10
Breast cancer
treatment of uncommon diseases
Pregnancy-associated breast cancer
Pregnancy-associated breast cancer (PABC) includes breast cancer diagnosed during pregnancy, up to 1 year after delivery, or during lactation. PABC is rare; it constitutes 0.2–3.8% of all breast cancers, occurring in 1 in 10 000 to 1 in 3000 pregnancies.1 Nonetheless, PABC is the second most common malignancy in pregnant women (cervical cancer is the most common). As more women delay childbearing, the incidence of PABC may increase.2
Age for age, compared to non-pregnant women with breast cancer, pregnant women with breast cancer tend to be diagnosed at later stages.3 The aetiology of this phenomenon is unclear. One possible explanation is that PABC may have a more aggressive biology. Alternatively, the significant anatomical and physiological changes in the breast during pregnancy may delay diagnosis.
Pathology
Histologically, PABC and non-PABC are similar. Compared to non-PABC, PABC tends to be oestrogen- and progesterone-receptor negative, while data on expression of human epidermal growth factor receptor (HER-2)/neu are conflicting.4,5 PABC stains strongly for Ki67 and p53; the clinical significance of these findings is undetermined.6
Clinical presentation
The majority of PABCs present as a palpable breast mass. Gravid breasts undergo significant ductal and lobular proliferation under the influence of elevated levels of oestrogen, progesterone, prolactin and chorionic gonadotrophin. Mammary blood flow increases by 180%; the breast may become nodular and double in weight and volume.7 Therefore, clinical breast examinations may be difficult, and may contribute to a delay in diagnosis and consequential poorer prognosis. Moreover, the differential diagnosis of breast mass in pregnant women is broad, including lactating adenoma, fibroadenoma, cystic disease, lobular hyperplasia, galactocoele, abscess, lipoma, hamartoma and PABC; 70–80% of breast biopsies in pregnant women are benign.8
Because PABC is rare, detailed evaluations of risk factors for PABC development are lacking. One study of 383 patients, including 192 patients with PABC, found that patients with PABC were 300% more likely to have a family history of breast cancer than age-matched, non-pregnant, non-lactating counterparts.3
Diagnosis
As in all women, dominant breast masses in pregnant women should be investigated with imaging and biopsies. While screening mammography is not routine for pregnant women, diagnostic mammography may be useful and can be performed safely with the use of abdominal shielding. Despite the fact that women with PABC are young and have dense, proliferative breasts, diagnostic mammograms detect 78–90% of palpable PABCs.9
However, core-needle biopsy is not without risk. In addition to bleeding, milk fistulas are known rare complications.10 Therefore, core-needle biopsy ought to be used with caution for the diagnoses of centrally located lesions in lactating women.
Metastatic work-up for non-PABC and PABC are similar and are guided by clinical stage and constellation of symptoms. Chest radiography with abdominal shielding is safe. Computed tomography (CT) scans are generally discouraged due to high radiation exposure. While magnetic resonance imaging (MRI) does not utilise ionising radiation – and, in fact, has been utilised in foetal imaging in utero – gadolinium is known to cross the placenta and to affect foetuses adversely in animals and is not recommended.5,11 No studies have investigated the role of position emission tomography (PET) in pregnant patients; in fact, pregnancy is often an exclusion criterion. Bone scans using lower doses of radioisotope have been performed in pregnant women, but are not generally recommended.11
Treatment (Fig. 10.1)
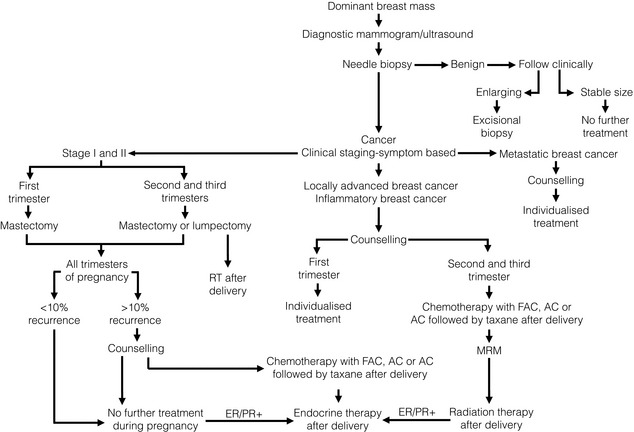
Figure 10.1 Management algorithm for the pregnant woman with a dominant breast mass. ER/PR+, oestrogen and progesterone receptor positive; FAC, fluorouracil, doxorubicin and cyclophosphamide; MRM, Modified Radical Mastectomy.
A landmark report of 2565 patients demonstrated no differences in congenital abnormalities between pregnant women who did and did not undergo surgery.12 However, surgery during pregnancy was associated with an increased rate of spontaneous abortion. The risk of spontaneous abortion is highest in the first trimester, and the patient and surgeon may choose to defer surgery until after the 12th week of pregnancy.
Axillary staging
Previously, sentinel lymph node biopsy (SLNB) was not recommended in gravid or lactating women. Lymphazurin blue, patent blue V and methylene blue, the most commonly used dyes, have not been studied in pregnant women and their safety has not been established. Technetium-99 m, the most commonly used radioactive tracer, involves low doses of radiation (1.85–3.7 MBq). Gentilini et al. found that sentinel lymph nodes were successfully identified in all patients, foetal radiation exposure was minimal and there were no adverse foetal outcomes.13 Although there are no medical contraindications to lymphatic mapping with radioactivity during pregnancy, it remains to be seen if this procedure will be widely adopted.
Systemic therapy
In general, chemotherapy is contraindicated during the first trimester, for it exposes the foetus to an increased risk of abortion and malformation. In a small series, Ebert et al. noted that all the abortions that occurred in women during pregnancy were in those women who had received chemotherapy during the first trimester of pregnancy; while chemotherapy during the first trimester resulted in a 14–19% risk of foetal malformation, chemotherapy during the second trimester resulted in a 1.3% risk.14 A prospective cohort study of 24 women, who received 5-fluorouracil, doxorubicin and cyclophosphamide during the second and third trimesters, found no congenital malformations, or short- or long-term complications in the infants; there were no stillbirths, miscarriages or perinatal deaths. Except for one child with Down’s syndrome, the vast majority of infants demonstrated ‘normal development’ and eventually grew into functional school-aged children.15,16
During pregnancy, methotrexate should be avoided due to a risk of associated abnormalities. A review examining the use of taxanes, vinorelbine and trastuzumab demonstrated that taxanes and vinorelbine were safe for mother and offspring in the limited experience available, but trastuzumab was associated with anhydramnios in 50% of cases.17 Endocrine therapy is not recommended during pregnancy. Tamoxifen has been associated with spontaneous abortions, teratogenicity and foetal demise.18
In general, for women with PABC with positive lymph nodes, chemotherapy is ideally administered after the first trimester, but within 6 weeks of surgery. For patients diagnosed early in the first trimester, this may be impossible. For women with PABC, negative lymph nodes and low-risk cancers (where the survival benefit of chemotherapy is 5% or less), treatment during pregnancy is usually avoided. For women with PABC also having negative lymph nodes and high-risk cancers, treatment decisions must be made on an individual basis. Chemotherapy ought to be stopped 3 weeks prior to delivery to avoid myelosuppression and septic complications in mothers and their offspring.6
Termination of and future pregnancy
Historically, the prognosis of PABC was considered so dismal that therapeutic abortion was advocated for all women. However, compared to women who terminated their pregnancies, women who continued their pregnancies did not demonstrate worsened overall or disease-free survivals.1,5 There are currently no formal recommendations for therapeutic abortions in patients with PABC. However, as discussed, therapeutic abortion can simplify treatment in patients with locally advanced and inflammatory breast cancers. Women should be counselled that chemotherapy may adversely affect future fertility.
Though fertility may be hindered by patient age and chemotherapy, retrospective studies demonstrated that pregnancy after diagnosis and treatment of breast cancer was safe. Gemignani et al. reported that compared to patients treated for PABC who did not become pregnant, those who did had equivalent or better survival,1 although this may be a result of selection bias. A population-based study conducted by the Danish Breast Cancer Cooperative Group found no difference in survival amongst 371 patients with PABC who became pregnant after treatment and 9865 patients with PABC who did not.19
Despite the fact that the rate of relapse is fairly constant for the first 10 years after treatment, some experts advocate waiting 2–5 years for future pregnancies,6 contending that this affords a period during which recurrences can become manifest – up to 2 years for high-risk cancers, up to 5 years for low-risk cancers. However, a population-based report showed that for women with localised disease and good prognosis, conception 6 months after completion of treatment was unlikely to reduce survival.20
Prognosis
Because of the anatomical and physiological changes of the gravid and lactating breast, PABC tends to be diagnosed at a more advanced stage. In one study, fewer than 20% were diagnosed prior to delivery, median tumour size at diagnosis was 3.5 cm, and 62% of PABC patients had lymph-node metastases compared to 39% of matched controls.1 Additionally, relative to non-PABC patients, PABC patients are more likely to have larger tumours, vascular invasion and distant metastasis.4
Historically, it was believed that, compared to non-PABC, PABC was intrinsically more aggressive and thereby carried a worse prognosis. However, recent studies have demonstrated this may not be correct. A review of 104 PABC and 564 non-PABC patients demonstrated that PABC had a more advanced T classification, N classification and stage.21 Moreover, the two groups did not demonstrate any differences in locoregional recurrence, distant metastases or overall survival. For patients with PABC, the timing of diagnosis and treatment was the only factor associated with overall survival; those who had prompt diagnosis and treatment had better overall survival than those who had delayed diagnosis and treatment. In an older study, Gemignani et al. found that, for stage I and stage II disease, PABC and non-PABC patients had similar survivals.1 However, compared to non-PABC, there was a trend towards a worse prognosis in stage III and stage IV PABC. Similarly, with negative lymph nodes, PABC patients had the same 5-year survival as non-PABC controls; however, for patients with positive lymph nodes, PABC and non-PABC patients had 5-year survivals of 47% and 59%, respectively.
Male breast cancer (Fig. 10.2)
In the 14th century, John of Arderne, an English surgeon, reported the first case of male breast cancer (MBC).22 He cared for a priest who had progressive nipple–areolar ulceration. Almost certainly he died of this disease.
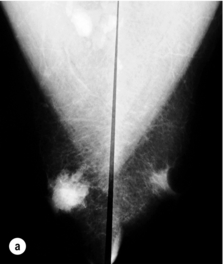
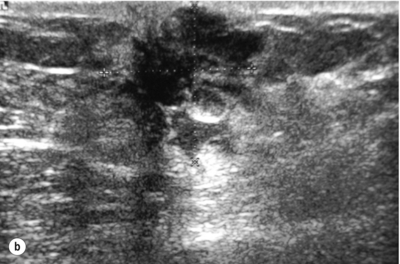
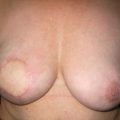
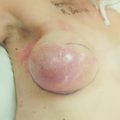
Figure 10.2 (a) Mammogram of male breast cancer in right breast. (b) Ultrasound of male breast cancer in right breast.
In the West, MBC accounts for less than 1% of all breast cancers and for 0.1% of cancer deaths in men.23 These rates have remained stable in the second half of the 20th century.24 However, rates of MBC have recently increased.25 Though a complete explanation is elusive, this is likely due in part to an increase in the number of men diagnosed with ductal carcinoma in situ (DCIS) between 1973 and 2001.26 Interestingly, there is an MBC ‘belt’ that extends from the Atlantic to the Indian Oceans in sub-Saharan Africa.27 In Tanzania, 6% of breast cancers are diagnosed in men.
Pathology
Almost all pathological types of breast cancer found in women have been described in men. Ninety per cent of MBCs are invasive; of these, 80% are ductal, 5% are papillary and 1% are lobular.28 The scarcity of lobular carcinoma probably reflects the paucity of terminal lobular units in male mammary tissue. Paget’s disease of the breast and inflammatory breast cancer are as frequent in men as they are in women.29 Less common subtypes, i.e. medullary, mucinous, squamous and tubular, have also been reported in men, but at lower frequencies than in women. Ten per cent of MBCs are non-invasive; almost all are DCIS,28 and most are of the papillary subtype and of low or intermediate grade.30 Lobular carcinoma in situ is rare.
When matched for age, stage and grade, men with breast cancer have a higher rate of oestrogen receptor positivity than women with breast cancer. A 25-year review of the Surveillance Epidemiology and End Results (SEER) database, which included 2357 MBCs, demonstrated oestrogen and progesterone positivity in 90% and 80% of breast cancers in men, and in 76% and 67% of breast cancers in women.31 Data on the overexpression of erbB-2 or HER-2/neu, p53, bcl-2, cyclin-D1 and epidermal growth factor receptor in MBC are limited, conflicting and inconclusive.
Clinical presentation
Though MBC can occur at any age, its incidence increases with age. The average age of diagnosis of breast cancer in men is approximately 10 years later than in women. Eighty-five per cent of MBCs present as a painless mass32 and most MBCs are centrally located, with up to 50% demonstrating nipple retraction, discharge, pain or ulceration.33 The time from the onset of symptoms to diagnosis is longer in men than in women – approximately 22 months. As a result, men often present at later stages.34
There are multiple risk factors for the development of MBC. Hormonal misbalance is one of these. Men with a history of undescended testes, congenital inguinal hernia, orchiectomy, orchitis, testicular injury, infertility, Klinefelter’s syndrome, and obesity and cirrhosis (both of which induce a hyper-oestrogenic milieu) are at elevated risk.35,36 In particular, Klinefelter’s syndrome, which is characterised by a 47XXY karyotype, small testes and gynaecomastia, is associated with a 50-fold elevated risk.
Family history is another important risk factor. Between 15% and 20% of male patients with breast cancer have a family history of disease. The odds ratio for MBC increases with a positive family history and continues to increase as the number of affected first-degree relatives increases.37,38 These factors probably reflect an increasing risk of BRCA1 and BRCA2 mutations, which predispose both men and women to breast cancer. Previous reports have focused on the association between MBC and BRCA2 mutations. However, a recent report suggests that BRCA1 and BRCA2 carry equivalent elevations in risk.39 Male BRCA2 mutation carriers have a 6.3% cumulative risk of breast cancer.37,38 In the United States, 4% of MBCs are in BRCA2 mutation carriers; in Iceland, where a ‘founder effect’ is present, 40% of MBCs carry a mutation in BRCA2.37
As in women, radiation exposure is a risk factor for the development of breast cancer in men.36 One evaluation of a national database of 324 799 men without breast cancer and 121 men with breast cancer found that a history of a bone fracture and obesity were associated with the development of MBC; relative risk ratios were 2.2 and 1.79 respectively.40 Though gynaecomastia in and of itself was once thought to be a risk factor, it is no longer considered to be.34,36 Indeed, it may be the case that obesity is associated with both gynaecomastia and MBC.
Treatment
In a retrospective review, Scott-Conner et al. found significant differences in the treatment of matched men and women with breast cancer.41 Given anatomical considerations, compared to women, men are less likely to undergo breast-conserving surgery. Also, compared to women, men who undergo breast-conserving surgery are less likely to receive adjuvant radiotherapy. Men are also less likely to receive adjuvant chemotherapy.
Local therapy
Axillary staging guidelines are similar for men and for women. If axillary lymph nodes are clinically negative, SLNB should be performed. Sensitivity and specificity of SLNB in women and men are similar.42 Clinically positive nodes should undergo core biopsy or FNA for histological confirmation, which will allow axillary dissection without an SLNB.
Adjuvant therapy
The same guidelines used to make recommendations for adjuvant therapy in women with breast cancer should be used in men. Two comprehensive reviews confirmed the benefits of adjuvant chemotherapy and hormonal treatment in MBC.43,44 Although no trials have been conducted to determine indications for postmastectomy radiation for MBC, it has been reported to reduce rates of local recurrence, and guidelines for women are usually applied for men.45
Treatment of metastatic disease
For hormone receptor-positive metastatic MBC, endocrine therapy is the mainstay of treatment. In the past, ablative surgeries, including orchiectomy, were used. A variety of hormonal therapies have been used, including exogenous steroids, androgens, anti-androgens, oestrogens, progestins, aminoglutethimide and tamoxifen, and may improve survival.46,47 Tamoxifen is the drug of choice. In men, the testes produce 20% and the periphery produces 80% of circulating oestrogens. Thus, aromatase inhibitors pose two potential problems in MBC, mitigating their efficacy. Firstly, they only partially block oestrogen production. Secondly, decreased levels of circulating oestrogens induce the hypothalamus to stimulate testicular hormone production. This may, in part, account for therapeutic resistance in aromatase inhibitors.48 A 2010 consensus conference concluded that aromatase inhibitors should only be used in men in conjunction with surgical or medical orchiectomy.49 A phase II trial examining the use of an aromatase inhibitor combined with goserelin acetate for androgen suppression is ongoing. For hormone receptor-negative MBC, chemotherapy is the mainstay of therapy.46
Prognosis
Overall, despite delays in presentation, diagnosis and treatment, stage for stage, women and men with breast cancer have the same prognosis, which continues to improve as new diagnostic methods and treatments evolve.50–52 The same factors predict outcome: node status, tumour size and grade, and hormone receptor status. As for women with breast cancer, axillary lymph node status – negative versus positive, and number of positive nodes – is the most important prognostic factor in men.53 In a study of 335 cases of MBC, node-negative patients had a 5-year survival rate of 90%, whereas node-positive patients had a 5-year survival rate of 65%.54 Also, 10-year survival for patients with one to three positive nodes was 44%; with four or more positive nodes, it was 14%.53 Giordano et al. reported that, for MBC, rates of 5-year survival were 76% for grade 1 tumours, 66% for grade 2 tumours and 43% for grade 3 tumours.31 Patients with oestrogen receptor- and progesterone receptor-positive tumours had significantly improved survival compared to those with oestrogen receptor- and progesterone receptor-negative tumours,55 reflecting differences in both biology and treatment.
Paget’s disease of the breast (Fig. 10.3)
In 1856, Alfred-Armand-Louis-Marie Velpeau, a French surgeon and anatomist, described a clinical condition in which patients suffered from bleeding and crusting ulcerations of the nipple and areola.56 Velpeau believed that this condition was primarily dermatological in nature, failing to recognise that more than 95% of women with what we now know as Paget’s disease of the breast have an underlying malignancy. Nearly two decades later, Sir James Paget, the British surgeon and pathologist, described this association.57 He described ‘an eczematous change in the skin of the nipple preceding an underlying mammary cancer’.
Pathology
In Paget’s disease of the breast, the epithelium of the nipple–areola complex displays two characteristic findings. First, Pagetoid cells are present – the sine qua non of Paget’s disease of the breast. These are large, round or oval cells with enlarged pleomorphic and hyperchromatic nuclei, prominent nucleoli and abundant, clear, pale cytoplasm. Second, reactive changes in the epidermis and dermis, such as lymphocytic infiltration and angiogenesis, are also seen, giving rise to the hyperaemic, exudative appearance characteristic of Paget’s disease of the breast.58
At present, there are two competing theories as to the pathogenesis of Paget’s disease of the breast. One suggests that Pagetoid cells are keratinocytes that have undergone malignant transformation. According to this theory, Paget’s disease of the breast represents an in situ carcinoma of the skin.59,60 This theory is supported by the observation that, often, overlying skin changes and underlying malignancy are discontinuous. Another theory suggests that cells migrate along basement membranes and enter the epidermis and dermis of the nipple–areola complex.61 Pagetoid cells and underlying carcinomas demonstrate similar immunohistochemical staining patterns, supporting this theory that it is cells from the cancer that migrate.62
Clinical presentation
Paget’s disease of the breast occurs in both men and women. However, it is rare, accounting for 0.7–4.9% of breast malignancies.59 Interestingly, age-adjusted rates of Paget’s disease of the breast peaked in 1985 and have steadily decreased from 1.31 to 0.64 per 100 000.63 The clear cause of this epidemiological phenomenon remains elusive, but it may be the case that as screening programmes are more widely available, clinically occult breast malignancies are detected prior to the development of dermatological nipple–areolar changes.
The first symptomatic manifestations are sensory – for example, lymphocytic infiltration and angiogenesis, which can produce burning or itching. Dermatological changes follow – crusty, erythematous flaking, and irregular, raised, scaly skin lesions may develop. Classically, these start within the nipple, spread to the areola and ultimately spread to the surrounding skin. This contrasts with eczema, which starts in the areola. As the disease progresses, bleeding, ulceration and destruction of the nipple–areola complex occur. Nipple discharge is rare and is usually a result of advanced local disease, rather than a consequence of Paget’s disease. In approximately half of patients with Pagetoid changes of the nipple, an underlying malignancy is palpable.64 Rarely, the underlying malignancy tethers the nipple– areola complex or adjacent skin, causing retractions and deformity of the natural contour of the breast.
In the absence of a palpable mass – in part because it is rare and has an innocuous appearance – misdiagnosis of Paget’s disease as eczema and treatment with topical steroids is common.60,65 Typically, the interval from the development of symptoms to definitive diagnosis and treatment is 10–12 months.58
Diagnosis
Eczematmous changes of the nipple–areola complex in the absence of a history suggestive of contact dermatitis should be biopsied. A punch biopsy can be performed and allows sampling of the epidermis, dermis and subdermis. If non-diagnostic, excisional biopsy is more invasive, but provides more tissue. Histology reveals Pagetoid cells. On immunohistochemistry, these cells stain for CK7, CAM-5.2, AE1/AE3 and S100; they do not stain for HMB-45 or keratins, differentiating them from melanoma.66 Almost 90% of Paget’s cells are HER-2 positive.
As with all breast cancers, bilateral mammography and ultrasound are the initial steps in the imaging work-up. If a lesion is seen, it is investigated in standard fashion. However, the sensitivity of mammography in this setting is limited; in one series, mammography detected only 32% of underlying carcinomas.67 Breast MRI may be valuable in diagnosing clinically and mammographically occult underlying malignancies in patients with Paget’s disease of the breast, with case reports and series demonstrating identification of carcinomas.68,69 In a series of eight cases with a normal mammogram, MRI identified an underlying carcinoma in half.67 Moreover, Morrogh et al. showed that MRI demonstrated the extent of occult disease in six of seven patients, thereby guiding surgical planning and the decision whether to pursue total mastectomy or breast conservation.67,70
Treatment (Table 10.1)
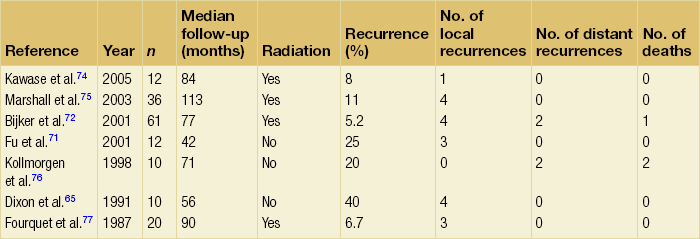
Surgery is the mainstay of treatment for Paget’s disease of the breast – except for patients with significant comorbidities. For all patients, resection of the nipple–areola complex is necessary; small studies have demonstrated that preservation of the nipple–areola resulted in unacceptably high rates of local recurrence.71 In patients with an identifiable primary tumour, the choice between total mastectomy and central lumpectomy is determined by the extent of disease. If disease is confined to the central breast, multiple small studies have demonstrated low rates of local recurrence after excision to negative margins and adjuvant radiotherapy. After a median follow-up of 6.4 years, one prospective study of 61 patients demonstrated a local recurrence rate of 5.2% after central lumpectomy to clear margins and 50 Gy of adjuvant radiotherapy.72 When cancer is present at a distance from the nipple–areola complex, mastectomy is the treatment of choice. In the absence of an identifiable tumour within the breast, Paget’s disease is considered to be ductal carcinoma in situ of the nipple–areolar region and can be treated with excision alone or excision and radiotherapy. In one small study of 48 patients, 40% of patients with Paget’s disease of the breast treated with excision alone experienced a local recurrence after 4.6 years of median follow-up.65
Other breast malignancies
Constituting 0.28% of all primary melanomas, primary melanoma of the breast is exceedingly infrequent.78 Melanoma is more likely to present in the breast as a result of a metastasis from a non-mammary primary tumour.79
Surgical excision is the primary treatment for melanoma of the breast. In the past, mastectomy was considered the procedure of choice. Currently, it is recommended that patients undergo wide-local excision to margins determined by the depth of the lesion. SLNB is indicated for thin melanomas with poor prognostic features and for all intermediate and deep melanomas.80 Clinically positive nodes and positive sentinel nodes should prompt axillary dissection.
Primary breast lymphoma (Fig. 10.4)
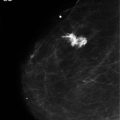
Primary breast lymphoma (PBL) constitutes 0.14% of all breast malignancies and 0.60–2.00% of all non-Hodgkin’s lymphomas. It arises from mammary lymphoid tissue and, by definition, is localised to the breast and its draining lymph node basins.81,82 The most common subtype is diffuse large B-cell lymphoma.81,82 PBLs are categorised using the Working Classification and the Ann Arbor Classification of lymphomas.
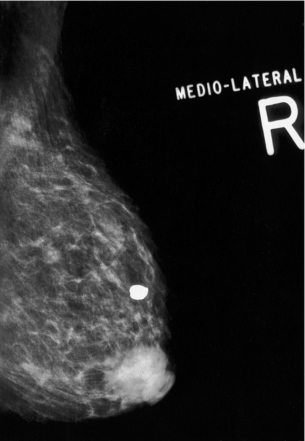
Figure 10.4 Mammogram of a primary breast lymphoma in the subareolar space. The appearance is indistinguishable from that of a primary adenocarcinoma.
The mean age of patients with PBL is 65 years.81,82 These lesions often present as a painless, enlarging and rubbery mass. Because mammographic and sonographic findings tend to be non-specific, PBL is usually not suspected (in the absence of constitutional symptoms) until a core-needle biopsy is obtained. Once the diagnosis is known, a complete history and physical examination should be obtained, focusing on constitutional symptoms and evaluation of lymph node basins. Additionally, CT of the chest, abdomen and pelvis, and bone marrow biopsy, are needed for adequate staging, though according to one pathological review, gene rearrangement analysis precluded the need for bone-marrow biopsy and histological evaluation in certain populations.81
In general, surgery is neither necessary nor recommended for PBL. According to single-institution reports, surgery either alone or in conjunction with radiation results in increased rates of local, regional or distant recurrence, and decreased rates of survival.82,83 Surgery is no longer indicated for PBL except for situations in which the core-needle biopsy proves to be non-diagnostic. Chemotherapy directed to the histological type and targeted radiotherapy are the mainstays of treatment, and have proven to be modestly effective.84–86 The role of rituximab continues to be evaluated.87
As with all lymphomas, the International Prognostic Index is helpful in predicting the aggressiveness of PBLs.88 The 2-year overall survival of mammary lymphomas is 63%, which is consistent with the overall survival of extramammary lymphomas.81 Immunohistochemical studies of PBLs have shown that tumours co-expressing bcl-6 and CD-10 have a better prognosis than those that do not.83
Angiosarcoma of the breast (Fig. 10.5)
Angiosarcoma of the breast is rare, constituting 0.05% of all breast malignancies.89 Primary angiosarcoma of the breast occurs sporadically; secondary angiosarcoma of the breast occurs in the setting of a history of radiotherapy and lymphoedema.89 For an angiosarcoma of the breast to be considered radiotherapy induced, it must occur within the radiated field and have a long latency. On average, the time interval from completion of radiotherapy to diagnosis of angiosarcoma of the breast is 75 months.90 The first report of post-radiotherapy angiosarcoma of the breast was in 1981.91 The incidence of angiosarcoma of the breast may be increasing – and may continue to increase – as more and more patients with breast cancer undergo breast-conserving therapy with irradiation.92
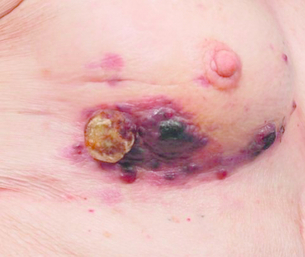
Figure 10.5 Appearance of a radiation-induced angiosarcoma on the inferior-lateral aspect of a breast treated for invasive ductal carcinoma with lumpectomy and radiotherapy.
Primary angiosarcoma of the breast usually presents as a mass, while post-radiotherapy angiosarcoma of the breast usually presents as multifocal, painless, red, blue, purple or black skin nodules (Fig. 10.5).93 The differential diagnosis of early skin lesions is fairly broad and includes benign radiation-associated skin changes, recurrent or de novo adenocarcinoma of the breast, and melanoma. Interestingly, the right breast is twice as likely to be affected as the left.88
Generally, mammography, ultrasound and MRI are not helpful.94 Full-thickness punch, or incisional or excisional biopsy are diagnostic; FNA is not. Two molecular markers, factor VIII-related antigen and CD-34, are positive in most angiosarcomas, distinguishing angiosarcomas from other sarcomas.92
Staging of angiosarcomas of the breast mirrors staging of other sarcomas; it is based on tumour size, grade and depth. As in other sarcomas, grade is the most important determinant of prognosis. Whereas most primary angiosarcomas are low grade, most radiotherapy-induced angiosarcomas are high grade.93
Treatment consists of total mastectomy with wide resection of involved skin. The microscopic extent of disease is often greater than is clinically evident; every effort should be made to achieve widely negative skin margins. Axillary staging is not recommended since most sarcomas do not metastasise to regional lymph nodes. Adjuvant treatment follows recommendations for other sarcomas.95 Recent case reports contend benefit for adjuvant radiotherapy, and in certain patients it may be considered.89,95
In one study of 58 patients, almost half were dead at an average follow-up period of 15 months.90 In another study of 69 patients, the recurrence rate was 55% at a median follow-up of 40 months; the overall survival was 61% at 60 months.95 In a third study of 49 patients, the local recurrence rate was 24% at 35 months, the distant recurrence rate was 58.5% at 34 months and the mortality was 44% at 29 months. These reports indicate that the prognosis is poor.
Metastasis to the breast
Metastasis to the breast is uncommon, accounting for 0.5–6.6% of breast malignancies.96,97 The contralateral breast is the most common primary source.92 Other primary sources include lymphoma, melanoma, rhabdomyosarcoma and small-cell lung cancer.79
Treatment is directed at the primary malignancy. Prognosis is poor: 80% of patients die within 1 year.79
References
1. Gemignani, M.L., Petrek, J.A., Borgen, P.I., Breast cancer and pregnancy. Surg Clin North Am. 1999;79(5):1157–1169. 10572556
2. Andersson, T.M., Johansson, A.L., Hsieh, C.C., et al, Increasing incidence of pregnancy-associated breast cancer in Sweden. Obstet Gynecol. 2009;114(3):568–572. 19701036
3. Ishida, T., Yokoe, T., Kasumi, F., et al, Clinicopathologic characteristics and prognosis of breast cancer patients associated with pregnancy and lactation: analysis of case–control study in Japan. Jpn J Cancer Res. 1992;83(11):1143–1149. 1483929
4. Guinee, V.F., Olsson, H., Moller, T., et al, Effect of pregnancy on prognosis for young women with breast cancer. Lancet. 1994;343(8913):1587–1589. 7911917
5. Barnes, D.M., Newman, L.A., Pregnancy-associated breast cancer: a literature review. Surg Clin North Am 2007; 87:417–430. 17498535
6. Keleher, A.J., Theriault, R.L., Gwyn, K.M., et al, Multidisciplinary management of breast cancer concurrent with pregnancy. J Am Coll Surg. 2002;194(1):54–64. 11800340
7. Scott-Conner, C.E., Schorr, S.J., The diagnosis and management of breast problems during pregnancy and lactation. Am J Surg. 1995;170(4):401–405. 7573738
8. Woo, J.C., Yu, T., Hurd, T.C., Breast cancer in pregnancy: a literature review. Arch Surg. 2003;138(1):91–99. 12511159
9. Liberman, L., Giess, C.S., Dershaw, D.D., et al, Imaging of pregnancy-associated breast cancer. Radiology. 1994;191(1):245–248. 8134581
10. Schackmuth, E.M., Harlow, C.L., Norton, L.W., Milk fistula: a complication after core breast biopsy. Am J Roentgenol. 1993;161(5):961–962. 8273635
11. Loibl, S., von Minckwitz, G., Gwyn, K., et al, Breast carcinoma during pregnancy. International recommendations from an expert meeting. Cancer. 2006;106(2):237–246. 16342247
12. Duncan, P.G., Pope, W.D., Cohen, M.M., et al, Fetal risk of anesthesia and surgery during pregnancy. Anesthesiology. 1986;64(6):790–794. 3717642
13. Gentilini, O., Cremonesi, M., Toesca, A., et al, Sentinel lymph node biopsy in pregnant patients with breast cancer. Eur J Nucl Med Mol Imaging. 2010;37(1):78–83. 19662412
14. Ebert, U., Loffler, H., Kirch, W., Cytotoxic therapy and pregnancy. Pharmacol Ther. 1997;74(2):207–220. 9336023
15. Berry, D.L., Theriault, R.L., Holmes, F.A., et al, Management of breast cancer during pregnancy using a standardized protocol. J Clin Oncol. 1999;17(3):855–861. 10071276
16. Hahn, K.M., Johnson, P.H., Gordon, N., et al, Treatment of pregnant breast cancer patients and outcomes of children exposed to chemotherapy in utero. Cancer. 2006;107(6):1219–1226. 16894524
17. Mir, O., Berveiller, P., Ropert, S., et al, Emerging therapeutic options for breast cancer chemotherapy during pregnancy. Ann Oncol. 2008;19(4):607–613. 17921242
18. Isaacs, R.J., Hunter, W., Clark, K., Tamoxifen as systemic treatment of advanced breast cancer during pregnancy – Case report and literature review. Gynecol Oncol. 2001;80(3):405–408. 11263941
19. Kroman, N., Jensen, M.B., Wohlfahrt, J., et al, Pregnancy after treatment of breast cancer – a population-based study on behalf of Danish Breast Cancer Cooperative Group. Acta Oncol. 2008;47(4):545–549. 18465320
20. Ives, A., Saunders, C., Bulsara, M., et al, Pregnancy after breast cancer: population based study. Br Med J. 2007;334(7586):194. 17158581
21. Beadle, B.M., Woodward, W.A., Middleton, L.P., et al, The impact of pregnancy on breast cancer outcomes in women ≤ 35 years. Cancer. 2009;115(6):1174–1184. 19204903
22. Love, S.M., Linsey, K. Dr. Susan Love’s breast book, 5th ed. Philadelphia: Da Capo Press; 2010.
23. Weir, H.K., Thun, M.J., Hankey, B.F., et al, Annual report to the nation on the status of cancer, 1975–2000, featuring the uses of surveillance data for cancer prevention and control. J Natl Cancer Inst. 2003;95(17):1276–1299. 12953083
24. La Vecchia, C., Levi, F., Lucchini, F., Descriptive epidemiology of male breast cancer in Europe. Int J Cancer. 1992;51(1):62–66. 1563846
25. Stang, A., Thomssen, C., Decline in breast cancer incidence in the United States: what about male breast cancer? Breast Cancer Res Treat. 2008;112(3):595–596. 18176840
26. Anderson, W.F., Devesa, S.S., In situ male breast carcinoma in the Surveillance, Epidemiology, and End Results database of the National Cancer Institute. Cancer. 2005;104(8):1733–1741. 16138363
27. Sasco, A.J., Lowenfels, A.B., Pasker-de Jong, P., Review article: epidemiology of male breast cancer. A meta-analysis of published case–control studies and discussion of selected aetiological factors. Int J Cancer. 1993;53(4):538–549. 8436428
28. Stalsberg, H., Thomas, D.B., Rosenblatt, K.A., et al, Histologic types and hormone receptors in breast cancer in men: a population-based study in 282 United States men. Cancer Causes Control. 1993;4(2):143–151. 8386948
29. Giordano, S.H., Buzdar, A.U., Hortobagyi, G.N., Breast cancer in men. Ann Intern Med. 2002;137(8):678–687. 12379069
30. Hittmair, A.P., Lininger, R.A., Tavassoli, F.A., Ductal carcinoma in situ (DCIS) in the male breast: a morphologic study of 84 cases of pure DCIS and 30 cases of DCIS associated with invasive carcinoma – a preliminary report. Cancer. 1998;83(10):2139–2149. 9827718
31. Giordano, S.H., Cohen, D.S., Buzdar, A.U., et al, Breast carcinoma in men: a population-based study. Cancer. 2004;101(1):51–57. 15221988
32. Ribeiro, G., Male breast carcinoma – a review of 301 cases from the Christie Hospital & Holt Radium Institute, Manchester. Br J Cancer. 1985;51(1):115–119. 3966965
33. Goss, P.E., Reid, C., Pintilie, M., et al, Male breast carcinoma: a review of 229 patients who presented to the Princess Margaret Hospital during 40 years: 1955–1996. Cancer. 1999;85(3):629–639. 10091736
34. Pant, K., Dutta, U., Understanding and management of male breast cancer: a critical review. Med Oncol. 2008;25(3):294–298. 18074245
35. Lynch, H.T., Kaplan, A.R., Lynch, J.F., Klinefelter syndrome and cancer. A family study. JAMA. 1974;229(7):809–811. 4367182
36. Thomas, D.B., Jimenez, L.M., McTiernan, A., et al, Breast cancer in men: risk factors with hormonal implications. Am J Epidemiol. 1992;135(7):734–748. 1350708
37. Thorlacius, S., Olafsdottir, G., Tryggvadottir, L., et al, A single BRCA2 mutation in male and female breast cancer families from Iceland with varied cancer phenotypes. Nat Genet. 1996;13(1):117–119. 8673089
38. Friedman, L.S., Gayther, S.A., Kurosaki, T., et al, Mutation analysis of BRCA1 and BRCA2 in a male breast cancer population. Am J Hum Genet. 1997;60(2):313–319. 9012404
39. Brose, M.S., Rebbeck, T.R., Calzone, K.A., et al, Cancer risk estimates for BRCA1 mutation carriers identified in a risk evaluation program. J Natl Cancer Inst. 2002;94(18):1365–1372. 12237282
40. Brinton, L.A., Richesson, D.A., Gierach, G.L., et al, Prospective evaluation of risk factors for male breast cancer. J Natl Cancer Inst. 2008;100(20):1477–1481. 18840816
41. Scott-Conner, C.E., Jochimsen, P.R., Menck, H.R., et al, An analysis of male and female breast cancer treatment and survival among demographically identical pairs of patients. Surgery. 1999;126(4):775–781. 10520928
42. Port, E.R., Fey, J.V., Cody, H.S., 3rd., et al, Sentinel lymph node biopsy in patients with male breast carcinoma. Cancer. 2001;91(2):319–323. 11180077
43. Fentiman, I.S., Fourquet, A., Hortobagyi, G.N., Male breast cancer. Lancet. 2006;367(9510):595–604. 16488803
44. Agrawal, A., Ayantunde, A.A., Rampaul, R., et al, Male breast cancer: a review of clinical management. Breast Cancer Res Treat. 2007;103(1):11–21. 17033919
45. Schuchardt, U., Seegenschmiedt, M.H., Kirschner, M.J., et al, Adjuvant radiotherapy for breast carcinoma in men: a 20-year clinical experience. Am J Clin Oncol. 1996;19(4):330–336. 8677899
46. Jaiyesimi, I.A., Buzdar, A.U., Sahin, A.A., et al, Carcinoma of the male breast. Ann Intern Med. 1992;117(9):771–777. 1416579
47. Ribeiro, G., Swindell, R., Adjuvant tamoxifen for male breast cancer (MBC). Br J Cancer. 1992;65(2):252–254. 1739625
48. Doyen, J., Italiano, A., Largillier, R., et al, Aromatase inhibition in male breast cancer patients: biological and clinical implications. Ann Oncol. 2010;21(6):1243–1245. 19861576
49. Korde, L.A., Zujewski, J.A., Kamin, L., et al, Multidisciplinary meeting on male breast cancer: summary and research recommendations. J Clin Oncol. 2010;28(12):2114–2122. 20308661
50. Anderson, W.F., Jatoi, I., Tse, J., et al, Male breast cancer: a population-based comparison with female breast cancer. J Clin Oncol. 2010;28(2):232–239. 19996029
51. Cutuli, B., Le-Nir, C.C., Serin, D., et al, Male breast cancer. Evolution of treatment and prognostic factors. Analysis of 489 cases. Crit Rev Oncol/Hematol. 2010;73(3):246–254. 19442535
52. Miao, H., Verkooijen, H.M., Chia, K.S., et al, Incidence and outcome of male breast cancer: an international population-based study. J Clin Oncol. 2011;29(33):4381–4386. 21969512
53. Cutuli, B., Lacroze, M., Dilhuydy, J.M., et al, Male breast cancer: results of the treatments and prognostic factors in 397 cases. Eur J Cancer. 1995;31A(12):1960–1964. 8562148
54. Guinee, V.F., Olsson, H., Moller, T., et al, The prognosis of breast cancer in males. A report of 335 cases. Cancer. 1993;71(1):154–161. 8416712
55. Donegan, W.L., Redlich, P.N., Lang, P.J., et al, Carcinoma of the breast in males: a multiinstitutional survey. Cancer. 1998;83(3):498–509. 9690543
56. Velpeau, A.-A.-L.-M. A treatise on diseases of the breast and mammary region. London: Sydenham Society; 1856.
57. Paget, J. On disease of the mammary areola preceding cancer of the mammary gland. St Bartholomews Hosp J. 1874; 10:87–89.
58. Sakorafas, G.H., Blanchard, K., Sarr, M.G., et al, Paget’s disease of the breast. Cancer Treat Rev 2001; 27:9–18. 11237774
59. Lagios, M.D., Westdahl, P.R., Rose, M.R., et al, Paget’s disease of the nipple. Alternative management in cases without or with minimal extent of underlying breast-carcinoma. Cancer. 1984;54(3):545–551. 6329506
60. Kothari, A.S., Beechey-Newman, N., Hamed, H., et al, Paget disease of the nipple: a multifocal manifestation of higher-risk disease. Cancer. 2002;95(1):1–7. 12115309
61. Jahn, H., Osther, P.J., Nielsen, E.H., et al, An electron microscopic study of clinical Paget’s disease of the nipple. Acta Pathol Microbiol Immunol Scand. 1995;103(9):628–634. 7488383
62. Fu, W., Lobocki, C.A., Silberberg, B.K., et al, Molecular markers in Paget disease of the breast. J Surg Oncol. 2001;77(3):171–178. 11455553
63. Chen, C.Y., Sun, L.M., Anderson, B.O., Paget disease of the breast: changing patterns of incidence, clinical presentation, and treatment in the US. Cancer. 2006;107(7):1448–1458. 16933329
64. Kaelin, C. Paget’s disease. In: Harris J.R., Lippman M.E., Morrow M., et al, eds. Diseases of the breast. 2nd ed. Baltimore: Lippincott, Williams & Wilkins; 2000:227–283.
65. Dixon, A.R., Galea, M.H., Ellis, I.O., et al, Paget’s disease of the nipple. Br J Surg. 1991;78(6):722–723. 1648987
66. Hitchcock, A., Topham, S., Bell, J., et al, Routine diagnosis of mammary Paget’s disease. A modern approach. Am J Surg Pathol. 1992;16(1):58–61. 1370192
67. Morrogh, M., Morris, E.A., Liberman, L., et al, MRI identifies otherwise occult disease in select patients with Paget disease of the nipple. J Am Coll Surg. 2008;206(2):316–321. 18222386
68. Echevarria, J.J., Lopez-Ruiz, J.A., Martin, D., et al, Usefulness of MRI in detecting occult breast cancer associated with Paget’s disease of the nipple–areolar complex. Br J Radiol. 2004;77(924):1036–1039. 15569646
69. Frei, K.A., Bonel, H.M., Pelte, M.-F., et al, Paget disease of the breast: findings at magnetic resonance imaging and histopathologic correlation. Invest Radiol. 2005;40(6):363–367. 15905723
70. Dominici, L.S., Lester, S., Liao, G.S., et al, Current surgical approach to Paget’s disease. Am J Surg. 2012;204(1):18–22. 22036205
71. Fu, W., Mittel, V.K., Young, S.C., Paget disease of the breast: analysis of 41 patients. Am J Clin Oncol. 2001;24(4):397–400. 11474272
72. Bijker, N., Rutgers, E.J., Duchateau, L., et al, Breast-conserving therapy for Paget disease of the nipple: a prospective European Organization for Research and Treatment of Cancer study of 61 patients. Cancer. 2001;91(3):472–477. 11169928
73. Dalberg, K., Hellborg, H., Wärnberg, F., Paget’s disease of the nipple in a population based cohort. Breast Cancer Res Treat. 2008;111(2):313–319. 17952590
74. Kawase, K., Dimaio, D.J., Tucker, S.L., et al, Paget’s disease of the breast: there is a role for breast-conserving therapy. Ann Surg Oncol. 2005;12(5):391–397. 15915373
75. Marshall, J.K., Griffith, K.A., Haffty, B.J., et al, Conservative management of Paget disease of the breast with radiotherapy: 10- and 15-year results. Cancer. 2003;97(9):2142–2149. 12712465
76. Kollmorgen, D.R., Varanasi, J.S., Edge, S.B., et al, Paget’s disease of the breast: a 33-year experience. J Am Coll Surg. 1998;187(2):171–177. 9704964
77. Fourquet, A., Campana, F., Vielh, P., et al, Paget’s disease of the nipple without detectable breast tumor: conservative management with radiation therapy. Int J Radiat Oncol Biol Phys. 1987;13(10):1463–1465. 3040645
78. Ariel, I.M., Caron, A.S., Diagnosis and treatment of malignant melanoma arising from the skin of the female breast. Am J Surg. 1972;124(3):384–390. 4115719
79. Bartella, L., Kaye, J., Perry, N.M., et al, Metastases to the breast revisited: radiological–histopathological correlation. Clin Radiol. 2003;58(7):524–531. 12834635
80. Bedrosian, I., Faries, M.B., Guerry, D.T., et al, Incidence of sentinel node metastasis in patients with thin primary melanoma (< or = 1 mm) with vertical growth phase. Ann Surg Oncol. 2000;7(4):262–267. 10819365
81. Topalovski, M., Crisan, D., Mattson, J.C., Lymphoma of the breast. A clinicopathologic study of primary and secondary cases. Arch Pathol Lab Med. 1999;123(12):1208–1218. 10583925
82. Kuper-Hommel, M.J., Snijder, S., Janssen-Heijnen, M.L., et al, Treatment and survival of 38 female breast lymphomas: a population-based study with clinical and pathological reviews. Ann Hematol. 2003;82(7):397–404. 12764549
83. Fruchart, C., Denoux, Y., Chasle, J., et al, High grade primary breast lymphoma: is it a different clinical entity? Breast Cancer Res Treat. 2005;93(3):191–198. 16172797
84. Jeanneret-Sozzi, W., Taghian, A., Epelbaum, R., et al, Primary breast lymphoma: patient profile, outcome and prognostic factors. A multicentre Rare Cancer Network study. BMC Cancer 2008; 8:86. 18380889
85. Ryan, G., Martinelli, G., Kuper-Hommel, M., et al, Primary diffuse large B-cell lymphoma of the breast: prognostic factors and outcomes of a study by the International Extranodal Lymphoma Study Group. Ann Oncol. 2008;19(2):233–241. 17932394
86. Martinelli, G., Ryan, G., Seymour, J.F., et al, Primary follicular and marginal-zone lymphoma of the breast: clinical features, prognostic factors and outcome: a study by the International Extranodal Lymphoma Study Group. Ann Oncol. 2009;20(12):1993–1999. 19570964
87. Aviles, A., Neri, N., Nambo, M.J., The role of genotype in 104 cases of diffuse large B-cell lymphoma primary of breast. Am J Clin Oncol. 2012;35(2):126–129. 21325938
88. The International Non-Hodgkin’s Lymphoma Prognostic Factors Project, A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med. 1993;329(14):987–994. 8141877
89. Nascimento, A.F., Raut, C.P., Fletcher, C.D., Primary angiosarcoma of the breast: clinicopathologic analysis of 49 cases, suggesting that grade is not prognostic. Am J Surg Pathol. 2008;32(12):1896–1904. 18813119
90. Rao, J., DeKoven, J.G., Beatty, J.D., et al, Cutaneous angiosarcoma as a delayed complication of radiation therapy for carcinoma of the breast. J Am Acad Dermatol. 2003;49(3):532–538. 12963926
91. Maddox, J.C., Evans, H.L., Angiosarcoma of skin and soft tissue: a study of forty-four cases. Cancer. 1981;48(8):1907–1921. 7197190
92. Monroe, A.T., Feigenberg, S.J., Mendenhall, N.P., Angiosarcoma after breast-conserving therapy. Cancer. 2003;97(8):1832–1840. 12673708
93. Scow, J.S., Reynolds, C.A., Degnim, A.C., et al, Primary and secondary angiosarcoma of the breast: the Mayo Clinic experience. J Surg Oncol. 2010;101(5):401–407. 20119983
94. Glazebrook, K.N., Magut, M.J., Reynolds, C., Angiosarcoma of the breast. Am J Roentgenol. 2008;190(2):533–538. 18212243
95. Sher, T., Hennessy, B.T., Valero, V., et al, Primary angiosarcomas of the breast. Cancer. 2007;110(1):173–178. 17541936
96. Paulus, D.D., Libshitz, H.I., Metastasis to the breast. Radiol Clin North Am. 1982;20(3):561–568. 7111707
97. Amichetti, M., Perani, B., Boi, S., Metastases to the breast from extramammary malignancies. Oncology. 1990;47(3):257–260. 2342767