Chapter 8 Breast Cancer Treatment-Related Imaging and the Postoperative Breast
Combined Clinical and Imaging Workup of Breast Abnormalities
For patients with nonpalpable findings on screening mammography, workup always includes diagnostic mammography. For suspicious calcifications alone, the radiologist usually obtains magnification mammograms, often not needing ultrasound. An exception might be extensive pleomorphic microcalcifications, in which ultrasound might be used to search for masses within the area that could be indicative of invasive cancer, prompting biopsy. However, if there is an image-detected mass, area of architectural distortion, or palpable mass, the radiologist usually uses both mammography and ultrasound to evaluate the abnormality, estimate its size, and direct later biopsy. Breast MRI may be valuable in selected cases, as discussed in Chapter 7. Ideally, the radiologist correlates all physical and imaging findings in the report to form a composite picture of all potential abnormalities and their level of suspicion on mammography, ultrasound, and MRI.
Breast Cancer Diagnosis and Treatment
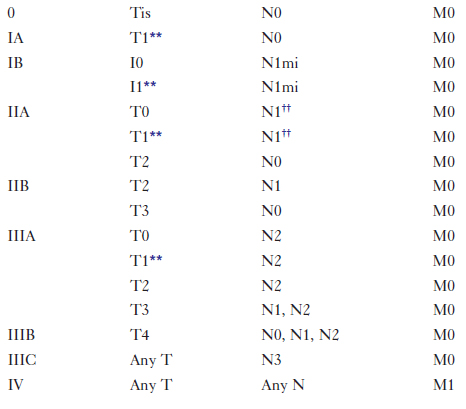
When a combined clinical and imaging workup leads to a breast cancer diagnosis, treatment planning usually involves a consideration of surgery, chemotherapy, and radiation therapy, with the goal to remove all the cancer from the breast, optimize chances for locoregional control, and eradicate occult foci of metastatic disease via systemic treatment (e.g., hormone therapy, chemotherapy), if indicated. The team of breast imagers, surgeons, medical oncologists, pathologists, radiation oncologists, and breast reconstruction surgeons plan the sequence in which surgery, chemotherapy, and radiation occurs. The pathology report is a key component on which treatment is based. The report states tumor histology; size; estrogen, progesterone, and her2neu receptor status; and lymph node involvement. Traditionally, breast tumors are staged using the TNM (tumor, lymph node, metastasis) Classification on Breast Cancer from the American Joint Committee on Cancer (currently in the 7th edition) (Table 8-1). The treatment plan is based on this classification. A clinical decision algorithm is also available from the National Comprehensive Cancer Network regarding the full spectrum of care; Adjuvant! Online is an Internet-based tool that provides guidance regarding prognosis and the potential benefit of different chemotherapy protocols. Additional tests based on tumor gene signatures are emerging (OncoType DX and MammaPrint) and are the subject of two large randomized trials, one in the United States and the other in Europe. Gene expression profiling may play an increasingly important role in the future; preliminary data suggest improvement in separating high- and low-risk patients.
In general, surgeons perform mastectomy when the entire cancer cannot be excised with a good cosmetic result (as just discussed), if the woman has a contraindication to radiotherapy, or if it is the patient’s desire. Usually, patients are offered ipsilateral breast reconstruction with an autologous tissue flap or a tissue expander after mastectomy, unless there is a medical contraindication to reconstruction (e.g., multiple co-morbidities). Because the contralateral breast is often larger than the reconstructed breast, patients may also need reduction mammoplasty on the contralateral side. Characteristic appearances of reduction mammoplasty and breast reconstruction are discussed in Chapter 9.
If the patient has breast-conserving surgery, she usually undergoes postsurgical whole-breast irradiation to achieve control of residual microscopic disease. Relative contraindications to radiation therapy include pregnancy, previous radiation therapy, and collagen vascular disease (Box 8-1). Axillary nodal involvement is not a contraindication. Six randomized trials of lumpectomy and radiation therapy showed that the frequency of local recurrence and overall survival rates are generally comparable to mastectomy. However, IBTRs are reported in 5% of patients at 5 years and in 10% to 15% at 10 years after completion of therapy. Treatment failures (i.e., IBTR) usually undergo salvage mastectomy.
Invasive IBTR usually occurs in the lumpectomy site or quadrant within the first 7 years, but rarely earlier than 18 months after treatment. IBTR after 7 years will more likely occur in any quadrant, not necessarily at the original site, and is usually considered a new cancer. IBTR near the original lumpectomy site is associated more frequently with systemic relapse than IBTR in other quadrants, which more often reflect a new primary tumor. IBTR is considered more likely in women who have invasive ductal cancer with an extensive intraductal component, residual disease in the breast, extensive DCIS, lymphatic or vascular invasion, or multicentricity, and is more common in younger women (Box 8-2).
Evaluation of Axillary Lymph Nodes
ALND is also problematic from the standpoint of side effects. It exposes patients to the risk of major complications such as lymphedema, shoulder dysfunction, and sensory changes in and around the axilla. To address this problem, routine level I/level II ALND (Table 8-2) has evolved to use the SLN biopsy as an initial screen for nodal involvement in patients who are clinically node-negative.
| Level | Location |
|---|---|
| I | Infralateral to lateral edge of the pectoralis minor muscle |
| II | Behind the pectoralis minor muscle |
| III | Between the pectoralis minor and subclavius muscles (Halsted ligament) |
SLN biopsy was initially described for patients with penile cancer, but did not attract much attention until it was broadly adopted for use in melanoma patients. SLN biopsy is performed by injecting a tracer material, either a radionuclide, blue dye, or both into the breast either preoperatively or perioperatively and by looking for evidence of the tracer in one or more sentinel nodes (Box 8-3).
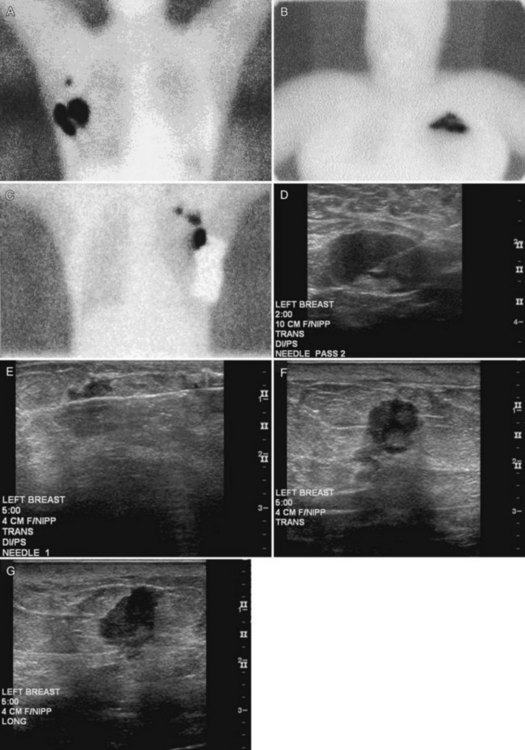
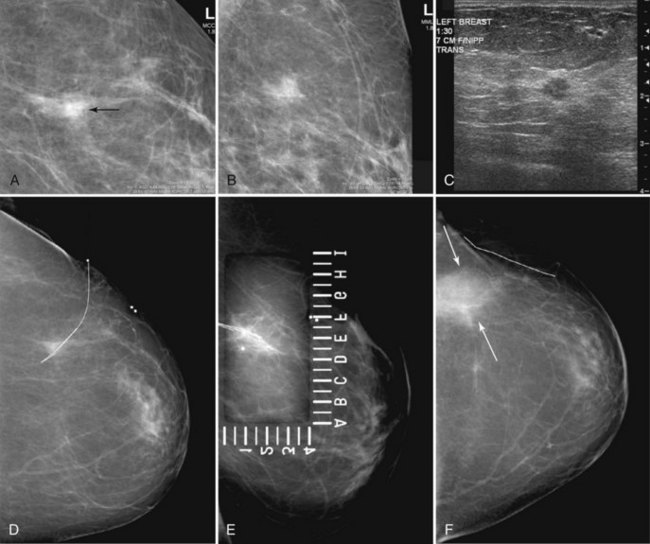
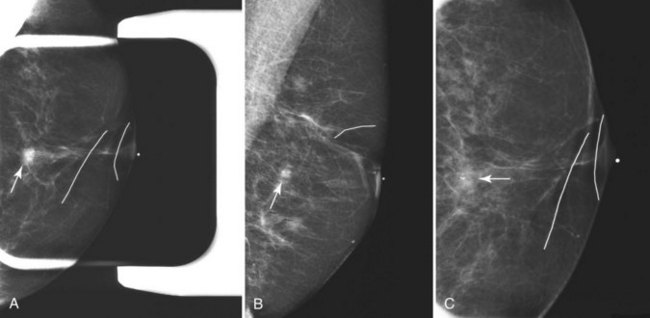
Preoperative lymphoscintigraphy is used in some facilities to assist preoperative localization of sentinel lymph nodes in the axilla or in extra-axillary sites (Fig. 8-1A to C). Most commonly these extra-axillary sites will be in the supraclavicular, infraclavicular, or internal mammary groups. If tracer does not identify an axillary SLN, the surgeon may choose to harvest an SLN from one of these other sites. Some facilities do not remove an internal mammary SLN or other nonaxillary SLN due to the very low frequency of isolated positive biopsies (usually <3%) and the relatively few cases that would result in meaningful changes in prognosis or therapy. Perhaps not surprisingly, institutions that harvest both axillary and internal mammary sentinel lymph nodes have demonstrated a poorer prognosis when lymph nodes at both sites are involved.
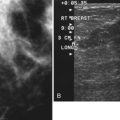
One preoperative axillary imaging method that has gained a following is axillary lymph node ultrasound with percutaneous FNA of suspicious nodes (see Fig. 8-1D to G). Although this test is not a routine part of the initial breast imaging evaluation, there is a new appreciation for preoperative evaluation of ipsilateral axillary lymph nodes in the setting of breast cancer. Axillary ultrasound is particularly helpful when the results of clinical examination of the axilla are suspicious for cancer. Several studies have recently been published using ultrasound-guided FNA or core biopsy to document nodal involvement preoperatively, thus allowing the surgeon to bypass SLN biopsy. This can obviate several known issues with intraoperative assessment of sentinel lymph nodes, such as the time needed to harvest one or more nodes, the intraoperative time needed for pathology to evaluate the node and, most important, the potential for false-negative touch preparation or frozen section at the time of surgery, which can lead to reoperation at a later date.
Clinical and Breast Imaging Factors in Determining Appropriate Local Therapy: Lumpectomy or Mastectomy
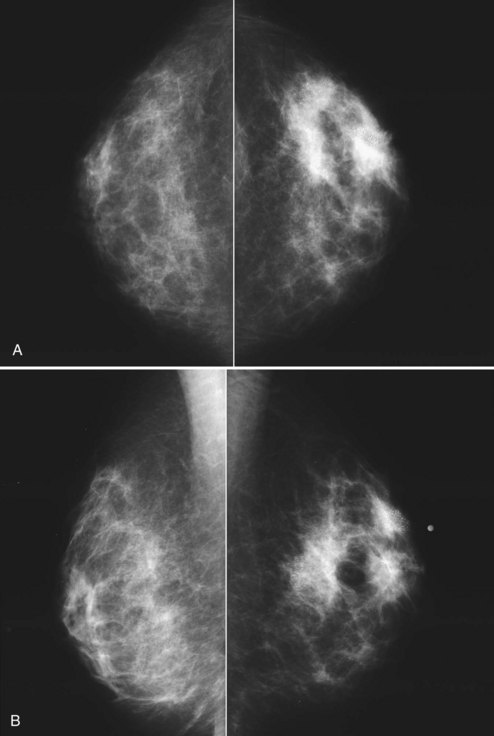
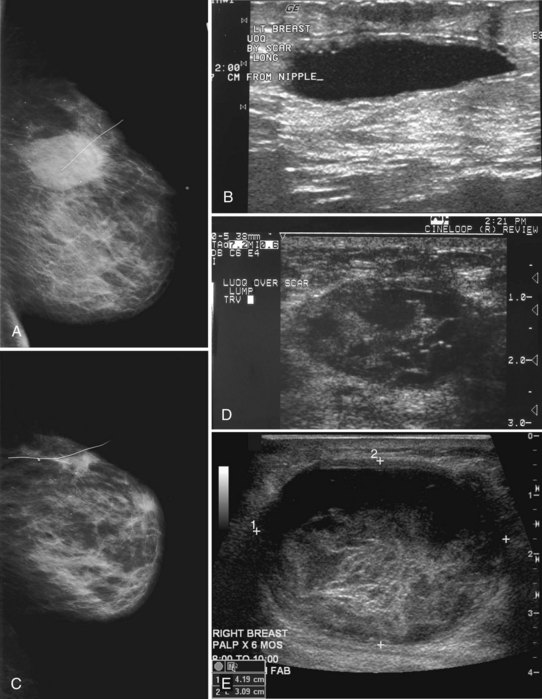
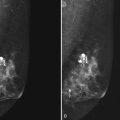
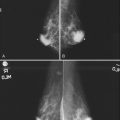
The breast imager plays a critical role in aiding the surgeon to make the right therapeutic choice by showing how much cancer is in the breast. There is virtually no disagreement that patients with a unifocal DCIS or invasive cancer may be treated with breast conservation therapy if the entire tumor can be removed with a good cosmetic result and if there are no relative contraindications to radiation therapy (i.e., pregnancy, collagen vascular disease, poorly defined or multicentric disease) or prior radiotherapy involving the breast (Fig. 8-2).
Preoperative Imaging
Mammography, ultrasound, and MRI for tumor extent are important tools for selecting appropriate breast conservation therapy candidates and planning surgery (Table 8-3). Mammography is the mainstay for determining extent of disease. Mammography identifies diffuse or multicentric disease by finding suspicious breast masses and pleomorphic calcifications. Mammography also can identify benign, extensive, innumerable bilateral calcifications that could hide early tumor recurrence. Such calcifications are a relative contraindication to breast conservation therapy. Furthermore, mammography finds DCIS that is invisible to MRI. Specifically, approximately 25% of DCIS cases are false-negative on MRI and are discovered only by visualizing pleomorphic calcifications on the mammogram.
Table 8-3 Breast Imaging Relating to Breast-Conserving Therapy
| Timing | Reason | Technique(s) |
|---|---|---|
| Preoperative | Ipsilateral tumor extent and contralateral tumor |
MRI, magnetic resonance imaging; SLN, sentinel lymph node; US, ultrasound.
Modified from Dershaw DD: The conservatively treated breast. In Bassett LW, Jackson VP, Fu KL, Fu YS, editors: Diagnosis of diseases of the breast. Philadelphia, 1997, WB Saunders, p. 553.
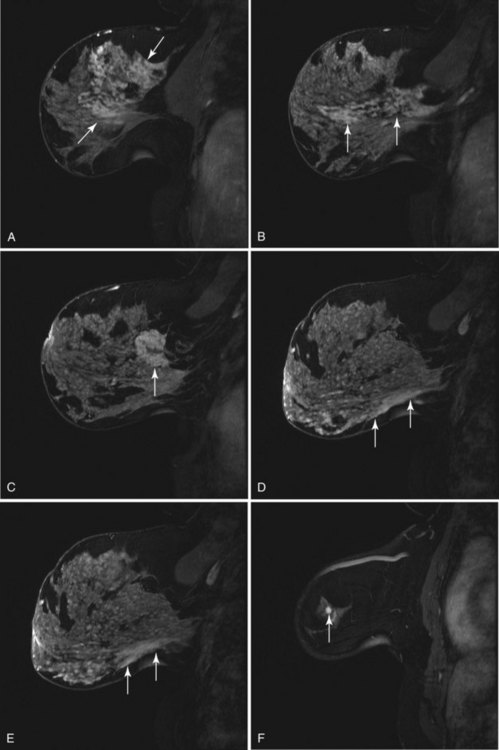
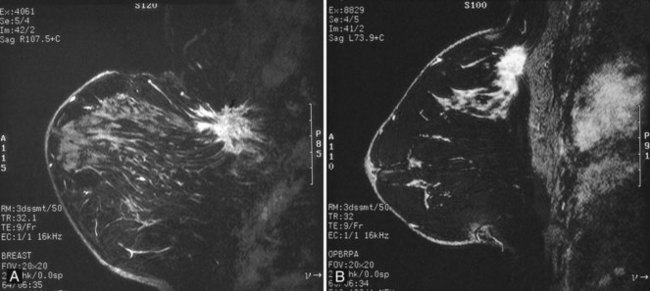
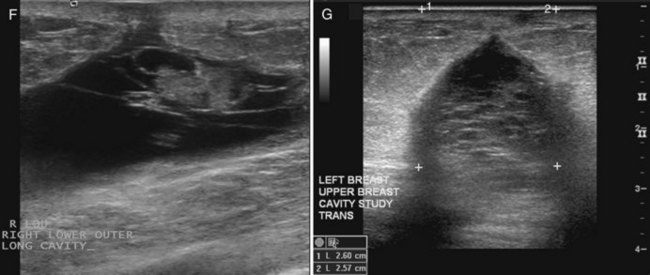
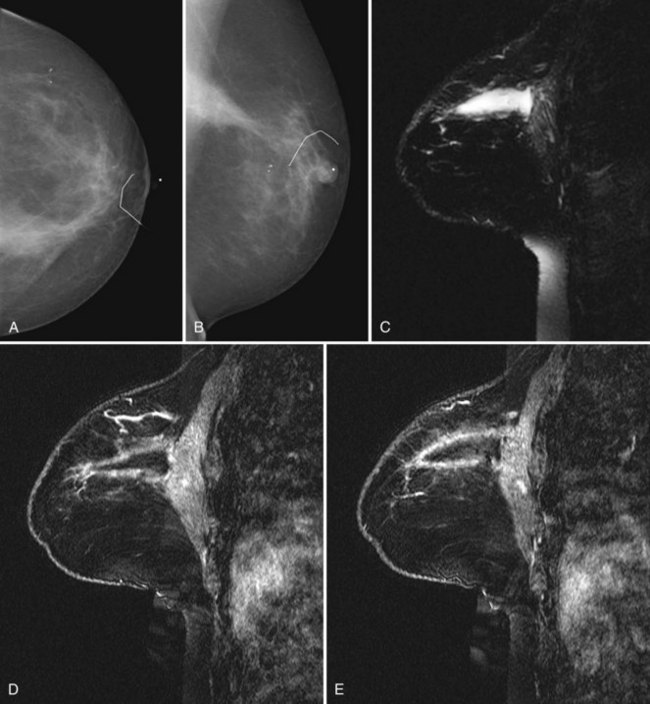
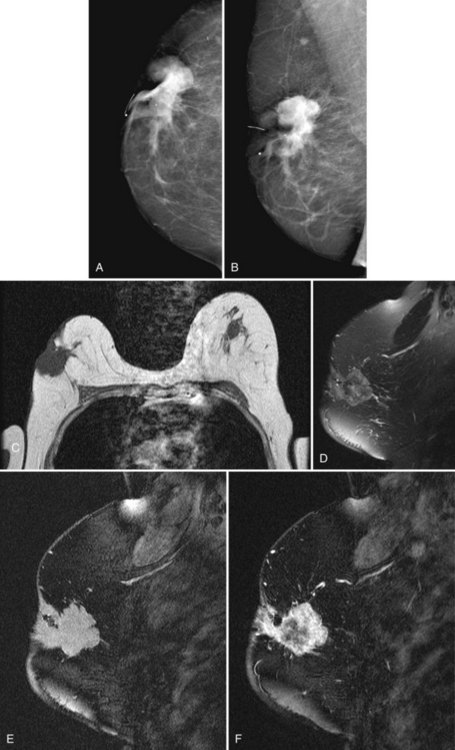
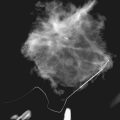
On the other hand, MRI has been especially useful in predicting tumor extent before the first surgical procedure (Fig. 8-3). Some investigators have claimed particular effectiveness of MRI in women with invasive lobular carcinoma or showing tumor invasion into the pectoralis muscle or chest wall (Fig. 8-4). With respect to invasive lobular carcinoma, several studies have suggested that MRI may be more effective in detecting the extent of disease than physical examination, mammography, and ultrasound. However, false-negative studies in these series have led to mixed opinions regarding the routine use of MRI in staging invasive lobular carcinoma.
Chest wall tumor invasion on MRI was shown by obliteration of the fat plane between the tumor and the pectoralis muscle, with muscle enhancement, and was proven in 5 of 5 cases at surgery (Morris et al, 2000). No muscle involvement was seen at surgery when muscle enhancement was absent in 14 of 14 cases.
MRI also helps exclude candidates for APBI when it finds more than one focus of cancer. Bedrosian and colleagues (2003) reported a 95% tumor detection rate with MRI and a change in surgical management in 26% (69/267) of patients requiring wider/separate excision or mastectomy, with pathologic verification in 71% (49/69).
Normal Postoperative Imaging Changes after Breast Biopsy or Lumpectomy
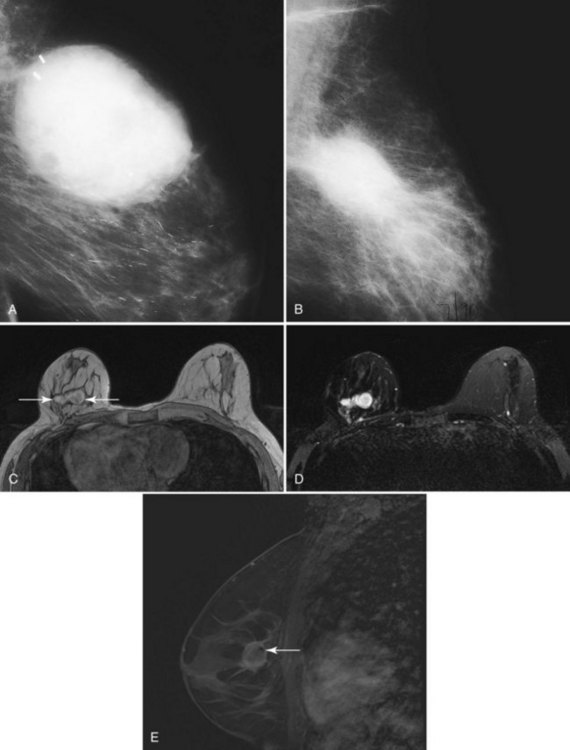
As a rule, mammograms are not often obtained immediately after diagnostic surgical excisional biopsy. However, in the rare cases when a mammogram is obtained within a few days of surgery, mammography shows a round or oval mass in the postoperative site representing a seroma or hematoma, with or without air. This mass represents the biopsy cavity, filled with fluid that should resolve over time (Fig. 8-5A and B). The adjacent breast tissue shows thickening of trabeculae in subcutaneous fat and increased density caused by local edema or hemorrhage. Skin thickening at the incision is usually present. On MRI the biopsy site is filled with blood or seroma. The fluid in the biopsy cavity is high signal intensity on T2-weighted noncontrast fat-suppressed images (see Fig 8-5C to E).
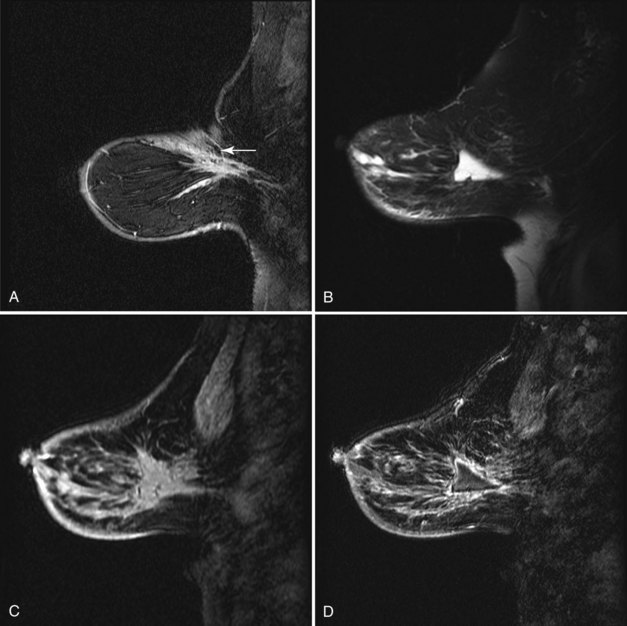
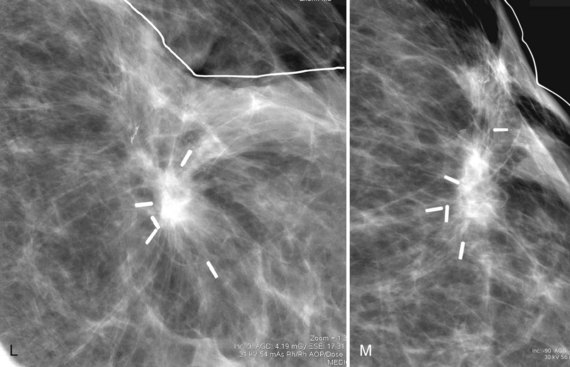
Over the subsequent weeks, the postoperative site resorbs the air and fluid collection; the collection is replaced by fibrosis and scarring, with residual focal skin thickening and breast edema. On MRI the immediate postbiopsy cavity is a fluid-filled structure with surrounding normal healing tissue enhancement for up to 18 months after the biopsy. The biopsy cavity shows high signal intensity, architectural distortion, and a scar that can simulate cancer (Fig. 8-6 and Box 8-4). The biopsy site usually contains fluid from the seroma, which will be bright on T2-weighted images on MRI. Rim enhancement around the biopsy site is normal even if there is no residual tumor and is due to healing. In the ipsilateral axilla, reactive lymph nodes may develop that cannot be distinguished from metastatic disease (Fig. 8-7). MRI after surgery may reveal cancer at the margin edge by showing clumped enhancement or an eccentric residual mass. Although immediate postbiopsy MRI for cancer staging may depict cancer at the biopsy margin, it is more often used to look for cancer elsewhere in the breast away from the biopsy site.
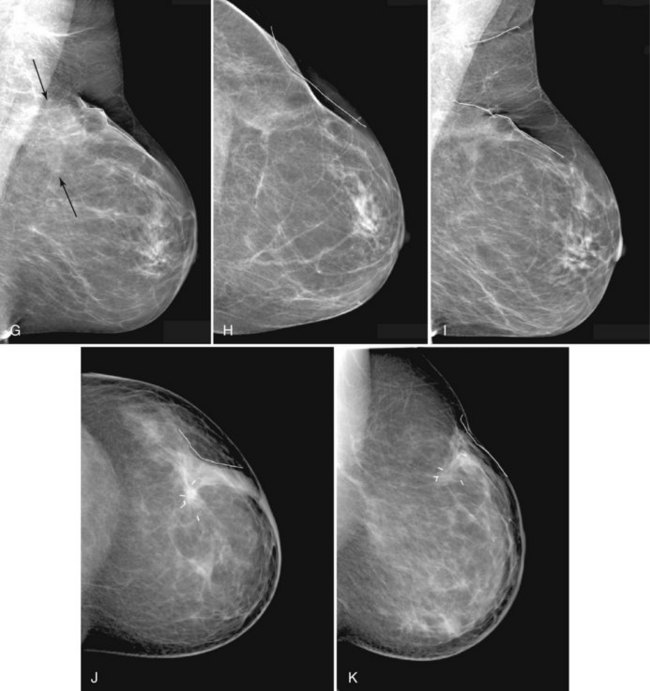
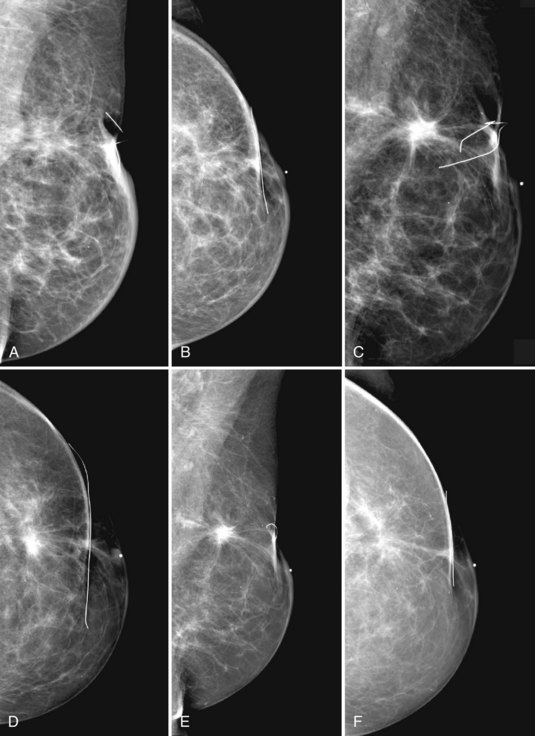
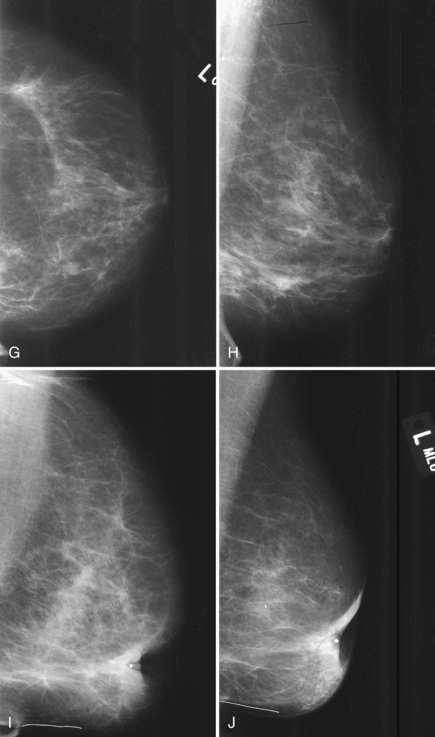
Normal postoperative findings on mammography include architectural distortion, increased density, and parenchymal scarring in at least 50% of patients (Box 8-5). These findings diminish in severity over time (Fig. 8-8A to I). After 3 to 5 years, the findings should be stable on subsequent mammograms. On the mammogram, in 50% to 55% of cases, the biopsy cavity resolves so completely that it leaves no scar or distortion in the underlying breast parenchyma, and only comparison with prebiopsy mammograms indicates that breast tissue is missing. In other cases, the scar appears as a chronic architectural distortion or a spiculated mass more evident on one projection than the other.
Box 8-5
Normal Postoperative Findings for Benign Disease
Increased focal density (edema) near the biopsy site (early)
Oval fluid or fluid/air collection (early)
Complete resolution of biopsy findings (late; 50% to 55% of all cases)
Time when findings resolve: 3 to 5 years after biopsy
Postoperative findings seen after 3 to 5 years (45% to 50% of all cases)
Data from Brenner RJ, Pfaff JM: Mammographic changes after excisional breast biopsy for benign disease, AJR Am J Roentgenol 167:1047–1052, 1996; and Sickles EA, Herzog KA: Mammography of the postsurgical breast, AJR Am J Roentgenol 136:585–588, 1981.
The remaining 45% to 50% of patients continue to have variable mammographic findings ranging from spiculated masslike scars to slight architectural distortion (see Fig. 8-8J and K). In still other, more rare cases, seroma cavities persist, appearing as a round or oval mass.
Postbiopsy scars often have a spiculated masslike appearance that can simulate cancer. Spiculated masses should be viewed with suspicion unless one knows that a biopsy was performed in that location. For this reason, it is important to document the date and location in the breast of previous biopsies on the breast history sheet. Some facilities also place a linear metallic scar marker directly on the skin’s biopsy scar before taking the mammogram to show the previous biopsy site. On the mammogram, the linear metallic scar marker on the skin will be near the underlying scar. The skin scar may not be immediately adjacent to the scar inside the breast because the skin is compressed away from the underlying breast parenchyma during the mammogram. If a spiculated mass is seen far from the metallic scar marker, that mass might be cancer rather than a scar. The radiologist reviews the preoperative mammograms to see where the biopsy occurred and correlates the prebiopsy and current mammograms to make this determination (see Fig. 8-8L and M).
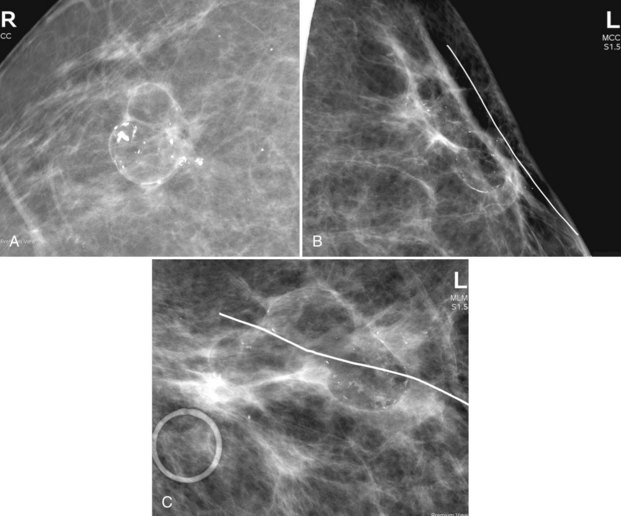
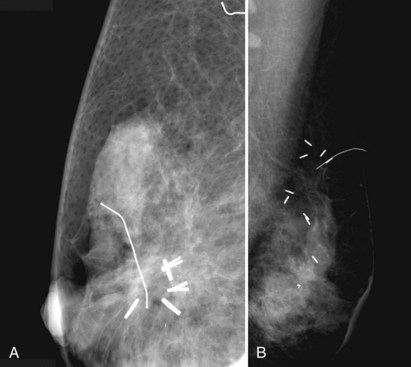
Fat necrosis is common after a breast biopsy and usually appears as a radiolucent lipid-filled mass. Mammography is pathognomonic for fat necrosis if it shows lipid cysts or typical calcified eggshell-type rims around a radiolucent center (Fig. 8-9A). The fat necrosis, lipid cyst, and calcifications usually form in the scar, so these findings should be located near any linear metallic scar markers on the skin (see Fig. 8-9B and C).
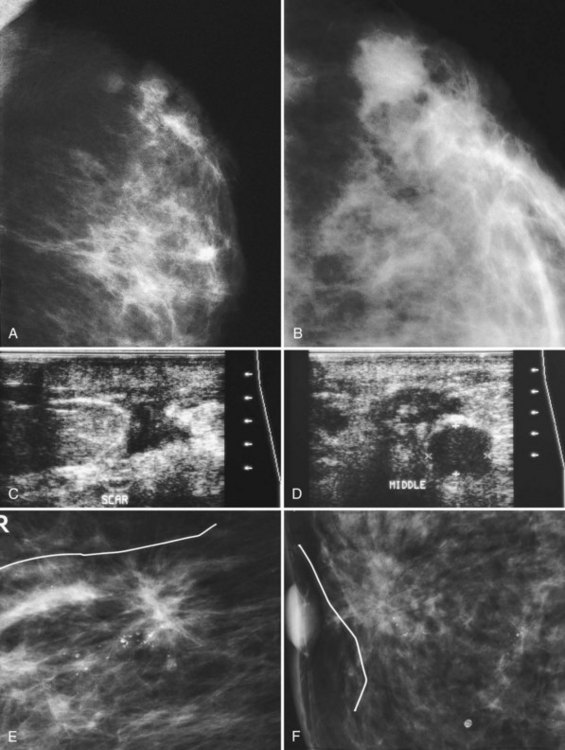
On ultrasound, the immediate postoperative site shows a seroma or hematoma, breast edema, and focal skin thickening. The fluid collection occasionally contains air. More commonly, the seroma is completely filled with fluid, sometimes containing septa or debris that has varying appearances on ultrasound (Fig. 8-10). Usually the incision can be traced from the biopsy cavity up to the skin and is shown as a linear scar that disturbs the normal breast architecture (Box 8-6).
Later, the fluid in the biopsy cavity resolves and only the fibrotic scar remains. In these cases, ultrasound shows the scar as a hypoechoic spiculated mass that simulates breast cancer, but it should correlate with the postoperative site (Fig. 8-11). Correlating biopsy histories and the physical finding of a scar on the skin distinguishes normal postoperative scarring from cancer. On ultrasound, the spiculated scar often has a “tail” that extends from the scar to the skin, representing the healing biopsy cavity and its adjacent subcutaneous tissue anastomosis (Fig. 8-12).
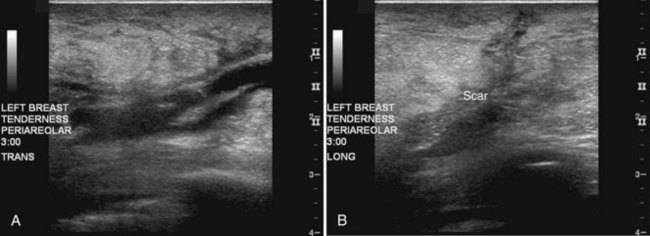
Figure 8-12 Older biopsy scars on ultrasound. Eight months after biopsy and radiation therapy, transverse (A) and longitudinal (B) scans show fluid in the scar, but note that the margins are less sharp than those in Figure 8-11, indicating scar healing and fluid resorption. Note the typical appearance of breast edema on ultrasound in part A, shown by the indistinct skin line, gray fat between the skin line and the scar, and dark linear fluid-filled lymphatics in the subcutaneous tissues.
Whole-Breast, External Beam Radiotherapy and Accelerated Partial Breast Irradiation
APBI is a treatment option for selected women with limited early stage breast cancer after breast-conserving surgery. APBI occurs over a significantly shorter period (accelerated) than WB-XRT and targets the tumor bed and defined margin (partial breast) rather than the whole breast (Box 8-7).
Box 8-7 Whole-Breast, External Beam Radiotherapy and Accelerated Partial Breast Irradiation
Breast Imaging before Re-Excision Lumpectomy or Radiotherapy
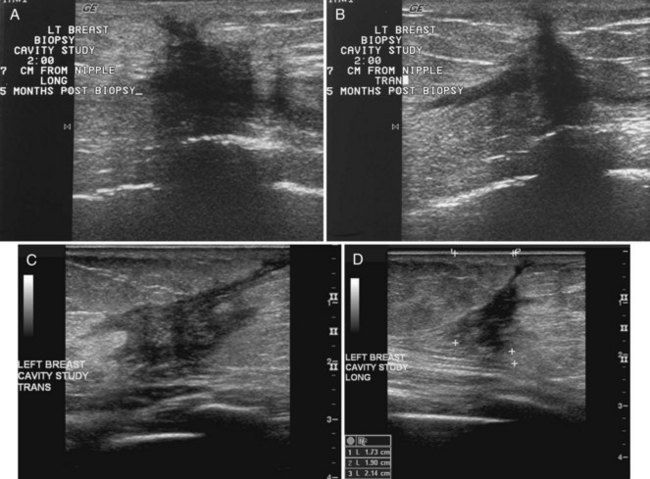
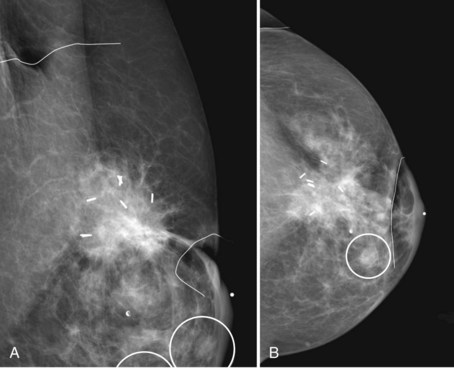
Patients who have undergone therapeutic excision demonstrating tumor-free margins may still benefit from additional imaging. This is particularly true for women whose lesions presented initially as microcalcifications. Such imaging helps determine the completeness of tumor excision. There is some controversy in the literature as to whether preradiotherapy mammography of the affected breast should become standard to identify possible multifocal disease before committing to radiotherapy. Because the main concern with breast conservation is local recurrence, and because IBTR may affect overall survival, physicians may order postbiopsy mammograms to determine the completeness of tumor excision if there is any question about residual gross disease or residual microcalcifications (Fig. 8-13). These mammograms are particularly appropriate before the initiation of breast irradiation to make sure there is no residual tumor.
Normal Imaging Changes after Radiation Therapy
Mammography
Recommendations for follow-up mammography after radiation therapy vary by institution. Most facilities obtain a unilateral mammogram immediately after the conclusion of radiation therapy, with further follow-up bilateral mammograms at 6- to 12-month intervals. Obtaining a mammogram relatively soon after completion of radiation therapy establishes a baseline for future reference (Fig. 8-14A to F).
Normal lumpectomy and WB-XRT changes alter the normal mammogram. These include the usual postbiopsy changes in the surgical site plus diffuse skin thickening and breast edema from WB-XRT (Box 8-8; see also Fig. 8-14G to J). Unlike normal focal postoperative edema, breast edema from WB-XRT encompasses the entire breast and not just the region around the postoperative site. On physical examination, the breast commonly shows peau d’orange, a large swollen areola or nipple, occasional brownish or red skin, and occasional breast tenderness and swelling. Skin thickening in the immediate postradiation therapy period is due to breast edema from small-vessel damage; later, it is due to fibrotic change. Findings of breast edema are most obvious when compared with the contralateral side or older mammograms.
Box 8-8 Mammographic Findings after Breast Conservation and Radiation Therapy
The findings are worst at 6 months, diminish, and then stabilize at 2 to 3 years.
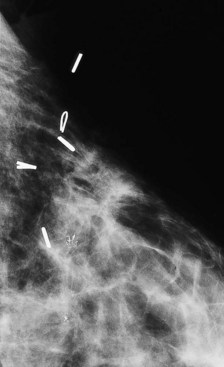
After completion of whole-breast irradiation, many facilities use an electron beam boost to sterilize the operative site. Some facilities line the cavity with radiopaque markers to guide the electron beam boost and use x-ray imaging for guidance (Fig. 8-15). Other facilities use breast ultrasound to delineate and mark the skin over the breast biopsy cavity for the electron beam boost.
In about 25% of women, calcifications develop in the treated breast at the biopsy site; these calcifications can be extreme if the radiation therapy was done many years ago with higher orthovoltage radiation compared to current therapy (Fig. 8-16A and B). Although most of these calcifications will be due to benign dystrophic calcification, fat necrosis, or calcifying suture material, magnification views of calcifications in the biopsy site are required to distinguish them from the pleomorphic calcifications of cancer recurrence. Fat necrosis may be evident if it has dystrophic appearance or forms around a radiolucent center. The dystrophic calcifications in fat necrosis can simulate malignancy, but magnification orthogonal projections may show the beginnings of the typical curvilinear shape of fat necrosis not evident on only one view. Careful inspection of the previous mammogram may also help by showing that the calcifications are forming around a radiolucent center of fat. Sometimes, when there are no distinguishing features to diagnose dystrophic or fat necrosis calcifications, these calcifications cannot be distinguished from cancer and prompt biopsy (see Fig. 8-16C to I).
Because investigators have been exploring the use of APBI in lieu of standard WB-XRT, the higher doses per fraction used with APBI have led to a variety of findings in several phase I/phase II studies. Some groups have reported a higher incidence of post-treatment calcifications, leading to fairly high rates of early postlumpectomy biopsy. It has not been clear from these studies whether a lower threshold for biopsy was set because of the experimental nature of APBI. Other studies have suggested that the incidence of calcifications, attributed to asymptomatic fat necrosis, increases with time after APBI. There has been no systematic evaluation of mammographic findings after APBI either with prior phase I or phase II studies, or from the limited reports from phase III studies published to date. It is fairly clear, however, that APBI will likely become a standard treatment option for at least some women with early-stage breast cancer pursuing breast conservation. Because radiologists may see the findings of a large seroma cavity extending from the skin surface to the chest wall (Fig. 8-17) or increased calcifications in and around the lumpectomy cavity resulting from fat necrosis, it will be important to contrast this to “normal” postbiopsy changes in which the scar is small (Fig. 8-18). Recognition of post-ABPI findings will minimize both false-positive, as well as false-negative, interpretations of post-APBI mammograms as this new paradigm emerges.
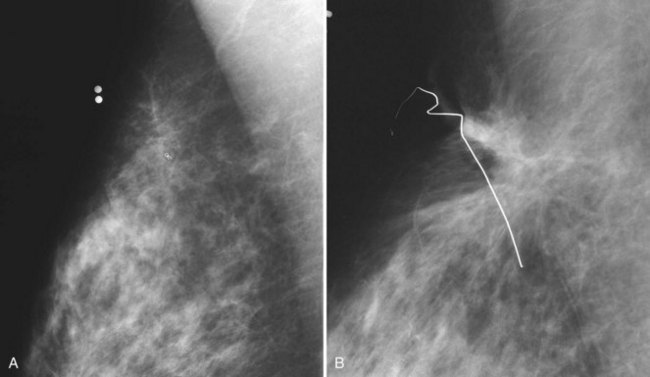
Figure 8-18 Scarring at a biopsy site. A, A prebiopsy cropped mediolateral oblique (MLO) view shows a clip where cancer was biopsied by stereotaxis; two BBs show the previous needle entry site. B, A postbiopsy cropped MLO view shows limited architectural distortion and skin deformity at the biopsy scar after definitive cancer surgery. Contrast part B to the scarring after accelerated partial breast irradiation in Figure 8-17. Cancer recurrences display pleomorphic calcifications, increasing density, or masslike change in the scar, which are not present here. Contrast part B to the cancer recurrence in Fig. 8-20A.
MRI
On MRI, the breast biopsy scar enhances for up to 18 months and is a common cause for false-positive readings. Normal postbiopsy fat necrosis produces a spiculated mass that enhances rapidly and washes out on the kinetic curve; this common false-positive MRI finding can result in biopsy unless the radiologist investigates the patient’s history and correlates the MRI to the mammogram (Fig. 8-19).
Treatment Failure or Ipsilateral Breast Tumor Recurrence
Treatment failures detected on mammography manifest as new pleomorphic calcifications or masses developing in the biopsy site. At times, a breast cancer recurrence is hard to distinguish from the normal postbiopsy scar, which mimics cancer. However, unlike cancer recurrences, the normal postbiopsy scar becomes smaller and less apparent over time on the mammogram, with stabilization at 2 to 3 years. Central fat necrosis may produce a radiolucent center in the biopsy cavity. Thus, it is not normal if the scar grows in size or becomes denser or more masslike. The radiologist suspects recurrent carcinoma and prompts biopsy if the “scar” develops new pleomorphic calcifications or becomes more dense, if the “scar” edge becomes rounder, or if the “scar” grows (Fig. 8-20).
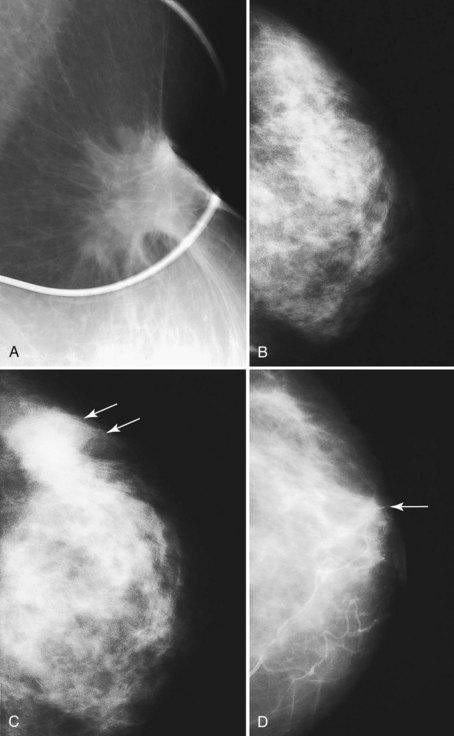
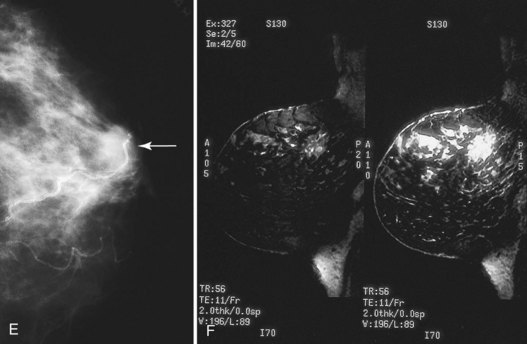
Figure 8-20 Breast cancer occurring at a biopsy site. A, A postbiopsy spot mediolateral oblique view shows architectural distortion, skin retraction, and deformity at a biopsy scar. Unlike normal biopsy scars, which have a little density in their central part, this spot view shows a moderately masslike area in the scar, which was invasive ductal cancer. Contrast this tumor with the normal postbiopsy scar in Figure 8-18B. B, In another patient, a postbiopsy craniocaudal (CC) view shows architectural distortion in the outer portion of the breast after biopsy and radiation therapy for cancer. C, Five years later, a developing density (arrows) is present in the outer part of the breast. Biopsy showed recurrent cancer. D, In a third patient, a postbiopsy CC view shows minimal architectural distortion near the nipple after biopsy and radiation therapy for cancer (arrow). E, The next year a developing mass (arrow) was noted in the biopsy site. Biopsy showed recurrent cancer. F, Sagittal 3-D spectral-spatial excitation magnetization transfer noncontrast-enhanced (left) and contrast-enhanced (right) magnetic resonance images show segmental enhancement in a cancer recurrence in the upper part of the breast long after biopsy and radiation therapy.
Recurrent tumor in the irradiated breast may arise at the site of the original tumor or elsewhere in the breast (Fig. 8-21). Recurrences at the original tumor site are usually due to failure to eradicate the original cancer and represent true treatment failures; they occur sooner than a tumor developing elsewhere in the breast. Tumors developing outside the treated area occur at the same rate as tumors forming in the contralateral breast and represent new cancers. Breast irradiation does not lead to an increased incidence of breast cancer in the opposite breast or in the boosted area of the treated breast.
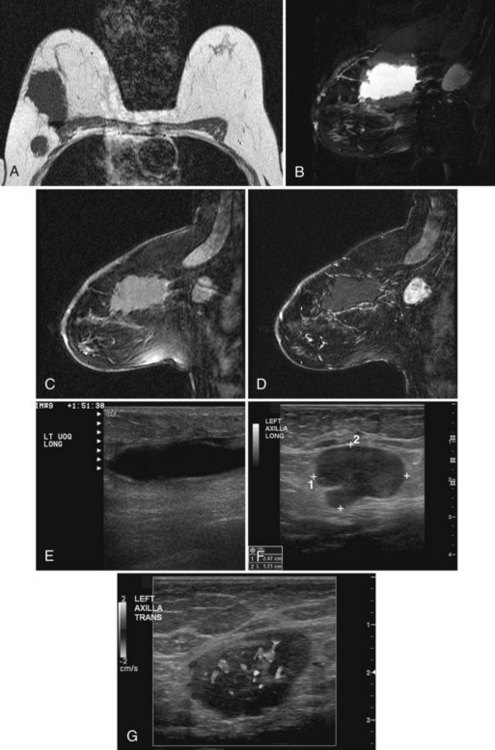
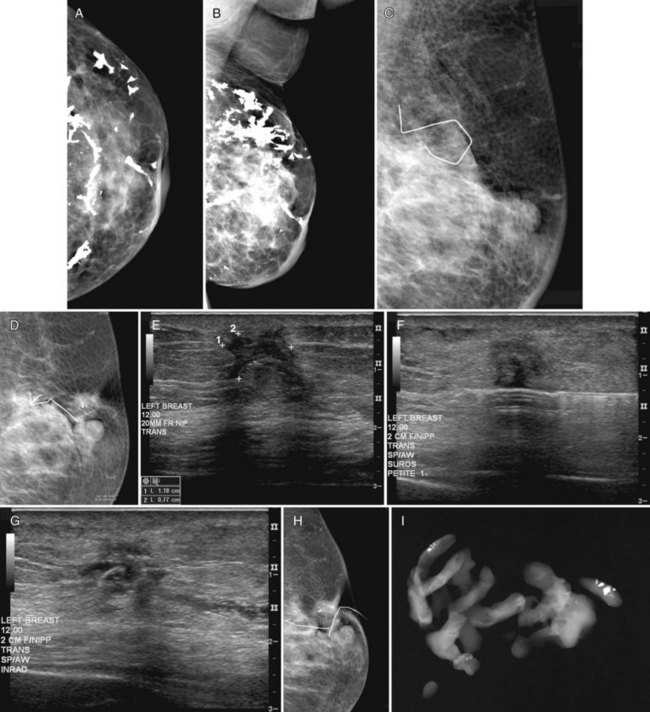
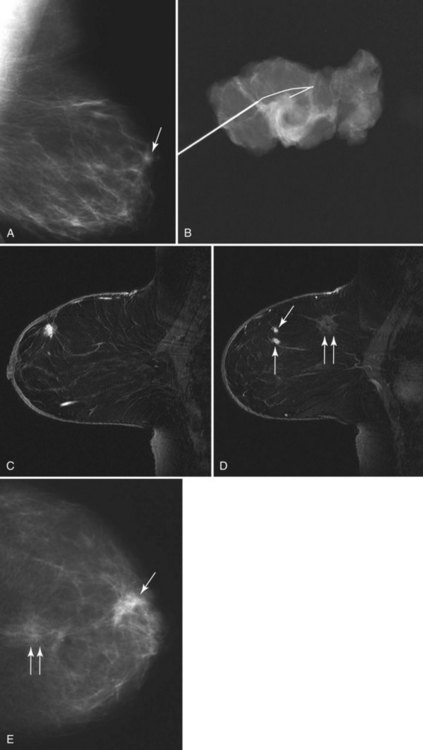
Recurrent disease is diagnosed by mammography or physical examination. About half of the recurrences are detected by mammography and half by physical examination. Those that are mammographically detected usually contain pleomorphic microcalcifications or masses (Box 8-9). Palpable recurrences are usually manifested as masses, are more frequently invasive cancer, and may be displayed on the mammogram as developing densities or masses. On ultrasound, an IBTR shows as a mass separate from or in continuity with the biopsy scar (Fig. 8-22A to D) if it occurs near the original biopsy site.
On mammography, breast cancer recurrences contain pleomorphic calcifications (see Fig. 8-22E and F) or are shown as masses with or without calcifications. This is why radiologists investigate any new mass, because even benign new solid masses may represent a new cancer (Figs. 8-23 to 8-25).
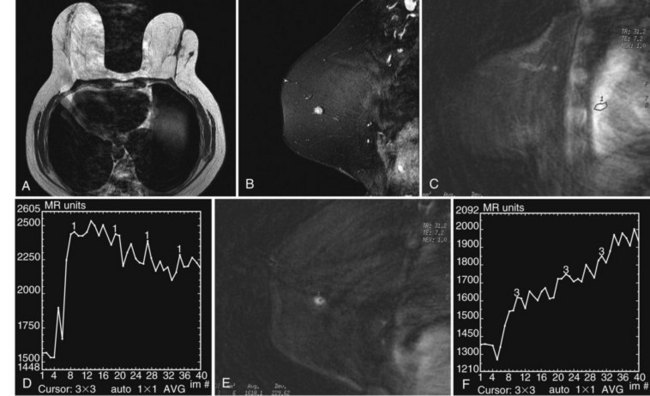
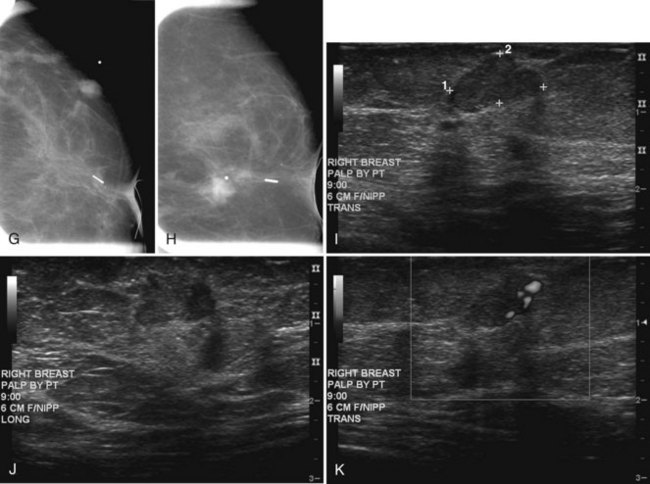
Figure 8-24 Atypical ipsilateral breast tumor recurrence on magnetic resonance imaging (MRI), mammography, and ultrasound. A, Axial nonfat-suppressed T1-weighted localizer shows architectural distortion in skin from the prior biopsy for cancer and a small round, low signal intensity mass in the outer right breast. B, Sagittal postcontrast 3-D spectral-spatial excitation magnetization transfer (3DSSMT) shows an enhancing round mass against a fatty background. Spiral dynamic sagittal MRI with an ROI over the heart (C) and dynamic kinetic curve (D) show that there was a good contrast bolus, as shown by the rapid initial rise and washout. Spiral dynamic sagittal MRI with an ROI over the outer breast mass (E) and kinetic curve (F) show a benign mass characterized by a slow initial rise and persistent late phase. However, because the mass was not present previously, it was considered suspicious. In the same patient, craniocaudal (G) and mediolateral (H) views show architectural distortion and a marker in the retroareolar region after biopsy and radiation therapy for cancer. In the outer left breast, lateral to the scar, there is a palpable oval mass, marked by a BB and corresponding to the mass seen on MRI in Figure 8-24B. I, Transverse ultrasound over the mass shows an oval lobulated solid mass that shows microlobulated margins on longitudinal scan (J) and marked vascularity on color Doppler ultrasound (K). Biopsy showed invasive ductal cancer and the patient underwent mastectomy.
Mastectomy
After mastectomy, breast reconstruction options include an implant, a latissimus dorsi flap with a tissue expander when significant breast skin has been lost, or a transverse rectus abdominis myocutaneous (TRAM) flap or one of its derivative procedures, such as a deep inferior epigastric perforator (DIEP) flap. Images of reconstructed breasts are shown in Chapter 9. In the case of skin-sparing subcutaneous mastectomy, the surgeon removes the breast tissue as for simple (total) mastectomy but preserves the nipple–areolar complex and inserts a tissue expander. Unless there is a medical contraindication to breast reconstruction, patients who choose mastectomy are always offered breast reconstruction with a tissue expander or autologous tissue flap. Imaging of the reconstructed breast is typically not performed after expander or implant placement or after autologous tissue reconstruction.
Sometimes, reduction mammoplasty may be required on the contralateral, unaffected breast to achieve symmetry with the treated breast. The appearances of breasts reconstructed with autologous tissue and contralateral normal breasts that have undergone reduction mammoplasty are characteristic and should not be mistaken for cancer. These are shown in Chapter 9.
Key Elements
Immediate postsurgical breast changes on mammography include increased density (local edema), oval or round masses (seroma/hematoma) with or without air, and skin thickening.
The fluid in the surgical site resolves over the next few weeks and months in most cases.
Postsurgical changes diminish in severity over time and are stable at 3 to 5 years.
From 50% to 55% of patients undergoing surgical breast biopsy for benign disease have no mammographic findings at 3 years.
The remaining 45% to 50% of patients show architectural distortion, parenchymal changes (scarring) that may be spiculated, or increased density that can simulate breast cancer.
To determine whether a spiculated density on the mammogram is a postbiopsy scar or cancer, it is important to correlate the postsurgical site with the location of the spiculated finding.
Fat necrosis occurring in a biopsy site is visualized as radiolucent lipid-filled masses, with occasional curvilinear calcifications forming around the lucent center.
On ultrasound, the immediate postsurgical site appears as a fluid-filled mass representing the seroma; it occasionally displays septa, debris, or fluid tracking up to the skin incision.
If only the fibrotic scar remains, ultrasound reveals a hypoechoic spiculated mass that simulates breast cancer, but it should correlate with the postoperative site.
On MRI, the immediate postbiopsy cavity is a fluid-filled structure with surrounding tissue enhancement.
Postbiopsy scarring enhancement persists for up to 18 months and should then subside.
Breast tumors are staged by the TNM (tumor, lymph node, metastasis) classification of breast cancer from the American Joint Committee on Cancer.
Local control of breast cancer requires surgical eradication of tumor by mastectomy or lumpectomy, followed by radiotherapy.
The breast imager aids the surgeon in selecting candidates for breast-conserving surgery by determining the extent of tumor.
Relative contraindications to radiation therapy include previous radiation therapy, pregnancy, collagen vascular disease, and multicentric or diffuse disease.
Ipsilateral breast tumor recurrences are reported in 5% of women at 5 years and in 10% to 15% at 10 years after completion of therapy.
Treatment failures after breast conservation are managed by salvage mastectomy.
The sentinel lymph node biopsy technique identifies the lymph node most likely to harbor metastasis. Radionuclide tracers or blue dye is injected into the breast and later carried into the breast lymphatics draining the tumor or biopsy cavity.
A “hot” node, a blue node, or an abnormal palpable node identified at surgery is a sentinel lymph node.
The sentinel lymph node may be examined by hematoxylin and eosin staining, as well as by immunohistochemistry staining for low-molecular-weight cytokeratins.
The sentinel lymph node may be identified at surgery even with nonvisualization of a sentinel lymph node at lymphoscintigraphy.
MRI has been used for predicting the extent of tumor before the initial breast cancer surgical procedure, with some false-negative results in women with invasive lobular carcinoma and ductal carcinoma in situ.
If preoperative needle localization has been performed, specimen radiography or specimen sonography is used to determine whether the suspicious finding has been adequately removed.
As needed, postbiopsy mammograms determine the completeness of tumor excision, are particularly appropriate before the initiation of breast irradiation, and should be performed if residual tumor is suspected.
Most facilities obtain a unilateral mammogram immediately after the conclusion of radiation therapy, with further follow-up bilateral mammograms at 6- to 12-month intervals.
Breast edema from whole breast radiation therapy encompasses the entire breast and is manifested as diffuse increased parenchymal density, skin thickening, and trabecular thickening in subcutaneous fat.
Postsurgical and postradiation therapy changes usually decrease somewhat over a period of 2.5 to 3 years or may remain stable.
Calcifications in the biopsy site in an irradiated breast represent fat necrosis, dystrophic calcifications, calcifying suture material, or breast cancer recurrence.
Chemotherapy changes the MRI enhancement pattern of the breast by diminishing enhancement of normal breast parenchyma and tumor alike.
On MRI, suspicious postchemotherapy kinetic late-phase plateau or washout curve patterns can change to a benign persistent late-phase pattern despite the presence of viable breast cancer.
Recurrent cancer on mammography shown by pleomorphic microcalcifications is frequently ductal carcinoma in situ.
Palpable recurrences are usually manifested as mammographic masses and are more frequently invasive cancers.
Breast reconstruction includes an implant, a latissimus dorsi flap, or a transverse rectus abdominis myocutaneous flap.
Breast cancer recurrence in an unreconstructed mastectomy site is usually detected by physical examination.
Abe H, Schmidt RA, Kulkarni K, et al. Axillary lymph nodes suspicious for breast cancer metastasis: sampling with US-guided 14-gauge core-needle biopsy—clinical experience in 100 patients. Radiology. 2009;250:41-49.
Alazraki NP, Styblo T, Grant SF, et al. Sentinel node staging of early breast cancer using lymphoscintigraphy and the intraoperative gamma-detecting probe. Semin Nucl Med. 2000;30:56-64.
Al-Hallaq HA, Mell LK, Bradley JA, et al. Magnetic resonance imaging identifies multifocal and multicentric disease in breast cancer patients who are eligible for partial breast irradiation. Cancer. 2008;113:2408-2414.
American College of Radiology. ACR Breast Imaging Reporting and Data System, Breast Imaging Atlas. Reston, VA: American College of Radiology. 2003.
American Joint Committee on Cancer. AJCC cancer staging manual, ed 7. New York: Springer; 2010.
Bauer TW, Spitz FR, Callans LS, et al. Subareolar and peritumoral injection identify similar sentinel nodes for breast cancer. Ann Surg Oncol. 2002;9:169-176.
Bedrosian I, Mick R, Orel SG, et al. Changes in the surgical management of patients with breast carcinoma based on preoperative magnetic resonance imaging. Cancer. 2003;98:468-473.
Bedrosian I, Reynolds C, Mick R, et al. Accuracy of sentinel lymph node biopsy in patients with large primary breast tumors. Cancer. 2000;88:2540-2545.
Bevilacqua JL, Gucciardo G, Cody HS, et al. A selection algorithm for internal mammary sentinel lymph node biopsy in breast cancer. Eur J Surg Oncol. 2002;28:603-614.
Birdwell RL, Smith KL, Betts BJ, et al. Breast cancer: variables affecting sentinel lymph node visualization at preoperative lymphoscintigraphy. Radiology. 2001;220:47-53.
Bleicher RJ, Ciocca RM, Egleston BL, et al. Association of routine pretreatment magnetic resonance imaging with time to surgery, mastectomy rate, and margin status. J Am Coll Surg. 2009;209:180-187.
Bleicher RJ, Morrow M. MRI and breast cancer: role in detection, diagnosis, and staging. Oncology (Williston Park). 2007;21:1521-1533.
Boughey JC, Middleton LP, Harker L, et al. Utility of ultrasound and fine-needle aspiration biopsy of the axilla in the assessment of invasive lobular carcinoma of the breast. Am J Surg. 2007;194:450-455.
Brennan ME, Houssami N, Lord S, et al. Magnetic resonance imaging screening of the contralateral breast in women with newly diagnosed breast cancer: systematic review and meta-analysis of incremental cancer detection and impact on surgical management. J Clin Oncol. 2009;27:5640-5649.
Brenner RJ, Pfaff JM. Mammographic features after conservation therapy for malignant breast disease: serial findings standardized by regression analysis. AJR Am J Roentgenol. 1996;167:171-178.
Brenner RJ, Pfaff JM. Mammographic changes after excisional breast biopsy for benign disease. AJR Am J Roentgenol. 1996;167:1047-1052.
Budrukkar A. Accelerated partial breast irradiation: an advanced form of hypofractionation. J Cancer Res Ther. 2008;4:46-47.
Chen PY, Vicini FA, Benitez P, et al. Long-term cosmetic results and toxicity after accelerated partial-breast irradiation: a method of radiation delivery by interstitial brachytherapy for the treatment of early-stage breast carcinoma. Cancer. 2006;106:991-999.
Choi YJ, Ko EY, Han BK, et al. High-resolution ultrasonographic features of axillary lymph node metastasis in patients with breast cancer. Breast. 2009;18:119-122.
Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366(9503):2087-2106.
Cody HS3rd, Fey J, Akhurst T, et al. Complementarity of blue dye and isotope in sentinel node localization for breast cancer: univariate and multivariate analysis of 966 procedures. Ann Surg Oncol. 2001;8:13-19.
Cody HS3rd, Urban JA. Internal mammary node status: a major prognosticator in axillary node-negative breast cancer. Ann Surg Oncol. 1995;2:32-37.
Cote RJ, Peterson HF, Chaiwun B, et al. Role of immunohistochemical detection of lymph-node metastases in management of breast cancer, for the International Breast Cancer Study Group. Lancet. 1999;354:896-900.
Cox BW, Horst KC, Thornton S, Dirbas FM. Impact of increasing margin around the lumpectomy cavity to define the planning target volume for 3D conformal external beam accelerated partial breast irradiation. Med Dosim. 2007;32:254-262.
Crivellaro M, Senna G, Dama A, et al. Anaphylaxis due to patent blue dye during lymphography, with negative skin prick test. J Investig Allergol Clin Immunol. 2003;13:71-72.
Damle S, Teal CB. Can axillary lymph node dissection be safely omitted for early-stage breast cancer patients with sentinel lymph node micrometastasis? Ann Surg Oncol. 2009;16(12):3215-3216.
Dang CM, Zaghiyan K, Karlan SR, Phillips EH. Increased use of MRI for breast cancer surveillance and staging is not associated with increased rate of mastectomy. Am Surg. 2009;75:937-940.
Denison CM, Ward VL, Lester SC, et al. Epidermal inclusion cysts of the breast: three lesions with calcifications. Radiology. 1997;204:493-496.
Dershaw DD, Shank B, Reisinger S. Mammographic findings after breast cancer treatment with local excision and definitive irradiation. Radiology. 1987;164:455-461.
Dirbas FM. Accelerated partial breast irradiation: where do we stand? J Natl Compr Canc Netw. 2009;7:215-225.
Dirbas FM, Jeffrey SS, Goffinet DR. The evolution of accelerated, partial breast irradiation as a potential treatment option for women with newly diagnosed breast cancer considering breast conservation. Cancer Biother Radiopharm. 2004;19:673-705.
Dowlatshahi K, Fan M, Anderson JM, Bloom KJ. Occult metastases in sentinel nodes of 200 patients with operable breast cancer. Ann Surg Oncol. 2001;8:675-681.
Dowlatshahi K, Fan M, Snider HC, Habib FA. Lymph node micrometastases from breast carcinoma: reviewing the dilemma. Cancer. 1997;80:1188-1197.
Dupont EL, Kuhn MA, McCann C, et al. The role of sentinel lymph node biopsy in women undergoing prophylactic mastectomy. Am J Surg. 2000;180:274-277.
Evans SB, Kaufman SA, Price LL, et al. Persistent seroma after intraoperative placement of MammoSite for accelerated partial breast irradiation: incidence, pathologic anatomy, and contributing factors. Int J Radiat Oncol Biol Phys. 2006;65:333-339.
Fischer U, Kopka L, Grabbe E. Breast carcinoma: effect of preoperative contrast-enhanced MR imaging on the therapeutic approach. Radiology. 1999;213:881-888.
Fisher B, Dignam J, Wolmark N, et al. Lumpectomy and radiation therapy for the treatment of intraductal breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-17. J Clin Oncol. 1998;16:441-452.
Freedman GM, Fowble BL, Nicolaou N, et al. Should internal mammary lymph nodes in breast cancer be a target for the radiation oncologist? Int J Radiat Oncol Biol Phys. 2000;46:805-814.
Garcia-Barros M, Paris F, Cordon-Cardo C, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300:1155-1159.
Godinez J, Gombos EC, Chikarmane SA, et al. Breast MRI in the evaluation of eligibility for accelerated partial breast irradiation. AJR Am J Roentgenol. 2008;191:272-277.
Golshan M, Martin WJ, Dowlatshahi K. Sentinel lymph node biopsy lowers the rate of lymphedema when compared with standard axillary lymph node dissection. Am Surg. 2003;69:209-212.
Gorechlad JW, McCabe EB, Higgins JH, et al. Screening for recurrences in patients treated with breast-conserving surgery: is there a role for MRI? Ann Surg Oncol. 2008;15:1703-1709.
Goyal S, Khan AJ, Vicini F, et al. Factors associated with optimal cosmetic results at 36 months in patients treated with accelerated partial breast irradiation (APBI) on the American Society of Breast Surgeons (ASBrS) MammoSite Breast Brachytherapy Registry Trial. Ann Surg Oncol. 2009;16:2450-2458.
Heywang SH, Hilbertz T, Beck R, et al. Gd-DTPA enhanced MR imaging of the breast in patients with postoperative scarring and silicone implants. J Comput Assist Tomogr. 1990;14:348-356.
Hill AD, Tran KN, Akhurst T, et al. Lessons learned from 500 cases of lymphatic mapping for breast cancer. Ann Surg. 1999;229:528-535.
Holwitt DM, Swatske ME, Gillanders WE, et al. Scientific Presentation Award: The combination of axillary ultrasound and ultrasound-guided biopsy is an accurate predictor of axillary stage in clinically node-negative breast cancer patients. Am J Surg. 2008;196:477-482.
Horst KC, Ikeda DM, Birdwell RL, et al. Breast magnetic resonance imaging alters patient selection for accelerated, partial breast irradiation. Proceedings of the American Society for Therapeutic Radiology and Oncology 47th annual meeting. Int J Radiat Oncol Biol Phys. 2005;63(Suppl 1):S4-S5.
Houssami N, Hayes DF. Review of preoperative magnetic resonance imaging (MRI) in breast cancer: should MRI be performed on all women with newly diagnosed, early stage breast cancer? CA Cancer J Clin. 2009;59:290-302.
Huvos AG, Hutter RV, Berg JW. Significance of axillary macrometastases and micrometastases in mammary cancer. Ann Surg. 1971;173:44-46.
Jannink I, Fan M, Nagy S, et al. Serial sectioning of sentinel nodes in patients with breast cancer: a pilot study. Ann Surg Oncol. 1998;5:310-314.
Jothy Basu KS, Bahl A, Subramani V, et al. Normal tissue complication probability of fibrosis in radiotherapy of breast cancer: accelerated partial breast irradiation vs conventional external-beam radiotherapy. J Cancer Res Ther. 2008;4:126-130.
Jozsef G, Luxton G, Formenti SC. Application of radiosurgery principles to a target in the breast: a dosimetric study. Med Phys. 2000;27:1005-1110.
Karamlou T, Johnson NM, Chan B, et al. Accuracy of intraoperative touch imprint cytologic analysis of sentinel lymph nodes in breast cancer. Am J Surg. 2003;185:425-428.
Keshtgar MR, Ell PJ. Clinical role of sentinel lymph node biopsy in breast cancer. Lancet Oncol. 2002;3:105-110.
Kiluk JV, Ly QP, Meade T, et al. Axillary recurrence rate following negative sentinel node biopsy for invasive breast cancer: long-term follow-up. Ann Surg Oncol. Sept. 24, 2009.
Krag D, Weaver D, Ashikaga T, et al. The sentinel node in breast cancer—a multicenter validation study. N Engl J Med. 1998;339:941-946.
Kuzmiak CM, Zeng D, Cole E, Pisano ED. Mammographic findings of partial breast irradiation. Acad Radiol. 2009;16(7):819-825.
Lacour J, Le M, Caceres E, et al. Radical mastectomy versus radical mastectomy plus internal mammary dissection. Ten year results of an international cooperative trial in breast cancer. Cancer. 1983;51:1941-1943.
Lagios MD. Clinical significance of immunohistochemically detectable epithelial cells in sentinel lymph node and bone marrow in breast cancer. J Surg Oncol. 2003;83:1-4.
Langer I, Guller U, Viehl CT, et al. Axillary lymph node dissection for sentinel lymph node micrometastases may be safely omitted in early-stage breast cancer patients: long-term outcomes of a prospective study. Ann Surg Oncol. 2009;16(12):3366-3374.
Lehman CD, DeMartini W, Anderson BO, Edge SB. Indications for breast MRI in the patient with newly diagnosed breast cancer. J Natl Compr Canc Netw. 2009;7:193-201.
Liberman L. Lymphoscintigraphy for lymphatic mapping in breast carcinoma. Radiology. 2003;228:313-315.
Liberman L, Van Zee KJ, Dershaw DD, et al. Mammographic features of local recurrence in women who have undergone breast-conserving therapy for ductal carcinoma in situ. AJR Am J Roentgenol. 1997;168:489-493.
Lu WL, Jansen L, Post WJ, et al. Impact on survival of early detection of isolated breast recurrences after the primary treatment for breast cancer: a meta-analysis. Breast Cancer Res Treat. 2009;114:403-412.
Mamounas EP. Sentinel lymph node biopsy after neoadjuvant systemic therapy. Surg Clin North Am. 2003;83:931-942.
Mariani L, Salvadori B, Marubini E, et al. Ten year results of a randomised trial comparing two conservative treatment strategies for small size breast cancer. Eur J Cancer. 1998;34:1156-1162.
Martin RC, Derossis AM, Fey J, et al. Intradermal isotope injection is superior to intramammary in sentinel node biopsy for breast cancer. Surgery. 2001;130:432-438.
McCarter MD, Yeung H, Fey J, et al. The breast cancer patient with multiple sentinel nodes: when to stop? J Am Coll Surg. 2001;192:692-697.
McCarter MD, Yeung H, Yeh S, et al. Localization of the sentinel node in breast cancer: identical results with same-day and day-before isotope injection. Ann Surg Oncol. 2001;8:682-686.
McMasters KM, Chao C, Wong SL, et al. Sentinel lymph node biopsy in patients with ductal carcinoma in situ: a proposal. Cancer. 2002;95:15-20.
McMasters KM, Tuttle TM, Carlson DJ, et al. Sentinel lymph node biopsy for breast cancer: a suitable alternative to routine axillary dissection in multi-institutional practice when optimal technique is used. J Clin Oncol. 2000;18:2560-2566.
McMasters KM, Wong SL, Martin RC2nd, et al. Dermal injection of radioactive colloid is superior to peritumoral injection for breast cancer sentinel lymph node biopsy: results of a multi-institutional study. Ann Surg. 2001;233:676-687.
Mendelson EB. Evaluation of the postoperative breast. Radiol Clin North Am. 1992;30:107-138.
Montague ED. Conservation surgery and radiation therapy in the treatment of operable breast cancer. Cancer. 1984;53(Suppl):700-704.
Montague ED, Fletcher GH. Local regional effectiveness of surgery and radiation therapy in the treatment of breast cancer. Cancer. 1985;55(Suppl):2266-2272.
Morris EA, Schwartz LH, Drotman MB, et al. Evaluation of pectoralis major muscle in patients with posterior breast tumors on breast MR images: early experience. Radiology. 2000;214:67-72.
Morrow M. Should routine breast cancer staging include MRI? Nat Clin Pract Oncol. 2009;6:72-73.
Mortellaro VE, Marshall J, Singer L, et al. Magnetic resonance imaging for axillary staging in patients with breast cancer. J Magn Reson Imaging. 2009;30:309-312.
Mumtaz H, Hall-Craggs MA, Davidson T, et al. Staging of symptomatic primary breast cancer with MR imaging. AJR Am J Roentgenol. 1997;169:417-424.
Nathanson SD, Wachna DL, Gilman D, et al. Pathways of lymphatic drainage from the breast. Ann Surg Oncol. 2001;8:837-843.
Nelson JC, Beitsch PD, Vicini FA, et al. Four-year clinical update from the American Society of Breast Surgeons MammoSite brachytherapy trial. Am J Surg. 2009;198(1):83-91.
Offersen BV, Overgaard M, Kroman N, Overgaard J. Accelerated partial breast irradiation as part of breast conserving therapy of early breast carcinoma: a systematic review. Radiother Oncol. 2009;90:1-13.
Ollila DW, Klauber-DeMore N, Tesche LJ, et al. Feasibility of breast preserving therapy with single fraction in situ radiotherapy delivered intraoperatively. Ann Surg Oncol. 2007;14:660-669.
Orel SG, Schnall MD, Powell CM, et al. Staging of suspected breast cancer: effect of MR imaging and MR-guided biopsy. Radiology. 1995;196:115-122.
Orel SG, Troupin RH, Patterson EA, Fowble BL. Breast cancer recurrence after lumpectomy and irradiation: role of mammography in detection. Radiology. 1992;183:201-206.
Pendas S, Dauway E, Giuliano R, et al. Sentinel node biopsy in ductal carcinoma in situ patients. Ann Surg Oncol. 2000;7:15-20.
Pernas S, Gil M, Benitez A, et al. Avoiding axillary treatment in sentinel lymph node micrometastases of breast cancer: a prospective analysis of axillary or distant recurrence. Ann Surg Oncol. 2010;17:772-777.
Philpotts LE, Lee CH, Haffty BG, et al. Mammographic findings of recurrent breast cancer after lumpectomy and radiation therapy: comparison with the primary tumor. Radiology. 1996;201:767-771.
Polgar C, Strnad V, Major T. Brachytherapy for partial breast irradiation: the European experience. Semin Radiat Oncol. 2005;15:116-122.
Rao R, Lilley L, Andrews V, et al. Axillary staging by percutaneous biopsy: sensitivity of fine-needle aspiration versus core needle biopsy. Ann Surg Oncol. 2009;16:1170-1175.
Sandrucci S, Mussa A. Sentinel lymph node biopsy and axillary staging of T1–T2 N0 breast cancer: a multicenter study. Semin Surg Oncol. 1998;15:278-283.
Schwartz GF, Giuliano AE, Veronesi U. Proceedings of the consensus conference of the role of sentinel lymph node biopsy in carcinoma of the breast, 19–22 April 2001, Philadelphia. Breast J. 2002;8:124-138.
Shen P, Glass EC, DiFronzo LA, Giuliano AE. Dermal versus intraparenchymal lymphoscintigraphy of the breast. Ann Surg Oncol. 2001;8:241-248.
Sickles EA, Herzog KA. Mammography of the postsurgical breast. AJR Am J Roentgenol. 1981;136:585-588.
Singletary SE, Greene FL. Revision of breast cancer staging: the 6th edition of the TNM Classification. Semin Surg Oncol. 2003;21:53-59.
Smith BD, Arthur DW, Buchholz TA, et al. Accelerated partial breast irradiation consensus statement from the American Society for Radiation Oncology (ASTRO). Int J Radiat Oncol Biol Phys. 2009;74:987-1001.
Steinhoff MM. Axillary node micrometastases: detection and biologic significance. Breast J. 1999;5:325-329.
Tendulkar RD, Chellman-Jeffers M, Rybicki LA, et al. Preoperative breast magnetic resonance imaging in early breast cancer: implications for partial breast irradiation. Cancer. 2009;115:1621-1630.
Tuli R, Christodouleas J, Roberts L, et al. Prognostic indicators following ipsilateral tumor recurrence in patients treated with breast-conserving therapy. Am J Surg. 2009;198:557-561.
Turner RR, Ollila DW, Krasne DL, Giuliano AE. Histopathologic validation of the sentinel lymph node hypothesis for breast carcinoma. Ann Surg. 1997;226:271-278.
Veronesi U, Luini A, Del Vecchio M, et al. Radiotherapy after breast-preserving surgery in women with localized cancer of the breast. N Engl J Med. 1993;328:1587-1591.
Veronesi U, Orecchia R, Luini A, et al. Full-dose intraoperative radiotherapy with electrons during breast-conserving surgery: experience with 590 cases. Ann Surg. 2005;242:101-106.
Veronesi U, Paganelli G, Viale G, et al. Sentinel lymph node biopsy and axillary dissection in breast cancer: results in a large series. J Natl Cancer Inst. 1999;91:368-373.
Veronesi U, Paganelli G, Viale G, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med. 2003;349:546-553.
Veronesi U, Zurrida S, Mazzarol G, Viale G. Extensive frozen section examination of axillary sentinel nodes to determine selective axillary dissection. World J Surg. 2001;25:806-808.
Vicini FA, Kestin L, Huang R, Martinez A. Does local recurrence affect the rate of distant metastases and survival in patients with early-stage breast carcinoma treated with breast-conserving therapy? Cancer. 2003;97:910-919.
Vicini FA, Remouchamps V, Wallace M, et al. Ongoing clinical experience utilizing 3D conformal external beam radiotherapy to deliver partial-breast irradiation in patients with early-stage breast cancer treated with breast-conserving therapy. Int J Radiat Oncol Biol Phys. 2003;57:1247-1253.
Wazer DE, Kaufman S, Cuttino L, et al. Accelerated partial breast irradiation: an analysis of variables associated with late toxicity and long-term cosmetic outcome after high-dose-rate interstitial brachytherapy. Int J Radiat Oncol Biol Phys. 2006;64:489-495.
Yarbro JW, Page DL, Fielding LP, et al. American Joint Committee on Cancer prognostic factors consensus conference. Cancer. 1999;86:2436-2446.
Zafrani B, Fourquet A, Vilcoq JR, et al. Conservative management of intraductal breast carcinoma with tumorectomy and radiation therapy. Cancer. 1986;57:1299-1301.
8-1. Fill in the contraindications to whole-breast radiation therapy.
_________________________________________
_________________________________________
_________________________________________
_________________________________________
_________________________________________
_________________________________________
_________________________________________
8-3. Fill in the sentinel lymph node biopsy identification techniques.
_________________________________________
_________________________________________
8-4. Fill in the enhancement on MRI after biopsy.
_________________________________________
_________________________________________
8-5. Fill in the normal postoperative findings for benign disease.
_________________________________________
_________________________________________
_________________________________________
_________________________________________
_________________________________________
8-6. Fill in the postbiopsy ultrasound findings.
_________________________________________
_________________________________________
8-8. Fill in the mammographic findings after breast conservation and radiation therapy.
_________________________________________
_________________________________________
_________________________________________
_________________________________________
_________________________________________
_________________________________________
_________________________________________
8-10. Fill in the TNM staging classification for breast cancer.
| _________ | _____________________________________ |
| _________ | _____________________________________ |
| _________ | _____________________________________ |
| _________ | _____________________________________ |
| _________ | _____________________________________ |
| _________ | _____________________________________ |
| _________ | _____________________________________ |
| _________ | _____________________________________ |
| _________ | _____________________________________ |
| _________ | _____________________________________ |
| _________ | _____________________________________ |
| _________ | _____________________________________ |
| _________ | _____________________________________ |
| _________ | _____________________________________ |
| _________ | _____________________________________ |
| _________ | _____________________________________ |
| _________ | _____________________________________ |
| _________ | _____________________________________ |
| _________ | _____________________________________ |
| _________ | _____________________________________ |
| _________ | _____________________________________ |
| _________ | _____________________________________ |
| _________ | _____________________________________ |
| _________ | _____________________________________ |
| _________ | _____________________________________ |
| _________ | _____________________________________ |
| _________ | _____________________________________ |
| _________ | _____________________________________ |
| _________ | _____________________________________ |
| _________ | _____________________________________ |
| _________ | _____________________________________ |
| _________ | _____________________________________ |
| _________ | _____________________________________ |
| _________ | _____________________________________ |
| _________ | _____________________________________ |
| _________________________________________________ | |
| _________ | _____________________________________ |
| _________ | _____________________________________ |
| _________ | _____________________________________ |
| _________ | _____________________________________ |
| _________ | _____________________________________ |
| _________ | _____________________________________ |
| _________ | _____________________________________ |
| _________ | _____________________________________ |
| _________ | _____________________________________ |
| _________ | _____________________________________ |
| _________ | _____________________________________ |
| _________ | _____________________________________ |
| _________ | _____________________________________ |
| _________ | _____________________________________ |
| _________ | _____________________________________ |
| _________ | _____________________________________ |
Anatomic Stage/Prognostic Group
8-11. Fill in the location of lymph nodes draining the breast.
| LEVEL | LOCATION |
|---|---|
| _________ | _____________________________________________ |
| _________ | _____________________________________________ |
| _________ | _____________________________________________ |
8-12. Fill in the breast imaging relating to breast-conserving therapy.
| TIMING | REASON | TECHNIQUE |
|---|---|---|
| _________ | ________________ | ________________ |
| ________________ | ||
| ________________ | ________________ | |
| _________ | ________________ | ________________ |
| ________________ | ||
| ________________ | ________________ | |
| ________________ | ||
| _________ | ________________ | ________________ |
| ________________ | ||
| _________ | ________________ | ________________ |
| ________________ | ________________ | |
| ________________ | ________________ | |
| ________________ |