CHAPTER 7 Breast Cancer Mimics
A variety of processes, both benign and malignant, may mimic primary breast carcinoma.1–4 Many of these can be distinguished from breast cancer on the basis of imaging findings alone. However, some may ultimately require histopathologic confirmation. The most common benign causes of masses in women are fibroadenomas and breast cysts.5 High-quality imaging, including diagnostic mammography and high-resolution ultrasound and strict adherence to interpretive criteria can, for the most part, distinguish fibroadenomas and cysts from breast cancer. However, because there is sufficient overlap in the appearance of benign and malignant lesions, a new or enlarging solid mass that is not classically benign (e.g., hamartoma or lipoma) requires biopsy. In addition to lesions related to the duct-lobular system, mimics of primary breast cancer may also be caused by a wide spectrum of pathologic disorders arising in mesenchymal structures of the mammary gland. These include tumors arising in the stroma of the breast that are breast specific, such as pseudoangiomatous stromal hyperplasia (PASH) and phyllodes tumors, as well as tumors arising from non-breast-specific stromal structures, including fibrous tissue, vascular structures, lymphoid tissue, nerves, and skin. These non-breast-specific tumors include focal fibrosis, fibromatosis, malignant fibrohistiocytomas, vascular malformations, angiosarcomas, neurofibromas, lymphomas, and liposarcomas. In addition, cancer mimics may also be caused by inflammatory processes (foreign body reaction, mastitis, and abscess), trauma (hematoma, fat necrosis), lactational changes, and metastasis from extramammary malignancies.
EPITHELIAL BREAST LESIONS
Sclerosing Adenosis
Although most breast calcifications are benign, clustered microcalcifications that do not appear classically benign on mammography (e.g., milk of calcium, vascular or rim calcifications), usually require biopsy. Sclerosing adenosis, a form of fibrocystic change, is a frequent mimicker of breast carcinoma. The mammographic findings of sclerosing adenosis include microcalcifications, a circumscribed mass, an ill-defined mass, a spiculated mass, focal asymmetry, and focal architectural distortion.6–8
Papillomas
Benign papillomas are another common breast lesion that can mimic carcinoma. Typically they present with bloody nipple discharge and occur in perimenopausal women. Mammographically, they present as single or multiple circumscribed or irregular masses, with or without microcalcifications. Sonographically, they may present as a complex intracystic lesion, intraductal lesion, or a homogeneous solid lesion. Because papillomas cannot be distinguished from papillary carcinoma or ductal carcinoma on the basis of clinical features or imaging features, biopsy is necessary.9
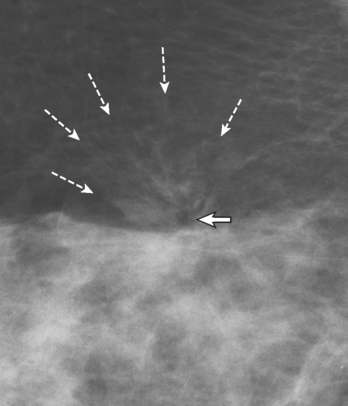
Radial Scars
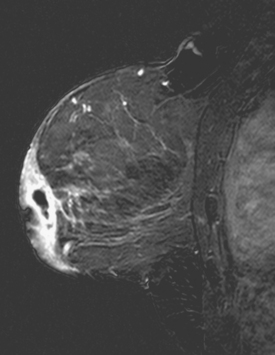
Radial scar (RS), also known as a complex sclerosing lesion, is a benign lesion that is often mistaken for carcinoma because of its spiculated appearance (Figure 1).
The pathogenesis of RS remains obscure. It has been postulated that these lesions arise as a result of unknown injury, leading to fibrosis and retraction of surrounding breast tissue. Although imaging features such as lack of a central mass and long, thin, radiating spicules help to suggest the diagnosis of RS, because they are commonly associated with atypia and malignancy, biopsy is necessary.10–11
LESIONS OF THE BREAST STROMA (BREAST SPECIFIC)
Pseudoangiomatous Stromal Hyperplasia
Pseudoangiomatous stromal hyperplasia is a mesenchymal lesion composed of myofibroblasts, which sometimes includes glandular components. It is frequently a microscopic incidental finding in breast biopsies but may occasionally present as a round or oval circumscribed or partially circumscribed mass mammographically.12–15 The sonographic appearance may be variable, with most presenting as a solid, hypoechoic mass without posterior acoustic shadowing. They typically occur in premenopausal women and pathologically may be mistaken for angiosarcoma.
Phyllodes Tumor
Phyllodes tumor, also called cystosarcoma phyllodes, are unusual fibroepithelial tumors composed of epithelium and a spindle cell stroma and can exhibit a wide range of clinical behavior. Radiographically they present as a rapidly growing, hypoechoic, circumscribed mass.16 They are classified as benign, borderline, or malignant based on histopathologic features. However, histologic classification does not always predict outcome. The prognosis of phyllodes tumors is favorable, with local recurrence in about 15% of patients overall and distant recurrence in about 5% to 10%.
LESIONS OF THE BREAST STROMA (NONBREAST–SPECIFIC)
Focal Fibrosis
Focal fibrosis, also known as fibrous mastopathy, is similar to pseudoangiomatous stromal hyperplasia. These lesions typically occur in premenopausal women and present as a circumscribed mass, irregular mass, or focal asymmetry and are hypoechoic on ultrasound. They are composed of dense collagenous stroma with sparse glandular and vascular elements.17–18
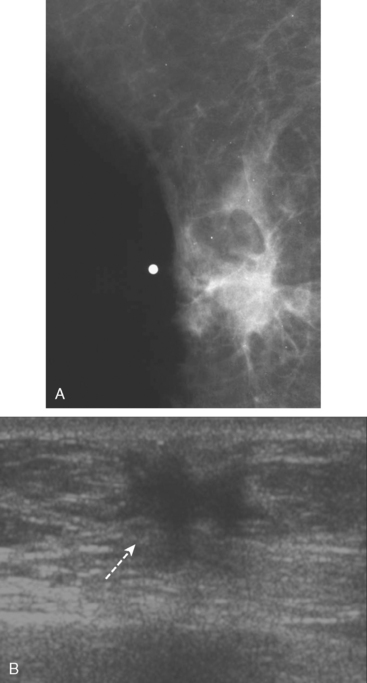
Fibromatosis
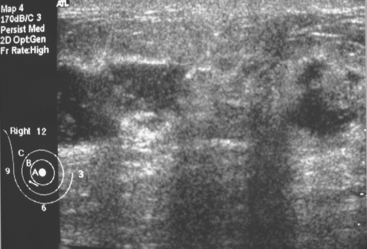
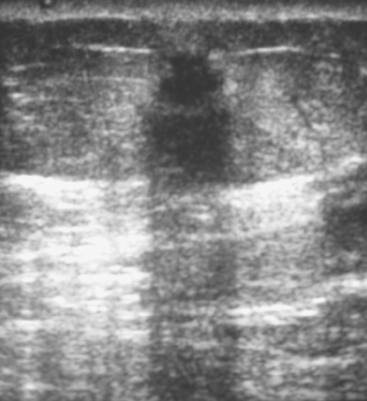
Fibromatosis, also known as extra-abdominal desmoid tumor, is an extremely rare low-grade infiltrative tumor composed of well-differentiated fibroblasts. The lesions tend to develop in the pectoralis fascia and may be fixed, causing retraction of the pectoralis muscle, skin, or nipple. On mammography, fibromatosis usually appears as a spiculated mass simulating carcinoma.19 On ultrasound, the lesions manifest as an irregular hypoechoic mass with posterior acoustic shadowing (Figure 2). Biopsy is necessary for diagnosis. Although fibromatosis does not metastasize, there is a high rate of local recurrence.
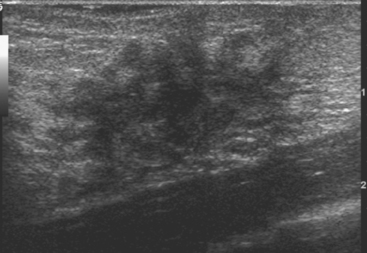
Diabetic Fibrous Mastopathy
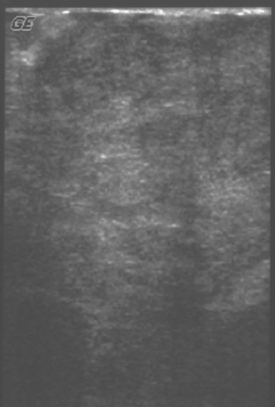
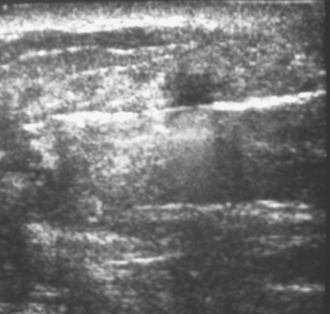
Diabetic mastopathy is an unusual form of stromal fibrosis with lymphocytic infiltration, which typically occurs in premenopausal women with juvenile-onset insulin-dependent diabetes (type 1). Patients present with solitary or multiple nontender, hard masses. Mammography shows generalized dense tissue, and ultrasound shows an irregular hypoechoic mass with posterior acoustic shadowing (Figure 3).
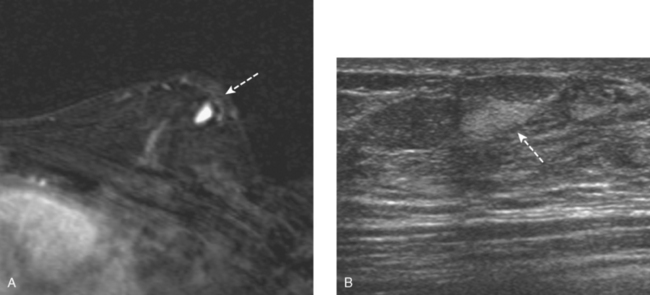
Vascular Tumors
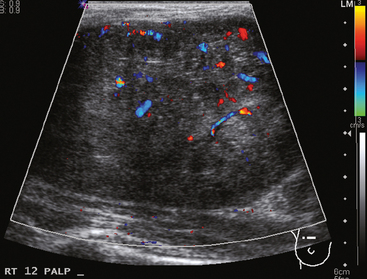
Vascular tumors of the breast are uncommon and include hemangiomas and angiosarcomas.20 Hemangiomas are extremely rare, are usually smaller than 2 cm, and can be differentiated from dermal hemangiomas by their distinct separation from the epidermis. Mammographically, breast hemangiomas are small, well-defined, lobulated masses. On ultrasound, their appearance may be variable, being either circumscribed hypoechoic masses, mixed echogenicity masses, or ill-defined hyperechoic masses (Figure 4). Angiosarcomas of the breast are more common than hemangiomas and are usually larger in size. Radiographically, they present as an ill-defined calcified or noncalcified hypoechoic mass. They may occur in the chest wall as a rare complication following radiation therapy for primary breast cancer.
Neural Tumors
Breast tumors of neural origin are rare and include granular cell tumors and neurofibromas. Granular cell tumors are uncommon benign tumors of probable Schwann cell origin that occasionally present as a breast mass. They occur more frequently in the upper inner quadrant, corresponding to the cutaneous sensory territory of the supraclavicular nerve. Granular cell tumors occur in middle-aged, premenopausal women, usually as an irregular or spiculated hypoechoic mass with posterior acoustic shadowing. Wide local excision is generally the preferred treatment because these tend to recur if incompletely excised. Neurofibromas are benign peripheral nerve sheath tumors, usually occurring in the subcutaneous tissue and occasionally are found in the breast. Mammographically they appear as multiple, well-defined, benign-appearing cutaneous masses. On ultrasound, they appear as well-defined hypoechoic masses with posterior acoustic enhancement, located in the subcutaneous tissue. Clinical history usually makes the diagnosis obvious. Neurofibromas occurring in the breast are rare, are usually associated with neurofibromatosis type 1 in the pediatric patient and occur in a periareolar location.
INFECTION AND INFLAMMATION
Granulomatous Mastitis
Granulomatous mastitis of the breast is a rare entity that often presents as a palpable mass. The clinical and imaging findings may be confused with inflammatory carcinoma of the breast. The diagnosis should be suspected in patients from endemic regions. In addition to infectious causes such as tuberculosis and fungus, granulomatous mastitis may also be due to autoimmune diseases, such as sarcoidosis, or it may be idiopathic in nature.21–23
MISCELLANEOUS BREAST LESIONS
Fat Necrosis
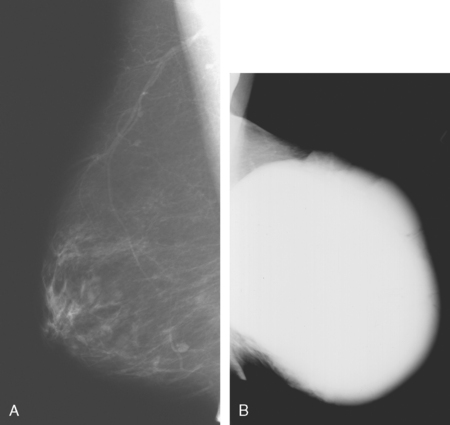
Fat necrosis is a benign, nonsuppurative process related to breast trauma, usually from prior surgical procedures. On mammography, fat necrosis can produce changes that are similar in appearance to malignancy (Figure 5). It can appear as a hypoechoic, shadowing mass with spiculated margins that may contain indeterminate microcalcifications and can cause skin retraction.24–25 The presence of central fat or dystrophic rim calcifications is usually diagnostic. Correlation of the imaging finding with the clinical history can aid in the diagnosis.
Metastasis
Metastatic disease should be considered when there is bilateral axillary adenopathy or multiple bilateral irregular solid masses. The most common metastatic lesions in the breast, in order of frequency, are due to lymphoma; melanoma (Figure 6); lung, ovarian, renal cell, and cervical carcinoma; and leukemia.26
Metastatic involvement of the breast from an extramammary malignancy is uncommon, with an incidence of between 0.8% and 6.6%. Rhabdomyosarcoma is the most common extramammary malignancy to metastasize to the breast in the pediatric age group but is rare in adults.
1 Iglesias A, Arias M, Santiago P, et al. Benign breast lesions that simulate malignancy: magnetic resonance imaging with radiologic-pathologic correlation. Curr Probl Diagn Radiol. 2007;36(2):66-82.
2 Porter GJ, Evans AJ, Lee AH, et al. Unusual benign breast lesions [review]. Clin Radiol. 2006;61(7):562-569.
3 Harvey JA. Unusual breast cancers: useful clues to expanding the differential diagnosis. Radiology. 2007;242(3):683-694.
4 Pojchamarnwiputh S, Muttarak M, Na-Chiangmai W, et al. Benign breast lesions mimicking carcinoma at mammography. Singapore Med J. 2007;48(10):958-968.
5 Foster ME, Garrahan N, Williams S. Fibroadenoma of the breast: a clinical and pathological study. J R Coll Surg Edinb. 1998;33:16-19.
6 Cyrlak D, Carpenter PM, Rawal NB. Breast imaging case of the day: florid sclerosing adenosis. RadioGraphics. 1999;19:245-247.
7 Nielsen NS, Nielsen BB. Mammographic features of sclerosing adenosis presenting as a tumour. Clin Radiol. 1986;37:371-373.
8 Günhan-Bilgen I, Memis A, Ustün EE, et al. Sclerosing adenosis: mammographic and ultrasonographic findings with clinical and histopathological correlation. Eur J Radiol. 2002;44:232-238.
9 Lam WW, Chu WC, Tang AP, et al. Role of radiologic features in the management of papillary lesions of the breast. AJR Am J Roentgenol. 2006;186(5):1322-1327.
10 Ciatto S, Morrone D, Catarzi S, et al. Radial scars of the breast: review of 38 consecutive mammographic diagnoses. Radiology. 1993;187:757-760.
11 Kennedy M, Masterson AV, Kerin M, et al. Pathology and clinical relevance of radial scars: a review. J Clin Pathol. 2003;56(10):721-724.
12 Powell CM, Cranor ML, Rosen PP. Pseudoangiomatous stromal hyperplasia: a mammary stromal tumor with myofibroblastic differentiation. Am J Surg Pathol. 1995;19:270-277.
13 Polger MR, Denison CM, Lester S, et al. Pseudoangiomatous stromal hyperplasia: mammographic and sonographic appearances. AJR Am J Roentgenol. 1996;166:349-352.
14 Cohen MA, Morris EA, Rosen PP, et al. Pseudoangiomatous stromal hyperplasia: mammographic, sonographic, and clinical patterns. Radiology. 1996;198:117-120.
15 Mercado CL, Naidrich SA, Hamele-Bena D. Pseudoangiomatous stromal hyperplasia of the breast: sonographic features with histopathologic correlation. Breast J. 2004;10:427-432.
16 Telli ML, Horst KC, Guardino AE, et al. Phyllodes tumors of the breast: natural history, diagnosis, and treatment [review]. J Natl Compr Cancer Netw. 2007;5(3):324-330.
17 Venta LA, Wiley EL, Gabriel H, et al. Imaging features of focal breast fibrosis: mammographic-pathologic correlation of noncalcified breast lesions. AJR Am J Roentgenol. 1999;173(2):309-316.
18 Goel NB, Knight TE, Pandey S, et al. Fibrous lesions of the breast: imaging-pathologic correlation. RadioGraphics. 2005;25(6):1547-1559.
19 Schwarz GS, Drotman M, Rosenblatt R, et al. Fibromatosis of the breast: case report and current concepts in the management of an uncommon lesion [review]. Breast J. 2006;12(1):66-71.
20 Glazebrook KN, Morton MJ, Reynolds C. Vascular tumors of the breast: mammographic, sonographic, and MRI appearances. AJR Am J Roentgenol. 2005;184:331-338.
21 Schelfout K, Tjalma WA, Cooremans ID, et al. Observations of an idiopathic granulomatous mastitis [review]. Eur J Obstet Gynecol Reprod Biol. 2001;97(2):260-262.
22 Bakaris S, Yuksel M, Ciragil P, et al. Granulomatous mastitis including breast tuberculosis and idiopathic lobular granulomatous mastitis. Can J Surg. 2006;49(6):427-430.
23 Ozturk M, Mavili E, Kahriman G, et al. Granulomatous mastitis: radiological findings. Acta Radiol. 2007;48(2):150-155.
24 Hogge JP, Robinson RE, Magnant CM, et al. The mammographic spectrum of fat necrosis. RadioGraphics. 1995;15:1347-1356.
25 Soo MS. Fat necrosis in the breast: sonographic features. Radiology. 1998;206:261-296.
26 Mihai R, Christie-Brown J, Bristol J. Breast metastases from colorectal carcinoma. Breast. 2004;13:155-158.
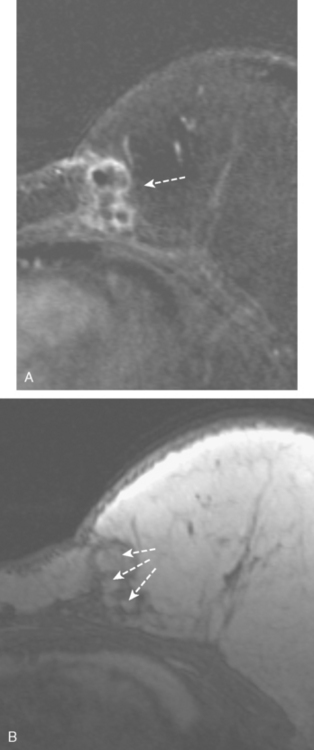
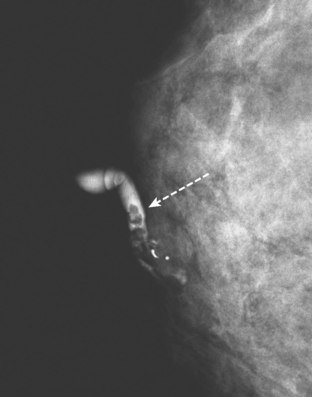
CASE 1 Papilloma presenting with bloody nipple discharge; ductogram and ultrasound
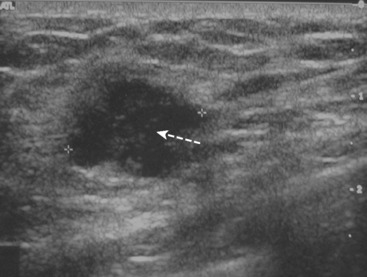
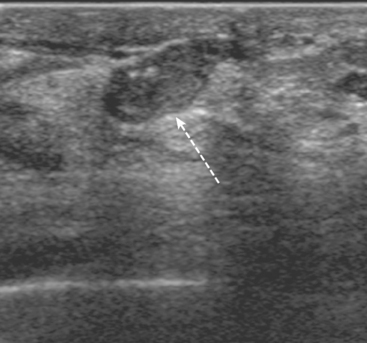
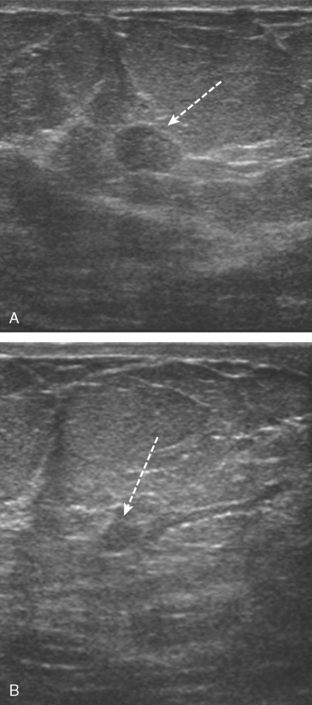
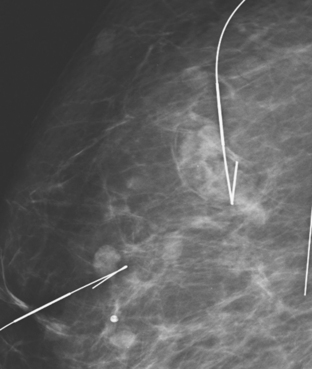
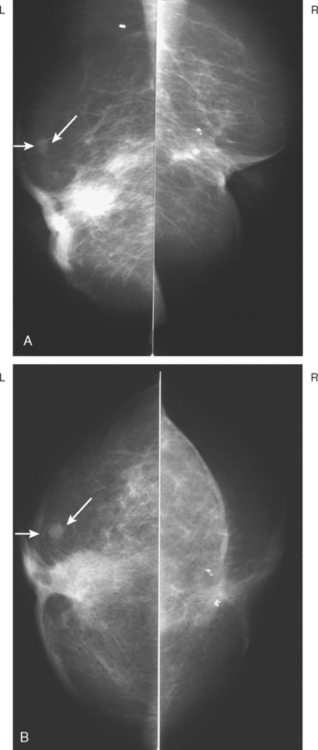
A 72-year-old woman presented with intermittent spontaneous right breast bloody nipple discharge for 2 weeks. A ductogram revealed an intraductal filling defect in the subareolar right breast (Figure 1). The intraductal mass was also demonstrated on ultrasound (Figure 2). Ultrasound-guided core needle biopsy yielded a diagnosis of benign papilloma. Subsequent needle localization and excisional biopsy confirmed the diagnosis of benign intraductal papilloma.
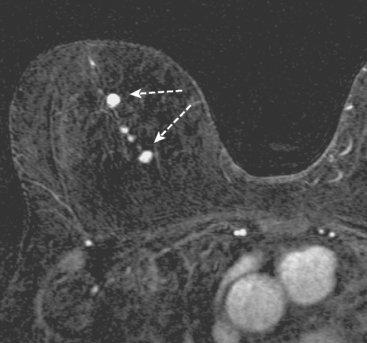
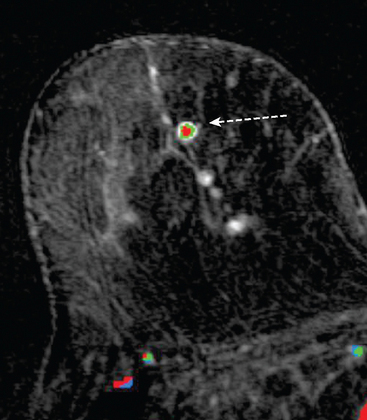
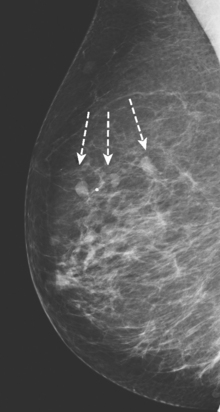
CASE 2 Multiple papillomas
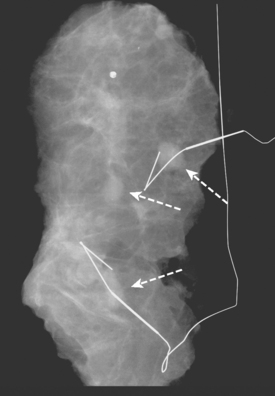
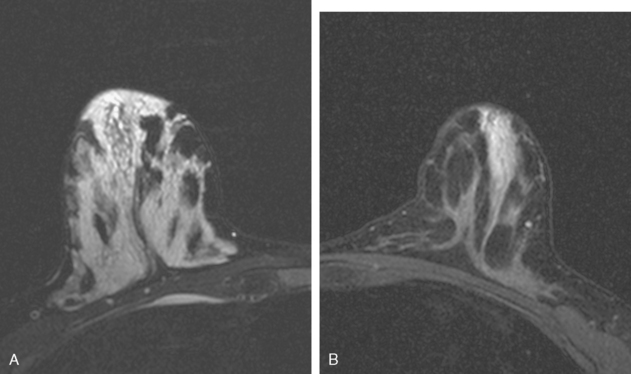
A 69-year-old woman with a right breast nipple discharge underwent MRI evaluation. The MRI showed multiple enhancing masses in the superior right breast that were oriented in a ductal pattern (figure 1 and figure 2). Correlation with the patient’s mammogram revealed that the masses seen on MRI corresponded to multiple masses in the superior right breast on mammography (Figure 3). The masses had slowly increased in size over several years. The appearance on ultrasound suggested that these were intraductal masses (Figure 4). Needle localization and excisional biopsy were performed (figure 5 and figure 6). Pathology revealed multiple benign papillomas.
TEACHING POINTS
This case illustrates that benign papillomas may exhibit malignant features on MRI. In this example, multiple masses are seen in a ductal orientation, mimicking DCIS. In addition, papillomas may display a suspicious washout kinetic pattern, concerning for invasive carcinoma. Because of the overlap in the imaging appearance of papillomas and carcinoma, biopsy is required to establish the diagnosis.
CASE 3 Large phyllodes tumor (ultrasound)
A 39-year-old woman was evaluated for an enlarging right breast lump. This had initially developed about 8 months before, as a golf ball–sized mass that had been noted a month after breast trauma. The findings were thought to be post-traumatic, and the patient was reassured. No imaging was performed.
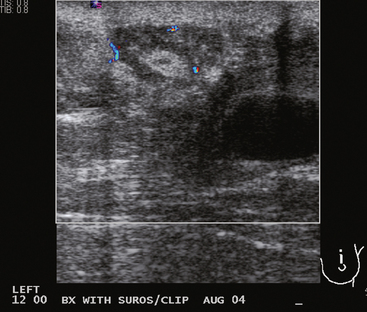
The mass never resolved. At the time of imaging evaluation, the patient noted an increase in size of the mass over the preceding 2 months. On physical examination, a mobile, tender, palpable, 8-cm mass occupied the entire upper breast, distorting the breast and stretching the overlying skin. Sonographic evaluation showed a 10-cm, solid, vascular mass with circumscribed margins (Figures 1, 2, and 3). Biopsy with ultrasound guidance obtained a pathologic result of biphasic neoplasm with cellular stroma, with differential diagnosis of cellular fibroadenoma versus phyllodes tumor.
CASE 4 Large phyllodes tumor
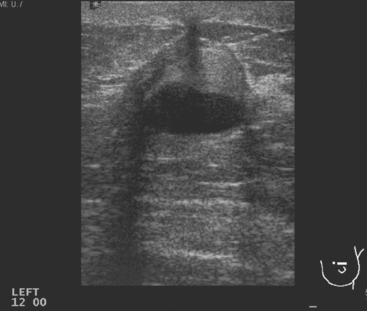
A 70-year-old woman presented with a large palpable mass replacing much of her left breast (Figure 1). Ultrasound showed a corresponding hypoechoic solid mass (Figure 2). Core biopsy yielded a diagnosis of a benign phyllodes tumor. Because of the size of the lesion, the patient underwent left mastectomy.
TEACHING POINTS
Phyllodes tumors of the breast are unusual fibroepithelial tumors composed of epithelium and a spindle cell stroma and can exhibit a wide range of clinical behavior. They are classified as benign, borderline, or malignant, based on histopathologic features. However, histologic classification does not always predict outcome. Immunohistochemical features of phyllodes tumors may help in predicting their clinical outcome. The prognosis of phyllodes tumors is favorable, with local recurrence in about 15% of patients overall and distant recurrence in about 5% to 10%. It may be difficult to distinguish a phyllodes tumor from a fibroadenoma on core needle biopsy. When a rapidly growing mass is detected and core biopsy reveals a cellular fibroadenoma, phyllodes tumor should be suspected. Wide excision, which may require mastectomy, is the definitive primary therapy for phyllodes tumors.
CASE 5 Multifocal breast abscesses
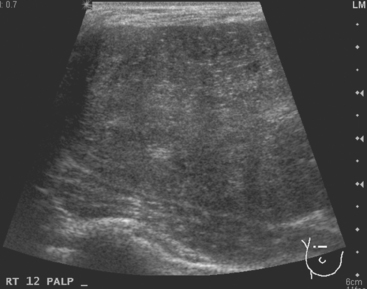
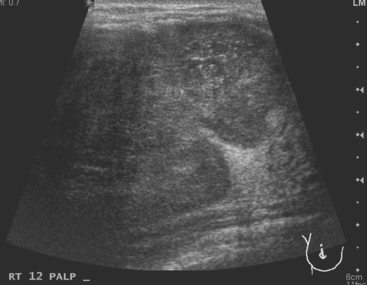
A 37-year-old woman developed two right palpa-ble breast masses, which were exquisitely tender. There was no erythema. Mammographic and sonographic evaluations were performed. Mammography showed a dense, ill-defined lower outer quadrant mass. Ultrasound showed two hypoechoic, similar-appearing masses with angular, irregular margins (Figure 1). Ultrasound-guided core needle biopsies of both masses returned diagnoses of acute mastitis, with acute neutrophilic infiltrate, edema, and focal chronic inflammation. There was no evidence of malignancy.
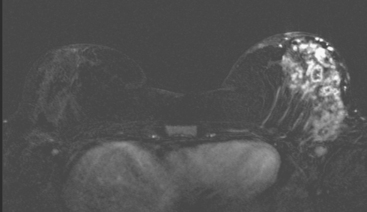
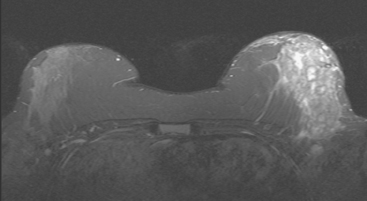
CASE 6 Granulomatous mastitis
A 61-year-old woman presented from South America, with a history of chronic left breast pain, swelling, and erythema. An MRI showed diffuse enhancement of the left lateral breast (Figures 1 and 2). Subsequent biopsy and cytologic evaluations confirmed a diagnosis of granulomatous mastitis from tuberculosis (TB) of the breast.
CASE 7 Lymphocytic mastitis
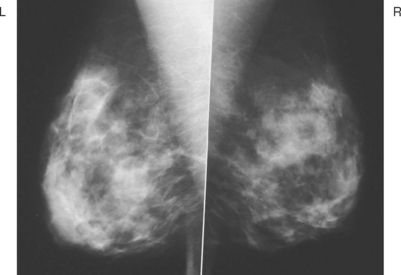
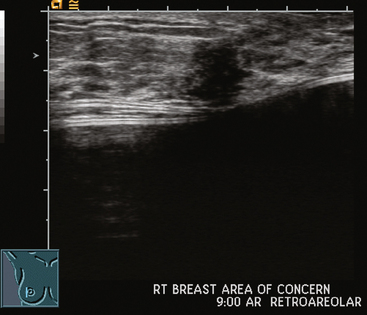
A 47-year-old healthy woman developed a left retroareolar tender mass and a palpable 2-cm lump. She was sent for ultrasound for a suspected cyst. Ultrasound showed increased vascularity in the para-areolar region, but no discrete mass. No mammographic abnormality or change was seen (Figure 1). The surgeon’s physical examination noted tender induration without a discrete mass of the upper areola, extending 1 to 2 cm beyond the areola. A core needle biopsy was obtained in the office, and a 1-week trial of antibiotics was given. The needle biopsy specimen showed acute and chronic inflammation. A mixed inflammatory infiltrate of neutrophils, plasma cells, and lymphocytes was noted. Improvement was noted on antibiotics, but the tenderness subsequently recurred and antibiotics were restarted, without complete resolution.
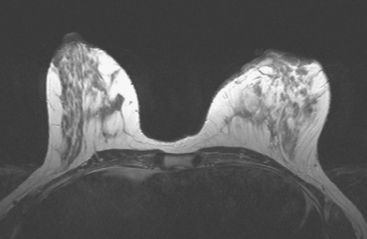
Breast MRI was obtained to better define the extent of the process (Figures 2, 3, 4, 5, 6, and 7). Intense mass-like enhancement of the medial left breast skin and retroareolar region was found, surrounding a fluid collection. Excisional biopsy removed a region of apparent granulation tissue and surrounding induration. The specimen showed chronically inflamed granulation tissue with adjacent fibrosis. Varying degrees of periductal, perilobular, and perivascular lymphocytic inflammation were noted, consistent with lymphocytic mastitis.
TEACHING POINTS
Inflammatory processes can mimic the imaging features of breast cancers. Compare the MRI findings in this case to those of Case 10 in Chapter 6, in which similar enhancement of the skin and nipple are due to recurrent breast cancer. Generally, the clinical features allow differentiation. This patient had a waxing and waning clinical course of recurrent tenderness, without fluctuation or drainage, which did not resolve with multiple courses of antibiotics. On initial imaging with ultrasound, no drainable fluid collection was seen. Breast MRI showed a fluid collection, which was small relative to the impressive, sheet-like confluent enhancement of the medial skin, subjacent breast parenchyma, and retroareolar region.
CASE 8 New fat necrosis mass mimicking recurrence
A 73-year-old woman, previously treated for bilateral breast cancer with lumpectomies and radiation therapy, underwent routine mammographic surveillance. This was 11 years after treatment for right breast cancer and 3 years after treatment for the left, a T1N0, estrogen receptor–positive tumor. Mammography showed a new 8-mm mass with indistinct margins in the upper outer left breast (Figure 1). Sonography identified a corresponding abnormality, a 6-mm hypoechoic, shadowing mass with spiculated margins (Figure 2). Recurrent breast cancer was suspected, and the lesion was biopsied with ultrasound guidance (Figure 3).
Pathology demonstrated benign fat necrosis with hyalinized sclerosis. Subsequent evaluations have shown postbiopsy changes and stable postoperative findings (Figures 4 and 5); no evidence of recurrence has been identified after 2 years of follow-up.
CASE 9 Fibrosis mimicking recurrence in implant-reconstructed breast cancer patient
Ultrasound of the palpable periareolar mass showed a very hypoechoic, taller-than-wide, irregularly marginated mass (Figure 1). This was biopsied with ultrasound guidance, with 14-gauge core needle biopsy technique.
Pathology showed no carcinoma, with sclerosis, fibrosis, and foreign body giant cell reaction found.
TEACHING POINTS
This patient had implants placed in the prior year and had been treated with breast conservation for early-stage IDC 10 years before. The ultrasound appearance of this mass is highly suspicious and raised the specter of recurrent breast cancer in this patient. Fortunately, 14-gauge core needle biopsy confirmed a satisfactory alternate diagnosis. Fibrosis and foreign body giant cell reaction, presumably related to the patient’s implant surgery, produced a mass that mimics recurrent breast cancer. See Case 7 in Chapter 6 for a very similar-appearing ultrasound mass, which did represent a recurrence.
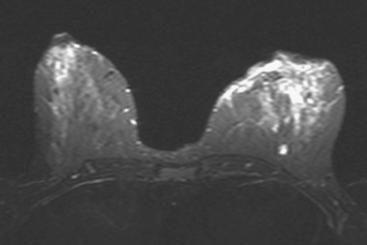
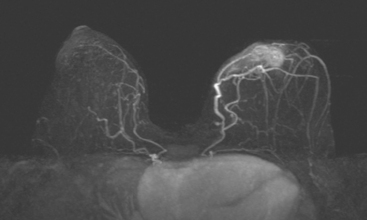
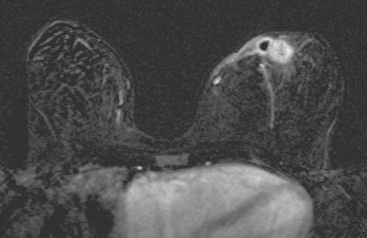
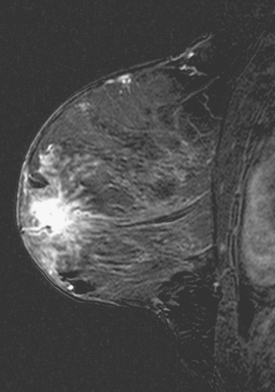
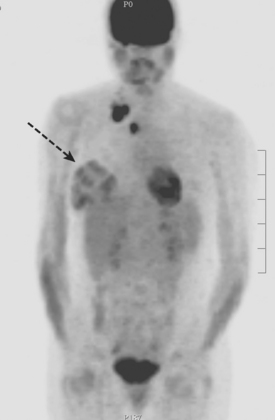
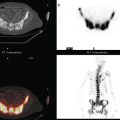
CASE 10 Lactational asymmetry on PET
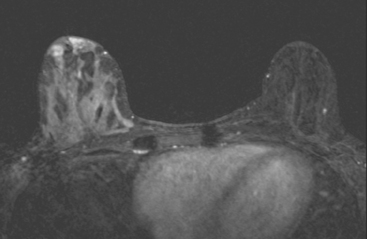
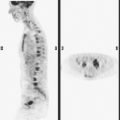
A 38-year-old woman with a recent diagnosis of lymphoma underwent a staging positron emission tomography (PET) scan. The patient was breast-feeding at the time. The PET scan demonstrated the known lymphoma within the upper thorax (Figure 1). In addition, diffuse uptake was seen involving the right breast. A subsequently performed breast MRI showed diffuse non-mass-like enhancement of the right breast, as well as breast edema (Figures 2 and 3). Upon questioning, it became apparent that the patient was breast-feeding only from the right breast, owing to difficulty on the left. The imaging findings of unilateral benign lactational change correlated with the history. No further breast workup or follow-up was required.
TEACHING POINTS
This case illustrates benign lactational breast changes, which should not be confused with a malignant process. In this example, although there is diffuse uptake on the PET scan and enhancement on the MRI, the imaging features are consistent with a benign process, given the absence of mass effect, parenchymal distortion, or secondary signs of malignancy, such as skin thickening or adenopathy. In addition to lactational changes, infection and trauma may mimic malignancy. This case also emphasizes the importance of clinical history in establishing such alternative diagnoses.