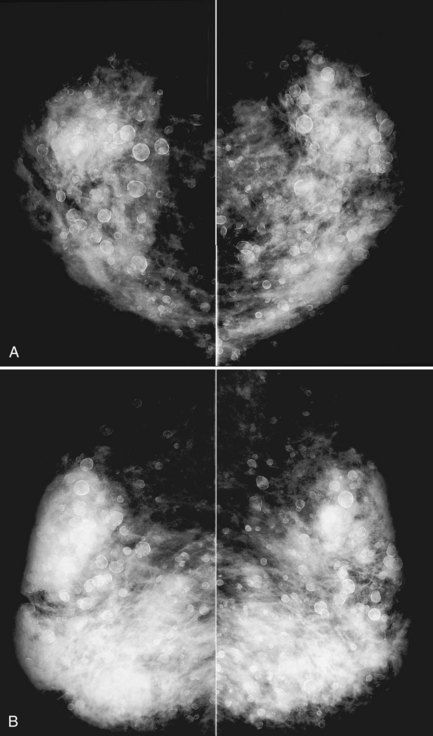
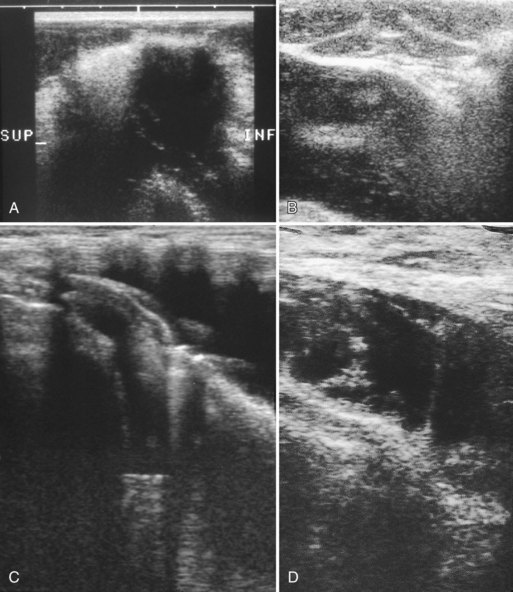
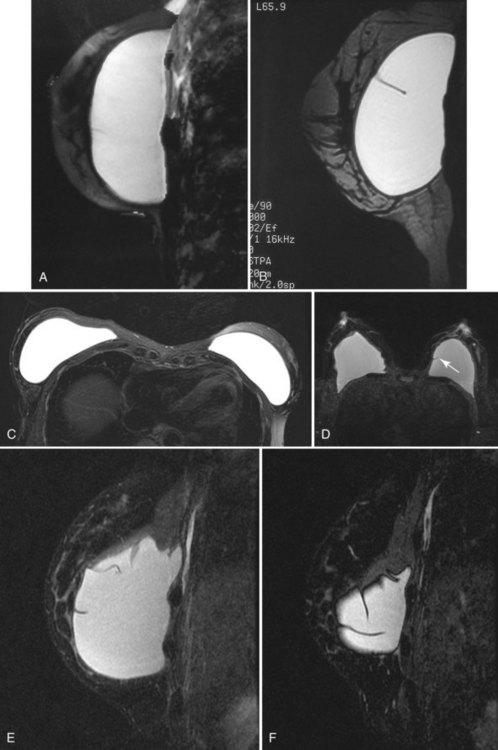
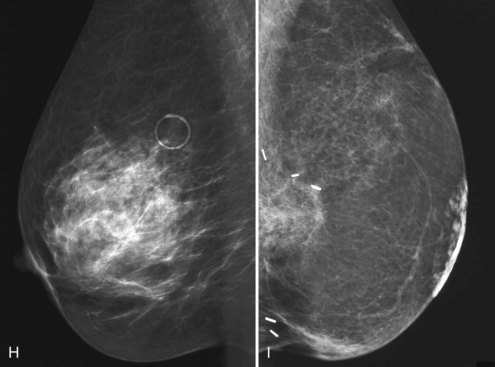
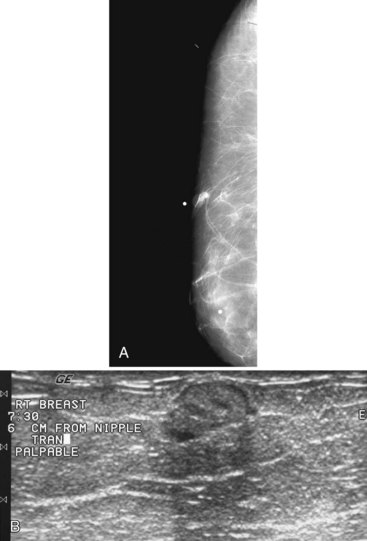
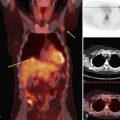
A retrospective review of screening mammograms in 350 asymptomatic women with breast implants showed an incidence of asymptomatic implant rupture of 5%. Mammography shows extracapsular rupture as silicone extravasation outside the implant envelope with blobs of silicone in the breast tissue (Fig. 9-8A and B), within implant ducts, or as a contour abnormality caused by extruded silicone in contiguity with the implant (Table 9-3). After extracapsular rupture, the surgeon removes the implant. Removal of all extravasated silicone is often impossible without removing much of the breast tissue, so the surgeon may leave some extravasated silicone in the breast. Later, when a new silicone implant is placed, residual silicone from the old ruptured implant makes it impossible to tell on mammography if the new implant has ruptured (see Fig. 9-8C). Silicone within axillary lymph nodes implies extravasation of silicone outside the fibrous capsule, because the silicone has traveled to the lymph nodes. This means that there must be an extracapsular rupture as well (see Fig. 9-8D).
Silicone implant contour lobulation indicates either capsular contracture, herniation of an intact implant envelope through a break in the surrounding fibrous capsule, or a contained implant leak (Table 9-4). Because intracapsular rupture is defined as implant rupture with silicone gel still contained within an intact fibrous capsule, mammography may show an intracapsular rupture as a normal-looking or bulging implant, depending on the shape of the fibrous capsule. Because both implant lobulation and a contained leak have the same mammographic appearance, radiologists use ultrasound and MRI to make the diagnosis of a rupture (see Fig. 9-8E and F). Mammography cannot identify intracapsular ruptures when the implant contour is normal, nor can it show posterior implant ruptures on the chest wall.
| Imaging Modality | Sign | Differential Diagnosis |
|---|---|---|
| Mammography | Implant contour deformity |
Direct silicone or paraffin injections were used overseas for breast augmentation; free silicone, paraffin, or other materials were injected directly into the breast tissue. The injections are foreign bodies and therefore result in multiple tiny round eggshell-type calcifications that obscure the underlying breast tissue. Because these silicone or injection granulomas may become quite hard, both physical examination and mammography of the underlying tissue are nearly impossible (Fig. 9-9). Ultrasound of patients with silicone injections shows multiple areas of snowstorm or echodense noise that cast shadows throughout the breast and obscure the breast tissue, thus rendering evaluation for breast cancer difficult.
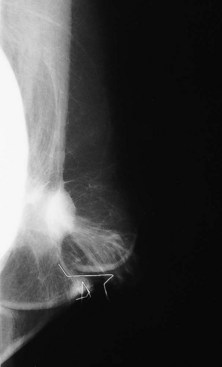
Some women have their implants removed and not replaced. When surgeons remove the implants, they often leave the fibrous capsules in the breasts. After implant removal the mammogram shows distortion from the implant cavity, usually located on the chest wall. The implant cavity may become unapparent on mammography, may scar, or may fill with fluid and look like a mass (Fig. 9-10).
Ultrasound
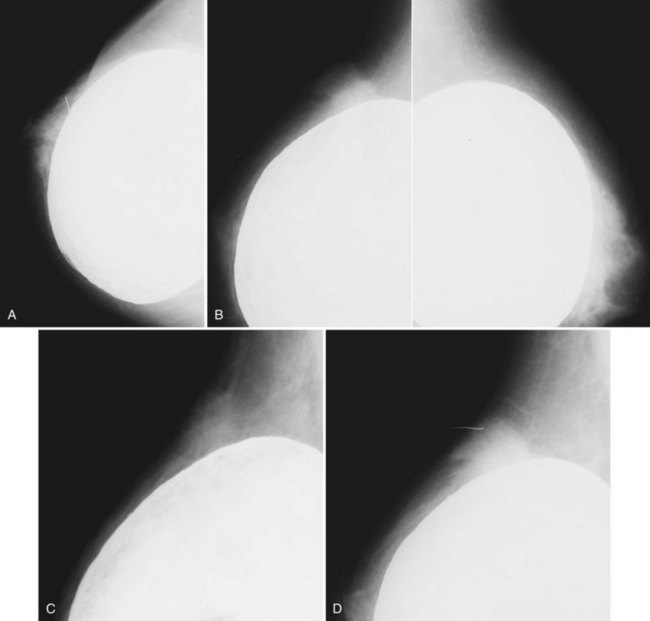
Ultrasound is an adjunct to mammography in the diagnosis of ruptured breast implants. The normal single-lumen implant has a smooth echogenic edge, and the inside of the implant is anechoic, similar to a cyst. The implant may have infoldings of the intact envelope, called radial folds, which look like white lines extending to the periphery of the envelope. Minor contour abnormalities and short radial folds are incidental findings. Normal reverberation artifacts on ultrasound appear as short gray echoes in the near-field of an intact implant. Reverberation artifacts are the same width as the breast tissue anterior to the implant and are easily distinguished from ruptures (Fig. 9-11).
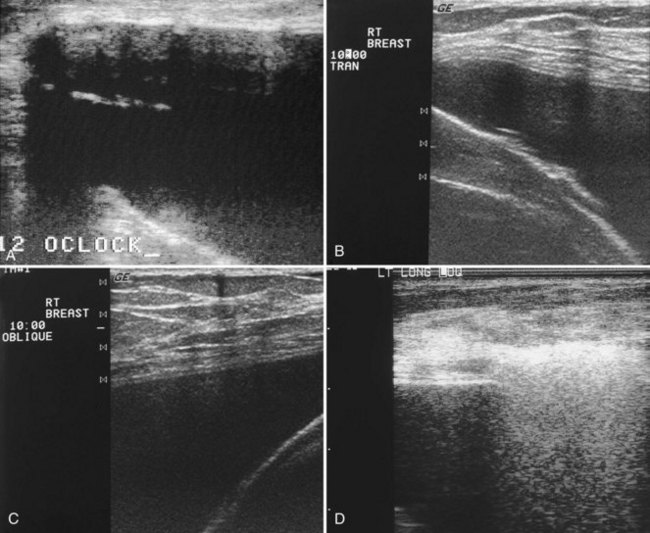
Ultrasound signs of rupture have varying sensitivities of 25% to 65% and specificities of 57% to 98%. Ultrasound is less expensive than MRI and is more cost-effective than MRI in diagnosing ruptures. On ultrasound, extracapsular rupture has the classic snowstorm sign or echodense noise, a characteristic echogenic finding caused by the slow velocity of sound in silicone with respect to the surrounding breast parenchyma. Snowstorm, or echodense noise, looks like air in the bowel, has an intense echogenic appearance, and obscures all findings beneath it (Fig. 9-12A to E). It can be distinguished from edge artifact by scanning at different angles, because snowstorm produces the echogenic snowstorm appearance when scanned from all angles whereas edge artifact changes or disappears.
Another sign of extracapsular rupture is a hypoechoic mass corresponding to large globules of silicone extruded away from the implant (see Fig. 9-12F). In this situation, so much silicone is extruded that the silicone glob appears as a hypoechoic mass, similar to the intact implant. To ensure the correct diagnosis, the sonographer places a skin marker on the hypoechoic mass and repeats the mammogram to correlate the silicone on the radiograph with the ultrasound finding. Silicone or paraffin injections have an appearance similar to extracapsular rupture and are characterized by echodense noise. Usually there is so much artifact from the snowstorm with silicone/paraffin injections that the ultrasound is nondiagnostic.
Intracapsular rupture means that the envelope is ruptured but the silicone is still inside an intact fibrous capsule. This means that there will be no snowstorm, unless silicone has leaked into the surrounding tissue (making it an extracapsular rupture). Intracapsular rupture produces stepladder sign, which represents the collapsing ruptured implant shell within the intact fibrous capsule. The stepladder sign is characterized by multiple thin echogenic lines within the implant that do not extend to the periphery of the implant. The thin lines represent echoes of the collapsing implant wall folding in on itself. The stepladder sign is seen with both intracapsular and extracapsular rupture (Fig. 9-13).
Magnetic Resonance Imaging
In the setting of ruptured implants, MRI distinguishes breast tissue from leaking silicone, contrasting fat and water in glandular tissue from silicone in the implant, and various signs of implant rupture (Fig. 9-14). MRI techniques that evaluate silicone breast implants include T1-weighted spin-echo imaging, gradient echo imaging, T2-weighted fast spin-echo (FSE) imaging, short tau inversion recovery (STIR) imaging, and the modeled three-point Dixon technique, which yield sensitivities and specificities of 95% to 98% and 50% to 93%, respectively (Box 9-4). The modeled three-point Dixon chemical shift technique has the distinct advantage of allowing selective imaging of silicone by separation of the signal of silicone from that of fat and water based on the chemical shift of silicone (1.3–1.6 parts per million lower than that of lipid). Inversion recovery fast spin-echo (IRFSE) sequences combine the speed of a T2-weighted FSE sequence with homogeneous fat suppression.
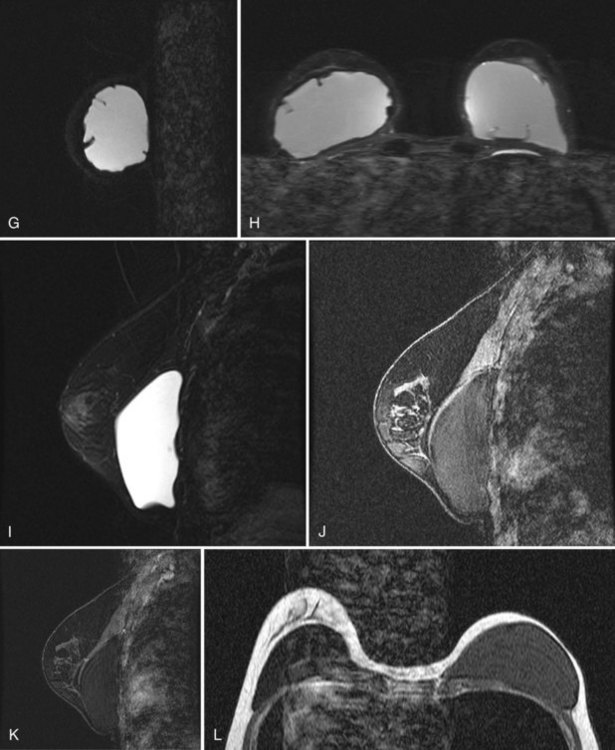
Patients are scanned prone in a dedicated breast coil to diminish breathing artifacts from chest wall movement. The implant is usually scanned in the axial and sagittal/oblique planes to look at all implant contours and to see radial folds versus ruptured envelopes. MRI of a normal single-lumen silicone implant shows high signal from the silicone with a smooth oval implant border. Minor implant bulges or herniations, short and long radial folds are noted as incidental findings. Radial folds are dark lines that extend to the periphery of the implant and represent folds in the implant envelope (Fig. 9-15). Reactive fluid around the implant and water droplets are classified as nonspecific findings but are noted in the report, particularly if the findings are marked or implant infection is suspected. MRI of a normal saline implant shows the intact implant envelope filled with water.
Intracapsular rupture is diagnosed by the linguine sign, which consists of dark lines inside the implant that do not extend to the periphery. The linguine is the curvy noodle-shaped dark lines of the collapsing ruptured implant shell contained within an intact fibrous capsule (Fig. 9-16). Another indication of intracapsular rupture is the teardrop or keyhole sign, which represents silicone outside the implant envelope within a short radial fold outside the implant envelope. Other signs of intracapsular rupture include an intracapsular mottled appearance of the silicone or a dark subcapsular line paralleling the implant shell that cannot be traced to the periphery of the implant. A subcapsular line represents incomplete shell collapse.
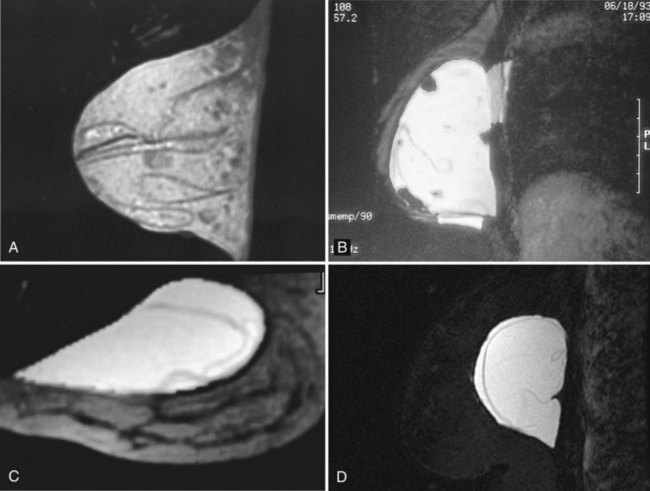
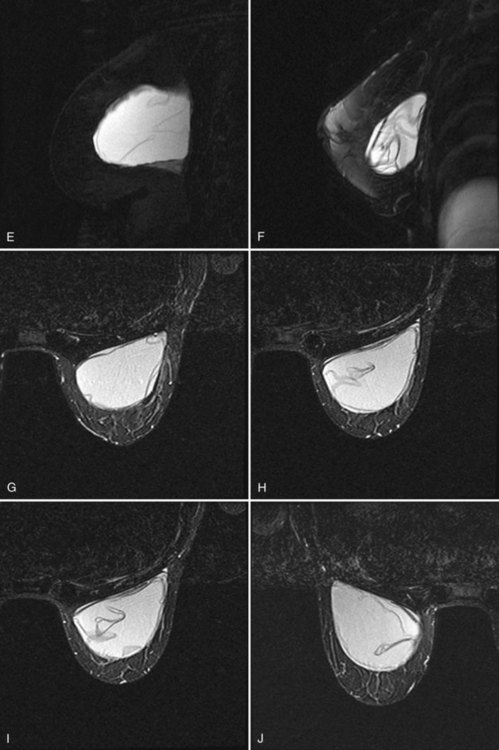
Figure 9-16 Intracapsular rupture findings on magnetic resonance imaging (MRI). A, Linguine sign on sagittal three-point Dixon MRI, with water droplets. B, Linguine sign, water droplets (round low signal findings in the implant), and keyhole sign on sagittal MRI. C, Linguine sign on axial MRI. D, Subcapsular line and keyhole sign on sagittal three-point Dixon MRI. E, Linguine sign, subcapsular line, and keyhole sign on sagittal MRI. F, Linguine sign on sagittal MRI. G, Axial MRI shows subcapsular lines from an intracapsular rupture. H and I, Two superior slices on axial MRI show subcapsular lines, linguine sign, and keyhole sign from intracapsular rupture. J, Intracapsular rupture on the contralateral side on axial MRI. Contrast the intracapsular rupture to the normal radial folds of the intact implant shown in Figure 9-15H.
Silicone outside the fibrous capsule, within the breast parenchyma or axilla, represents extracapsular rupture (Fig. 9-17). Signs of intracapsular rupture will always be present with the finding of extracapsular rupture. Severe gel bleed is diagnosed if a thin coating of silicone is identified around the periphery of the implant but the implant is intact.
In another series of 30 patients with 59 implants, MRI had a sensitivity of 100%, a specificity of 63%, a positive predictive value of 71%, a negative predictive value of 100%, and an accuracy of 81% in the detection of rupture/bleed. The linguine sign was the most sensitive (93%) and specific (65%) finding for rupture. The nonspecific sign of water droplets within the implant had a sensitivity of 92% and a low specificity of 44% (Box 9-5). Linear extension of silicone along the chest wall and the presence of reactive fluid were neither sensitive nor specific for rupture. Nonspecific signs such as contour deformity (77%, 10/13), water droplets (54%, 7/13), and reactive fluid (23%, 3/13) were common. In this series, MRI was shown to be more sensitive and accurate than mammography and ultrasound in detecting breast implant rupture or bleed.
The modest MRI specificity noted in most series was predominantly due to pitfalls in imaging interpretation—namely, misinterpreting contour abnormalities and overinterpreting findings within the implant. Knowing the implant type before imaging is crucial for accurate interpretation (Fig. 9-18) because one can overdiagnose ruptures if complex multilumen implants, stacked implants, or a history of previous ruptures is present (see Table 9-4).
Finally, patients should understand that breast MRI for the diagnosis of implant rupture is not the same as for the diagnosis of breast cancer. Specifically, implant MRI examinations do not use intravenous contrast, which is essential for the diagnosis of breast cancers (Box 9-6).
Breast Reconstruction
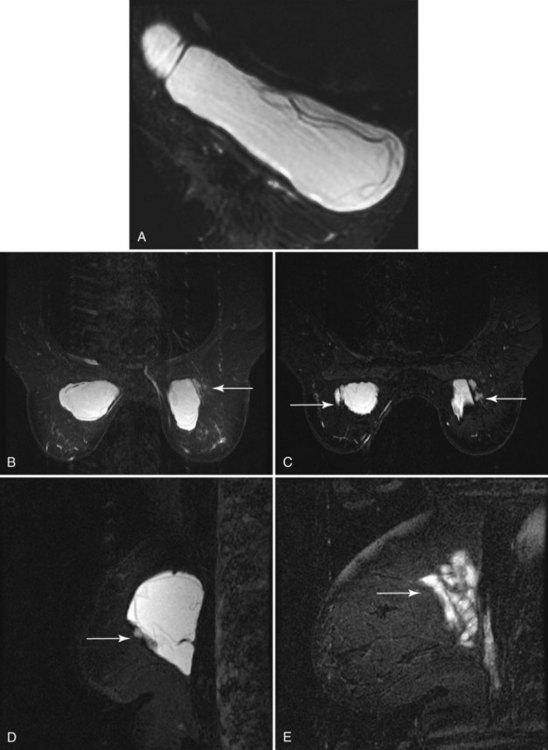
In the case of TRAM, latissimus dorsi, and free flap reconstructions, fat and muscle are transferred to the mastectomy site with attachments to vascular structures and shaped to form a breast. Traditionally, autologous flaps are not imaged by mammography, but mammography can be helpful in evaluating these structures when there are clinical questions. The most common findings in autologous flaps are fat centrally, with or without density from muscle fibers around the edges of the TRAM or latissimus dorsi flaps (Fig. 9-19A to E). Common mammographic findings in TRAM flaps are calcifications from fat necrosis, benign dermal calcifications, calcified hematoma, and clustered microcalcifications. Areas of increased or decreased density without calcifications are also common and appear to be related to postsurgical changes and fat necrosis. A nipple can be reconstructed out of skin and tattooed to provide color similar to the contralateral side. Rarely, the tattoo can be seen on mammography (see Fig. 9-19F to I).
Reduction Mammoplasty
The reduction mammoplasty mammogram shows characteristic skin thickening over the lower breast in the region of the scars, most evident on the mediolateral oblique or mediolateral view. The breast ducts terminate lower than the replaced nipple because the nipple has been moved to a higher location. The lower portion of the breast shows architectural distortion, and the overall pattern of the lower portion of the breast will be distorted from scarring. Depending on the amount of tissue removed from various areas of the breast, the breast parenchymal pattern can be patchy and much different from the prereduction mammogram (Figs. 9-20 and 9-21).
Reduction mammoplasty or any breast surgery can result in focal fat necrosis or oil cysts that have a characteristic appearance, or they may be atypical and form a palpable mass (Fig. 9-22). Epidermal inclusion cysts can also form in biopsy scars and produce a dense smooth round or oval mass near the skin surface but not connected to it. These masses represent epidermal cells that are displaced into breast tissue during biopsy. The epithelial cells can grow and form a round benign-appearing mass. In the case of fat necrosis, breast ultrasound may be helpful, but biopsy may be needed.
Key Elements
No scientific evidence has shown a definite association between silicone breast implants and connective tissue disorders or breast cancer.
In the United States, silicone breast implants are approved for breast reconstruction after mastectomy, and saline implants are approved for breast augmentation.
Breast implants may have single or multiple lumens, each containing silicone or other materials in the different shells.
Implants are placed in subpectoral or subglandular (above the pectoral muscle) locations.
Fibrous capsules form around all implants, sometimes becoming hard or calcified and impairing the implant’s look and feel.
Implant complications include rupture, infection, hematoma, and capsular contraction.
Closed capsulotomy, or manual breaking of a hardened fibrous capsule, can result in implant rupture.
Implant rupture is classified as intracapsular (silicone contained in the fibrous capsule) or extracapsular (silicone outside the fibrous capsule).
Symptoms associated with silicone implant rupture are nonspecific and include axillary, breast, or chest wall masses; pain; and changes in breast size, shape, or texture.
Mammography includes standard and implant-displaced craniocaudal and mediolateral or mediolateral oblique views of each breast, for a total of four views of each breast.
Approximately 5% of asymptomatic women have implant ruptures detected on screening mammography.
Extracapsular ruptures appear on mammography as silicone outside the implant in breast tissue, lymph nodes, or ducts or as a deformity in implant contour.
Direct silicone or paraffin injections are used outside the United States for augmentation and cause eggshell-type calcifications or dense masses on mammography, snowstorm or echodense noise on ultrasound, and hard palpable silicone granuloma masses on physical examination.
Ultrasound of extracapsular rupture shows the snowstorm sign, or echodense noise.
Ultrasound of intracapsular rupture shows the stepladder sign.
MRI of extracapsular rupture shows blobs of silicone outside the implant and signs of intracapsular rupture.
MRI of intracapsular rupture shows the linguine sign, subcapsular lines, teardrops, or the keyhole sign.
Nonspecific findings on MRI are water droplets, reactive fluid, and implant contour abnormalities.
False-positive findings of rupture on ultrasound and MRI are due to intact multiple-lumen implants simulating the stepladder and linguine signs.
False-positive findings of rupture on all imaging methods include previous rupture with implant replacement but without removal of all intraparenchymal silicone.
To avoid false-positive diagnoses of rupture, know the implant type and whether previous rupture and removal of the implant have occurred.
Autologous tissue reconstructions consist of transverse rectus abdominis or latissimus dorsi myocutaneous flaps performed as a pedicle or a free flap.
Mammographic findings of autologous tissue reconstruction include fat and muscle and, commonly, calcifications from fat necrosis and densities from postsurgical changes.
Cancer in reconstructed breasts is often detected by physical examination, with occasional mammographic findings of suspicious masses or calcifications.
Reduction mammoplasty produces a characteristic distortion of the lower portion of the breast and scarring, with relocation of the nipple higher on the breast.
Ahn CY, DeBruhl ND, Gorczyca DP, et al. Comparative silicone breast implant evaluation using mammography, sonography, and magnetic resonance imaging: experience with 59 implants. Plast Reconstr Surg. 1994;94:620-627.
Ahn CY, Shaw WW, Narayanan K, et al. Residual silicone detection using MRI following previous breast implant removal: case reports. Aesthetic Plast Surg. 1995;19:361-367.
Baker JLJr. Augmentation mammaplasty. In: Owsley JQJr, Peterson RA, editors. Symposium on Aesthetic Surgery of the Breast, 1978. St Louis: CV Mosby, 1978.
Beahm EK, Walton RL. Discussion. Patient satisfaction with mastectomy breast reconstruction: a comparative evaluation of DIEP, TRAM, latissimus flap, and implant techniques. Plast Reconstr Surg. 2010;125(6):1596-1598.
Beekman WH, Hage JJ, Taets van Amerongen AH, Mulder JW. Accuracy of ultrasonography and magnetic resonance imaging in detecting failure of breast implants filled with silicone gel. Scand J Plast Reconstr Surg Hand Surg. 1999;33:415-418.
Berg WA, Caskey CI, Hamper UM, et al. Diagnosing breast implant rupture with MR imaging, US, and mammography. Radiographics. 1993;13:1323-1336.
Berg WA, Caskey CI, Hamper UM, et al. Single- and double-lumen silicone breast implant integrity: prospective evaluation of MR and US criteria. Radiology. 1995;197:45-52.
Breiting VB, Holmich LR, Brandt B, et al. Long-term health status of Danish women with silicone breast implants. Plast Reconstr Surg. 2004;114:217-228.
Brown SL, Middleton MS, Berg WA, et al. Prevalence of rupture of silicone gel breast implants revealed on MR imaging in a population of women in Birmingham, Alabama. AJR Am J Roentgenol. 2000;175:1057-1064.
Carlson GW, Moore B, Thornton JF, et al. Breast cancer after augmentation mammaplasty: treatment by skin-sparing mastectomy and immediate reconstruction. Plast Reconstr Surg. 2001;107:687-692.
Cher DJ, Conwell JA, Mandel JS. MRI for detecting silicone breast implant rupture: meta-analysis and implications. Ann Plast Surg. 2001;47:367-380.
Chung KC, Wilkins EG, Beil RJJr, et al. Diagnosis of silicone gel breast implant rupture by ultrasonography. Plast Reconstr Surg. 1996;97:104-109.
Cicchetti S, Leone MS, Franchelli S, Santi PL. [Evaluation of the tolerability of Hydrogel breast implants: A pilot study,]. Minerva Chir. 2002;57:53-57.
Collis N, Coleman D, Foo IT, Sharpe DT. Ten-year review of a prospective randomized controlled trial of textured versus smooth subglandular silicone gel breast implants. Plast Reconstr Surg. 2000;106:786-791.
Collis N, Sharpe DT. Silicone gel-filled breast implant integrity: a retrospective review of 478 consecutively explanted implants. Plast Reconstr Surg. 2000;105:1979-1989.
Collis N, Litherland J, Enion D, Sharpe DT. Magnetic resonance imaging and explantation investigation of long-term silicone gel implant integrity. Plast Reconstr Surg. 2007;120:1401-1406.
Cunningham B. The Mentor Core Study on silicone MemoryGel breast implants. Plast Reconstr Surg. 2007;120(7 Suppl 1):19S-32S.
Cunningham B. The Mentor Study on contour profile gel silicone MemoryGel breast implants. Plast Reconstr Surg. 2007;120(7 Suppl 1):33S-39S.
Destouet JM, Monsees BS, Oser RF, et al. Screening mammography in 350 women with breast implants: prevalence and findings of implant complications. AJR Am J Roentgenol. 1992;159:973-981.
Di Benedetto G, Cecchini S, Grassetti L, et al. Comparative study of breast implant rupture using mammography, sonography, and magnetic resonance imaging: correlation with surgical findings. Breast J. 2008;14:532-537.
Eidelman Y, Liebling RW, Buchbinder S, et al. Mammography in the evaluation of masses in breasts reconstructed with TRAM flaps. Ann Plast Surg. 1998;41:229-233.
Eklund GW, Busby RC, Miller SH, Job JS. Improved imaging of the augmented breast. AJR Am J Roentgenol. 1988;151:469-473.
Fajardo LL, Roberts CC, Hunt KR. Mammographic surveillance of breast cancer patients: should the mastectomy site be imaged? AJR Am J Roentgenol. 1993;161:953-955.
Fajardo LL, Harvey JA, McAleese KA, et al. Breast cancer diagnosis in women with subglandular silicone gel-filled augmentation implants. Radiology. 1995;194:859-862.
Feng LJ, Amini SB. Analysis of risk factors associated with rupture of silicone gel breast implants. Plast Reconstr Surg. 1999;104:955-963.
Ganott MA, Harris KM, Ilkhanipour ZS, Costa-Greco MA. Augmentation mammoplasty: normal and abnormal findings with mammography and US. Radiographics. 1992;12:281-295.
Gorczyca DP. MR imaging of breast implants. Magn Reson Imaging Clin North Am. 1994;2:659-672.
Gorczyca DP, Schneider E, DeBruhl ND, et al. Silicone breast implant rupture: comparison between three-point Dixon and fast spin-echo MR imaging. AJR Am J Roentgenol. 1994;162:305-310.
Gui GP, Kadayaprath G, Tan SM, et al. Long-term quality-of-life assessment following one-stage immediate breast reconstruction using biodimensional expander implants: the patient’s perspective. Plast Reconstr Surg. 2008;121:17-24.
Harris KM, Ganott MA, Shestak KC, et al. Silicone implant rupture: detection with US. Radiology. 1993;187:761-768.
Hayes MK, Gold RH, Bassett LW. Mammographic findings after the removal of breast implants. AJR Am J Roentgenol. 1993;160:487-490.
Heden P, Nava MB, van Tetering JP, et al. Prevalence of rupture in inamed silicone breast implants. Plast Reconstr Surg. 2006;118:303-312.
Heden P, Bone B, Murphy DK, et al. Style 410 cohesive silicone breast implants: safety and effectiveness at 5 to 9 years after implantation. Plast Reconstr Surg. 2006;118:1281-1287.
Heden P, Bronz G, Elberg JJ, et al. Long-term safety and effectiveness of style 410 highly cohesive silicone breast implants. Aesthetic Plast Surg. 2009;33:430-436.
Helvie MA, Bailey JE, Roubidoux MA, et al. Mammographic screening of TRAM flap breast reconstructions for detection of nonpalpable recurrent cancer. Radiology. 2002;224:211-216.
Helvie MA, Wilson TE, Roubidoux MA, et al. Mammographic appearance of recurrent breast carcinoma in six patients with TRAM flap breast reconstructions. Radiology. 1998;209:711-715.
Henriksen TF, Holmich LR, Fryzek JP, et al. Incidence and severity of short-term complications after breast augmentation: results from a nationwide breast implant registry. Ann Plast Surg. 2003;51:531-539.
Herborn CU, Marincek B, Erfmann D, et al. Breast augmentation and reconstructive surgery: MR imaging of implant rupture and malignancy. Eur Radiol. 2002;12:2198-2206.
Hogge JP, Zuurbier RA, de Paredes ES. Mammography of autologous myocutaneous flaps. Radiographics. 1999;19(Spec No):S63-S72.
Holmich LR, Friis S, Fryzek JP, Vejborg IM, et al. Incidence of silicone breast implant rupture. Arch Surg. 2003;138:801-806.
Holmich LR, Fryzek JP, Kjoller K, et al. The diagnosis of silicone breast-implant rupture: clinical findings compared with findings at magnetic resonance imaging. Ann Plast Surg. 2005;54:583-589.
Holmich LR, Vejborg I, Conrad C, et al. The diagnosis of breast implant rupture: MRI findings compared with findings at explantation. Eur J Radiol. 2005;53:213-225.
Hsu W, Sheen-Chen SM, Eng HL, Ko SF. Mammographic microcalcification in an autogenously reconstructed breast simulating recurrent carcinoma. Tumori. 2008;94:574-576.
Hulka BS, Kerkvliet NL, Tugwell P. Experience of a scientific panel formed to advise the federal judiciary on silicone breast implants. N Engl J Med. 2000;342:812-815.
Ikeda DM, Borofsky HB, Herfkens RJ, et al. Silicone breast implant rupture: pitfalls of magnetic resonance imaging and relative efficacies of magnetic resonance, mammography, and ultrasound. Plast Reconstr Surg. 1999;104:2054-2062.
Janowsky EC, Kupper LL, Hulka BS. Meta-analyses of the relation between silicone breast implants and the risk of connective-tissue diseases. N Engl J Med. 2000;342:781-790.
Kang BJ, Jung JI, Park C, et al. Breast MRI findings after modified radical mastectomy and transverse rectus abdominis myocutaneous flap in patients with breast cancer. J Magn Reson Imaging. 2005;21:784-791.
Kessler DA. The basis of the FDA’s decision on breast implants. N Engl J Med. 1992;326:1713-1715.
Kirkpatrick WN, Jones BM. The history of Trilucent implants, and a chemical analysis of the triglyceride filler in 51 consecutively removed Trilucent breast prostheses. Br J Plast Surg. 2002;55:479-489.
Kreymerman P, Patrick RJ, Rim A, et al. Guidelines for using breast magnetic resonance imaging to evaluate implant integrity. Ann Plast Surg. 2009;62:355-357.
Kwek JW, Choi H, Ma J, Miller MJ. Gel-gel double-lumen silicone breast implant: mimic of intracapsular implant rupture. AJR Am J Roentgenol. 2006;187:W436-W437.
Lee JM, Georgian-Smith D, Gazelle GS, et al. Detecting nonpalpable recurrent breast cancer: the role of routine mammographic screening of transverse rectus abdominis myocutaneous flap reconstructions. Radiology. 2008;248:398-405.
Leibman AJ, Kossoff MB, Kruse BD. Intraductal extension of silicone from a ruptured breast implant. Plast Reconstr Surg. 1992;89:546-547.
Marotta JS, Widenhouse CW, Habal MB, Goldberg EP. Silicone gel breast implant failure and frequency of additional surgeries: analysis of 35 studies reporting examination of more than 8,000 explants. J Biomed Mater Res. 1999;48:354-364.
Mason AC, White CS, McAvoy MA, Goldberg N. MR imaging of slipped stacked breast implants: a potential pitfall in the diagnosis of intracapsular rupture. Magn Reson Imaging. 1995;13:339-342.
McKeown DJ, Hogg FJ, Brown IM, et al. The timing of autologous latissimus dorsi breast reconstruction and effect of radiotherapy on outcome. J Plast Reconstr Aesthet Surg. 2009;62:488-493.
McLaughlin JK, Lipworth L, Murphy DK, Walker PS. The safety of silicone gel-filled breast implants: a review of the epidemiologic evidence. Ann Plast Surg. 2007;59:569-580.
Middleton MS. Magnetic resonance evaluation of breast implants and soft-tissue silicone. Top Magn Reson Imaging. 1998;9:92-137.
Mitnick JS, Vazquez MF, Plesser K, Colen SR. “Ductogram” associated with extravasation of silicone from a breast implant. AJR Am J Roentgenol. 1992;159:1126-1127.
Mitnick JS, Vazquez MF, Plesser K, et al. Fine needle aspiration biopsy in patients with augmentation prostheses and a palpable mass. Ann Plast Surg. 1993;31:241-244.
Monticciolo DL, Nelson RC, Dixon WT, et al. MR detection of leakage from silicone breast implants: value of a silicone-selective pulse sequence. AJR Am J Roentgenol. 1994;163:51-56.
Monticciolo DL, Ross D, Bostwick J3rd, et al. Autologous breast reconstruction with endoscopic latissimus dorsi musculosubcutaneous flaps in patients choosing breast-conserving therapy: mammographic appearance. AJR Am J Roentgenol. 1996;167:385-389.
Mund DF, Wolfson P, Gorczyca DP, et al. Mammographically detected recurrent nonpalpable carcinoma developing in a transverse rectus abdominis myocutaneous flap. A case report. Cancer. 1994;74:2804-2807.
Munker R, Zorner C, McKiernan D, Opitz J. Prospective study of clinical findings and changes in 56 Trilucent implant explantations. Aesthetic Plast Surg. 2001;25:421-426.
Muzaffar AR, Rohrich RJ. The silicone gel-filled breast implant controversy: an update. Plast Reconstr Surg. 2002;109:742-748.
Palmon LU, Foshager MC, Parantainen H, et al. Ruptured or intact: what can linear echoes within silicone breast implants tell us? AJR Am J Roentgenol. 1997;168:1595-1598.
Patani N, Devalia H, Anderson A, Mokbel K. Oncological safety and patient satisfaction with skin-sparing mastectomy and immediate breast reconstruction. Surg Oncol. 2008;17:97-105.
Piza-Katzer H, Pulzl P, Balogh B, Wechselberger G. Long-term results of MISTI gold breast implants: a retrospective study. Plast Reconstr Surg. 2002;110:1425-1465.
Rieber A, Schramm K, Helms G, et al. Breast-conserving surgery and autogenous tissue reconstruction in patients with breast cancer: efficacy of MRI of the breast in the detection of recurrent disease. Eur Radiol. 2003;13:780-787.
Rivero MA, Schwartz DS, Mies C. Silicone lymphadenopathy involving intramammary lymph nodes: a new complication of silicone mammaplasty. AJR Am J Roentgenol. 1994;162:1089-1090.
Rizkalla M, Duncan C, Matthews RN. Trilucent breast implants: a 3-year series. Br J Plast Surg. 2001;54:125-127.
Rizkalla M, Webb J, Chuo CB, Matthews RN. Experience of explanation of Trilucent breast implants. Br J Plast Surg. 2002;55:117-119.
Rosculet KA, Ikeda DM, Forrest ME, et al. Ruptured gel-filled silicone breast implants: sonographic findings in 19 cases. AJR Am J Roentgenol. 1992;159:711-716.
Saint-Cyr M, Nagarkar P, Schaverien M, et al. The pedicled descending branch muscle-sparing latissimus dorsi flap for breast reconstruction. Plast Reconstr Surg. 2009;123:13-24.
Shaikh N, LaTrenta G, Swistel A, Osborne FM. Detection of recurrent breast cancer after TRAM flap reconstruction. Ann Plast Surg. 2001;47:602-607.
Silverstein MJ, Handel N, Gamagami P, et al. Mammographic measurements before and after augmentation mammaplasty. Plast Reconstr Surg. 1990;86:1126-1130.
Silverstein MJ, Handel N, Gamagami P. The effect of silicone-gel-filled implants on mammography. Cancer. 1991;68(Suppl):1159-1163.
Soo MS, Kornguth PJ, Walsh R, et al. Intracapsular implant rupture: MR findings of incomplete shell collapse. J Magn Reson Imaging. 1997;7:724-730.
Spear SL, Mardini S. Alternative filler materials and new implant designs: what’s available and what’s on the horizon? Clin Plast Surg. 2001;28:435-443.
Stevens WG, Pacella SJ, Gear AJ, et al. Clinical experience with a fourth-generation textured silicone gel breast implant: a review of 1012 Mentor MemoryGel breast implants. Aesthet Surg J. 2008;28(6):642-647.
Stewart NR, Monsees BS, Destouet JM, Rudloff MA. Mammographic appearance following implant removal. Radiology. 1992;185:83-85.
Stralman K, Mollerup CL, Kristoffersen US, Elberg JJ. Long-term outcome after mastectomy with immediate breast reconstruction. Acta Oncol. 2008;47:704-708.
Tugewell P, Wells G, Peterson J, et al. Do silicone breast implants cause rheumatologic disorders? A systematic review for a court-appointed national science panel. Arthritis Rheum. 2001;44:2477-2484.
Wong JS, Ho AY, Kaelin CM, et al. Incidence of major corrective surgery after post-mastectomy breast reconstruction and radiation therapy. Breast J. 2008;14:49-54.
Young VL, Bartell T, Destouet JM, et al. Calcification of breast implant capsule. South Med J. 1989;82:1171-1173.
Young V, Watson M. Breast implant research: where we have been, where we are, where we need to go. Clin Plast Surg. 2001;28:451-483.
9-1. Fill in the implant types.
_________________________________________________
_________________________________________________
_________________________________________________
_________________________________________________
_________________________________________________
_________________________________________________
9-2. Fill in the mammography of implants.
9-3. Fill in the untoward effects of breast implants.
_________________________________________________
_________________________________________________
_________________________________________________
_________________________________________________
9-4. Fill in the MRI techniques for implants.
_________________________________________________
_________________________________________________
_________________________________________________
9-5. Fill in the nonspecific findings on MRI.
_________________________________________________
_________________________________________________
9-6. Fill in the reducing false-positive MRI studies.
9-7. Fill in the Baker classification.
| RUPTURE TYPE | SILICONE LOCATION | ENVELOPE STATUS |
|---|---|---|
| _______________________________________ | _______________________________________ | _______________________________________ |
| _______________________________________ | _______________________________________ | _______________________________________ |
| _______________________________________ | _______________________________________ | _______________________________________ |
9-9. Fill in the image findings with rupture.
9-10. Fill in the false-positive imaging findings for rupture.
| IMAGING MODALITY | SIGN | DIFFERENTIAL DIAGNOSIS |
|---|---|---|
| Mammography | _____________________________________ | _____________________________________ |
| _____________________________________ | ||
| _____________________________________ | ||
| _____________________________________ | _____________________________________ | |
| Ultrasound | _____________________________________ | _____________________________________ |
| _____________________________________ | ||
| MRI | _____________________________________ | _____________________________________ |
| _____________________________________ | _____________________________________ |