Chapter 61 Breast Cancer
Stages I and II
Approximately 70% to 80% of patients with stage I or II invasive breast cancer are technically candidates for breast-conserving therapy (BCT).1,2 Six major randomized trials comparing mastectomy with BCT using modern radiotherapy techniques found no differences in disease-free or overall survival rates between the two approaches3–8 ![]() (see web-only Table 61-1 available on the Expert Consult website), which were confirmed by a meta-analysis including additional studies.9
(see web-only Table 61-1 available on the Expert Consult website), which were confirmed by a meta-analysis including additional studies.9
Many questions remain regarding optimal patient evaluation and selection for BCT, irradiation techniques and doses, factors affecting complications and cosmetic outcome, follow-up, and treatment of locoregional recurrences. Several texts discuss these topics in much greater depth.10–13
Pretreatment Evaluation
Imaging
Mammography should always be performed before treatment, although it does not always accurately delineate the pathologic extent of disease. Magnetic resonance imaging (MRI) has substantial false-positive and false-negative rates.14 It may be most valuable for selected patient subgroups, such as patients with tumors larger than 2 cm, infiltrating lobular histologic characteristics, or positive axillary nodes.15 One small study found a lower risk of local failure in patients undergoing pretreatment MRI,16 but two larger studies with longer follow-up did not.17,18
Postoperative mammograms rarely show residual calcifications in patients with uninvolved microscopic margins.19 In a series from Philadelphia, postoperative mammographic imaging made no difference to the recurrence rate for patients with ductal carcinoma in situ (DCIS).20 Postoperative MRI has limited accuracy in assessing the presence or amount of residual disease.21
Pathologic Evaluation
The most common approach to microscopic margin assessment is surgical removal of a single specimen, which the pathologist rolls in India ink and sequentially sections (as one would slice a loaf of bread) perpendicular to its long axis. Some pathologists “shave” each face of a specimen; the margin is considered involved when tumor is seen in one of the shaved margin slides. These approaches may have quite different clinical implications.22,23 Some surgeons take additional “cavity” specimens after removing the main specimen24; their value is uncertain. The role of intraoperative margin assessment is also uncertain.25
Multidisciplinary Review
One quarter to half of prior community hospital radiologic and pathologic interpretations may be changed on review by specialists.26,27 Management strategies also often differ substantially between physicians and institutions. Hence, it is preferable to have a multidisciplinary review of cases before treatment plans are finalized.
Surgical Techniques
Breast-Conserving Surgery
Technical aspects of breast surgery are discussed in depth elsewhere.10,12,13 Studies differ on whether wider gross tissue resection margins reduce local failure rates.28,29 Patients with large cancers or lesions in areas where resection can cause substantial breast distortion may benefit from “oncoplastic” excision.30,31 Reduction mammoplasty has been used prior to radiation therapy for patients with very large breasts with excellent results.32
Axillary Dissection
The axillary nodes were divided by Berg33 into three anatomic “levels”: level I, lateral and inferior to the border of the pectoralis minor muscle; level II, under the pectoralis minor muscle; and level III (also called the infraclavicular nodes), medial and superior to the border of the pectoralis minor muscle. Randomized trials found no differences in axillary failure or survival rates between “limited” dissection (removal of level I or level I and II nodes only) and “complete” dissection (removal of level I, II, and III nodes), but limited dissection caused less morbidity.34–36
Axillary failures are rare in patients treated with dissection whether nodes are involved or not.33,37 Failure rates are higher in patients treated with inadequate surgery or in patients with eight or more positive nodes.38
Sentinel Node Biopsy
Sentinel node biopsy (SNB) uses injection of a radionuclide tracer or vital dye (or both) in the breast to guide the surgeon in determining which nodes to remove.39 Immediate and long-term complications are substantially lower for SNB compared with level I or II axillary dissection.40–42 False-negative rates of SNB are 0% to 12%, but the risk of axillary failure after negative SNB is very small.43–46 Treatment of patients with involved sentinel nodes is controversial and is discussed below. There were no differences in distant failure rates in two published randomized trials comparing axillary dissection and SNB.47,48 Results from many other trials are pending.
Breast-Conserving Surgery with Radiation Therapy
Patient Status
Pregnancy
Unterminated pregnancy is an absolute contraindication to breast irradiation because of the possible teratogenic and carcinogenic effects on the fetus of scattered radiation.49
Prior Treatment with Radiation Therapy
Although most physicians recommend mastectomy,50 there were no unusual acute or chronic sequelae among 12 women treated with conservative surgery and radiation therapy at the University of Pittsburgh 10 to 29 years after irradiation for Hodgkin’s disease or non-Hodgkin’s lymphoma,51 37 patients treated in Milan,52 or 2 patients treated in Ottawa, Canada.53 However, one of two such patients treated at Stanford University developed severe soft tissue necrosis.54 A few such patients have been successfully treated with partial-breast irradiation, albeit with short follow-up.55,56
Rheumatologic Disorders
The effect of rheumatologic disorders on the risk of developing acute and chronic complications after radiation therapy has been controversial. Some investigators have considered the presence of these disorders a relative or absolute contraindication to the use of radiation therapy.1 However, only three small studies have compared complication rates in patients with rheumatologic diseases with a matched “normal” population.57,58,59 These studies found no clear evidence of increased risk (including the one study focusing on patients treated with BCT58), except for patients with scleroderma. Therefore, the author believes that patients with other rheumatologic disorders can be treated safely but those with scleroderma and its variants should not receive radiation therapy if at all possible.
Breast Size
Women with large breasts have more acute skin reactions and long-term retraction and fibrosis than patients with smaller breasts. Results can be improved by using higher-energy photons,60 1.8-Gy daily fractions, and three-dimensional compensation. Treatment in the lateral decubitus or prone position may also be very helpful (see later discussion).
Prior Breast Augmentation
The risk of capsular fibrosis and other complications following irradiation of patients with prosthetically augmented breasts was 50% or higher in several series61,62 but very low in others.63–65 Local failure rates have also varied between studies, from none65 to 25%.63 The small number of patients in these studies makes it difficult to assess how radiation therapy technique and pretreatment evaluation may affect such outcomes. Given the limitations of mammography in the augmented breast, MRI should be used to help assess the spread of tumor within the breast. If the lesion seems limited after such imaging and margin assessment, it seems reasonable to offer such patients BCT with irradiation. Three-dimensional treatment planning may then help reduce the risk of complications (see section on Acute and Chronic Complications).
Patient Age
Patients younger than 35 to 40 years at diagnosis have higher local recurrence rates than older patients for unclear reasons.66 Despite this, mastectomy does not yield superior distant disease-free or breast cancer-specific survival rates.67,68,69 One study found a 5-year actuarial local failure rate of only 3% among 38 patients with margins wider than 2 mm whose tumor did not contain an EIC.70 In another series, there was no difference in the local failure rate between patients younger or older than age 45 years when margins were wider than 2 mm.71 In a study at the Beth Israel Deaconess Medical Center in Boston, the 8-year local failure rate for 54 patients age 40 years or younger with histologic grade 1 to 2 tumors was 7%, compared with 18% for 74 patients with grade 3 tumors.72 Systemic therapy also reduces local failure rates.66,73
Older patients tolerate radiation therapy well and have excellent local control rates.74,75
Genetic Factors
Many studies found little or no difference in ipsilateral local failure rates between patients with BRCA1 or BRCA2 gene mutations and unaffected patients.76–78,79,80 Other studies have suggested that BRCA1 or BRCA2 mutation carriers have increased late local failure rates, likely resulting from the development of new primary cancers.81–84 Tamoxifen or oophorectomy may reduce rates of both ipsilateral local failure79 and contralateral new primary cancers.79,85 A recent study found equal distant failure rates for 160 BRCA1 and BRCA2 patients treated with BCT and 213 patients treated with mastectomy, with a mean follow-up of 10 years.86 There is also no convincing evidence that contralateral prophylactic mastectomy improves the outcome.80,87,88
Patients with rare genetic syndromes associated with impairment of radiation damage repair, such as ataxia telangiectasia, are at substantial risk of complications from radiation therapy.89 However, patients heterozygous for BRCA1 or BRCA2 mutations do not seem to have increased toxicities.79,90 Complication rates for patients with ATM heterozygosity were not increased in some studies,91,92 but others suggested adverse results for patients with more than one ATM mutation93 or those with particular single mutations.94,95 Patients with polymorphisms of multiple radiation repair genes may also be at increased risk of complications.94,96–99
Some patients with genetic defects of DNA repair may also be at increased risk of radiation carcinogenesis. In-field cancers have been reported to occur within a few years of radiation therapy in several patients with Li-Fraumeni syndrome.100,101 Limited evidence suggests radiation therapy does not increase the risk of contralateral breast cancers in patients with ATM,102,103 BRCA1, or BRCA2 mutations, however.86
Clinical Factors
Means of Detection
Local recurrence rates are similar for patients with palpable and nonpalpable cancers.104 Patients with nipple discharge do not have higher local failure rates than other patients when resection margins are not involved.105
Tumor Size and Location
Tumor size does not influence recurrence rates after BCT.70 It is difficult, however, to achieve both acceptable cosmetic results and negative margins in most patients with tumors larger than 4 to 5 cm without using neoadjuvant chemotherapy (see Chapter 62).
A few studies suggest that patients with medial tumors have higher local recurrence rates,106,107 but this has not been confirmed. Patients with subareolar or periareolar lesions that do not directly extend to the nipple or areola have high local control rates following excision with negative margins without nipple-areolar resection.108,109 Even when nipple-areolar resection must be performed, the appearance and texture of the remaining breast are at least as good as these characteristics following reconstruction procedures, and sensation in the remaining breast mound is preserved. Local control rates also appear to be comparable to those achieved with mastectomy.110
Bilateral Breast Cancers
Patients with bilateral breast cancer (either synchronous or metachronous) can be treated successfully with BCT without an increased risk of complications,111,112 even when the ipsilateral internal mammary nodes (IMNs) were initially irradiated.113
Multiple Ipsilateral Primary Cancers
Few patients have more than one independent ipsilateral breast cancer at presentation. Only 6 of a consecutive series of 200 patients (3%) with palpable masses had two or more separate lesions on mammography in one series.114 Eight of 225 selected patients (4%) in another series had biopsy-proven additional cancers in a separate quadrant detected only on MRI.115 Only 3 of 183 mastectomy specimens (2%) in another study had multiple lesions without demonstrable continuity.116 The majority of apparently independent lesions in one recent study were clonal, indicating spread from a single site of origin.117 Local failure rates in patients with multiple ipsilateral primary tumors having negative margins are similar to those of patients with a single lesion.112,118–122
Pathologic Factors
Margin Status
A “positive,” or “involved,” margin is defined as invasive cancer or DCIS present at an inked surface when a “bread-loafing technique” is used (i.e., sequential sections of the specimen are obtained as one would slice a loaf of bread) or as tumor found anywhere in “shaved” margin specimens. Patients with involved margins generally have higher failure rates than those with uninvolved margins. Involvement by either invasive cancer or DCIS has similar implications.123,124
In a study from the Joint Center for Radiation Therapy (JCRT) in Boston123 with a median follow-up time of 127 months, the crude 8-year local failure rate was 14% for 122 patients with “focal” margin involvement (defined as DCIS and invasive cancer across all examined slides that could be encompassed by three or fewer low-power microscopic fields) and 27% for 66 patients with “extensive” involvement. In a study from Marseille, France, the risk of local failure was 14% (10 of 70) for patients with a single positive margin, compared with 36% (17 of 47) for patients with multiple involved margins with a median follow-up of 72 months.125
Many investigators have arbitrarily distinguished “negative” from “close” but uninvolved margins based on a minimum tumor-free margin width. However, only three published studies with long follow-up times have subdivided results according to sequential intervals71,123,126 (Table 61-1). They do not show consistent patterns between increasing margin width and lower local failure rates.
TABLE 61-1 Tumor-Free Margin Width and Risk of Local Failure Following Breast-Conserving Therapy with Radiation Therapy*
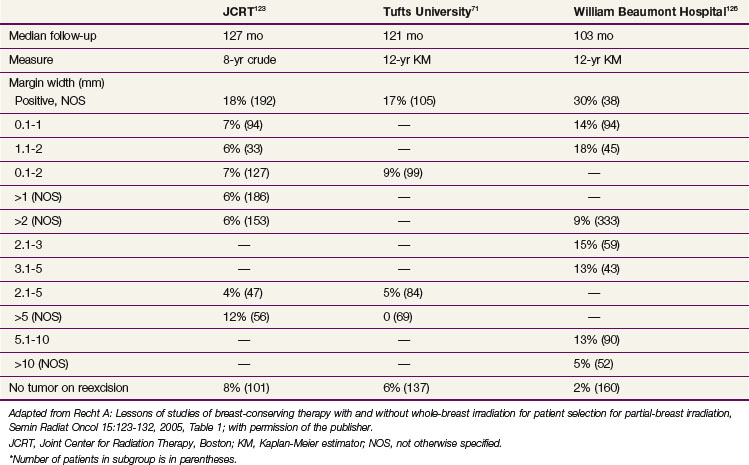
Margin width may still be important for certain patient subgroups, such as young patients. Chemotherapy and tamoxifen substantially reduce failure rates in patients with uninvolved margins as a whole,127,128 but this may be proportionally greater for patients with margins narrower than 1 to 2 mm.123,129
One study found that the volume of disease near uninvolved margins affected the risk of local recurrence.126 The number of excisions required to achieve uninvolved margins was not a risk factor for local failure in most,130,131 but not all,132 series.
Other Histologic Features
An extensive intraductal component (EIC) is defined when two features are simultaneously present for infiltrating ductal carcinomas: (1) intraductal carcinoma that is a prominent portion of the area of the primary mass (in an earlier definition, 25% or more) and (2) intraductal carcinoma clearly extending beyond the infiltrating margin or present in grossly normal adjacent breast tissue.133 Predominantly noninvasive tumors, with only focal areas of invasion, are also included in this category. (Some investigators have used other definitions, most of which probably describe the same entity.) Tumors with an EIC extend more widely through the breast than others.134 The presence of an EIC was a major risk factor for local failure in studies in which microscopic margins were not routinely examined, but its impact is uncertain when margin status has been taken into account.70,123,135
The presence of lymphovascular invasion has been an independent risk factor for local recurrence in most,136–138 though not all,139 studies.
Patients with infiltrating lobular carcinomas or mixed ductal and lobular features have recurrence rates comparable to those of patients with infiltrating ductal carcinomas.140–143 Results for rarer histologic subtypes also seem similar.143–147
Benign proliferative diseases do not appear to affect the risk of local failure.148,149 One study found a substantial risk of residual DCIS or invasive disease on reexcision when atypical ductal hyperplasia was at or near the margins, however.150
Studies conflict on whether finding lobular carcinoma in situ (LCIS) does151–153 or does not154–156 increase local failure rates.
Biologic Markers of Tumor Behavior
Patients with tumors that express the estrogen receptor have modestly lower local recurrence rates than patients with estrogen receptor-negative tumors when systemic therapy is not used. For example, the 10-year local recurrence rate for patients with node-negative, estrogen receptor-positive tumors in the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14 trial was 11%, compared with 15.3% for patients with estrogen receptor-negative tumors in the companion B-13 trial.157 Appropriate systemic therapy reduced this difference substantially (rates of 3.6% and 3.5%, respectively, in these trials).
The impact of c-ERBB-2/neu, or HER2, overexpression on the risk of local recurrence is uncertain.158,159 Several studies found similar local recurrence rates for patients with “triple-negative” cancers and patients with other combinations of estrogen receptor, progesterone receptor, and HER2,160–163 but such patients had modestly higher local failure rates in several recent series.164–166
The implications of gene expression profiles for local therapy are just beginning to be explored. The “wound signature score” discriminated between patients age 53 years or younger who had a high or low risk of recurrence in one study.167 The 10-year local failure rate after BCT was low (4% to 5%) for patients age 50 years or older with node-negative, estrogen receptor-positive tumors, regardless of the score of the 21-gene Oncotype DX test (Genomic Health, Redwood City, California), but for younger patients with a low score, the risk was 12.5%, compared with 27.7% and 26.5% for patients with intermediate- and high-risk scores, respectively (Mamounas E, oral presentation, San Antonio Breast Cancer Symposium, December 2005).168
Breast-Conserving Surgery without Radiation Therapy
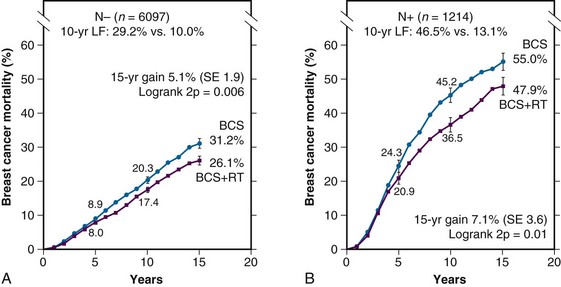
![]() Web-only Table 61-2 summarizes the largest randomized trials comparing conservative surgery alone and conservative surgery with radiation therapy for relatively unselected patients.5,169–174 These trials had broad entry criteria and required only very small microscopically tumor-free margins. In the most recently published meta-analysis of the Early Breast Cancer Trialists’ Collaborative Group, which included 7311 patients in these and similar trials, the risk of isolated local recurrence among patients not receiving radiation therapy was 32%, compared with 10.3% in those receiving radiation therapy.9 There was a statistically significant reduction in the 15-year breast cancer-specific mortality rate among patients randomized to irradiation, compared with the control arm (35.9% and 30.5% in the two arms, respectively; hazard ratio, 0.83). The overall mortality rate was also significantly reduced (40.5% and 35.2%, respectively). Of note, the absolute reduction in the breast cancer-specific mortality rate was greater for patients with positive axillary nodes than for those with negative nodes, reflecting the higher risk of local relapse without radiation therapy in the former group (Fig. 61-1).
Web-only Table 61-2 summarizes the largest randomized trials comparing conservative surgery alone and conservative surgery with radiation therapy for relatively unselected patients.5,169–174 These trials had broad entry criteria and required only very small microscopically tumor-free margins. In the most recently published meta-analysis of the Early Breast Cancer Trialists’ Collaborative Group, which included 7311 patients in these and similar trials, the risk of isolated local recurrence among patients not receiving radiation therapy was 32%, compared with 10.3% in those receiving radiation therapy.9 There was a statistically significant reduction in the 15-year breast cancer-specific mortality rate among patients randomized to irradiation, compared with the control arm (35.9% and 30.5% in the two arms, respectively; hazard ratio, 0.83). The overall mortality rate was also significantly reduced (40.5% and 35.2%, respectively). Of note, the absolute reduction in the breast cancer-specific mortality rate was greater for patients with positive axillary nodes than for those with negative nodes, reflecting the higher risk of local relapse without radiation therapy in the former group (Fig. 61-1).
WEB-ONLY TABLE 61-2 Randomized Trials Comparing Breast-Conserving Surgery Alone with Breast-Conserving Surgery and Radiation Therapy for Relatively Unselected Patients with Early-Stage Invasive Breast Cancer, with Median Follow-up Time Longer Than 5 Years*
Many investigators have tried to identify patient subgroups with an acceptably low risk of local recurrence following conservative surgery alone.175 Age older than 65 years,174 the absence of comedo or lobular features,169 and low histologic grade176 were associated with reduced failure rates in series not employing systemic therapy.
Several randomized trials (Table 61-2) and retrospective or prospective studies186,187 found low local failure rates in selected patients treated with conservative surgery and antihormonal therapy without irradiation. None of the randomized trials showed any differences in rates of distant metastases, breast cancer-specific mortality, or overall survival between the arms. Antihormonal therapy may be needed to obtain acceptably low failure rates even in such populations.178,188,189
TABLE 61-2 Local Failure Rates in Randomized Trials Treating Selected Patients with Breast-Conserving Surgery and Antihormonal Therapy with or without Radiotherapy
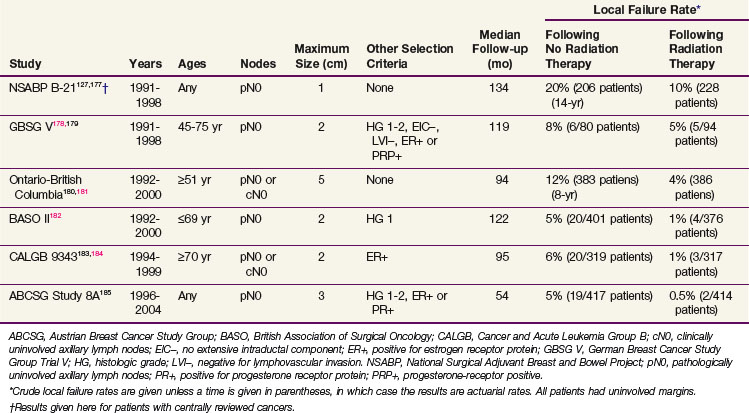
There are few data on how specific factors or their combinations affect outcome with conservative surgery with antihormonal therapy. There was no difference in local failure rates between patients ages 50 to 60 years, 60 to 70 years, or older than 70 years with tumors 1 cm or smaller with negative axillary nodes in one study in London.190 In the Ontario–British Columbia trial, at a median follow-up of 94 months the actuarial 8-year local recurrence rate for patients age 60 years or older with positive estrogen receptor or progesterone receptor was 7% for 237 patients with tumors 2 cm or smaller and 5% for 107 patients with tumors 1 cm or smaller treated with tamoxifen alone.181 In the Milan study, patients with infiltrating lobular carcinomas had a slightly higher local failure rate (12%, or 9 of 72 patients) compared with patients with infiltrating ductal histology (8%, or 18 of 231 patients).186 In a retrospective study of patients older than 70 years treated in Nottingham, England, with a limited median follow-up of 37.5 months, the risks of local failure were 33% (4 of 12 patients) for margin widths less than 1 mm, 12% (2 of 17 patients) for margins of 1 to 5 mm, and 2% (1 of 54 patients) for margins wider than 5 mm.191
Even patients with low failure rates may sometimes prefer to reduce their risk of local failure yet further by being irradiated.192,193 The effect of radiation therapy on patients’ quality of life is brief and limited.194 Therefore the decision to omit irradiation should be made by the patient and her physicians together, not unilaterally.
When to Use Nodal Irradiation
Axillary Irradiation
Some patients with clinically uninvolved (cN0) axillary nodes may have a low risk of regional nodal failure without specific axillary treatment181,184,186,195 or with breast irradiation alone.186,196–200 Axillary plus breast irradiation results in failure rates of 1% to 3%,199,201,202,203 very similar to those of axillary dissection. Axillary plus breast irradiation resulted in failure rates that were 1% to 2% lower than those of breast radiation therapy alone in two Italian trials.204,205 Axillary radiation therapy is not as effective for patients with clinically suspicious (cN1) nodes,33 who should undergo a level I or II dissection.
Axillary failure rates after dissection in patients with positive nodes are generally low if a minimum of 6 to 10 nodes are removed, unless there is extensive involvement.38,206 Failure rates may be 7% to 10% or higher in unirradiated patients with eight or more positive nodes or T2 to T3 tumors and four or more positive nodes38 or when a substantial proportion of the removed nodes is involved.207 Extracapsular tumor extension does not increase the risk of axillary failure.206,208
Management of patients with positive nodes on SNB is very controversial.37 The largest study of different approaches was conducted in the Netherlands.209 With a median follow-up of 56 months, the 5-year risk of axillary failure in patients with sentinel node metastases smaller than 0.2 mm was 1% among 396 patients who underwent completion axillary dissection, none of 54 patients treated with breast and axillary radiation therapy, and 2% among 345 patients receiving breast radiation therapy alone or (about one third of the cases) mastectomy without irradiation. For patients with sentinel node micrometastases (0.2 to 2 mm), respective failure rates were 1.1% of 793 patients, none of 94 patients, and 5% of 141 patients. Results from two randomized trials comparing different approaches are not yet available.
Supraclavicular Nodal Irradiation
Supraclavicular recurrences (which are often grouped with axillary apical or infraclavicular recurrences) occur in only a small percentage of patients with uninvolved nodes or 1 to 3 positive axillary nodes.37,38,210,211 Supraclavicular nodal failures occurred in 7% to 10% or more of patients with four or more pathologically positive axillary nodes in older studies,38,206,212,213 but several recent studies found rates of only 3% to 5%.214–216 Patients with a high proportion of recovered axillary nodes involved may also be at increased risk.207 Some studies suggest that lymphovascular invasion in the primary tumor may increase the risk in patients with negative or positive axillary nodes,206,210,215 though others do not.208,211 A study from Taiwan of 2658 consecutive patients found that high grade, four or more positive nodes, and positive nodes at axillary level II or III were associated with increased supraclavicular failure rates.217
Internal Mammary Nodal Irradiation
Internal mammary node (IMN) metastases occur in 5% or less of patients with pathologically negative axillary nodes.218,219 Reported rates are 20% to 50% in patients with a positive dissection. Tumor size and the number of involved nodes likely influence this risk.37 Results of the largest such study220 are shown in ![]() web-only Table 61-3, available on the Expert Consult website. The location of the primary tumor appears to have only a minor impact when tumor size and axillary nodal status have been taken into account.220,221
web-only Table 61-3, available on the Expert Consult website. The location of the primary tumor appears to have only a minor impact when tumor size and axillary nodal status have been taken into account.220,221
WEB-ONLY TABLE 61-3 Incidence of Internal Mammary Node Involvement in Relation to Axillary Node Involvement and Tumor Size (Fudan University, Shanghai, China, 1956 to 2003)
| Tumor Size, No. Involved Axillary Nodes | No. Patients | Internal Mammary Nodes Involved (%) |
|---|---|---|
| T1, N0 | 204 | 2.9 |
| T2, N0 | 493 | 4.9 |
| T3, N0 | 187 | 4.8 |
| T1, 1-3 | 57 | 10.5 |
| T2, 1-3 | 244 | 20.5 |
| T3, 1-3 | 97 | 19.6 |
| T1, 4-6 | 9 | 33.3 |
| T2, 4-6 | 89 | 31.5 |
| T3, 4-6 | 41 | 19.5 |
| T1, ≥7 | 12 | 25.0 |
| T2, ≥7 | 143 | 42.0 |
| T3, ≥7 | 103 | 42.7 |
Data from Huang O, Wang L, Shen K, et al: Breast cancer subpopulation with high risk of internal mammary lymph node metastasis. Analysis of 2,269 Chinese breast cancer patients treated with extended radical mastectomy. Breast Cancer Res Treat 107:379-387, 2008.
It is common to see drainage to the IMNs when lymphoscintigraphy is performed for SNB.221 Only 10% to 25% of such “hot spots” are involved pathologically, however.222–224
The value of treating the IMNs prophylactically has been controversial for decades.225 Clinical IMN recurrence is found in 1% or less of patients in nearly all studies and is rare even when the nodes are shown to be involved by biopsy.226 Retrospective studies have differed as to whether patients benefit from IMN irradiation.227–230 Randomized trials have not shown improved outcome after specific IMN treatment with either surgery or radiation therapy.231–233 Two large trials conducted by the European Organization for Research and Treatment of Cancer (EORTC)234 and the National Cancer Institute of Canada are closed, but outcome data are not yet available.
Treatment Techniques
Patient Position and Immobilization
Patients are traditionally irradiated in the supine position, but the lateral decubitus235 and prone positions236,237 may be especially useful for patients with very large breasts ![]() (see web-only Fig. 61-1, available on the Expert Consult website). Treatment in the prone position results in anterior displacement of the heart,238 which can increase the irradiated heart volume for some patients.239 These positions also have the disadvantages of reducing the coverage of the chest wall and of not allowing the use of nodal irradiation. Some patients are unable to tolerate lying in these positions.237 One study also reported an increased risk of acute skin reactions when using the prone position.240
(see web-only Fig. 61-1, available on the Expert Consult website). Treatment in the prone position results in anterior displacement of the heart,238 which can increase the irradiated heart volume for some patients.239 These positions also have the disadvantages of reducing the coverage of the chest wall and of not allowing the use of nodal irradiation. Some patients are unable to tolerate lying in these positions.237 One study also reported an increased risk of acute skin reactions when using the prone position.240
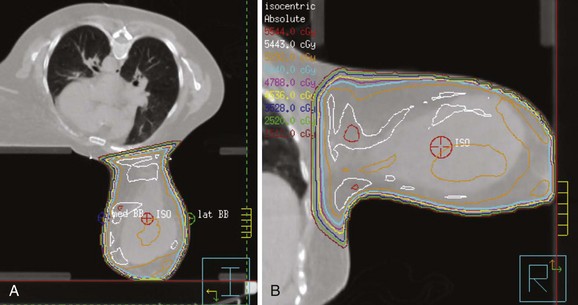
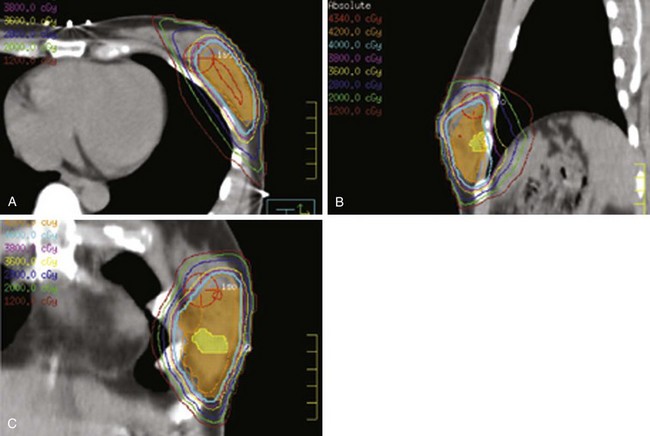
Web-Only Figure 61-1 Patient with large, pendulous breast treated in the prone position. A, Planning CT. B, Detailed isodose distribution; prescription dose was 5040 cGy.
Numerous immobilization techniques using specialized breast boards, custom-made foam cradles, and other techniques can reproduce the daily patient position to within 5 mm.241–243 There is no fully satisfactory way to immobilize the breast itself, especially a large breast, however. Netting is useful to move the breast tissue medially, and careful taping can help open skin folds.
Respiratory excursion of the breast in different directions is 2 to 6 mm.244 Respiratory gating or breath-holding techniques during deep inspiration may reduce the amount of heart irradiated.242,244–248
The effects of daily setup variation and normal respiratory motions on the cumulative dose distribution within the breast or at the excision cavity are small.244,249,250 Respiratory motion may result in a substantial effect on the doses given to the IMNs, however.244
Whole-Breast Irradiation
Traditional “standard” tangential field borders, based on surface anatomy, are designed to include nearly the entire volume of the breast with adequate margin for daily setup error (Table 61-3). Fluoroscopic simulation has been largely replaced by computed tomography (CT) simulation; there is substantial interphysician variation in delineating the breast, however.251,252,253 This may be reduced by the use of “standard” templates or atlases.254,255 Because the risk of local recurrence is quite low for patients whose treatment planning has been based on surface anatomy, it is unlikely that such variations are of substantial clinical importance.
Blocking or gantry rotation should be used to make the posterior field edges coplanar to reduce normal tissue exposure (Fig. 61-2). It may be acceptable to deviate from these field borders for selected patients (e.g., to move the medial entrance laterally in a patient with a lateral lesion to reduce heart and lung exposure).
Most patients are best treated with 6-MV photons. For patients with large breasts or medial-to-lateral separations (over 22 to 24 cm), it is preferable to use 10 = MV or higher energies alone or in combination with 6-MV photons to keep the maximum inhomogeneity less than 110%. Skin failures are rare, regardless of treatment energy,60 so that a bolus or beam spoiler does not need to be used routinely.
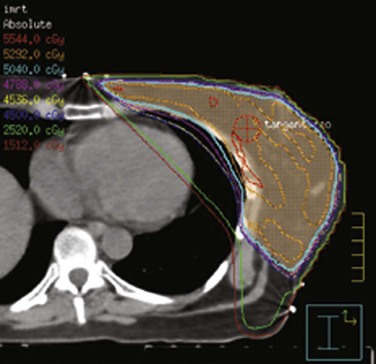
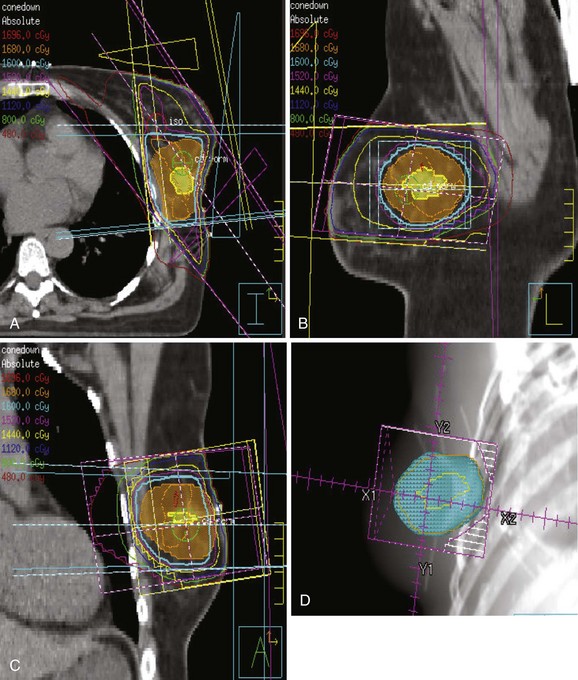
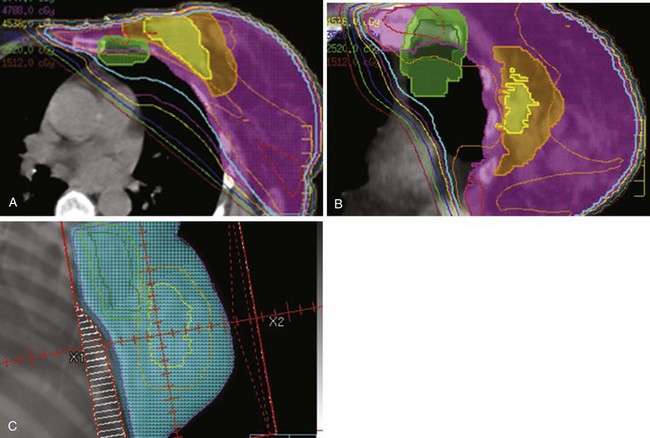
The breast–chest wall volume varies substantially in shape and transverse dimensions from superiorly to inferiorly. Although physical wedges generally result in satisfactory dose homogeneity in the central-axis plane, there are often very large, intense “hot spots” in the more superior and inferior planes. Inverse-planned intensity-modulated radiation therapy (IMRT),256,257 with or without treatment of regional lymph nodes,258–264 results in much more homogeneous dose distributions throughout the entire treatment volume. Simple forward-planned IMRT techniques using only a few additional segments produce nearly identical results for the great majority of patients265–268 (Fig. 61-3). Elimination of a physical medial wedge reduces scattered radiation exposure of the contralateral breast and other areas.269,270 Using additional gantry angles may reduce the heart dose ![]() (see web-only Fig. 61-2, available on the Expert Consult website), albeit at the price of decreased coverage of the breast volume271 or increased exposure of other normal tissues.259,263,264,272
(see web-only Fig. 61-2, available on the Expert Consult website), albeit at the price of decreased coverage of the breast volume271 or increased exposure of other normal tissues.259,263,264,272
Several randomized273,274 and retrospective and prospective studies275–277 of three-dimensionally compensated breast radiation therapy found it reduces acute and chronic toxicities. Hence, CT simulation and three-dimensional treatment planning and compensation should be routinely used when possible.
Breast Boost Volume and Techniques
In most institutions, the breast boost clinical target volume is defined by adding 1- to 2-cm circumferential margins around the tumor bed, with the anterior boundary extending just below the skin and at the anterior chest wall or pectoralis major muscle. The volume of the excision cavity decreases after surgery, particularly within the first 1 to 2 months.278,279,280–282 There are no data showing whether local control or cosmetic results would be different if the boost were planned soon after surgery or just before it is to be given. The volume defined by CT is generally larger than that defined solely by surgical clips.283,284 It can be difficult to delineate the extent of the cavity on CT scanning,285–288 and there is no agreement on exactly what features should be included in the target volume. Variation between radiation oncologists in defining the lumpectomy site can be reduced by establishing common guidelines and training.289 Nonetheless, small differences in the size or placement of the boost volume seem unlikely to have a major impact on the risk of recurrence in patients receiving whole-breast irradiation.290,291
Local control and cosmetic results are similar with interstitial implantation, photons, or electrons292,293 ![]() (see web-only Figs. 61-3 to 61-5, available on the Expert Consult website). Intraoperative photon and electron beams have also been used for this purpose.294–297 Hence, the radiation oncologist appears free to use whatever technique is most convenient and available.
(see web-only Figs. 61-3 to 61-5, available on the Expert Consult website). Intraoperative photon and electron beams have also been used for this purpose.294–297 Hence, the radiation oncologist appears free to use whatever technique is most convenient and available.
Supraclavicular and Axillary Nodal Irradiation
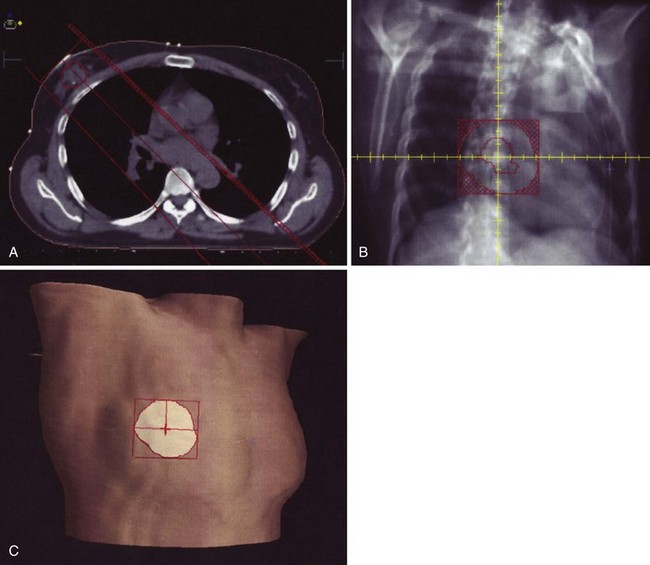
It is generally necessary to add a slightly obliqued anterior “third field” to treat the upper axillary and supraclavicular nodes, which are not adequately included in breast tangential fields.298–301 Both multiple-isocenter302–306 and single-isocenter307,308 approaches have been employed to match this field to the tangents. The former approaches use combinations of table, gantry, and collimator rotation and blocking to avoid “hot spots” resulting from field overlap or underdosing due to fields being too far apart (Fig. 61-4).
The “supraclavicular field” (i.e., lateral border at the coracoid process or medial border of the humeral head) includes the level III nodes in most patients, as well as the true supraclavicular nodes more medially. The inferior border is generally placed at the inferior edge of the clavicular head, which ensures coverage of these nodes.309 The “full axillary field” (also called a supraclavicular/axillary field) extends the lateral border of the supraclavicular field to split the humeral head, therefore including more soft tissue laterally. When CT simulation is employed, the subclavian and axillary vessels are used as surrogates for the position of the axillary nodes, and the sternocleidomastoid, pectoralis minor and major, and anterior scalene muscles and the clavicle are used to define the boundaries of the infraclavicular and supraclavicular nodal regions.310 The location of the superior border varies between institutions, from the top of the first rib radiographically, to the top of the shoulder, to the superior border of the thyroid cartilage. A block is usually placed along the transverse spinous pedicles to protect the spinal cord, larynx, and esophagus, but care must be taken because a recent study found that some supraclavicular recurrences were very close to the medial field border.311
Supraclavicular and full axillary field doses are traditionally prescribed to an arbitrary depth below the skin surface (3 cm and 5 cm, respectively) when two-dimensional treatment planning methods are used. However, the depth of the supraclavicular vessels varies substantially.310,312 The location of the axillary nodes also varies with arm position.313 Hence, CT planning should be used to define this depth when possible (Fig. 61-5).
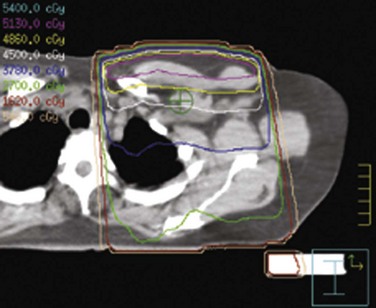
Figure 61-5 Dose distribution in a supraclavicular-axillary field, prescribed to 4500 cGy at a depth of 5 cm.
It is not clear how often a full axillary field should be used, particularly in patients undergoing a complete axillary dissection. For example, there were no axillary failures in patients with 10 or more positive nodes who received supraclavicular irradiation, whether the axilla was fully irradiated or not.314
The value of giving an axillary boost is uncertain. Because the nodes are fairly anterior, the use of a posterior axillary boost does not appear optimal.315 For individuals with very deep nodes or situations calling for doses above 45 to 50 Gy, however, it may be best to use opposed fields or three-dimensional conformal techniques to improve nodal coverage with acceptable inhomogeneity.316,317
Internal Mammary Nodal Irradiation
The majority of IMNs are found in the first three intercostal spaces between the posterior edge of the sternum and the parietal pleura, but some may be as low as the sixth intercostal space and some are located directly under the ribs.318,319 Half to three-quarters of the nodes are located medial to the internal mammary vessels in the upper two to three intercostal spaces, with most (but not all) nodes lateral to the vessels in the more inferior levels.319 The internal mammary vessels are usually visible at the sternal-pleural junction.320 Their depth and location from the midline vary substantially between patients.313 Therefore CT simulation should be used to plan IMN treatment. However, the maximum distances of the IMNs from the vessels have not been described, and, hence, the optimal expansions needed to define the clinical target volume are not known.
Anterior photon fields were commonly used to treat the IMNs in the past, but this increases the risk of late cardiac deaths.321 Heart exposure can be limited by using electron- or mixed-beam direct internal mammary fields,322–324 but this approach requires substantial care to achieve an acceptable junction with the tangential fields. The “partially wide tangent technique” includes the IMNs in the third interspace and above in tangential fields that ordinarily cross the midline, with the inferior portion of the fields blocked posteriorly to reduce the irradiated volumes of lung and heart325 ![]() (see web-only Fig. 61-6, available on the Expert Consult website). Such blocking may increase the risk of local failure for patients with inferiorly located tumors, however.326 Coverage of the IMNs may be improved and normal tissue doses reduced with the use of IMRT.316
(see web-only Fig. 61-6, available on the Expert Consult website). Such blocking may increase the risk of local failure for patients with inferiorly located tumors, however.326 Coverage of the IMNs may be improved and normal tissue doses reduced with the use of IMRT.316
Radiotherapy Dose and Schedule Parameters
Interval between Surgery and Start of Radiation Therapy
Local failure rates do not seem to be increased when radiation therapy is started up to 4 months after the last breast surgery.327–331,332 Prolonging the start of radiation therapy beyond this time, however, resulted in increased failure rates for patients with close margins (≤1 mm) in one randomized trial of the sequencing of chemotherapy and irradiation.333,334 Unfortunately, the effect of the tumor-free margin width was not reported in other such randomized trials.335–337
Whole-Breast Fractionation
Conventions for prescribing doses to the breast varied substantially from one institution to another in the “two-dimensional” era and only examined the central-axis plane, without the use of inhomogeneity corrections.338,339 Nor is there yet agreement on this in the three-dimensional era. (Differences in prescribing doses for boost fields are even more substantial.) Hence, the data below on dose response must be interpreted with caution.
Most centers in the United States and Western Europe have traditionally given whole-breast doses of 45 to 50 Gy in 1.8- to 2-Gy daily fractions. Centers in the United Kingdom, Canada, and occasionally elsewhere routinely used schemes with higher daily fraction sizes (e.g., 40 Gy in 15 or 16 fractions) with excellent results.340–344 Several randomized clinical trials have compared these and other fractionation schemes.345–347,348,349,350 Two are particularly important. A trial conducted from 1993 to 1996 in Ontario and Québec, Canada, included 1234 women with pathologically clear resection margins and negative axillary lymph nodes, who were randomly assigned to receive whole-breast irradiation of 50 Gy in 25 fractions or 42.5 Gy in 16 fractions.347,348 A boost was not given. With a median follow-up of 144 months, the 10-year rates of local recurrence in the two arms were 6.7% and 6.2%, respectively. Seventy percent of patients in each arm had excellent or good global cosmetic outcomes, with no difference in complication rates. The “START B” trial, conducted in the United Kingdom between 1999 and 2002, compared 50 Gy in 25 fractions with 40 Gy in 15 fractions in 2215 patients.349 A boost of 10 Gy in five fractions was given in 43% of patients. With a median follow-up of 6 years, the 5-year actuarial local failure rates were 3.3% and 2.2% in the two arms, respectively. Cosmetic results were slightly better in the 40-Gy arm. There was no difference between the arms in the risk of symptomatic rib fractures, symptomatic lung fibrosis, or ischemic heart disease.
Even more hypofractionated schemes have been tried.351–356 A randomized study at the Hôpital Necker (Paris) compared giving 45 Gy in 25 fractions, delivered in 5 weeks, to 23 Gy delivered as 5 Gy on days 1 and 3 and 6.5 Gy on days 15 and 17.351 With a minimum follow-up of 4 years, the locoregional recurrence rate in patients undergoing tumorectomy was 7% (4 of 56 patients) in the conventional fractionation arm, compared with 4% (2 of 45 patients) in the hypofractionation arm. The incidence of fibrosis and telangiectasias appeared greater in the hypofractionated group, although the severity of these was not described. The “FAST” trial, conducted from 2004 to 2007 in the United Kingdom, will help assess the place of such schemes.357,358
Total Dose to the Primary Tumor Site and Overall Treatment Time
Four randomized trials compared whole-breast radiation therapy alone with whole-breast irradiation plus a boost for patients with uninvolved margins.359–364 Absolute differences in local control rates were only 2% to 3% for their overall populations. The largest was conducted by the EORTC.362,364 This trial, however, defined margin status only in relationship to the invasive component of the tumor, ignoring DCIS, which severely limits the applicability of its results. Results of a central pathology review including about one-third of the entered patients found that the boost benefited patients with margins wider than 2 mm (reducing the 10-year failure rate from approximately 14% to 7% when the distance to invasive disease was assessed and from about 17% to 5% when DCIS was assessed).365 The boost did not have a statistically significant impact in the combined group of patients with either close (≤2 mm) or positive margins (approximately 11% and 9%, respectively, when invasive disease was considered, and approximately 14% and 11% when DCIS was examined). However, results were not presented separately for patients with close and positive margins, or for all patients with uninvolved margins together, regardless of width. Researchers also found that a boost decreased local failure rates significantly for “high-risk” groups characterized by high-grade invasive cancers or age younger than 50 years, but the results were not described according to margin status and the use of the boost simultaneously.
Giving doses above 60 Gy to the tumor bed may be of greater benefit for patients whose specimen resection margins are positive or not evaluated. In the EORTC randomized trial, patients with margins involved by invasive cancer were randomly allocated to receive either 76 Gy or 60 Gy.366 The rates of local failure at 10 years were 17.5% and 10.8%, respectively. However, 13% of patients in the high-dose arm developed severe fibrosis in the boost region, compared with 3% of the control group.
There are very few data on how the time to complete radiation therapy affects the risk of local recurrence. A retrospective study from Istanbul, Turkey, suggested that treatment interruptions or breaks of 8 days or more significantly increased this risk in patients treated with whole-breast irradiation plus a boost, most of whom also received systemic therapy.367 If radiation therapy is interrupted for more than 2 weeks, one may consider increasing the total dose to the primary tumor site, although the value of this is unknown. Several studies have investigated “concurrent boost” techniques, in which IMRT is used to give a higher daily dose to the boost volume than to the remainder of the breast.368,369 Such treatment appears to be well tolerated acutely, but long-term data on efficacy and complications are not yet available.
Nodal Irradiation Doses
Doses of 45 to 50 Gy result in a very low incidence of nodal failure in patients with clinically uninvolved nodes or involved nodes following axillary dissection.33,370 It is uncertain when, if ever, higher doses should be used in patients without gross residual disease. There is also little evidence regarding the nature of the dose-response relationship for suspiciously enlarged nodes.
Accelerated Partial-Breast Irradiation
As noted above, occult synchronous primary cancers are rare in patients with clinically unicentric cancers. Most residual tumor cells remaining after lumpectomy are located within a few centimeters of the excision cavity.134,371 Two thirds or more of local recurrences are at or near the site of the primary tumor, whether radiation therapy is used or not.372 Hence, treatment of only a more limited volume surrounding the tumor might be as effective as whole-breast irradiation for many patients. In principle, this approach might reduce complications associated with whole-breast irradiation and allow treatment to be completed more quickly.373
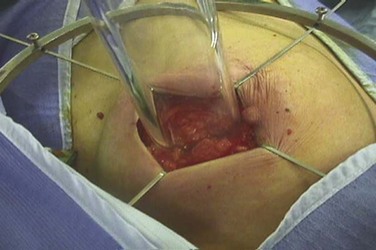
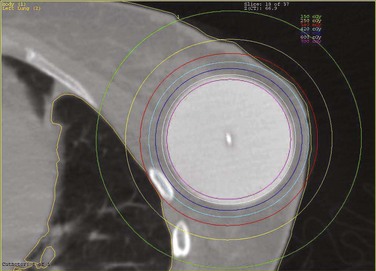
Among the approaches described to date for giving accelerated partial-breast irradiation (APBI) are the following: (1) low-dose-rate or high-dose-rate interstitial implantation374 ![]() (see web-only Fig. 61-7, available on the Expert Consult website); high-dose-rate brachytherapy using a balloon catheter with one or more source channels375,376 (see web-only Fig. 61-8); permanent interstitial implantation of radioactive seeds377 (see web-only Fig. 61-9); single-fraction intraoperative irradiation using 50-kV photons378 (see web-only Fig. 61-10), high-energy electrons379 (see web-only Fig. 61-11) or brachytherapy380; and three-dimensional conformal external beam radiation therapy (EBRT) using photons,381–383 mixed photons and electrons384 (see web-only Fig. 61-12), or protons.385,386
(see web-only Fig. 61-7, available on the Expert Consult website); high-dose-rate brachytherapy using a balloon catheter with one or more source channels375,376 (see web-only Fig. 61-8); permanent interstitial implantation of radioactive seeds377 (see web-only Fig. 61-9); single-fraction intraoperative irradiation using 50-kV photons378 (see web-only Fig. 61-10), high-energy electrons379 (see web-only Fig. 61-11) or brachytherapy380; and three-dimensional conformal external beam radiation therapy (EBRT) using photons,381–383 mixed photons and electrons384 (see web-only Fig. 61-12), or protons.385,386
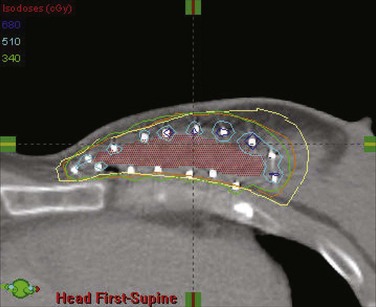
Web-Only Figure 61-7 Transverse CT image of a volume interstitial implant for partial-breast irradiation, with target volume (red color wash) and superimposed isodose distribution.
From Vicini FA, Arthur DW: Breast brachytherapy, North American experience. Semin Radiat Oncol 15:108-115, 2005, Figure 3; with permission of the publisher.
Web-Only Figure 61-8 Optimal placement of balloon brachytherapy device for partial-breast irradiation.
From Shah NM, Wazer DE: The MammoSite balloon brachytherapy catheter for accelerated partial breast irradiation, Semin Radiat Oncol 15:100-107, 2005, Figure 4; with permission of the publisher.
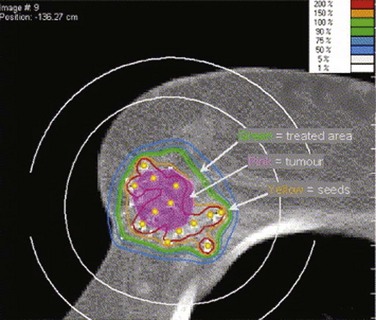
Web-Only Figure 61-9 Permanent palladium-103 (103Pd) seed implant for partial-breast irradiation, with superimposed dose distribution.
From Pignol JP, Keller B, Rakovitch E, et al: First report of a permanent breast 103Pd seed implant as adjuvant radiation treatment for early-stage breast cancer, Int J Radiat Oncol Biol Phys 64:176-181, 2006, Figure 3; with permission of the publisher.
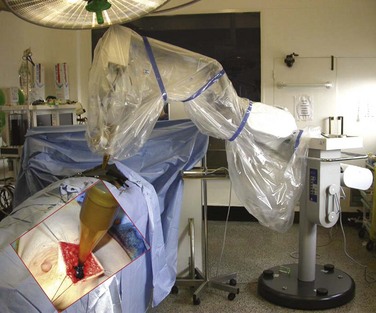
Web-Only Figure 61-10 Intraoperative partial-breast irradiation using the 50-kV intrabeam system, with the x-ray source in the breast wound (inset).
Vaidya JS, Tobias JS, Baum M, et al: TARGeted intraoperative radiotherapy (TARGIT). An innovative approach to partial-breast irradiation, Semin Radiat Oncol 15:84-91, 2005, Figure 2; with permission of the publisher.
Randomized Trials Comparing Whole-Breast and Accelerated Partial-Breast Irradiation
![]() Web-only Table 61-4 lists the randomized trials comparing partial-breast irradiation with conventional whole-breast irradiation. Three have been completed, with published results. Two of these, one conducted from 1982 to 1987 in Manchester, England, and one from 1986 to 1990 by the Yorkshire Breast Cancer Group in Leeds, England, did not assess microscopic margin status and used treatment techniques that make them of limited relevance to current practice.388,389 The National Institute of Oncology in Budapest, Hungary, conducted a trial from 1998 to 2004 that included 258 patients.396 All except one patient had microscopic margins of 2 mm or wider. Patients in the whole-breast irradiation arm received 50 Gy in 25 fractions without a boost, whereas patients in the APBI arm received either high-dose-rate implantation (36.4 Gy in 7 fractions over 1.5 weeks) or (if technically unsuited for an implant) electrons (50 Gy in 25 fractions over 5 weeks). With a median follow-up of 68 months, the 5-year actuarial local failure rates in the two arms were 3% and 5%, respectively. The rates of late grade 2 to 3 complications were 23% and 25%, respectively, and rates of excellent or good cosmetic results were 63% and 78%, respectively.
Web-only Table 61-4 lists the randomized trials comparing partial-breast irradiation with conventional whole-breast irradiation. Three have been completed, with published results. Two of these, one conducted from 1982 to 1987 in Manchester, England, and one from 1986 to 1990 by the Yorkshire Breast Cancer Group in Leeds, England, did not assess microscopic margin status and used treatment techniques that make them of limited relevance to current practice.388,389 The National Institute of Oncology in Budapest, Hungary, conducted a trial from 1998 to 2004 that included 258 patients.396 All except one patient had microscopic margins of 2 mm or wider. Patients in the whole-breast irradiation arm received 50 Gy in 25 fractions without a boost, whereas patients in the APBI arm received either high-dose-rate implantation (36.4 Gy in 7 fractions over 1.5 weeks) or (if technically unsuited for an implant) electrons (50 Gy in 25 fractions over 5 weeks). With a median follow-up of 68 months, the 5-year actuarial local failure rates in the two arms were 3% and 5%, respectively. The rates of late grade 2 to 3 complications were 23% and 25%, respectively, and rates of excellent or good cosmetic results were 63% and 78%, respectively.
Results of Nonrandomized Studies and Correlates of Local Recurrence
The only studies of APBI with a median follow-up of 5 years or more have employed interstitial implantation or balloon brachytherapy (Table 61-4). Local failure rates in most of these studies have been comparable to those achieved with conventional whole-breast irradiation. However, several series have had much higher failure rates, likely resulting from different patient selection criteria and treatment techniques.
TABLE 61-4 Results of Selected Studies of Accelerated Partial-Breast Irradiation with Median Follow-up of 5 Years or Longer
Experience with other forms of APBI is much more limited. So far, local control rates seem comparable to those of series using interstitial implants or balloon brachytherapy.373,408,409
There are few data on how the outcome of APBI varies in relation to patient, tumor, or clinical factors. In the Manchester trial, recurrence rates were quite similar in the two arms for patients with infiltrating ductal carcinomas (11% and 15%, respectively, for whole-breast vs. limited-field therapy); for patients with infiltrating lobular tumors, however, respective recurrence rates were 8% and 34%.388 The rate of local failure was 3% (4 of 130) for women younger than age 50 years treated with balloon brachytherapy in a large registry study, compared with 2% (21 of 1319) of older patients, with a median follow-up of 38 months.410 In a study from the University of Wisconsin with a median follow-up of 48.5 months, the risk of local failure was 2% in 183 patients in a “low-risk” group (≥50 years of age; node-negative, estrogen receptor-positive disease) and 4% in 90 patients with one or more “high-risk” criteria (≤50 years of age; estrogen receptor-negative disease, or one to three positive nodes).411 In the William Beaumont Hospital study, the risk of local failure was 36% (4 of 11) for patients with a tumor-free margin width of less than 2 mm, compared with 1% (1 of 77) for patients with margin widths of 2 to 10 mm or 1% (1 of 111) for those with margins of more than 10 mm.412,413 In the Radiation Therapy Oncology Group (RTOG) 96-17 trial, the risk of local failure was 14% (4 of 29) among patients not receiving systemic therapy, compared with 3% (2 of 70) among those who did.405
Complications
The most common long-term complication of APBI is fat necrosis, which occurs in 10% to 30% or more of patients by 2 to 3 years after interstitial implantation or balloon brachytherapy in some,403,404,406,414 but not all,410,415 series. Most are asymptomatic. Fibrosis occurs in a similar proportion of patients. Severe pain is rare.406 A few patients have had such severe fibrosis or discomfort that they have required mastectomy.405 Other toxicities include skin thickening and telangiectasias. In most studies, cosmetic results are good or excellent in 80% to 90% or more of patients, though some studies have lower rates.403
Few serious long-term toxicities have been seen so far in studies using intraoperative electron radiotherapy.379,416 Toxicity rates in general appear lower in patients treated with external beam APBI than with brachytherapy, but experience is more limited.409,417,418 Radiation pneumonitis has been reported following external beam APBI, which exposed large volumes of the ipsilateral lung to relatively low doses.419
Patients receiving chemotherapy after brachytherapy appear to have increased late toxicity and poorer cosmetic results.420–422 These problems can be reduced by delaying the start of chemotherapy by 3 weeks or more.421 A recent pilot study showed, however, that external beam partial-breast irradiation using once-daily fractionation could be safely given with concurrent dose-dense doxorubicin and cyclophosphamide.423
Conclusions
The optimal pretreatment evaluation, selection criteria, and technical parameters, including how to choose between the different methods for APBI, are not known. Several professional societies408,424,425 and expert consensus panels426 have promulgated guidelines for the use of APBI outside formal protocols, which, however, do not agree on all specifics. At present, the author prefers to limit such treatment to patients age 50 years or older with estrogen receptor-positive, node-negative invasive cancers or DCIS measuring 2 cm or smaller, with microscopic margins of 2 mm or wider.
Acute and Chronic Complications and Cosmetic Results
Acute Reactions and Their Management
Most patients will develop mild skin irritation or itching as radiation therapy progresses. Moisturizers can reduce these symptoms. Applying skin care products prior to irradiation does not appreciably increase the skin dose427; hence, patients may use such products whenever and as often as needed. The value of prophylactic skin treatment is much less clear. Two randomized trials found that washing with soap and water reduces the severity of skin reactions, compared with not washing at all or using water alone,428,429 but several trials showed no differences in reactions between patients using different skin care products when compared with each other or when no products were applied.430–433 One double-blind trial suggested that a cream including hyaluronic acid was more effective in preventing severe skin reactions than one containing only the base of polyethylene glycol, glycerol, and sorbitol.434 Another trial found that applying a potent steroid cream from the beginning of treatment decreased the intensity of the acute skin reaction.435 There were no data on whether this caused long-term changes in the skin, however.
About 10% to 15% of patients treated with radiation therapy alone or sequential chemotherapy and radiation therapy will develop moist desquamation (usually in the axilla or inframammary fold) during or shortly after completion of irradiation333; the rate may be 50% or higher for patients receiving concurrent chemoradiotherapy.436 Patients with only limited areas of moist desquamation can often continue radiation therapy without interruption. It may be desirable to temporarily switch from tangential fields to the boost field, rather than giving a complete treatment break. Treatment breaks of more than 1 week are rarely necessary. Reepithelialization need not be complete for treatment to resume. Skin care commonly used in this setting includes chlorhexidine gluconate or other antibacterial soaps to cleanse the area (also useful for patients developing folliculitis) and using nonadherent dressings to absorb drainage and maintain moisturization. In a randomized trial performed in Scotland, patients with moist desquamation took longer to heal when a hydrogel dressing was used than when a dry dressing was applied.433
Fatigue is very common during irradiation. It is generally mild and resolves within 2 months following completion of irradiation. A prospective study performed in Munich, Germany, did not find any factors correlated with its development or severity.437 A study in Newcastle, Australia, found that a higher baseline fatigue level and higher baseline neutrophil and red cell counts were predisposing factors.438
Subacute and Chronic Complications
Skin, Soft Tissue, and Bone Reactions
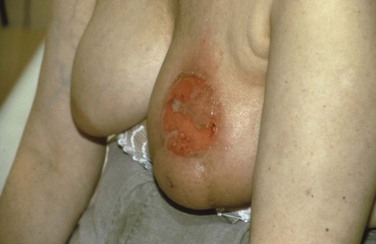
Patients may develop a skin “radiation recall” reaction when chemotherapy (particularly doxorubicin) is given following irradiation (Fig. 61-6). This is rare when chemotherapy begins more than 2 weeks after the completion of irradiation, however.436
Breast abscess or cellulitis develops in 5% to 10% of patients at any time after surgery.439,440 Cellulitis may be more frequent in patients with hematomas or seromas requiring drainage, in those in whom large volumes of breast tissue have been resected, or in those with arm edema.441,442 Sometimes cellulitis or abscess mimics an inflammatory-type recurrence,439,443 although the presence of pain, possible systemic symptoms of infection, and the time course of development usually make the distinction clear.
Rarely, patients develop patches of white or yellowish well-circumscribed sclerosis and induration weeks to years following radiation therapy, sometimes preceded by erythema. The patches may be surrounded by a more highly pigmented, bruise-like area. This phenomenon has been termed postirradiation morphea or circumscribed scleroderma.444,445
Fibrosis is common but generally mild. When severe, it can impair the cosmetic results.
Some individuals develop fat necrosis, which typically causes a painful or painless mass, sometimes accompanied by retraction or dimpling of the overlying skin.446 The skin may be thickened clinically and radiologically. Mammography usually reveals a spiculated, often poorly defined mass that may contain punctate or large, irregular calcifications. Cystic degeneration may eventually develop, resulting in a cavity that contains oily fluid or necrotic fat.
Patients commonly note intermittent transient breast pain, which can be sharp and shooting or dull and aching, lasting a few seconds or minutes, or rarely longer. Younger patients seem more likely to experience pain after BCT than older individuals.447 In a randomized trial in which patients were allocated to receive radiation therapy or not following conservative surgery, patients treated with radiation therapy had more breast discomfort initially, but with further follow-up the degree of discomfort became equal in both groups.448 Pain is generally mild or moderate, but in some patients it is sufficiently bothersome to require medication.447 Some patients with severe pain or fibrosis have responded to treatment with pentoxifylline, alone or in combination with vitamin E.449,450
Soft tissue necrosis is quite rare and generally associated with outmoded irradiation techniques.451
The incidence of rib fractures is related to the treatment energy and the total breast dose given.451 Today, this risk should be less than 1%.452
Arm Edema and Shoulder and Arm Function
Arm edema may cause psychological and functional morbidity, as well as predisposition to the development of cellulitis. Lymphangiosarcoma (Stewart-Treves syndrome) due to arm edema is extremely rare.453
The great majority of cases of arm edema develop in the first 3 years after treatment.454 Symptomatic arm edema occurs in at most 5% to 10% of patients when either SNB, level I to II axillary dissection, or radiation therapy alone is employed; these cases are usually mild.455,456 The incidence and severity of arm edema are substantially increased following a complete axillary dissection,35 especially when full axillary irradiation is also given.457–459 However, full axillary irradiation does not increase the risk of arm edema for patients having limited surgery.457–460 Arm edema rates were not increased by supraclavicular irradiation, even when patients underwent complete axillary dissection, in most studies,461–464 though in others they were increased.465,466 Several studies found that a posterior axillary boost increased the risk of arm edema.464,466–468
Few randomized trials have compared different approaches to the management of lymphedema.469 For example, a trial conducted in Edmonton, Alberta, Canada, found that manual lymphatic massage had no benefits in addition to compression bandaging, except perhaps in patients with mild lymphedema.470
Axillary irradiation may also cause changes in shoulder mobility and/or arm functioning.471 Such changes are rare in patients treated with doses of 50 Gy or less in 1.8- to 2-Gy fractions. Physiotherapy immediately following axillary dissection improved shoulder functioning in a randomized trial performed in Nijmegen, the Netherlands,472 though the role of such therapy at a later time or in patients with dysfunction due to radiation therapy has not been studied.
Neurologic Injuries
The most common neurologic complication due to axillary dissection is the intercostobrachial nerve syndrome, characterized by fairly constant paresthesias and dull, aching, or burning pain of the upper arm, shoulder, and axilla, and occasionally of the more anterior chest wall. This syndrome may be permanent, at least for the most severely affected individuals. Treatment tends to be ineffective, although occasional patients respond to nerve blocks or topical measures.473,474 Motor injury resulting from axillary dissection is unusual because the thoracodorsal, long thoracic, and pectoral nerves can be exposed and protected.
Classic radiation therapy brachial plexopathy is a chronic, progressive syndrome the onset of which is highly variable, from as soon as 3 months to as long as 26 years after irradiation.475 Patients usually present with sensory and/or motor symptoms of the ipsilateral limb. Pain can occur but is generally less prominent, which helps in distinguishing the syndrome from neoplastic plexopathy. A second, rarer type of radiation plexopathy appears more rapidly than classic chronic plexopathy (median, 4.5 months) and usually resolves spontaneously.476 The incidence of brachial plexopathy is low (0% to 5%) in patients receiving doses at the plexus of 50 to 54 Gy in 1.8- to 2-Gy fractions or 40 to 45 Gy in 2.5- to 3-Gy fractions.477 In the JCRT experience, both the radiation therapy dose and the use of chemotherapy affected this risk.451 Other studies using both nodal irradiation and chemotherapy had no patients who developed plexopathy, however.333,478 There is no clearly effective treatment.477
The presence of an enhancing mass on MRI is the most helpful finding in distinguishing between radiation-induced and malignant brachial plexopathy, particularly in individuals with severe pain.479–482 Some patients with an indeterminate MRI may have abnormal uptake of 18F-fluorodeoxyglucose (FDG) on positron emission tomography (PET), indicating malignancy.483
Pulmonary Effects
Radiographic infiltrates and localized interstitial fibrosis are common in patients treated with radiation therapy.484 These generally stabilize by 12 months after treatment, although some may resolve and some continue to progress for as long as 5 years. Pulmonary function tests worsen soon after radiation therapy but then recover partially.485
Clinical radiation pneumonitis is characterized by nonproductive cough, fever, and nonspecific infiltrates on chest x-ray.484 It generally appears from 4 to 9 months after completion of radiation therapy. Rarely, radiation therapy appears to precipitate a more generalized syndrome called bronchiolitis obliterans with organizing pneumonia.486,487 Prolonged courses of prednisone are often needed to treat symptoms when severe.
Clinical radiation pneumonitis is very rare in patients treated to the breast alone, whether chemotherapy is given or not.488,489,490 Pneumonitis occurs in about 5% or less of patients treated with a third nodal field when the average lung distance is 3 cm or less.490
There are conflicting data on whether the risk of pneumonitis is increased by chemotherapy (particularly when given concurrently with radiation therapy), which may be due to differences in details of such programs.436 Two studies found that tamoxifen increased the risk of radiologic pulmonary fibrosis following radiation therapy.491,492 In a series from the Fox Chase Cancer Center, however, tamoxifen did not affect the risk of clinical radiation pneumonitis.129
Cardiovascular Complications
Radiation therapy may cause either acute or chronic heart damage.493,494 Acute and subacute complications, such as pericarditis, are rare following breast radiation therapy.451
Radionuclide cardiac imaging suggests that the development of perfusion abnormalities correlates with increasing irradiated cardiac volume.495 The presence of defects has not yet been shown to predict the long-term risk of clinical heart disease, however.496 Such toxicity appears to be mainly due to coronary artery disease and may have a latency period of 10 years or more. Long-term increased cardiac death rates were found by meta-analyses321 and registry-based studies,497,498 which included patients treated mainly with outmoded techniques of radiation therapy. Other registry studies did not find such an effect or found substantial effects only for patients irradiated before 1980.499,500 For example, Giordano and colleagues499 found that, for 12,136 patients treated between 1985 and 1989, there was no significant difference in the 15-year cardiac mortality rates between patients treated to the left and right sides (5.8% and 5.2%, respectively). Several single-institution studies using modern irradiation techniques also did not show increased risks of cardiac morbidity and mortality at median follow-up times of 10 to 20 years.501–505 Some studies of patients treated after 1980, however, have found increased risks of cardiovascular mortality or morbidity.506,507 A study from the University of Pennsylvania found that the 10-year actuarial cumulative incidence of cardiac mortality was 1.9% for left-sided patients and 1.5% for right-sided patients; at 15 years, rates were 4.4% and 2.9%, respectively, and at 20 years, 6.4% and 3.6%, respectively.506
Risk factors predisposing patients to cardiac complications of radiation therapy are not well understood. The University of Pennsylvania investigators found a statistically significant interaction between hypertension and left-breast irradiation in the development of coronary artery disease that increased over time. A study from Amsterdam and Rotterdam found that smoking was the only significant factor interactive with radiation therapy, however.508 Of note, excess cardiac events occurred in patients treated with postmastectomy irradiation (which generally included the IMNs) but not patients treated with BCT. The volume of heart irradiated in the tangential fields was not a risk factor, however, in another study from the Netherlands with a median follow-up time of 16 years.507
Most studies have not shown an increased risk of anthracycline cardiac toxicity in irradiated patients receiving commonly used doses.452,509–512 So far it does not appear that there is a deleterious interaction between radiation therapy and trastuzumab, but follow-up times have been short.513,514 It is also uncertain whether irradiating the IMNs will increase the risk of toxicity from trastuzumab.515,516
Giving prophylactic irradiation to the supraclavicular nodes does not appear to increase the risk of stroke.517–519
Carcinogenesis
Patients age 45 years or younger at initial diagnosis appear to have a small increased risk of developing new contralateral primary cancers due to radiation therapy.520,521 In a study using the Connecticut Tumor Registry, the relative risk was 1.21 at a dose of 1 Gy (probably the minimum achievable with current techniques).520
Most cancers that develop within the radiation therapy fields are soft tissue sarcomas or angiosarcomas. These may appear as soon as 1 or 2 years after treatment but tend to occur after 8 to 10 years or longer.522,523 The excess incidence of soft tissue sarcomas is 9 to 14 cases per 100,000 patient-years of observation.524–527 In a series from the Institut Gustav-Roussy near Paris, the 10-year actuarial incidence was 0.2%, the 20-year incidence 0.43%, and the 30-year incidence 0.78%.525 The excess incidence of angiosarcomas in patients treated with BCT is 5 to 13 cases per 10,000 treated patients.522,527–529 Of interest, a registry study from Finland found the risk of angiosarcomas was increased in both irradiated and unirradiated breast cancer patients, compared with that expected.530
In one study, postmastectomy irradiation was associated with an estimated excess of seven to eight lung cancer cases per 10,000 women who survived more than 10 years from diagnosis.531 Such cancers are almost entirely limited to current or former smokers.532,533 More recent studies found no increased risk of lung cancer following BCT, perhaps because of the smaller volumes of lung treated.534,535
Radiation-induced leukemia appears very rare in patients not receiving chemotherapy.452 The risk of leukemia may be increased by radiation therapy by 0.3% to 0.5% in patients who receive standard-dose chemotherapy.536,537
Cosmetic Results
The great majority of patients have acceptable cosmetic results.292 Patients tend to have more favorable views of cosmetic outcome than do physicians.538,539 Cosmetic results tend to decline for the first 3 years after treatment but then remain stable.
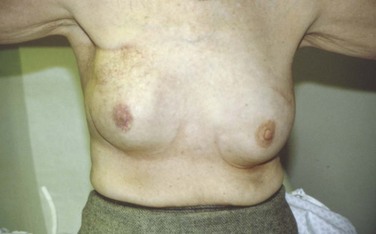
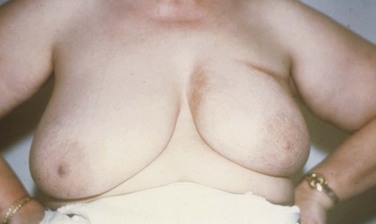
Cosmetic outcomes have improved over time due to changes in surgical and irradiation techniques (Fig. 61-7). However, results in different series are difficult to compare because there is no “standard” for describing cosmesis. Schemes for objectively measuring changes in breast size, shape, fibrosis, or contour have been devised,540,541 but these are not widely used. They also may not capture skin changes and other significant sequelae.
Surgery appears to be the most important factor affecting cosmetic outcome.292,542 Greater breast size has been associated with increased retraction after BCT543–545 ![]() (see web-only Fig. 61-7). This problem can be at least partially overcome, however, by the use of higher photon energies60 and by placing the patient in the prone or lateral decubitus position. Doses to the entire breast much above 50 Gy in 2-Gy fractions seem to worsen results,546 as does the use of large fraction sizes when doses above 45 Gy are given.547 Giving a breast boost worsens results modestly,364 although in the EORTC trial the cosmetic results obtained with implantation and external beam boosts were comparable.542 The use of a hockey-stick field or other techniques resulting in imperfectly matched field edges can cause substantial fibrosis and skin changes at areas of overlap
(see web-only Fig. 61-7). This problem can be at least partially overcome, however, by the use of higher photon energies60 and by placing the patient in the prone or lateral decubitus position. Doses to the entire breast much above 50 Gy in 2-Gy fractions seem to worsen results,546 as does the use of large fraction sizes when doses above 45 Gy are given.547 Giving a breast boost worsens results modestly,364 although in the EORTC trial the cosmetic results obtained with implantation and external beam boosts were comparable.542 The use of a hockey-stick field or other techniques resulting in imperfectly matched field edges can cause substantial fibrosis and skin changes at areas of overlap ![]() (see web-only Fig. 61-13, available on the Expert Consult website).
(see web-only Fig. 61-13, available on the Expert Consult website).
Cosmetic results appear to be similar whether chemotherapy is given before or after radiation therapy.333 The cosmetic results in patients receiving concurrent chemotherapy and radiation therapy tend to be inferior to those with sequential regimens, although not all studies agree on this point.436 Overall cosmetic results were unaffected by tamoxifen.129,548 In a study from the University of Pennsylvania, complication rates and cosmetic results were very similar whether tamoxifen was given concurrently or sequentially with irradiation, although breast edema was slightly more frequent in the former group (79% vs. 65%).549