CHAPTER 11 Breast Cancer Metastases to the Neural Axis
Breast cancer is the second most common primary tumor to metastasize to the central nervous system (CNS), after lung cancer.1,2,3 Breast cancer metastases to the neural axis typically occur late in the disease, with lung, liver, or bone metastases preceding the diagnosis of CNS metastases. The median interval between the diagnosis of breast cancer and brain metastases is 34 months. The historical 1-year survival rate after diagnosis of brain metastases is 20%.3
Based on data from the 1960s and 1970s, the incidence of clinically apparent brain metastases in women with stage IV breast cancer is 10% to 16%. However, autopsy data show the incidence to be greater and has been reported to be as high as 30%.1
Younger patients are at higher risk for CNS metastases. In an autopsy series of 1044 breast cancer patients, the median age of patients with CNS metastases was 5 years younger than patients without CNS metastases.1
Studies suggest a relationship between hormone receptor status and the incidence of CNS metastases.1,3,4 A study of 217 breast cancer patients found a difference in the rate of brain metastases between estrogen receptor–negative and –positive tumors (10% versus 4%). In a multivariate model, HER-2/neu overexpression was the strongest predictor of CNS relapse, with a 10-fold increase in the incidence of brain metastases (4.3% versus 0.4%). However, it is unclear whether HER-2/neu positive patients are actually at higher risk for CNS metastases or whether the natural history of their disease is affected by more effective systemic disease control with trastuzumab (Herceptin). HER-2 amplification occurs in 25% to 30% of breast cancers and correlates with decreased disease-free and overall survival rates. Treatment with Herceptin improves systemic disease control and overall survival of HER-2/neu positive patients with metastatic breast cancer. However, it does not cross the blood-brain barrier. The theory is that the natural history of the disease may be changed as a result of improved extra-CNS systemic disease control from Herceptin therapy, resulting in an increase in the incidence of CNS metastases. Circumstantial evidence for this includes the statistic that the incidence of CNS metastases in stage IV breast cancer patients who are treated with Herceptin is higher than historical norms, and ranges from 28% to 43%.
DISTRIBUTION OF CRANIAL METASTASES
Brain metastases result from hematogenous spread. Their predilection for the gray-white matter junction is thought to result from decreased vessel caliber at this level acting as a trap for embolized clumps of tumor cells. Similarly, there is a predilection of metastases to occur at intracranial vascular watershed levels. The distribution of metastases within brain compartments is roughly proportionate to the blood supply, with about 80% occurring in the cerebrum, 15% in the cerebellum, and 5% in the brainstem. Metastases occur most commonly at the junction of gray and white matter in the cerebral hemispheres, followed by deep gray matter (basal ganglia, thalami), intraventricular (choroid plexus), and cerebellum.3,6
CNS metastases can be categorized as intra-axial, extra-axial, or cerebrospinal fluid (CSF) dissemination. Breast cancer more commonly involves multiple compartments (parenchyma, meninges, bone) than other tumors. It is the solid tumor most commonly associated with leptomeningeal involvement, although this is considerably less common than parenchymal metastases, occurring in 5% to 16% of patients at autopsy.1 Breast cancer also metastasizes to the eye at a higher rate than other tumors. A small percentage of breast cancer metastases target the meninges or the skull diploic space. Growth of such lesions may be intracranial and can compress the brain surface, whereas metastases to the skull base dura or bone can exert mass effect on the brainstem or cranial nerves.
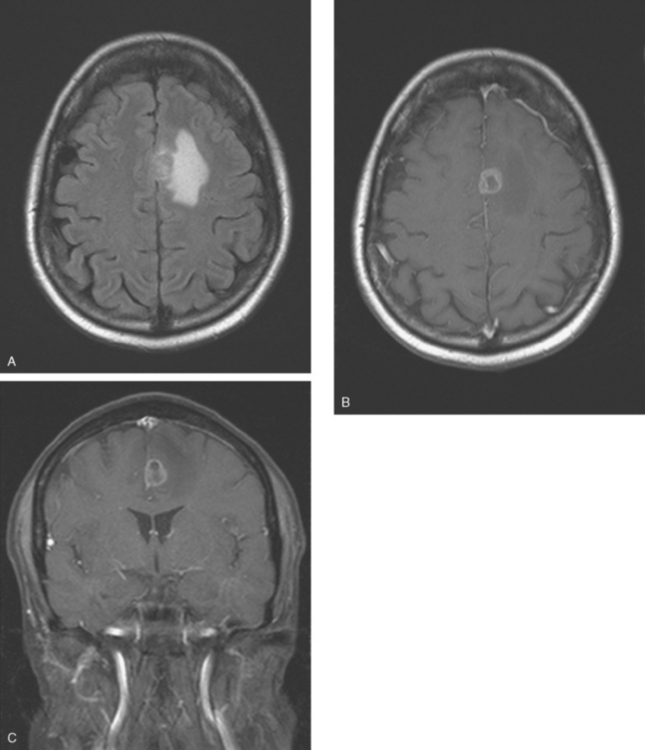
The most common imaging manifestation of intracranial metastatic disease is multiple intra-axial brain masses.6 These vary in size, shape, and enhancement patterns; the amount of associated edema can range from negligible to intense. Lesions can be solid and enhance uniformly (Figure 1), or can be cystic (Figure 2) or necrotic, with peripheral rim enhancement. Rim enhancement can be thin, thick, uniform, or irregular (Figure 3). Identifying single or multiple intracranial mass lesions in a breast cancer patient is highly suspicious for intracranial metastases, although it has mimicks with other diagnoses, including other neoplasms (metastases from other primaries, brain primary neoplasia, and CNS lymphoma), demyelinating disorders such as multiple sclerosis, multifocal infectious or granulomatous processes, and multifocal ischemic or vasculitic lesions.6
Extra-axial mass lesions may be formed by metastases to the skull or dura. Dural-based metastatic masses can be nodular and mass-like (Figure 4) or elongated and plaque-like (Figure 5). The underlying cerebral cortex may be compressed and edema incited in the underlying parenchyma. Morphologically, dural-based metastases may mimic meningiomas, or subdural collections.6
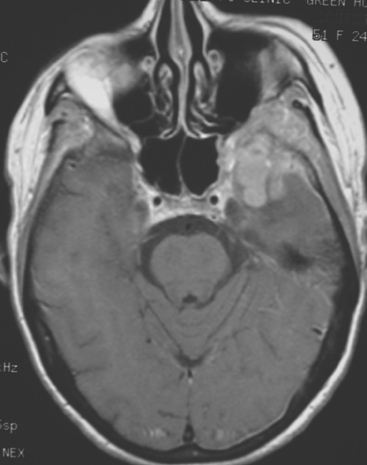
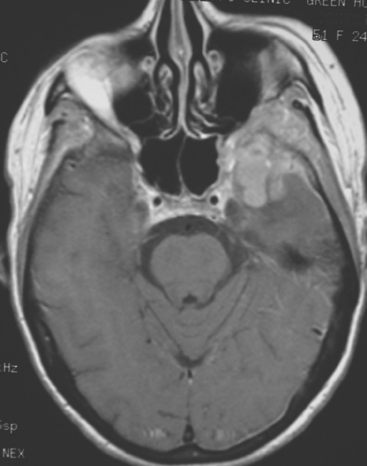
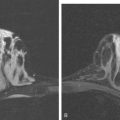
FIGURE 4 Enhanced axial T1-weighted MRI of a 51-year-old woman with metastatic breast cancer shows an enhancing, lobular, dural- and bone-based mass centered on the left sphenoid wing. The component extending into the left middle cranial fossa mimics a meningioma, being dural based and extra-axial, with well-defined margins and an enhancing dural tail. The underlying brain is compressed and edematous. Enhancing tumor also replaces the sphenoid wing bones, and on other levels, extended into the left orbit (not shown). See additional images from the same patient in Case 6 in this chapter.
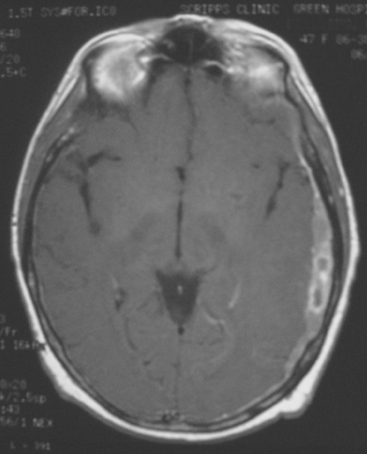
FIGURE 5 A 47-year-old woman with new-onset expressive aphasia and prior gamma knife therapy 7 months before for right posterior parietal metastasis. Enhanced axial T1-weighted MRI shows a localized region of plaque-like metastatic dural enhancement over the left cerebrum. Additional images on this case can be seen in Case 7 in this chapter.
Leptomeningeal tumor consists at pathologic studies of sheet-like growth of tumor on the brain, spinal cord, or nerve root surfaces. Three mechanisms are postulated for CSF dissemination of tumors.3 Tumor cells may rupture out of the subarachnoid space or pial vessels. Growing metastases in the brain cortex or pia may contact and be bathed by CSF, and thereby shed cells into it. Alternatively, hematogenous metastases to the choroid plexus may seed the CSF. Lobular carcinoma has been noted in clinical and autopsy series to be more likely to manifest as leptomeningeal tumorthan ductal carcinoma. The diagnosis can be suggested by characteristic imaging findings on gadolinium-enhanced MRI or can be established by CSF cytology. The imaging hallmarks are enhancement of the leptomeninges (pia and arachnoid) on the brain or cord surface, which can be confluent and sheet-like or nodular, with extension into sulci or along cranial nerves or around the cauda equina (Figure 6).
DETECTION OF CNS METASTASES BY IMAGING
Gadolinium-chelate contrast-enhanced MRI is acknowledged to be the most sensitive imaging modality for the diagnosis of neural axis metastases.7,9 Enhanced MRI is superior to enhanced CT for both brain parenchymal and brain and spine leptomeningeal disease due to its higher soft tissue contrast, higher sensitivity to contrast enhancement, direct multiplanar capability, and lack of artifacts related to bone. It particularly excels in demonstration of lesions in the posterior fossa and brainstem, where beam-hardening artifacts can be problematic on CT. MRI is also superior to CT in identifying multiple lesions, which is helpful in the differential diagnosis.6
The role of brain PET is limited in evaluating for metastases. Although brain metastases can be seen occasionally on whole-body PET scans (see Case 4 in this chapter), the sensitivity of PET for detection of brain metastases is inferior to MRI and is size dependent. The likelihood of detecting a 1-cm lesion with PET is reported to be about 40%. On a retrospective evaluation of whole-body PET performance in identifying brain metastases, Rohren and colleagues found that PET detected only 61% of lesions found by MRI.5
INDICATIONS FOR IMAGING
CNS metastases may be heralded clinically by focal neurologic symptoms, seizure activity, or symptoms reflecting increased intracranial pressure, such as headache, nausea, vomiting, and mental status changes.1,3 Focal neurologic symptoms may result from tumor-induced chemical changes causing neuron dysfunction and swelling, or from mass effect from compression of normal tissue. Tumor-induced electrical dysfunction can lead to seizure activity. Increased intracranial pressure may result from obstructive hydrocephalus, such as from mass effect from a parenchymal metastasis to the posterior fossa, or from communicating hydrocephalus due to leptomeningeal metastases.
TREATMENT OPTIONS
After corticosteroid treatment for edema, therapeutic choices for brain metastases include whole-brain radiation therapy (WBRT), neurosurgery, stereotactic radiosurgery (SRS), and chemotherapy.1,2,3 If there are multiple metastases, WBRT has been shown to improve survival and quality of life compared with corticosteroids alone. Median survival is 4 to 5 months. Most patients (75% to 85%) will have improvement of symptoms, with best palliation achieved of seizures and headache. Imaging responses to effective radiation therapy range from complete resolution to shrinkage in size of lesions, to decreased number of identifiable lesions, to alterations of morphology, with improved mass effect and associated edema (Figure 7).
SPINAL METASTASES
Metastases that compress the spinal cord most commonly present with pain at that level. With increased compression, symptoms progress to numbness below that level and eventually to difficulty ambulating. If untreated, there can be progression to paralysis. Bowel and bladder dysfunction may occur late.
Metastases to the cauda equina produce back pain and, variably, radicular symptoms.9
1 Lin NU, Bellon JR, Winer EP. CNS metastases in breast cancer. J Clin Oncol. 2004;22:3608-3617.
2 Chang EL, Lo S. Diagnosis and management of central nervous system metastases from breast cancer. Oncologist. 2003;8:398-410.
3 Parker EC, Kelly PJ. Brain metastases from breast cancer. In: Roses DF, editor. Breast Cancer. Philadelphia: Elsevier Churchill Livingstone; 2005:633-643.
4 Bendell JC, Domchek SM, Burstein HJ, et al. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer. 2003;97(12):2972-2977.
5 Rohren EM, Provenzale JM, Barboriak DP, et al. Screening for cerebral metastases with FDG PET in patients undergoing whole-body staging of non–central nervous system malignancy. Radiology. 2003;226:181-187.
6 Yock DH. Magnetic Resonance Imaging of CNS Disease: A Teaching File. St. Louis: Mosby, 2002;2-19. 101, 439
7 Sze G, Milano E, Johnson C, et al. Detection of brain metastases: comparison of contrast-enhanced MR with unenhanced MR and enhanced CT. AJNR Am J Neuroradiol. 1990;11:785.
8 Davis PC, Hudgins PA, Peterman SB, et al. Diagnosis of cerebral metastases: double-dose delayed CT vs contrast-enhanced MR imaging. AJNR Am J Neuroradiol. 1991;12:293.
9 Douglas AF, Cooper PR. Spinal column metastases from breast cancer. In: Roses DF, editor. Breast Cancer. Philadelphia: Elsevier Churchill Livingstone; 2005:644-652.
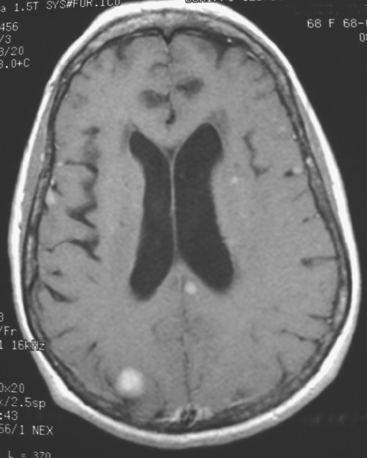
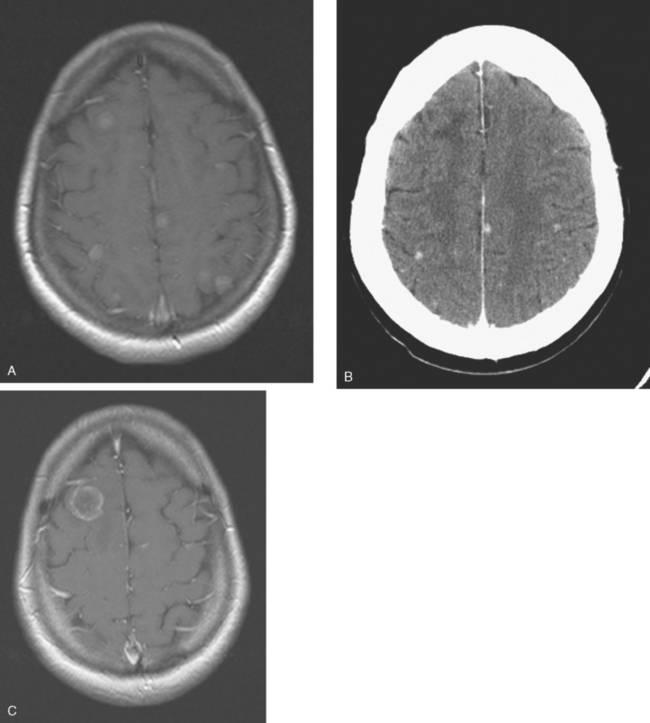
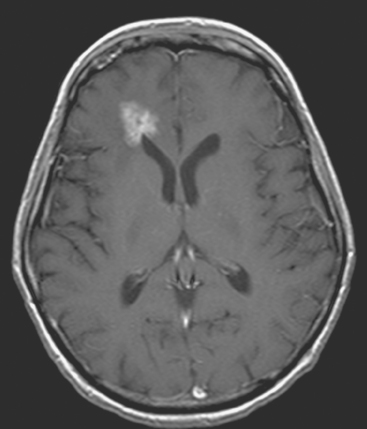
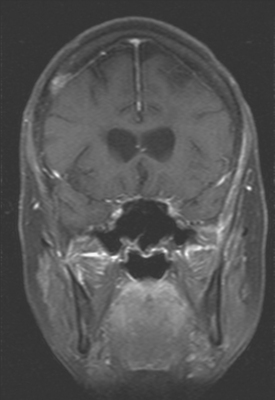
CASE 1 Multilocular thalamic cystic metastasis
A 43-year-old woman with metastatic breast cancer to the thorax developed nausea, one episode of vomiting, progressive headache, confusion, and speech difficulty.
Lung and nodal breast cancer metastases had been identified the year before with PET and CT evaluation for rising tumor markers. The diagnosis of recurrent breast cancer was confirmed by a lung nodule biopsy, which showed malignant adenocarcinoma, consistent with breast primary. The patient was begun on a regimen of docetaxel (Taxotere) and Gemzar. This was eventually discontinued because of pulmonary toxicity (see Case 12 in Chapter 10) and disease progression. The patient’s regimen was changed to capecitabine (Xeloda) for six cycles, with a good initial response, but subsequent regrowth.
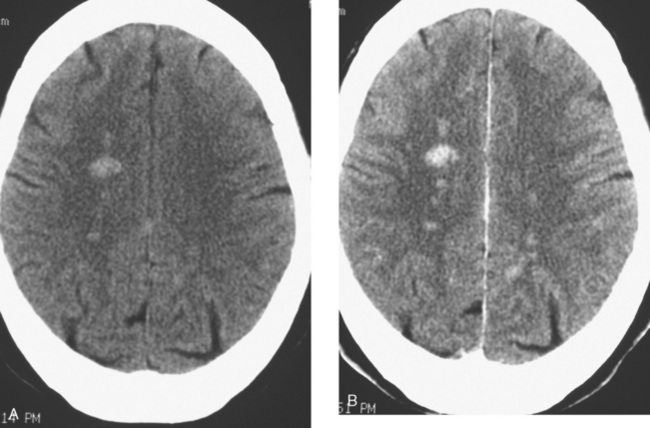
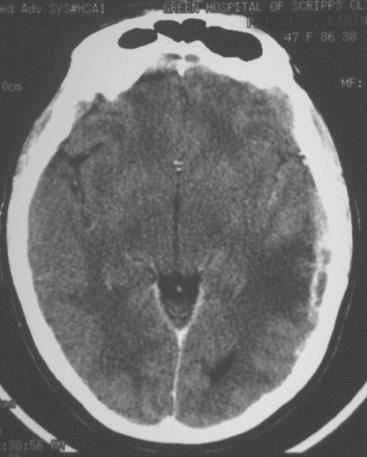
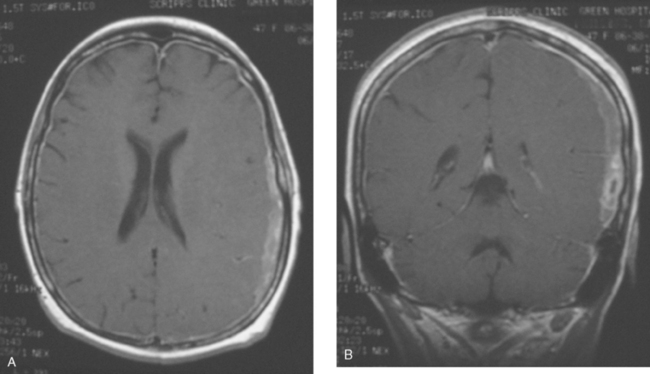
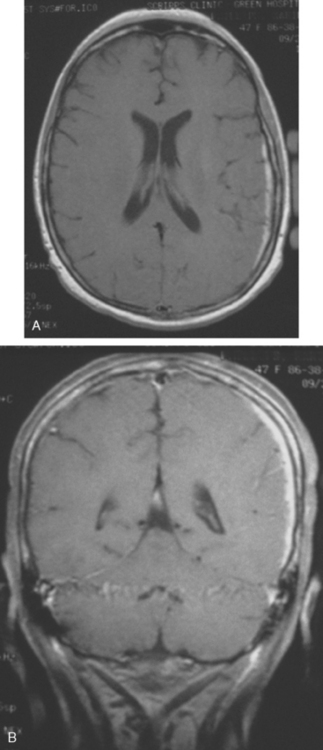
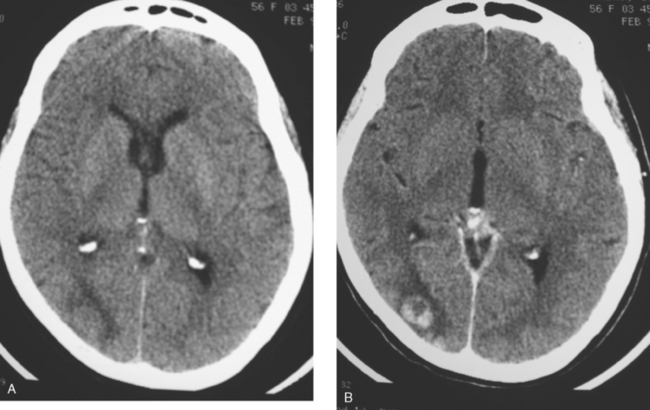
When the patient developed symptoms of headache, nausea, and mental status changes, a noncontrast head CT scan was obtained (Figure 1). The head CT identified a lesion and suggested that secondary hydrocephalus was developing. Unenhanced head CT excluded a hemorrhage. Brain MRI was obtained next, which better showed the internal structure of the mass, which was solitary (Figure 2).
TEACHING POINTS
Although hematogenous brain metastases classically favor a peripheral distribution, often at the gray-white matter junction, brain metastases not uncommonly occur in deeper locations, frequently the periventricular regions. Masses such as this, occurring adjacent to ventricles, can be difficult to distinguish from masses within ventricles. MRI is very helpful in this distinction, because of its multiplanar capability and better soft tissue contrast.The higher sensitivity of MRI is also useful in the evaluation of suspected metastases by identification or exclusion of additional lesions.
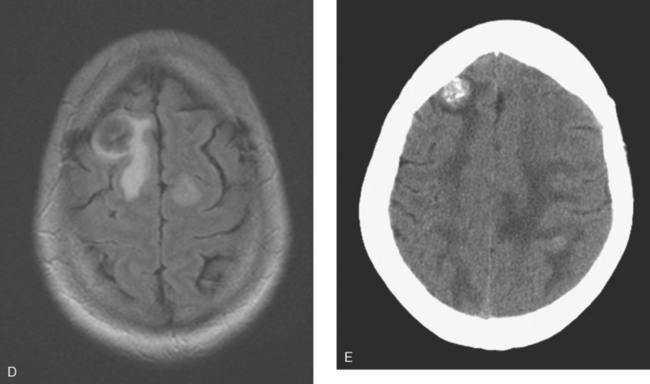
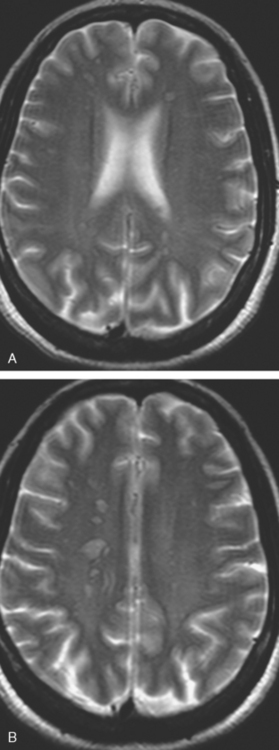
CASE 2 Brain metastases mimicking multiple sclerosis
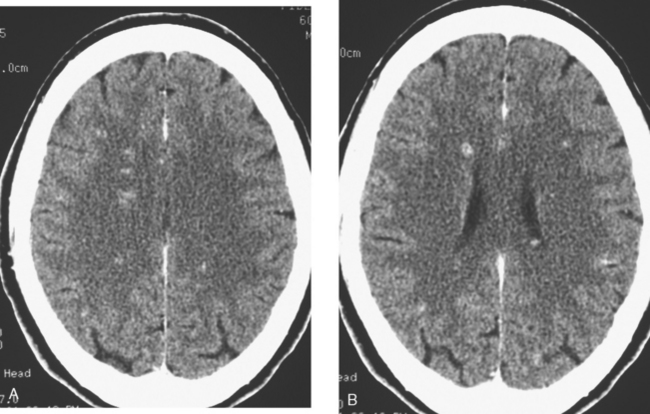
At the time of evaluation for intractable nausea and dry heaves, additional symptoms of dysphagia, coughing, anorexia, and weight loss were noted. Head CT, with and without contrast enhancement, showed small, enhancing periventricular white matter lesions, and a sclerotic clivus lesion (Figures 1, 2, and 3). MRI showed comparable findings (Figures 4, 5, 6, and 7).
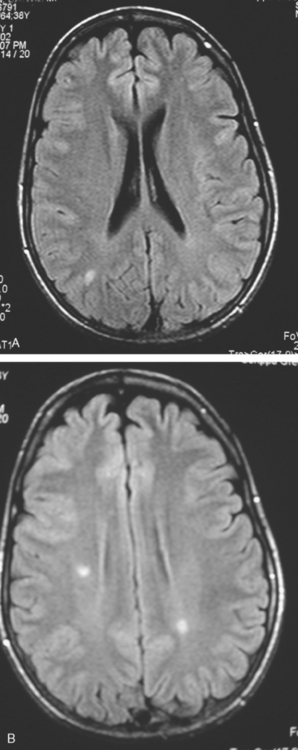
CASE 3 Brain metastases mimicking multiple sclerosis
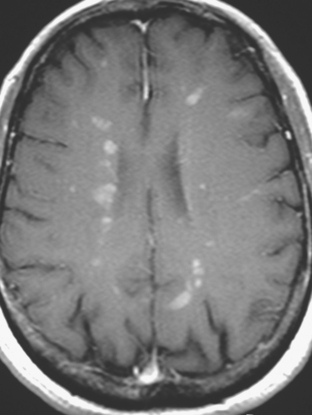
A 38-year-old woman treated for breast cancer 2 years earlier developed episodes of visual disturbance, with scintillating scotomas. Enhanced brain MRI was abnormal, with multiple enhancing elliptical white matter lesions, without edema (Figures 1 and 2). A differential diagnosis of brain metastases versus demyelinating process (multiple sclerosis) was considered.
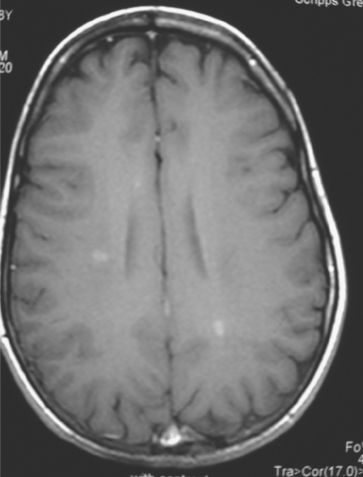
FIGURE 2 Enhanced axial T1-weighted sequence, at the same level as Figure 1B, shows enhancement of the lesions.
Repeat brain MRI 2 months later showed more numerous and larger enhancing brain lesions, with a more typical appearance for metastases than on the prior study (Figure 3).
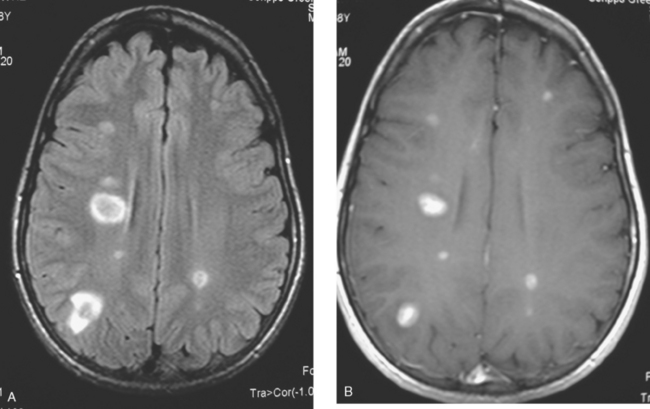
FIGURE 3 Repeat brain MRI, 2 months later, at levels corresponding to Figures 1B and 2 (A, axial FLAIR; B, enhanced axial T1-weighted) shows the lesions to be larger, with collars of perilesional edema demonstrated on FLAIR. Multiple similar appearing new enhancing lesions are seen in addition.
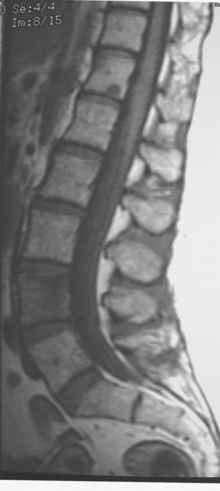
A cervical and thoracic spine MRI was obtained at the time of the initial brain MRI to evaluate complaints of back pain. Spine MRI showed metastatic involvement of T4 (Figure 4), and the patient underwent T3-T5 radiation for palliation of symptoms.
TEACHING POINTS
Intracranial lesions in patients with cancer histories most often represent brain metastases, although not invariably. The differential diagnosis includes primary brain neoplasia, infection, inflammation, infarction, and as raised in this case, demyelinating processes. In a randomized trial reported by Patchell and colleagues of 54 patients who underwenteither surgery and radiation therapy compared with radiation therapy after needle biopsy for treatment of single brain metastases from a variety of primaries, 11% were found to have an alternative diagnosis on pathology.
The bone metastasis depicted here at T4 shows typical imaging features (see Figure 4). The bone lesion conspicuity, based on replacement of the normal fatty marrow signal, is greatest on T1-weighted images. Bone lesion conspicuity is comparatively poor with T2-weighting where the lesion is essentially isointense to other vertebral levels. The expansion of the replaced body into the epidural space and mass effect on the cord are well depicted against the bright cerebrospinal fluid (CSF) signal afforded by T2-weighted technique. Contrast enhancement also decreases the conspicuity of the bony lesion, although depiction of the epidural extent, associated dural enhancement, and mass effect on the cord are improved.
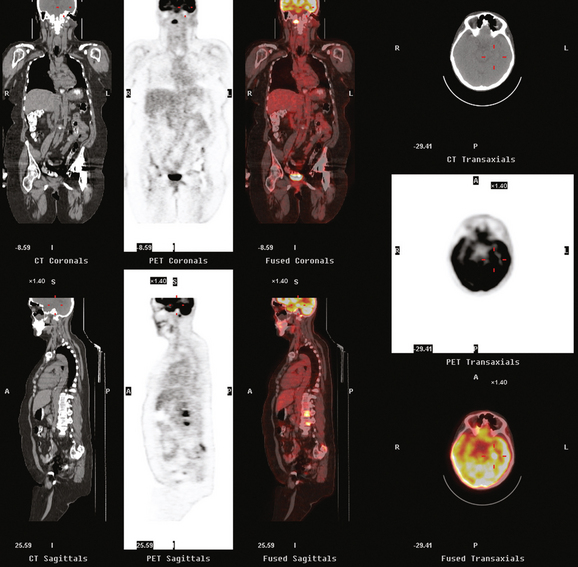
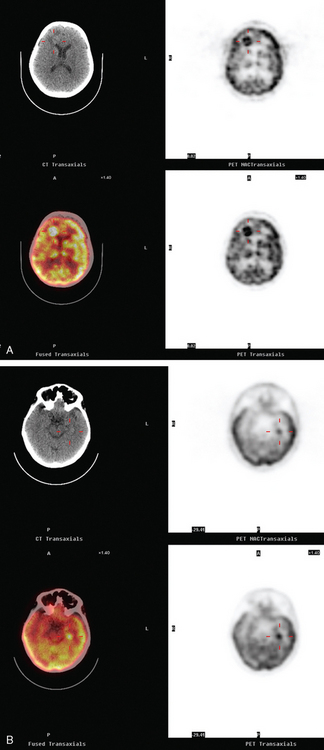
CASE 4 Brain metastases identified on PET in an asymptomatic metastatic breast cancer patient
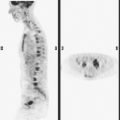
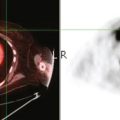
A 52-year-old woman on chemotherapy for metastatic breast cancer to bone was being followed with positron emission tomography (PET)/CT (Figure 1). Although she was asymptomatic from a neurologic standpoint, PET suggested new brain metastases (Figures 2 and 3). These were confirmed on enhanced brain MRI, which showed multiple additional lesions (Figures 4, 5, 6, 7, 8, 9, and 10).
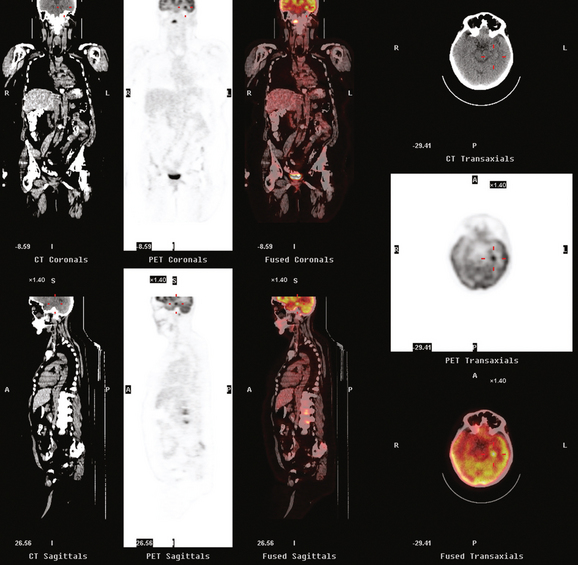
FIGURE 2 Three-plane PET/CT images at the same level as Figure 1, with the intensity “dialed down” to better visualize the brain parenchyma. The bone metastases are less well seen, but a small hypermetabolic metastasis can now be seen in the left temporal lobe of the brain (cursors).
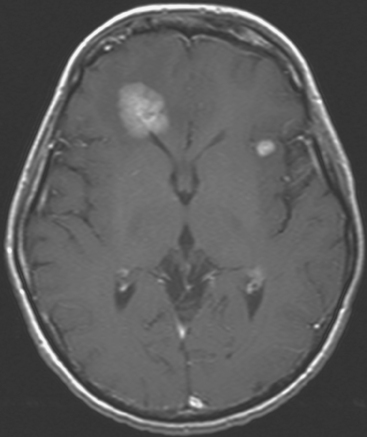
FIGURE 5 Axial enhanced, T1-weighted brain MRI shows two enhancing frontal lobe lesions. The larger of the two abuts the right frontal horn and corresponds to one of the two lesions first seen on PET imaging (see Figure 3A).
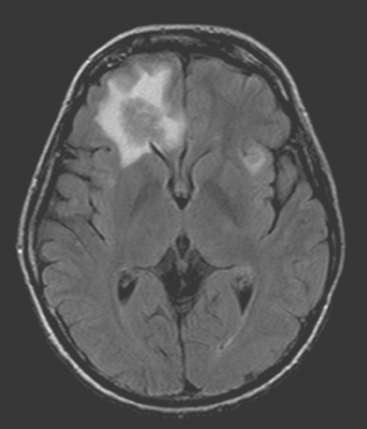
FIGURE 6 Axial FLAIR brain MRI at the same level as Figure 5, showing both isointense frontal lobe lesions to be surrounded by high signal vasogenic edema.
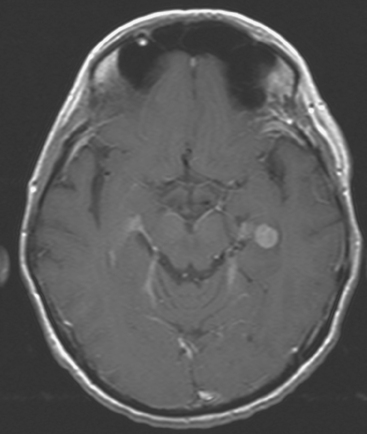
FIGURE 7 Axial enhanced, T1-weighted image through the temporal lobes shows a small, uniformly enhancing metastasis at the left temporal lobe level, representing the other lesion identified on PET (see Figures 2 and 3B).
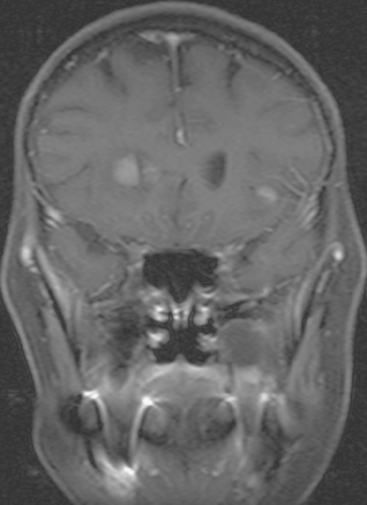
FIGURE 9 Enhanced, fat-saturated, T1-weighted coronal view through the frontal lobes shows the same two metastases seen in Figures 5 and 6.
The patient initially presented 16 months before with a greater than 1 year history of a central, firm, and retracted left breast mass involving the nipple, with fungation and drainage. The patient had not sought medical attention. Evaluations at presentation confirmed a locally advanced, poorly differentiated infiltrating ductal carcinoma, estrogen receptor and progesterone receptor positive, and HER-2/neu positive, involving axillary lymph nodes and metastatic to bone (see Case 1 in Chapter 12).
Palliative whole-brain radiation therapy was performed (Figure 11). The Navelbine was discon tinued, and Herceptin was held because of decline in left ventricular ejection fraction.
TEACHING POINTS
How frequently are brain metastases identified on whole-body PET? The answer is: not often enough to justify inclusion of the brain in whole-body fluorodeoxyglucose (FDG) PET studies performed forstaging purposes. Most of the information in the literature is derived from studies of lung cancer staging, but the same principles and limitations should apply to detection of breast cancer brain metastases. In general, even dedicated brain PET is not sensitive enough to be relied on over CT or MRI. A retrospective study of PET compared with enhanced brain MRI by Rohren and associates showed PET to identify only 61% of the metastases seen on MRI. The sensitivity of FDG PET for detecting brain metastases in this study was 75%, with specificity of 83%. Lesion size is a major factor in the ability of brain PET to depict metastases. The likelihood of detecting a 1-cm metastasis has been estimated to be 40%.
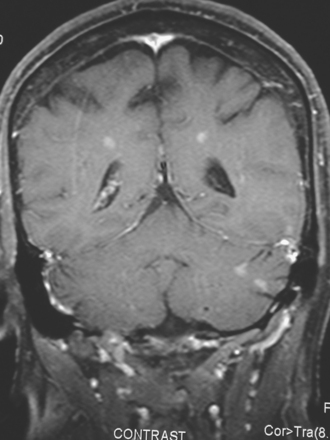
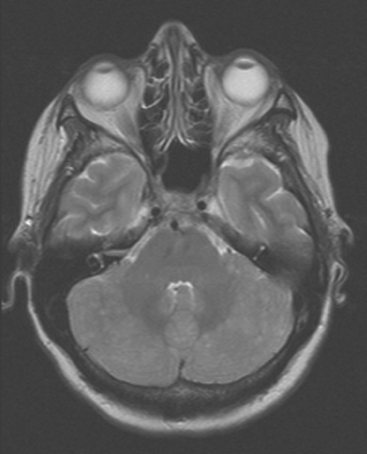
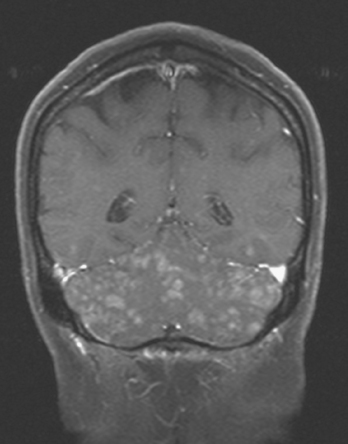
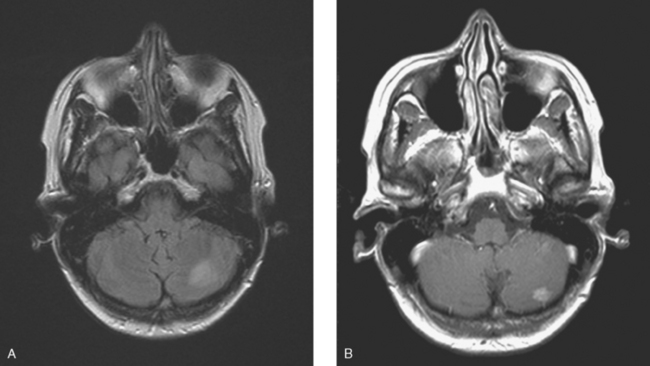
CASE 5 Unusual miliary pattern of brain metastases
A 57-year-old woman with locally recurrent and metastatic breast cancer to the pleura developed new symptoms of flashing visual disturbance, progressive lower extremity weakness, low back pain, nausea, occasional vomiting, and difficulty with balance.
Brain MRI obtained to evaluate the new neurologic symptoms showed an unusual pattern of metastases, with innumerable, tiny, miliary, enhancing nodules, concentrated in the cerebellum and scattered supratentorially (Figures 1, 2, 3, and 4). The patient was treated with whole-brain radiation therapy for palliation of symptoms.
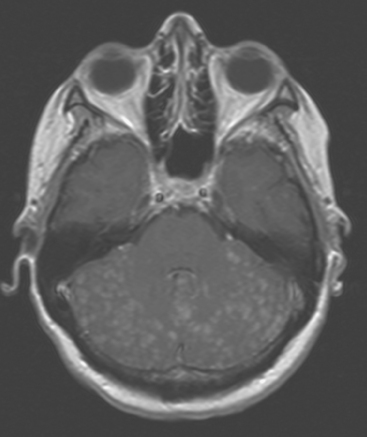
FIGURE 2 Enhanced, axial T1-weighted brain MRI shows innumerable, tiny, cerebellar foci of enhancement.
TEACHING POINTS
This is an unusual pattern of brain metastases, with innumerable tiny, enhancing foci without much associated edema. The scattered supratentorial distribution of lesions, favoring the gray matter–white matter junction, is fairly typical, but the disproportionate concentration of lesions in the cerebellum is distinctly unusual. The near confluency of the cerebellar lesions suggests leptomeningeal metastases as a possibility, although these lesions are more nodular than the typical appearance of leptomeningeal metastases.
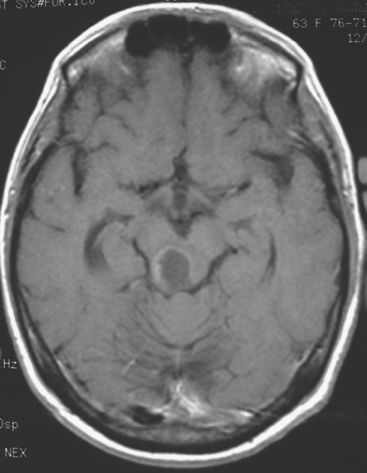
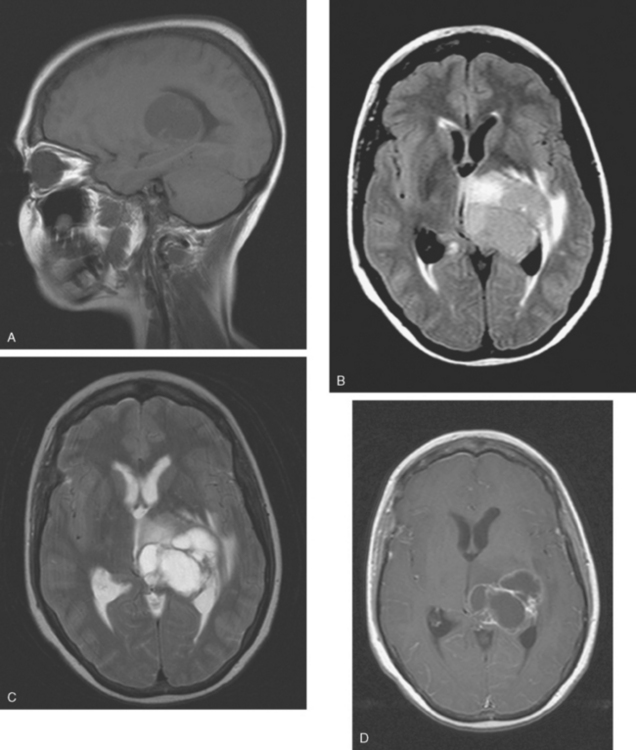
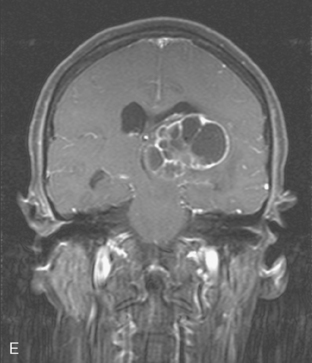
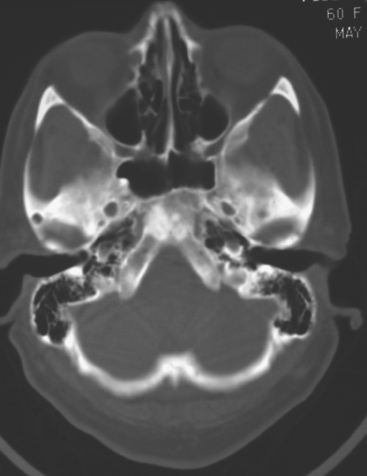
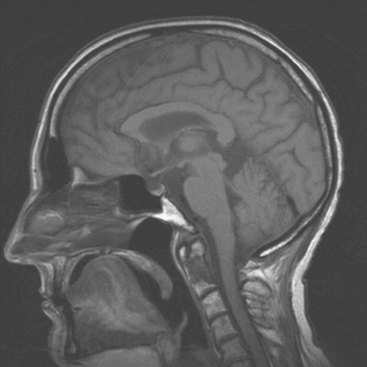
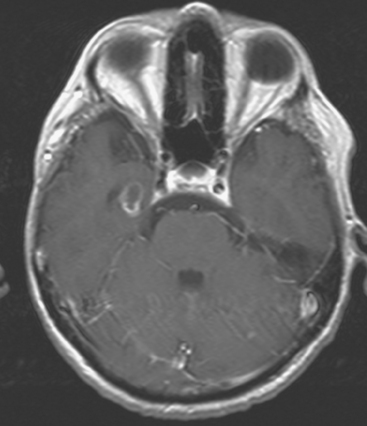
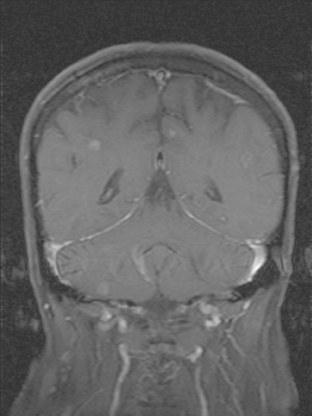
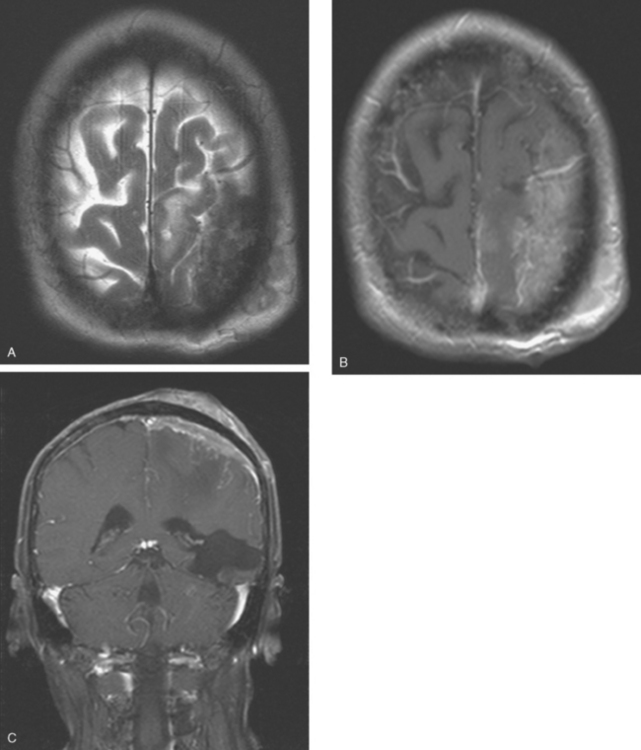
CASE 6 Dural-based sphenoid wing metastasis with orbital extension
When the patient developed confusion, she was evaluated with a head CT scan. This suggested calvarial, but no definite brain, metastases (although the study was compromised by motion artifact). About 3 weeks later, the patient developed aphasia. A brain MRI showed extensive calvarial and dural metastases, with intraorbital extension, as well as parenchymal metastases (Figures 1, 2, and 3). Whole-brain radiation therapy was given, with marked improvement in the aphasia. The patient died a month later.
TEACHING POINTS
This case illustrates the predilection of breast cancer to involve multiple intracranial compartments (parenchyma, meninges, bone). This patient had known bone metastases for 4 years by the time she developed neurologic symptoms. Although there are brain parenchymal metastases, the sphenoid-centered process, with encroachment on the middle cranial fossa and the secondary vasogenic edema, presumably is the culprit lesion.
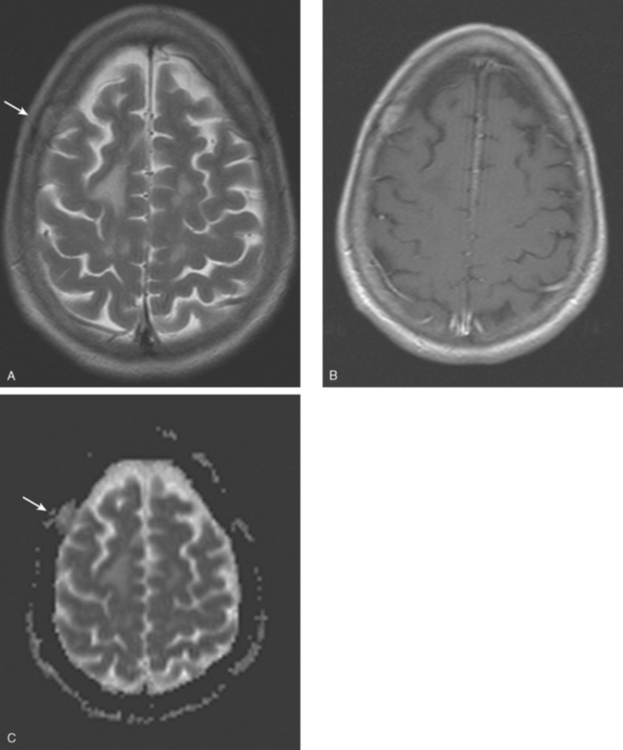
CASE 7 Plaque-like dural metastases
Brain MRI obtained to evaluate new symptoms of vertigo and headache showed two small adjacent enhancing cortical foci in the right posterior parietal lobe, with overlying dural thickening (Figures 1 and 2). This unifocal disease site was treated with gamma knife therapy.
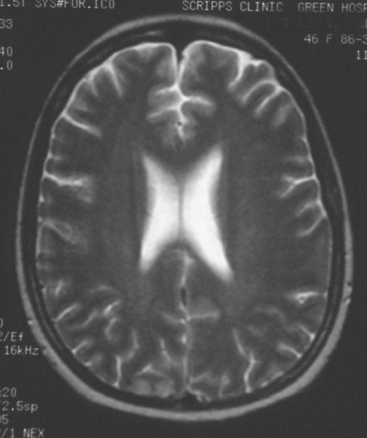
FIGURE 2 Axial T2-weighted MRI, from the same study, at the same level as Figure 1, shows increased cortical signal at the same level as the enhancement seen in Figure 1. Note also the generalized white matter hyperintensity, attributable in this case to prior treatment with high-dose chemotherapy (see Case 9 in this chapter for another example).
Seven months later, the patient developed new expressive aphasia and recurrent headache, as well as mild right upper extremity tingling. An enhanced head CT showed new extra-axial enhancement on the left, with subjacent parenchymal edema and swelling and mild midline shift (Figure 3). A small, separate, round, enhancing metastasis was seen in the right cerebellum, near the transverse sinus. Contrast-enhanced brain MRI confirmed the findings, which were interpreted as extensive dural metastases, with a separate right cerebellar paren chymal metastasis and diffuse skull metastases (Figures 4 and 5). The patient was treated with high-dose dexamethasone (Decadron) and whole-brain radiation therapy, with nearly complete resolution of her expressive aphasia.
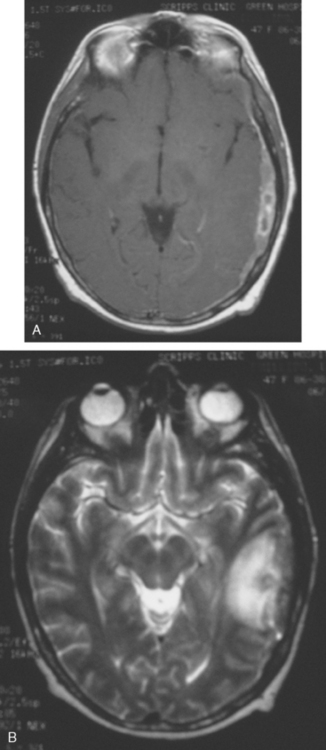
FIGURE 4 Axial brain MRIs at the same level as the CT scan in Figure 3 (A, enhanced T1-weighted; B, T2-weighted) show inhomogeneous extra-axial enhancement at the left temporoparietal level, with mass effect. The underlying edema is well seen on the T2-weighted image, and mass effect is seen as localized sulcal effacement and mild asymmetry of the sylvian fissure and lateral ventricular trigone. Skull metastases are seen as patchy areas of altered signal intensity in the diploic space, bright on T2 weighting and bright because of enhancement on T1-weighted, enhanced sequences.
Repeat MRI was obtained 3 months later (2 months after whole-brain radiation) and showed improvement in the dural thickening, with resolution of the subjacent parenchymal edema (Figure 6).
TEACHING POINTS
As is typical, this patient developed brain metastases late in the course of her disease. In this case, clinical symptoms of brain metastases developed 3.5 years after her initial diagnosis of breast cancer, which initially presented with stage IV disease, metastatic to thorax and bone. The median interval between the diagnosis of breast cancer and brain metastases is 34 months.
The more diffuse dural enhancement noted in this case could reflect diffuse dural metastatic disease or be reactive. The extent of the dural process and presence of edema were considered in the decision to treat with whole-brain radiation. After whole-brain radiation, the plaque-like dural-based mass decreased in thickness and much of the diffuse dural enhancement resolved. The vasogenic edema associated with the dural-based mass also resolved. The patient had an excellent clinical response, with nearly complete resolution of her presenting expressive aphasia. It is worth noting that administration of whole-brain radiation therapy decreases the incidence of subsequent CNS relapse and neurologic death.
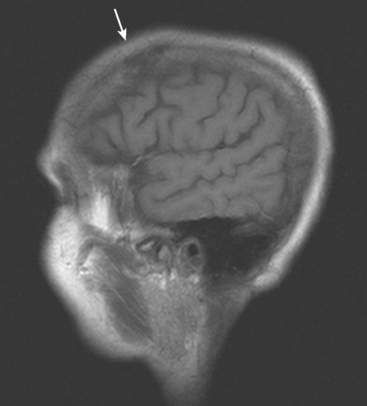
CASE 8 Skull metastases with extracranial and intracranial extension
A 69-year-old woman with stage IV metastatic breast cancer developed new weakness of right upper and lower extremities, and a mild headache. The patient had known skull metastases with scalp and dural-based components. These had been identified on brain MRI 9 months earlier when she developed forgetfulness and slurring of words. At that time, she was evaluated for radiation therapy, but deferred because her measurable disease (scalp masses) appeared to be responding to systemic therapy, her symptoms were mild, and there was concern that scalp radiation could compromise future whole-brain radiation. The patient had known extensive bony metastatic disease diagnosed 6 years earlier, and for which she had undergone palliative radiation to the pelvis and hips, as well as systemic treatment with samarium. Her original diagnosis of left breast cancer was 18 years before the onset of central nervous system (CNS) metastases. This was a T1cN1M0, left 1.9-cm infiltrating ductal carcinoma with 1 of 20 lymph nodes involved. She was treated with mastectomy as well as breast and regional lymphatic radiation.
When the patient developed symptoms of right-sided weakness, repeat brain MRI was obtained, which showed progression of metastases and increased mass effect and edema (Figures 1, 2, and 3). She was treated with dexamethasone and palliative whole-brain radiation therapy (WBRT) but had continued deterioration, with limited use of her right arm and inability to walk. Systemic therapy was discontinued in favor of hospice care.
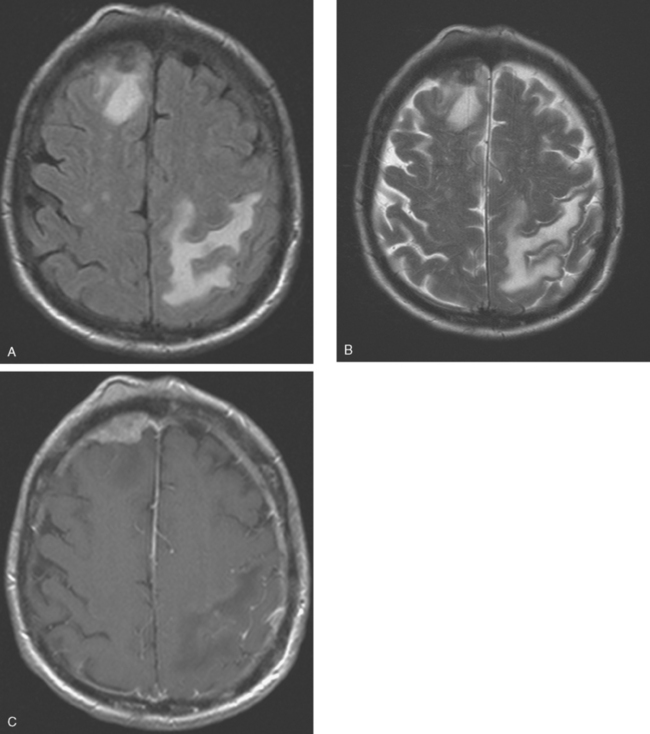
FIGURE 2 Axial brain MRIs from the same level (A, FLAIR; B, T2-weighted; C, enhanced, T1-weighted) shows a lobular, right frontal dural-based enhancing mass, with a similar-appearing subgaleal mass overlying the calvarium at the same level. The skull is extremely hypointense at this level, but patchy areas of enhancement are seen within the diploic space elsewhere. Edema accompanying the right frontal metastasis is seen on FLAIR and T2 weighting, as is edema at the left parietal level from another lesion (illustrated in Figure 3).
TEACHING POINTS
Although the dural-based component of these metastases resembles Case 7, the epicenter of disease in this case is in the skull, from which expansile soft tissue components extend into the scalp and intracranially, infiltrating the dura and underlying brain. This patient had known extensive, refractory bone metastases for 6 years before developing CNS symptoms.
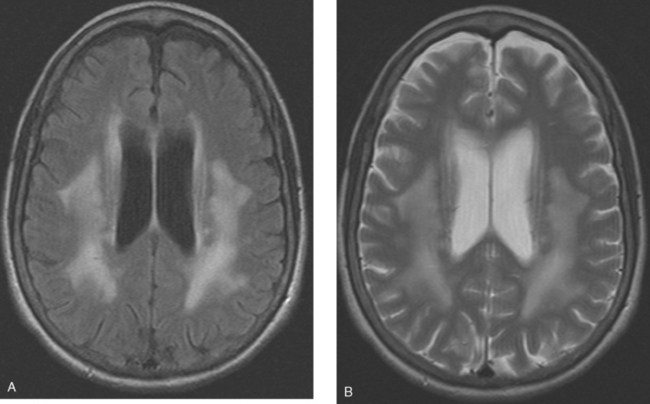
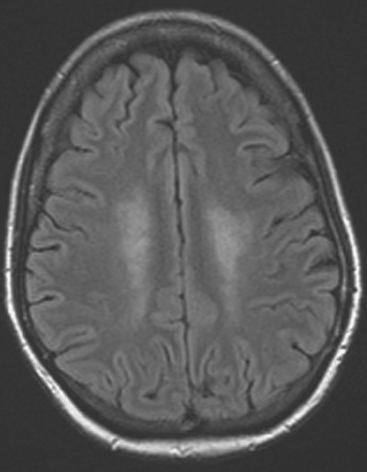
CASE 9 Skull metastasis and chemotherapy-induced leukoencephalopathy
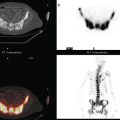
Oncologic evaluation included tumor markers, which were elevated (CEA and CA 27.29). Positron emission tomography (PET) and CT imaging showed hypermetabolic mediastinal and hilar adenopathy and bone metastases (see Case 7 in Chapter 8). The patient had been noted to be hoarse on physical examination, and PET showed asymmetrical muscular activity of the vocal cords.
Brain MRI obtained to evaluate the nausea showed no focal intracranial lesions or enhancement to suggest brain parenchymal metastases. There was instead extensive, confluent, symmetrical white matter hyperintensity, or leukoencephalopathy, seen on fluid-sensitive sequences (Figure 1). Skull metastases were also seen, including the right frontal level (Figures 2, 3, and 4).
TEACHING POINTS
In cancer patients, the pattern of diffuse symmetrical white matter edema seen here most commonly reflects radiation therapy effects. However, this patient had never been treated with brain radiation. This pattern of leukoencephalopathy is also known to occur after high-dose chemotherapy, which this patient had previously received. A study reported by Brown and associates compared spectra obtained from advanced breast cancer patients with white matter changes after treatment with high-dose chemotherapy and bone marrow transplantation with age- and sex-matched controls. There was no significant difference in spectral ratios of N-acetyl aspartate (NAA) to either creatine or choline in either short-echo or long-echo spectra. As NAA is considered to be reflective of neuronal structure and function, these results imply that the observed white matter changes do not reflect major neuronal or axonal injury and may reflect changes in free and bound water fraction resulting from chemotherapy. This result differs from spectroscopic studies of other white matter diseases, such as long-standing multiple sclerosis and adrenoleukodystrophy, in which significant decreases in NAA can be identified, presumably reflecting neuronal loss or dysfunction.
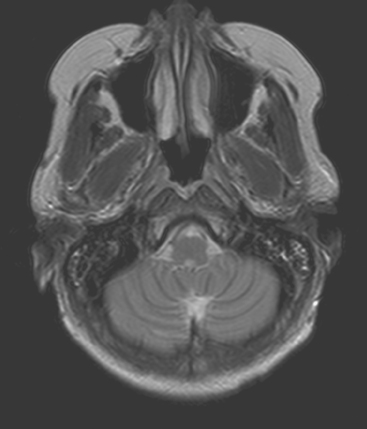
CASE 10 Recurrent brain metastasis; radiation-induced leukoencephalopathy
Chemotherapy with carboplatin, docetaxel (Taxotere), and trastuzumab (Herceptin) was initiated, with good response by imaging. Later the same year, brain metastases were identified and treated with WBRT, with MRI resolution of lesions. Sixteen months later, a single recurrent left cerebellar metastasis was found on MRI, obtained to evaluate recurrent headache (Figures 1, 2, and 3). This was treated with gamma knife therapy.
TEACHING POINTS
Typical imaging findings from prior WBRT are demonstrated here. The white matter edema is diffuse and fairly symmetric. No mass effect or abnormal enhancement was seen supratentorially. In this case, the patient previously had a complete imaging response to WBRT for multiple brain metastases (not shown). The white matter findings were first seen on the first post-treatment MRI, obtained 2 months after the conclusion of radiation therapy. Similar findings can be seen after chemotherapy, particularly high-dose therapy (see Case 9 in this chapter). Fluid signal intensity is seen in the mastoid air cells after treatment, another commonly observed effect of radiation treatment.
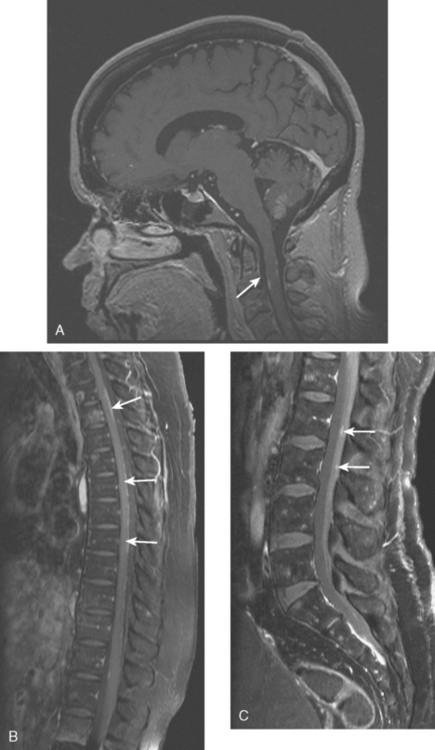
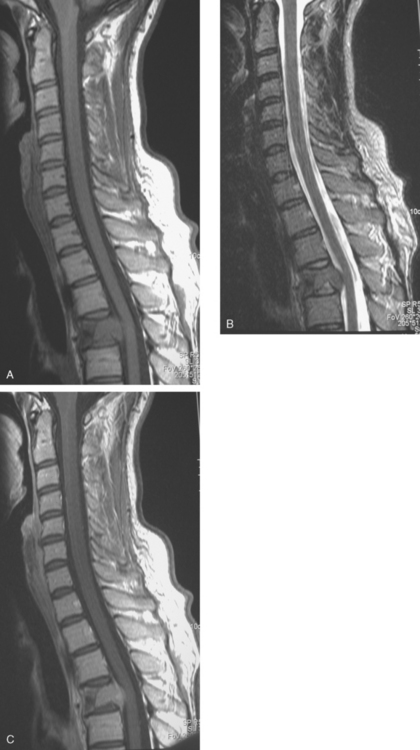
CASE 11 Spinal leptomeningeal carcinomatosis
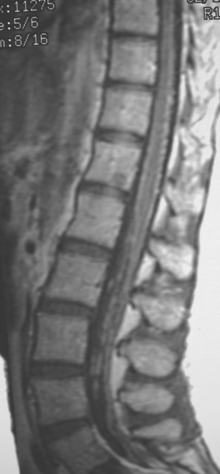
Eight months later, brain CT and spine MRI showed bone and brain metastases and diffuse pial enhancement, consistent with leptomeningeal carcinomatosis (Figures 1, 2, and 3).
TEACHING POINTS
This case nicely illustrates the role of contrast enhancement in evaluation of the spine for metastases. The high inherent soft tissue contrast of MRI is generally sufficient to detect bony metastases, which generally does not require the use of contrast media. As shown here, contrast enhancement of bone metastases can result in a loss of lesion conspicuity, if enhancing metastases become isointense to the normal fatty marrow. For this reason, many centers utilize fat-saturated, T1-weighted, postcontrast sequences. Administration of contrast material, although not contributing much toward detecting bone metastases, can be helpful in assessing the activity of treated lesions and in assessing the extent of epidural tumor.