Chapter 27 Breast Cancer
Epidemiology
Breast cancer remains the most common cancer diagnosis and the number two cause of cancer-related death in women in the United States, with an estimated 40,610 deaths in 2009.1,2 Leukemia is the leading cause of death in females 20 years and younger, and lung cancer is the leading cause of death in women 60 years and older.1 The latest data on worldwide estimated number of deaths from breast cancer totaled 519,000 in 2004, with 69% occurring in developing countries.3 Breast cancer survival rates vary greatly around the world, ranging from less than 40% in impoverished or developing nations to 80% and higher in North America.4 This disparity in breast cancer–related mortality is most likely related to the lack of screening and early-detection programs, as well as inadequate treatment facilities, in countries with limited healthcare resources, which results in a higher proportion of women presenting with late-stage disease.3
Screening mammography has been demonstrated to reduce breast cancer–specific mortality by 20% to 30% in randomized clinical trials. Between 1991 and 2005, the overall death rate from breast cancer decreased by 37% in the United States1; this decrease was most likely related to progress in treatment options and screening technology and the implementation of the Mammography Quality Standard Act of 1992, which established a national screening program. A national and government-funded mammography screening program is too costly for many countries because it requires a substantial financial investment to build the healthcare infrastructure required to maintain this program long term. However, the financial investment in preventive healthcare would be worthwhile because mammography screening in the community setting has been shown to reduce breast cancer mortality not only in the United States but also in other developed countries.5,6
In the United States, the segment of the population 65 years and older will increase as the baby boom generation, individuals born between 1946 and 1964, ages.7 Because breast cancer incidence increases with age—from 1 in 208 for women 39 years and younger to 1 in 16 for women 70 years and older2—the incidence of breast cancer is expected to increase as well, even though the incidence of newly diagnosed breast cancer cases decreased by about 3.5% per year from 2001 to 2004.1 This decrease in new breast cancer cases has been most strongly linked to a decrease in the use of hormone replacement therapy during this period.8,9
In addition to age and country of residence, other factors affect the incidence of and mortality from breast cancer in women worldwide. Such factors include race and ethnicity, with a higher incidence of breast cancer in African American and white women living in the United States than in women living in Asia, South America, or Eastern Europe. Asian American women born in the West have a higher risk of breast cancer than those who were born in Asia; this finding suggests that environmental and behavioral factors, rather than genetic predisposition alone, contribute to the risk of breast cancer.10 Studies identifying potentially modifiable risk factors for breast cancer provide better opportunities for breast cancer prevention among women who have an average-to-high risk of developing breast cancer.
Screening
Mammography
Mammography is utilized for two major purposes: (1) to screen asymptomatic women for breast cancer and (2) to evaluate breast problems or abnormalities. Although the United States does not have a government-funded screening program, the screening guidelines recommended by the National Cancer Institute advise that women 40 years and older who are at average risk of breast cancer undergo screening mammography every 1 to 2 years.11 Since the introduction of screening mammography, the number of deaths from breast cancer has declined in every age and racial group of women in the United States.12 Some investigators attribute this decline to mammography screening, whereas others believe it is related to advances in therapy. The 2002 Swedish screening trial reported that screening mammography reduced breast cancer mortality by 30%, supporting the concept that the decline in breast cancer mortality rates is related to the utilization of screening mammography.13 Today, the benefits of screening mammography for women between the ages of 50 and 69 years is universally accepted whereas screening for women between the ages of 40 and 49 years remains controversial despite the statistically significant reduction in breast cancer mortality14 and significant increase in survival rates15 reported.
Screening mammography consists of mediolateral oblique and craniocaudal views of each breast. In the United States, a standardized terminology for interpreting mammograms, the Breast Imaging Reporting and Data System (BI-RADS), has been developed by the American College of Radiology to help facilitate uniformity in mammography reporting across facilities.16
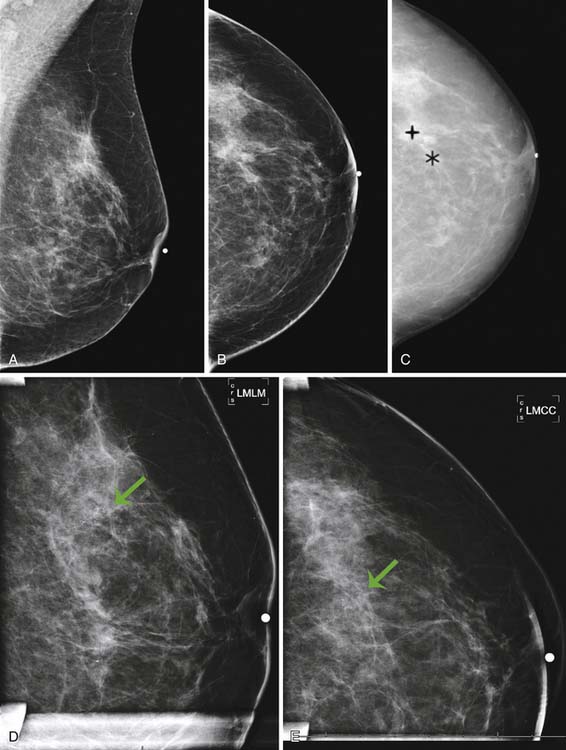
Currently, mammography studies are performed using either film screen or full-field digital mammography (FFDM) technologies. FFDM technology has slowly replaced film screen mammography because FFDM provides a lower radiation dose in large breasts; increased image quality associated with a higher contrast resolution; shorter examination times, and higher patient throughput; elimination of costs associated with film such as film storage, film, and processing; and facilitation of teleradiology for mammography.17 Furthermore, cancer detection rates with current FFDM technology are equivalent to those with film mammography.17 In addition to advancing detector and processing technology, advanced techniques that operate in conjunction with FFDM, such as digital tomosynthesis, computer-assisted diagnosis algorithms, and contrast-enhanced mammography, are current areas of research that may improve the detection of small early-stage cancers and decrease the false-positive recall rate (Figure 27-1).
The potential role of physician-performed screening ultrasonography, as an adjunct to mammography screening, has been evaluated in a multi-institutional trial that demonstrated many barriers to widespread implementation, such as high false-positive rates and low reimbursement rates.18 In high-risk populations, such as women with a genetic predisposition for breast cancer, MRI has been reported to have a higher sensitivity than either ultrasonography or mammography for the detection of breast cancer; therefore, MRI is a more appropriate adjunct to screening mammography in these cases.
Magnetic Resonance Imaging
Use of screening mammography in the general population has been shown to reduce mortality associated with breast cancer by at least 24%.19 Women at high risk of breast cancer owing to a known genetic predisposition should be engaged in aggressive surveillance and screening, which should begin at a younger age than screening for women in the general population because of the potential for early onset associated with familial breast cancer. Current surveillance protocols for screening women with a BRCA1 or BRCA2 mutation include a clinical breast examination every 6 months and annual mammography and MRI.20
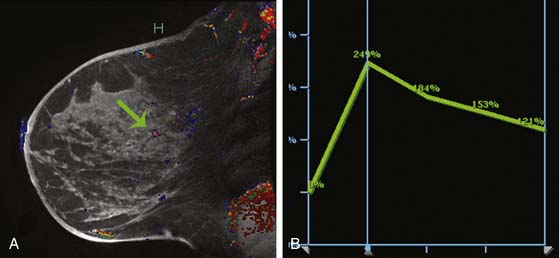
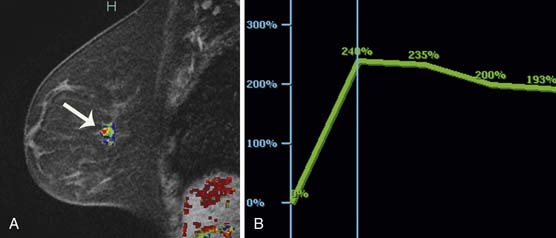
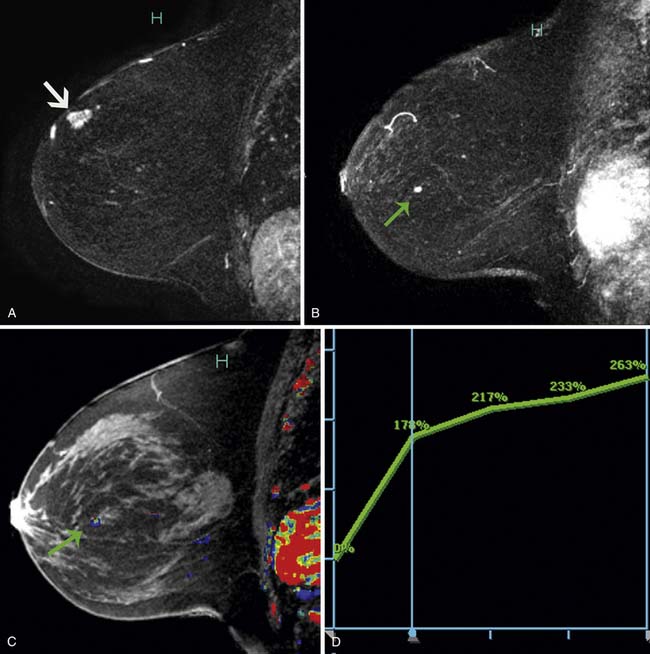
Multiple studies have demonstrated higher cancer detection rates with MRI than with mammography, although the differences were not statistically significant.21–23 Despite variations in breast cancer screening techniques between facilities, MRI is consistently reported to have a higher sensitivity (77-100%) than mammography (25-40%).24–26 MRI detects more breast cancers than either mammography or sonography.24–26 However, MRI also consistently demonstrates a higher false-positive rate and, thus, is associated with a higher biopsy rate—8.2%—than mammography (2.3%) or sonography (2.3%).26 The majority of breast cancers detected on MRI screening demonstrate the typical conspicuous morphologic and dynamic enhancing patterns expected for malignant lesions (Figure 27-2). However, in a trial studying patients at high risk of breast cancer, 20% of the breast cancers detected on MRI screening presented with non-masslike enhancement and 33% presented with an enhancing pattern typical for benign lesions.27 This lack of specificity associated with current morphologic and kinetic MRI features makes MRI detection of breast cancers in high-risk patients challenging. A study by Kuhl and coworkers28 compared clinical breast examination, mammography, sonography, and MRI in 529 study participants. The combination of mammography and MRI yielded the highest sensitivity (93%), which agrees with the findings from other published trials. In this trial, MRI and mammography had the same specificity (97%), as opposed to a higher specificity for mammography than MRI reported in prior studies. This improved specificity of MRI may be due to improved imaging techniques.28
While the role of MRI in breast screening programs continues to evolve, the full impact of modern MRI screening technology on mortality from breast cancer is still unknown. Patients for whom MRI is being considered should be informed of the current status of MRI in the detection of breast cancer, and appropriate management strategies are best determined on an individual basis. At the present time, no consensus exists as to the appropriateness of MRI as an adjunct to screening mammography in the general population.
Anatomy and Pathology
Anatomy
The breast is located in the superficial fascia and is attached to the skin by fibrous connective tissue and suspensory structures, called Cooper’s ligaments. Fibrous connective tissues interdigitate between the Cooper ligaments and the glandular tissue within the breast. Posterior to the breast tissue is the retromammary bursa, located between the deep layer of the superficial fascia and the deep layer of the pectoralis major muscle.29 The breast extends from the second or third rib to the sixth or seventh rib, or inframammary fold. The muscles that the breast rests on are the pectoralis major, serratus anterior, and external abdominal oblique muscles and the upper extent of the rectus sheath. The growth of the mammary gland is influenced by the levels of estrogen, progesterone, and adrenal corticoids; during pregnancy or lactation, growth of the mammary gland is also influenced by prolactin (from the adenohypophysis) and somatomammotropin (from the placenta).
Pathology
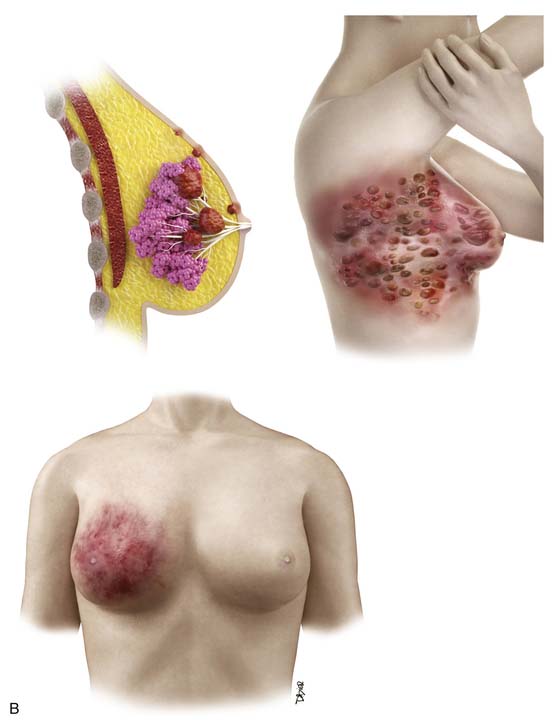
Breast cancer is a heterogeneous disease and has been traditionally categorized as in situ lesions (DCIS and lobular carcinoma in situ [LCIS]) or invasive cancers. The in situ or noninvasive disease encompasses a spectrum of lesions and is not as simple as the original definition implies30; in situ lesions develop in a linear progression from normal breast tissue to atypical ductal hyperplasia, DCIS, and invasive cancers. DCIS predominantly arises from the terminal ductal-lobular unit and may extend into the extralobular ducts.
The conventional pathologic classifications of invasive breast cancers by morphology include invasive ductal carcinoma, invasive lobular carcinoma, tubular carcinoma, mucinous carcinoma, medullary carcinoma, papillary carcinoma, metaplastic carcinoma, and other less common types. Tubular carcinoma accounts for 3% to 5% of all invasive breast carcinomas. It is a low-grade cancer with an excellent prognosis and usually occurs in older women.31 Mucinous or colloid carcinoma is characterized on histologic examination as having extracellular pools of mucin and low-grade tumor aggregates. Pure mucinous carcinoma also has a very favorable prognosis, with a 10-year survival rate of 90%.31 Medullary carcinoma is commonly seen on imaging as a circumscribed mass consistent with the distinctive smooth pushing border seen on pathology.32 Medullary carcinoma also has a favorable prognosis, but it is not observed as frequently as tubular and mucinous carcinomas. This type of carcinoma comprises a higher proportion of BRCA-related breast cancers than sporadic cases but still represents a minority of the breast cancers in women with BRCA mutations. Metaplastic carcinomas include tumors that show both epithelial and mesenchymal features. Although there are conflicting data on the biologic behavior of these tumors, many exhibit an aggressive clinical course with sarcoma-like behavior.33 Inflammatory breast cancer is a rare but aggressive disease with a poor prognosis; it is usually diagnosed clinically owing to the inflammatory symptoms. In addition to the type of breast cancer, the receptor or tumor marker status of the cancer (estrogen receptor [ER], progesterone receptor [PR], and human epidermal growth factor receptor 2 [HER2] receptor) is very important for determining the treatment options in patients with invasive breast carcinoma. The status of these receptors is also important for patients with in situ disease who are considering hormone therapy.
Even though genetic profiling has not been widely used in clinical practice, the research on this technology has resulted in a molecular classification of breast cancer with the hope of offering insight on the prognosis and response of tumors to specific therapy. Based on genetic profiling, breast cancers are divided into five subtypes: luminal A, luminal B, normal breast–like, HER2+, and basal-like carcinomas.34 Luminal A breast cancer is characterized by the high expression of ERs and PRs. These cancers tend to be of a lower grade and have a better prognosis than the other subtypes. Luminal B breast cancers not only express ERs and/or PRs but also overexpress HER2. They are more aggressive, with high tumor grade. The normal breast–like type or unclassified subtype is a triple-negative tumor with a molecular profile similar to that of basal-like carcinoma; however, normal breast–like carcinoma has a better prognosis than basal-like carcinoma. The HER2+ subtype includes ER-negative and ER-positive types and is often associated with DCIS. The basal-like breast carcinomas lack ER, PR, and HER2 expression and, therefore, are often called triple-negative tumors. These tumors show a high rate of p53 mutations and are common in BRCA mutation carriers. Triple-negative cancers tend to be more sensitive to chemotherapy but have a worse prognosis than other subtypes.2,35 However, not all triple-negative breast cancers are basal-like tumors. Some data suggest that 76% of triple-negative breast cancers are basal-like, and 77% of the basal-like tumors are triple negative.31 This molecular classification will likely be further modified as additional molecular features are discovered. Future classifications should help predict the sensitivity of particular tumors to targeted therapies, thereby improving the selection of patients for specific therapeutic approaches.
Key Points Pathology
• Breast cancer is a heterogeneous disease and can be in situ, invasive, or both.
• Invasive breast cancers are classified by morphology: ductal, lobular, tubular, mucinous, medullary, papillary, metaplastic, and others.
• Breast cancer subtypes are determined by gene profiling: luminal A, luminal B, normal, HER2+, and basal-like.
• ER, PR, HER2, and lymphovascular invasion status are important risk factors for relapse.
Clinical Presentation
The detection of cancer at an early stage, when the cancer is small, may reduce cancer-related mortality and improve survival. Cancers can be detected on palpation or examination of breast symptoms rather than with imaging screening, such as mammography or ultrasonography. In a national survey of 41,000 breast cancer patients, approximately 70% of the patients presented with a palpable breast mass.36 A more recent study of 592 breast cancer cases reported that the cancer was detected as a palpable mass and not at screening in 43% of the patients.37 The investigators evaluated the role of breast self-examination as a screening tool and reported that women who performed breast self-examinations did not have lower breast cancer mortality rates than women who did not practice breast self-examinations.37
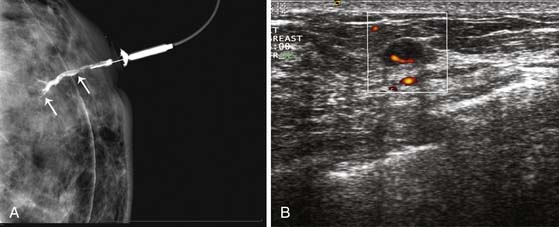
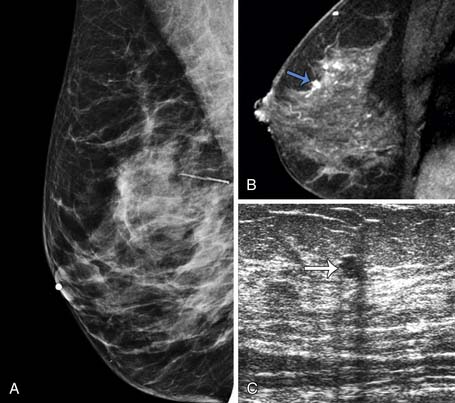
A clinical breast examination should evaluate breast symmetry and shape, palpable lumps, and skin changes. Some common clinical presentations of breast cancer include a breast mass, asymmetrical thickening or nodularity, nipple retraction, bloody nipple discharge, skin dimpling, and erythema. For a palpable breast mass, further evaluation on imaging and, possibly, a core needle or fine-needle aspiration biopsy would be needed. For asymmetrical thickening or nodularity, further evaluation with ultrasonography is needed and, possibly, mammography for women 30 years or older. For nonspontaneous nipple discharge and discharge from multiple ducts, the suspicion of breast cancer is low. These patients are advised to stop compression of the breast and elicitation of the nipple discharge. However, spontaneous nipple discharge with unilateral and single duct involvement, as well as discharge that is serous, sanguineous, or serosanguineous in nature, would require investigation with mammography, ultrasonography, MRI, or ductography to determine the etiology and rule out a malignancy (Figure 27-3). Skin changes in women older than 40 years would raise a concern of either infection or inflammatory breast cancer. Skin changes may include peau d’orange or skin thickening, Paget’s disease with scaling, eczema, or nipple excoriation. Other associated clinical symptoms of concern include lymphadenopathy and weight loss.
Staging Classification
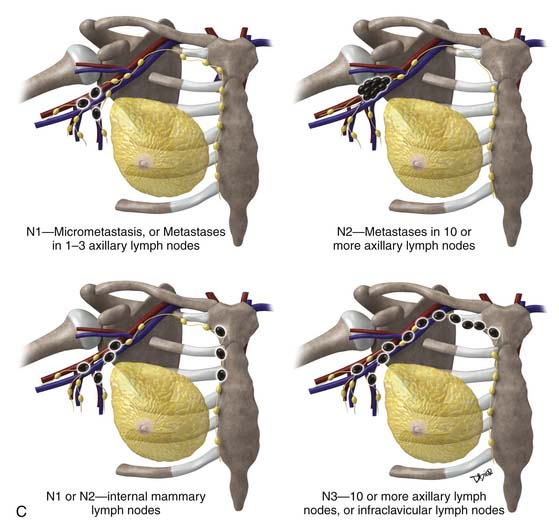
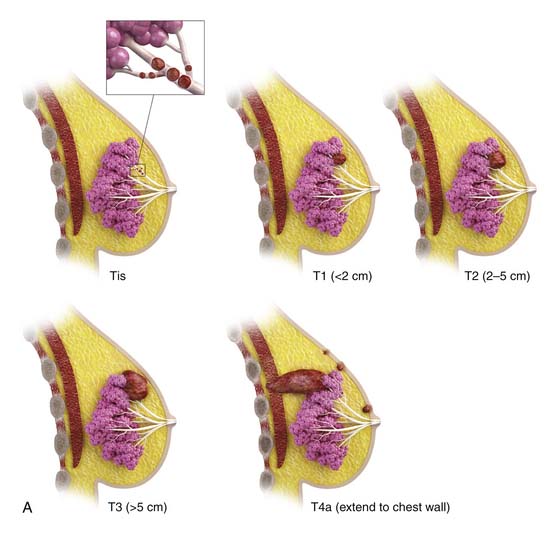
The most popular staging system for classifying breast cancer is the tumor-node-metastasis (TNM) system (Table 27-1), which includes the primary breast tumor size, the spread of cancer to the regional lymph nodes, and the spread of cancer to distant sites.38 The pathologic staging is based on the tumor size of the final pathology specimen. For multiple synchronous ipsilateral primary carcinomas, the largest tumor is used. Once the tumor size, node status, and presence of metastatic disease have been determined, one of the five breast cancer stages is assigned (Table 27-2). Stage 0 is assigned to precancerous lesions or carcinoma in situ with no local or distant metastasis; this stage is associated with a nearly 100% cure rate. Stage I is assigned to small cancers confined to the breast; patients with stage I disease have an excellent prognosis. Stage II cancers have regional lymph node metastases, and stage III breast cancers have large tumors or locally advanced disease at the time of initial diagnosis. Stages II and III are associated with a poor prognosis. Stage IV cancers have a distant metastasis and are associated with a poor survival (Figure 27-4).
Table 27-1 Tumor-Node-Metastasis Classification of Breast Cancer
| STAGE | CHARACTERISTICS |
|---|---|
| T | Primary Tumor |
| Tx | Primary tumor not assessed |
| To | Primary tumor not detected |
| Tis | DCIS, LCIS, Paget’s disease of nipple with no tumor. |
| T1 | Tumor 2 cm or smaller in greatest dimension |
| T1mic | Microinvasion ≤0.1 cm or smaller in greatest dimension |
| T1a | Tumor larger than 0.1 cm but 0.5 cm or less in greatest dimension |
| T1b | Tumor larger than 0.5 cm but 1 cm or less in greatest dimension |
| T1c | Tumor larger than 1 cm but 2 cm or less in greatest dimension |
| T2 | Tumor larger than 2 cm but 5 cm or less in greatest dimension |
| T3 | Tumor larger than 5 cm in greatest dimension |
| T4 | Tumor of any size with direct extension to chest wall or skin |
| T4a | Extension to chest wall, but not pectoralis muscle |
| T4b | Edema |
| T4c | T4a and 4b |
| T4d | Inflammatory carcinoma |
| N | Regional Lymph Nodes |
| pNx | Regional lymph nodes cannot be assessed |
| pN0 | No regional lymph node metastasis identified histologically |
| pN1 | Micrometastasis, or Metastases in one to three axillary lymph nodes with or without internal mammary nodes |
| pN2 | Metastases in 10 or more axillary lymph nodes, or Infraclavicular lymph nodes (level III), or Clinically detected internal mammary lymph nodes, or More than three axillary lymph nodes and internal mammary nodes, or Ipsilateral supraclavicular lymph nodes |
| pN3 | Metastases in 10 or more axillary lymph nodes, or Metastases to infraclavicular lymph nodes (level III) |
| M | Distant Metastases |
| M0 | No distant metastases |
| cM0 (i+) | Circulating tumor cells or microscopic tumor cells in bone marrow No clinical or radiologic distant metastasis |
| M1 | Distant metastases |
DCIS, ductal carcinoma in situ; LCIS, lobular carcinoma in situ.
Table 27-2 American Joint Committee on Cancer Stage Grouping System Classification for Breast Cancer
| STAGE GROUPING | |
|---|---|
| Stage 0 | Tis N0 M0 |
| Stage I | T1* N0 M0 |
| Stage IIA | T0 N1 M0 T1* N1 M0 T2 N0 M0 |
| Stage IIB | T2 N1 M0 T3 N0 M0 |
| Stage IIIA | T0 N2 M0 T1* N2 M0 T2 N2 M0 T3 N1 M0 T3 N2 M0 |
| Stage IIIB | T4 N0 M0 T4 N1 M0 T4 N2 M0 |
| Stage IIIC | Any T N3 M0 |
| Stage IV | Any T Any N M1 |
Tis, in situ; T1.
From Edge SB, Byrd DR, Compton CC, et al. Breast. AJCC Cancer Staging Manual. 7th ed. New York: Springer; 2010:347-369.
Patterns of Tumor Spread
Local recurrence, regional recurrence, and distant metastases occur when cancer cells remain after treatment or spread beyond the breast and axillary lymph nodes; the spread of cancer cells represents a major source of morbidity in breast cancer patients. Breast cancer cells can spread via the lymphatic system to the regional lymph nodes, involving the low axillary lymph nodes (level I) first, followed by the mid- (level II) and high axillary lymph nodes (level III). Level I lymph nodes are lateral to the lateral border of the pectoralis minor muscle. Level II lymph nodes are between the medial and the lateral borders of the pectoralis minor muscle, as well as the interpectoral lymph nodes (Rotter’s nodes). Level III lymph nodes are nodes medial to the medial margin of the pectoralis minor muscle and inferior to the clavicle. Therefore, the standard approach to staging lymph nodes via axillary nodal dissection often involves removal of the low and midaxillary lymph nodes. Involvement of the internal mammary chain is observed in approximately 20% of patients, often with large and deep medially located breast cancer, and tends to be associated with extensive metastasis.
Tumor cells may spread to distant sites via the lymphatic or circulatory system; the four major sites of distant metastasis for breast cancer are bone, lung, brain, and liver. Bone is the most common site of metastasis from most subtypes of breast cancer,39 and the presence of tumor cells in the bone marrow is a strong predictor for distant metastases. Cutaneous metastasis is not common, but breast carcinoma is the most common primary malignancy to spread to the skin and accounts for 24% of all cutaneous metastases.40
Imaging
Several modalities available to image the breasts include mammography, ultrasonography, ductography, and MRI. Once a woman has been diagnosed with breast cancer, cross-sectional imaging with computed tomography (CT), positron-emission tomography (PET), MRI, or bone scintigraphy are often indicated to image the rest of the body, especially when there are suspicious findings on the patient’s history or physical examination or if serologic tests for liver or bone function show elevated serum levels. Most often, using a combination of imaging modalities is crucial for the accurate staging of breast cancer. Here, an overview of the role of imaging in the diagnosis and staging of this common disease is presented.
Primary Tumor
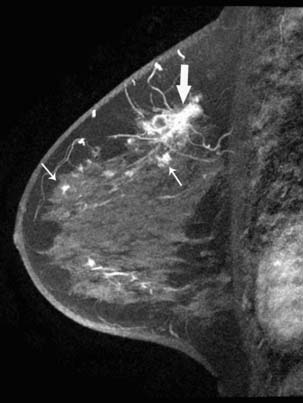
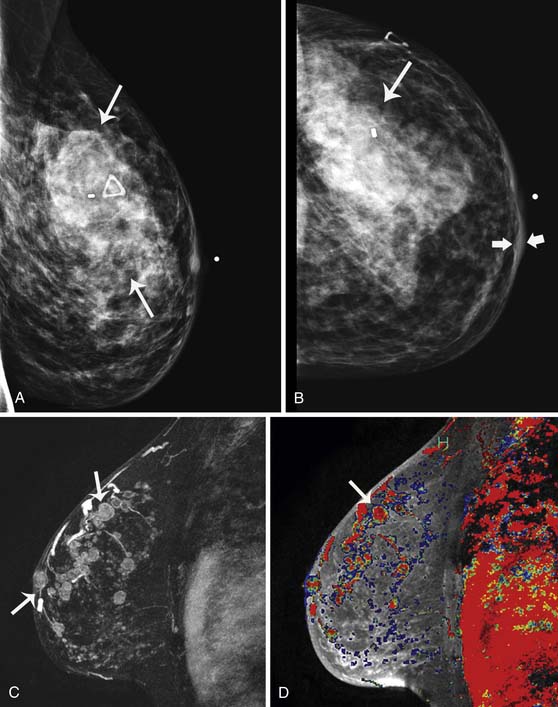
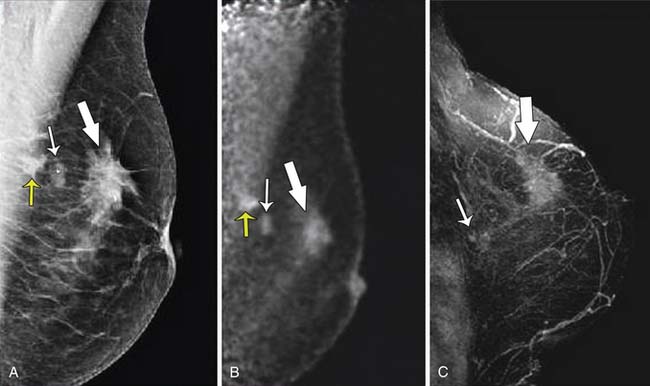
Breast imaging is used to detect primary or recurrent breast cancer, determine the extent of disease in the affected breast, evaluate regional lymph nodes, and identify distant metastatic disease. Cancer within the affected breast may be described as unifocal (Figure 27-5), multifocal (two or more lesions in the same quadrant [Figure 27-6]), or multicentric (lesions in two or more quadrants [Figure 27-7]). Currently, mammography, ultrasonography, and MRI are the primary modalities indicated in the detection and staging of breast cancer.
Mammography
Diagnostic mammography or problem-solving mammography is performed when a patient presents with a palpable finding or when a suspicious finding is detected with screening mammography. A radiopaque BB or skin marker is placed directly over the suspicious region before the mammogram is obtained. In addition to the mediolateral oblique and craniocaudal views, described earlier, additional views may be indicated to properly evaluate the abnormality. A 90-degree lateral view can assist in triangulating the abnormality. Spot compression views in craniocaudal and lateral projections are incorporated to evaluate a possible mass, asymmetrical density, or superimposition of normal breast parenchyma. Magnification views, commonly performed in craniocaudal and lateral projections, facilitate characterization of microcalcifications. Slowly, conventional analog technology is being replaced by digital mammography. However, standard mammography projections remain unchanged. With advances in digital acquisition and processing technology, digital tomosynthesis is being incorporated into mammography systems and may provide a solution to the problem of distinguishing overlapping structures in the breast, increasing the sensitivity and specificity of mammography for cancer detection and diagnosis.41 Clinical trials are currently under way to establish the efficacy of digital breast tomosynthesis and to define its role in future practice.
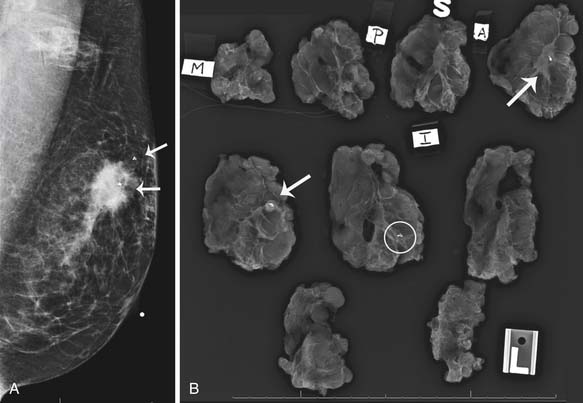
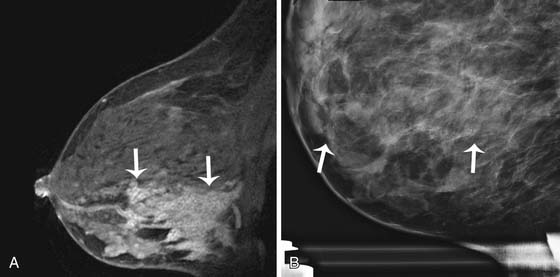
Mammography is also used to guide breast interventions, including a stereotactic-guided core biopsy using vacuum-assisted devices and an image-guided needle localization of a lesion prior to surgery. Immediately after resection of the targeted lesion, the biopsy or resected specimen can be imaged while the patient is still under anesthesia in order to verify the margins. This “specimen radiography” provides additional information in patients who had a complete response to preoperative chemotherapy because the only image-detectable finding is often the biopsy clip placed at the time of the initial biopsy or residual calcifications (Figure 27-8).
Sonography
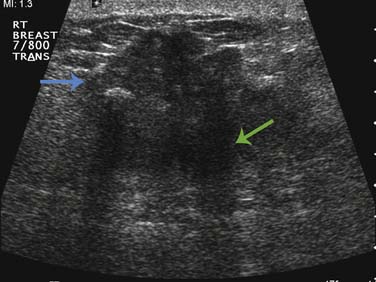
Currently, breast sonography is considered a diagnostic tool for characterization of lesions detected via mammography or clinical examination and for staging of newly diagnosed breast cancer. The Breast Imaging Reporting and Data System (BI-RADS) risk assessment categories should be adhered to in the reporting of breast ultrasound findings.16 A linear array transducer should be used to perform the examination, utilizing the highest center frequency (7.5-15 MHz) possible that still penetrates to the chest wall in order to provide high-resolution images of the breast. Both longitudinal and transverse planes are commonly used to characterize findings.42 However, a radial and orthogonal antiradial pattern has been demonstrated to be superior and is preferred.42 When investigating a suspicious finding, the entire ipsilateral breast should be scanned to determine the full extent of the index lesion and assessed for any satellite or synchronous lesions. Breast tumors are usually hypoechoic solid masses, with irregular margins, posterior acoustic shadowing, and internal vascularity on color Doppler imaging (Figure 27-9). Sonography of the ipsilateral axilla should be conducted for all newly diagnosed breast cancer cases to assess for morphologically abnormal nodes. Upon detection of an abnormal lymph node, confirmation of malignancy by ultrasonography-guided core biopsy or fine-needle aspiration biopsy may assist the surgeon in the decision to proceed directly to an axillary dissection as opposed to a sentinel node biopsy. A radiologic marker placement at the time of the ultrasonography-guided biopsy can aid in facilitating subsequent confirmation of proper node recovery or ensuring the complete removal of the breast cancer that has disappeared after the completion of neoadjuvant chemotherapy. At our institution, the infraclavicular and internal mammary regions are also evaluated during the staging sonographic examination of all new breast cancer cases (Figure 27-10).
Magnetic Resonance Imaging
Over the past several years, the increased use of preoperative breast MRI in the staging of breast cancers has been heavily scrutinized, and the role of MRI in staging remains a source of considerable discussion and research.43 In experienced hands, MRI used in conjunction with a computer-aided detection software can be helpful in defining the extent and size of the primary breast carcinoma and has been used to detect additional foci of malignancy within the ipsilateral breast in up to 16% of patients and malignancies that were occult on mammography within the contralateral breast in up to 4% of patients.44,45 A recently published meta-analysis of 19 studies and prospective randomized trials, such as the randomized Comparative Effectiveness of Magnetic Resonance Imaging in Breast Cancer trial, reported that MRI demonstrated no benefit on the reoperation rate but did result in more extensive breast surgery.44,46 However, the impact of these findings on patient management decisions and outcomes has yet to be rigorously evaluated. Although these publications indicated that the preoperative MRI is not necessarily indicated in every patient, the ability of MRI to detect additional tumor foci and contralateral cancer that are not detected via physical examination or mammography should not be discounted. The impact of preoperative MRI-based decisions on recurrent disease or overall survival remains unanswered and is a subject for future clinical research.
For less common and more aggressive invasive breast cancers, such as invasive lobular carcinoma or inflammatory breast cancer, MRI can provide useful information regarding tumor extension as well as involvement of skin, nipple, or chest wall.47,48 Because of the diffuse infiltrative growth pattern of invasive lobular carcinoma, a prebiopsy MRI may be helpful in accurately localizing the disease and facilitating a more effective biopsy and surgical plan (Figure 27-11). In patients who present with adenocarcinoma in the axilla without a diagnosis of a primary carcinoma, MRI has been demonstrated to be beneficial in identifying the primary tumor.49 When a primary breast lesion can be detected (Figure 27-12), proper staging can be performed, facilitating targeted therapy or consideration for breast-conservation therapy in conjunction with radiotherapy, depending on the stage of the carcinoma.
DCIS is a noninvasive malignancy, and the patient is often asymptomatic. However, DCIS is associated with increased risk of developing invasive carcinoma that can involve multiple sites and intervening normal tissue. The prevalence of DCIS diagnoses in the United States has increased with the introduction of screening mammography and currently accounts for 25% to 30% of all reported breast cancers. However, mammography tends to underestimate both the size and the extent of DCIS, especially when the DCIS is not associated with the characteristic pleomorphic microcalcifications.50 MRI, conversely, has a reported sensitivity of 67% to 100% for the detection DCIS whereas mammography has a sensitivity of 70% to 80%.51,52 Common MRI features indicative of DCIS include clumped or heterogeneous enhancement in a linear, ductal, or segmental distribution (Figure 27-13). Despite these highly specific morphologic features, current MRI techniques poorly differentiate benign proliferative disease from DCIS; thus, the specificity of MRI in the detection of DCIS remains low due to a high rate of false-negative findings.53 Ultimately, MRI may be useful in cases where mammography, sonography, and clinical findings are inconclusive and no focal finding is apparent; currently, approximately 95% of DCIS cases are diagnosed via calcifications identified on mammography.
Unconventional Breast Imaging
A high-resolution [18F]-fluorodeoxyglucose PET scanner, a positron-emission mammography flex device (Naviscan PET Systems Inc., San Diego, CA), was recently approved by the U.S. Food and Drug Administration (FDA) for imaging the breast or other small body parts in contrast to the whole body imaging of PET (Figure 27-14). In one published breast cancer study of 77 women with 92 lesions from four centers, positron-emission mammography was reported to have a sensitivity of 90%, with a specificity of 86% in detecting breast cancer.54
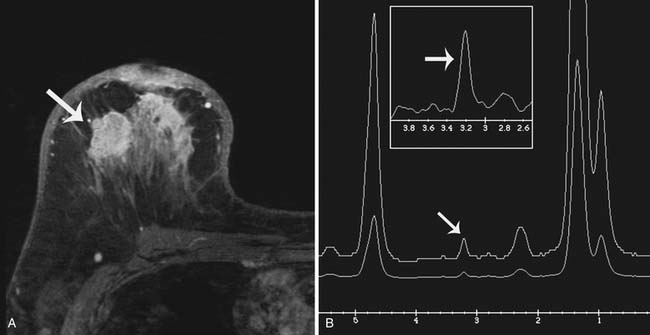
The purpose of breast MRS is to detect an increase in the concentration of choline, a metabolic byproduct of cellular proliferation, within a suspicious lesion because elevated choline concentration tends to be indicative of a malignant process. As an adjuvant tool to breast MRI, MRS may improve the specificity of MRI, reducing the number of MRI-detected lesions requiring biopsy.55 Currently, only single-voxel 1H MRS acquisition is commercially available for interrogating breast lesions. Such acquisitions are limited in resolution and are generally successful only in lesions greater than 1.5 cm; also, MRS allows the evaluation of only one lesion at a time.56 These and other acquisition and processing oriented technological hurdles currently limit the usefulness of MRS in breast imaging (Figure 27-15).
Numerous other emerging technologies, such as dedicated breast CT and optical coherence tomography, are still being developed and are currently at the preclinical or early clinical investigational stages. Very promising preliminary results have been demonstrated in studies performed with full-breast dedicated CT scanners that provide high-resolution, three-dimensional, cross-sectional imaging of the breast with a radiation dose similar to the dose of a two-view mammography; this type of CT study is likely to compete directly with digital mammography and tomography techniques in the future57 (Figure 27-16).
Nodal Disease
Evaluation of locoregional nodal disease is the single most important prognostic factor in the staging of newly diagnosed breast cancers, because this information can help guide local and systemic treatment decisions. Cancer cells in the lymphatic fluid from the breast can drain directly into the regional lymph nodes or can bypass the nodes and enter the bloodstream. The axillary lymph nodes are the nodes most commonly involved in breast cancer. The internal mammary lymph nodes may also be involved when the primary breast cancer is in the inner quadrants of the breast.58
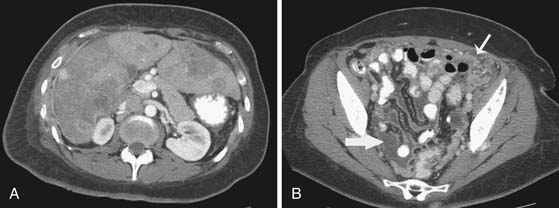
The status of the axillary lymph nodes has been traditionally evaluated with axillary lymph node dissection. However, axillary lymph node dissection is associated with lymphedema, nerve injury, paresthesias, and other morbidities. Sentinel lymph node biopsy, in place of axillary lymph node dissection, may be an alternative option for women with node-positive breast cancer at initial diagnosis because sentinel lymph node biopsy is associated with less morbidity. An on-going multi-institutional trial is evaluating sentinel lymph node biopsy as an alternative to axillary lymph node dissection for axillary staging after preoperative chemotherapy.59 Other clinicians are also considering the role of preoperative axillary sonography in combination with fine-needle aspiration biopsy or core needle biopsy in evaluating metastasis or residual nodal disease after preoperative chemotherapy. In a meta-analysis of 16 selected studies, axillary sonography combined with biopsy had a specificity of 88% to 98% in diagnosing axillary metastasis.60
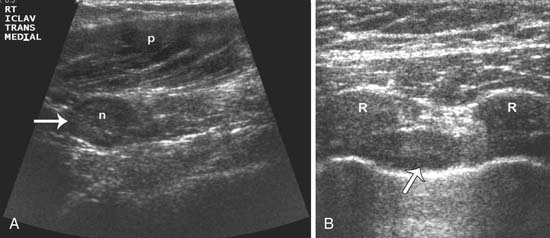
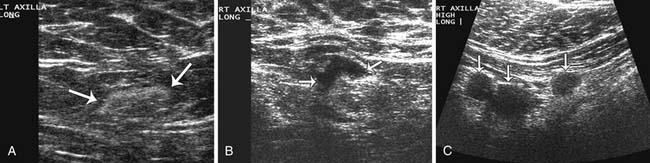
Sonographic evaluation of the regional lymph nodes, including the axillary, infraclavicular, and internal mammary regions, is a part of all staging breast sonography examinations performed at our institution. Lymph nodes are assessed for both size and morphology. A short-axis, or minimum diameter larger than 1 cm, has been suggested as the threshold criterion for an abnormal lymph node diagnosis.42 However, many nodes that are normal or fatty can measure over 1 cm, while a malignant node can be less than 1 cm. For this reason, the morphologic findings of the lymph node are crucial for an accurate diagnosis. Morphology suggestive of a malignant node includes a diffuse or focal hypoechoic and thickened cortex of 3 mm or more, partial or complete hilar compression or displacement, and perinodal invasion (Figure 27-17). If a suspicious lymph node is detected, a biopsy can be performed using a hypodermic needle (21- to 23-gauge attached to a 10-mL syringe). Papanicolaou staining or air-dried Diff-Quik staining is performed,61 and preliminary results can be available to the patient at the same appointment. The management of internal mammary nodes remains controversial, and many medical centers do not perform ultrasound-guided or surgical percutaneous biopsies owing to concerns about morbidity and the lack of a demonstrated impact on survival.
Metastatic Disease
Metastatic disease of the breast is a heterogeneous disease and ranges from a solitary metastasis to diffuse disease within multiple organs. Despite the medical advances in the detection and diagnosis of breast cancer at an early stage, approximately 30% of patients with early-stage breast cancer will develop a distant metastasis.62 A subset of oligometastatic disease refers to a few metastatic lesions within one organ and has a more optimistic prognosis than the cases with diffuse disease when treated with an intensified multidisciplinary therapeutic approach.63 Currently, we do not have a clear understanding of the patterns of spread or specific sites of recurrence. Advances in whole body imaging may aid in the early detection of metastases and in facilitating a better understanding of how and why breast cancer spreads in spite of therapy.
Bone Metastases
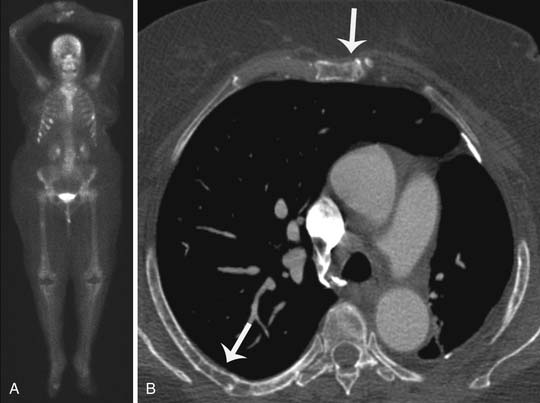
Metastases develop in approximately 69% of patients with advanced stage or recurrent breast cancer, and bone is the most common site of distant metastases from breast cancer.62 Imaging modalities commonly indicated for the detection and management of osseous metastases include plain-film radiography, skeletal scintigraphy, CT, MRI, PET, and single-proton emission radiography, as well as combination techniques such as PET/CT, dual-modality single-photon emission CT, and whole-body MRI.64 For a newly diagnosed breast cancer patient with no bone symptoms, skeletal scintigraphy is the modality of choice for screening for osseous metastases (Figure 27-18). If focal bony symptoms are present, plain film radiography of the symptomatic area(s) would be helpful to visualize the bony anatomy. If the radiography fails to identify osteolysis, CT can be performed to evaluate the cortex and associated soft tissue calcifications. CT is also preferred over MRI for rib lesions. When evaluation of bone marrow or soft tissue is indicated, high-contrast MRI is the preferred imaging modality. MRI carries the added advantage of being able to differentiate metastatic breast cancer from osteoporotic compression fractures (Figure 27-19). If radiography, CT, or MRI studies remain discordant in the presence of highly suspicious clinical symptoms, PET/CT is indicated.
Liver Metastasis
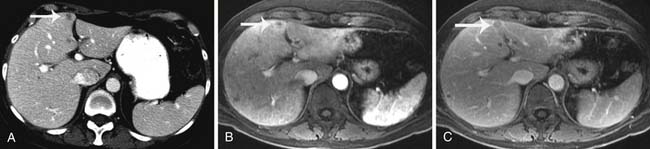
Liver metastasis from breast cancer is less common than bone metastasis, yet liver metastasis is observed in 55% to 75% of autopsies in patients with breast cancer.65 Common clinical presentation of liver metastasis includes hepatomegaly and abnormally elevated liver function tests. Imaging tests commonly incorporated to investigate these clinical symptoms include abdominal ultrasonography and contrast-enhanced CT, MRI, and PET studies.
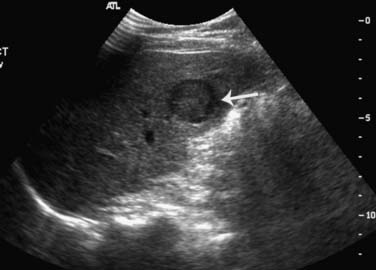
Ultrasonography is the least expensive and most widely available modality, with a reported sensitivity of 94% in the detection of liver lesions larger than 2 cm and 56% for smaller lesions.66 The examination of the liver should be performed with a sector or a curvilinear array transducer, with center frequencies between 2 and 5 MHz for deep penetration. Hepatic metastasis is usually presented as a hypoechoic focal lesion compared with normal liver parenchyma, or it may have a “target” appearance, displaying as an echogenic center surrounded by a hypoechoic halo (Figure 27-20). Diffuse hepatic metastases may present as a diffusely inhomogeneous liver parenchyma. Ultrasonography is primarily used for further characterization of liver lesions and for guiding biopsies; ultrasonography is not used as a surveillance tool for the detection of metastases owing to its poor ability in differentiating benign from malignant lesions, especially at medical centers where CT or MRI is available. However, ultrasonography is a strong alternative choice for patients with allergies or contraindications to CT or MRI contrast agents.
Unlike ultrasonography, CT is not operator-dependent, is easily reproducible, and can detect subcentimeter liver lesions. Hepatic metastases from breast cancer typically present as irregular, ill-defined, hypodense masses on the precontrast image and during the portal venous phase. The periphery of the mass may demonstrate mild enhancement. Against the background of hepatic steatosis, hepatic metastasis can be difficult to detect on CT, and MRI is a superior modality for this clinical scenario. The liver may be shrunken or have the appearance of cirrhosis due to diffuse disease related to periportal fibrosis. Hepatic steatosis can also be a posttreatment appearance from tamoxifen or cytotoxic drugs.67 Because of its usefulness in lesion detection and prevalence in interventional clinics, CT is also used to guide biopsies of suspicious lesions. For nonspecific liver CT findings, MRI may be useful in both confirming the presence of liver lesions and further characterization of the lesions.
Intrathoracic Metastasis
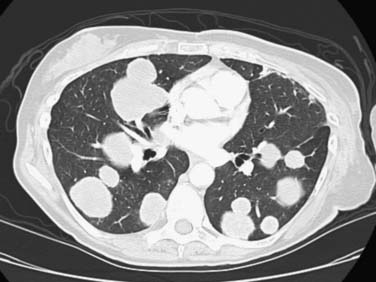
The thorax is a common site of metastasis and involves the lung, pleura or mediastinum, and airway. Pulmonary metastasis may be a single pulmonary mass, multiple pulmonary nodules, an airspace-pattern metastasis, a lymphangitic metastasis, or an endobronchial metastasis (Figure 27-21). The metastatic pulmonary nodules or masses can be irregular or circumscribed lesions and tend to be in the periphery of the lung. Centrally located metastases or mediastinal metastases can extend into the bronchial walls, resulting in endobronchial metastasis. The pulmonary metastases rarely cavitate or present as a consolidation or airspace pattern, mimicking pneumonia. Breast cancer cells can spread to the pulmonary lymphatic system, resulting in lymphangitic metastasis. This pattern of spread presents as a thickening and nodularity of the interlobular septa or Kerley B lines. Unilateral involvement is more common than bilateral involvement in metastatic breast cancer. Other intrathoracic metastases include pleural effusions, pleural thickening, or pleural-based masses. The effusion tends to be unilateral and ipsilateral to the primary breast cancer. Pleural metastasis is thought to be due to lymphatic dissemination of breast cancer.40 The heart and pericardium can be involved by extrathoracic primary carcinoma from the breast. Most patients receive a chest radiograph at the initial staging evaluation, even though CT better delineates the metastatic pulmonary lesions, the endobronchial lesions, and the pleural nodules.
Brain Metastasis
The most common intracranial malignancy is metastases, with lung and breast cancers as the most common primary cancers.68 Breast cancer cells spread to the brain via the bloodstream, and the location of the metastases parallels the intracranial blood flow, with the cerebral hemispheres being the most common location. Metastases can also involve the meninges, skull, and dura. Brain metastases may present as a solitary lesion or oligometastases. On MRI, the metastases tend to be hypointense to isointense to brain parenchyma on T1-weighted images and hyperintense with peritumoral edema on T2-weighted images. On contrast-enhanced T1-weighted images, the metastatic lesions are typically moderate to marked nodular-enhancing lesions or ringlike enhancing lesions with central nonenhancement. With advances in CT and MRI in detecting intracranial lesions and the longer survival of patients with breast cancer, the prevalence of brain metastases is increasing. Single intracranial metastatic lesions treated with surgery followed by whole brain radiation therapy have been reported to improve survival.69
Abdominal Metastasis
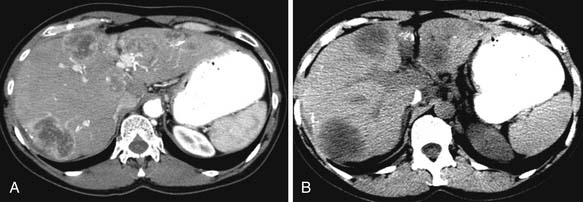
Besides the liver, other organs that breast cancer cells can spread to include the spleen, adrenal glands, lymph nodes, and peritoneum (Figure 27-22). Splenic metastases tend to be microscopic rather than macroscopic disease, and on CT, splenic metastases appear similar to liver metastases with irregular, ill-defined hypodense lesions. If there is diffuse disease, splenomegaly may also be present. Metastases to the adrenal glands are more common than to other endocrine organs, such as the thyroid or pituitary glands. Adrenal metastases demonstrate similar morphologic appearances as in the liver or spleen, with irregular, ill-defined margins and lower attenuation than the normal adrenal gland. The metastases can be unilateral or bilateral; however, a new lesion is suspicious for metastases. Lymph nodes in the abdomen that can be involved by breast cancer cells include the retrocrural, para-aortic, retroperitoneal, and intrapelvic nodes. Ascites and peritoneal spread to the bowel wall, mesentery, and omentum are not uncommon, especially with invasive lobular carcinoma.68 Other distant sites include the kidneys, ovaries, and uterus, although metastasis to the uterus is uncommon.
Treatment*
Surgical Treatment
Surgical management of breast cancer involves the appropriate extirpation of the primary tumor as well as assessment of the regional lymph nodes. The National Surgical Adjuvant Breast and Bowel Project (NSABP) B-4 and B-6 trials clearly established that less extensive surgery is as effective in curing patients of their disease as more extensive surgery and opened the door to breast-conserving therapy.33,70 Thus, most breast cancer patients have the option of undergoing breast-conserving surgery as an alternative to mastectomy for treatment of their primary tumor. Currently, oncologic exclusions to breast-conserving surgery include inflammatory breast cancer, multicentric breast cancer, and T4 tumors involving the skin. Breast-conserving surgery requires the complete removal of all macroscopic and microscopic disease, as determined by achieving negative margins; after surgery, the patient must undergo radiation therapy to the breast. Margin-positive excisions are clearly recognized as increasing the risk of local failure,71 which in turn may compromise long-term survival.72
The second component in the surgical management of breast cancer is the evaluation of regional lymph nodes to provide accurate regional staging and prognostic data. Traditionally, this was accomplished by axillary dissection with its attendant morbidities, in particular lymphedema and paresthesia. In 1994, however, sentinel node biopsy was introduced as an accurate and less morbid means of staging the axilla73 and has now become the standard practice for this purpose. Considerable debate remains about whether women with sentinel node metastasis require complete dissection because many will have no metastasis beyond the sentinel node.74 Among the underlying issues in this debate is whether axillary dissection has therapeutic value in addition to providing prognostic information. At a minimum, it is becoming clear that, even if a survival benefit to completion node dissection cannot be proved, in-basin recurrences are higher if completion node dissection is omitted.75,76 It is also theoretically possible that failure to remove additional foci of nonsentinel nodal disease may result in understaging of the patient and, potentially, undertreatment as well. Despite this ongoing debate, it should be noted that, although in individual cases a decision may be made to forego complete node dissection, the American Society of Clinical Oncology recommends node dissection for all women with micro- or macrometastasis in the sentinel lymph node.77
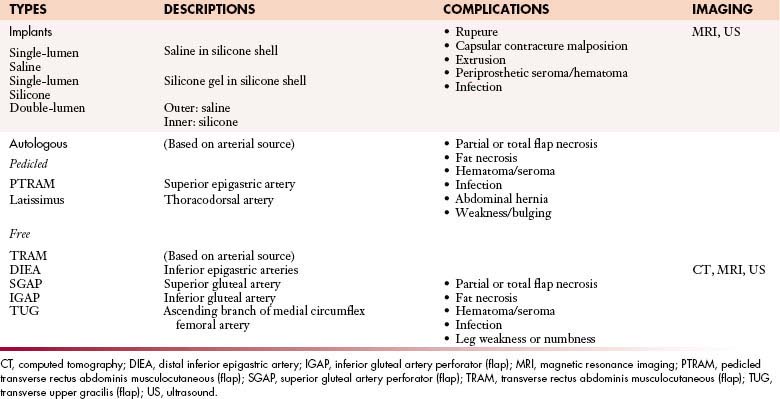
For women who require a mastectomy, breast reconstructive surgery is offered to improve the physical and psychosocial effects of the physical deformity caused by mastectomy. The two broad categories of breast reconstruction are implant-based reconstruction and autologous tissue–based reconstruction. The types of breast reconstruction are listed in Table 27-3.
For implant-based reconstruction, the surgeon initially creates a breast mound by placing a prosthesis or tissue expander within the mastectomy skin pocket extending to the pectoralis major muscle. Patients with tissue expanders should avoid undergoing an MRI owing to the presence of metal in the infusion port. The decision to use either a silicone- or a saline-filled implant is largely based upon the patient’s preference. Silicone implants offer a more natural appearance and feel whereas saline implants are perceived as a safer choice, even though studies have not shown a difference in the safety profiles of the two types of implants.
Autologous tissue–based reconstructions, also described as flap reconstruction, are the second major classification of breast reconstruction.78 Flaps are classified by both the source of their arterial perfusion and the method of their transfer (see Table 27-3). Pedicled flaps entail the transfer of skin and subcutaneous fat based on a vascular leash that remains attached to its source vessels. Free flaps are created when the vascular supply to the tissue is detached from the source vessels and the vessels are anastomosed under microscopic visualization to distant recipient vessels.79
Chemotherapy
Chemotherapy treatment for breast cancer depends on disease stage, patient symptoms, and tumor features such as receptor status and grade. Chemotherapy may be administered after surgical resection (adjuvant therapy) to reduce the risk of developing metastatic disease in high-risk patients. Adjuvant therapy reduces the risk of breast cancer recurrence by 30% to 50%.80,81 The administration of chemotherapy before surgical resection (neoadjuvant therapy) offers the same survival benefit as adjuvant therapy and is often considered in patients with locally advanced breast cancer to reduce tumor size, which results in a less extensive operative procedure in certain patients. Chemotherapy is also used to palliate symptoms in patients with metastatic breast cancer.
Many chemotherapy drugs have activity against breast cancer (Table 27-4). Anthracyclines, such as doxorubicin and epirubicin, are commonly used to treat breast cancer and exert their action by inhibiting topoisomerase II, an enzyme that relieves torsional stress during DNA replication and transcription.82 To increase cytotoxicity, anthracycline-based regimens usually include cyclophosphamide with or without fluoropyrimidines, such as 5-deoxyfluorouridine (5-FU).83 Taxanes, such as paclitaxel, docetaxel, and nab-paclitaxel (an albumin-bound formulation of paclitaxel that reduces hypersensitivity reactions), exert their cytotoxic effects by binding to alpha-tubulin, stabilizing microtubule formation, and blocking the progression of mitosis.84 Epothilones, such as ixabepilone, also bind to alpha-tubulin but have demonstrated activity in taxane-resistant cell lines.85 Vinca alkaloids, such as vinorelbine, interfere with mitosis by exerting their effects on microtubule assembly.86 Nucleoside analogues, such as 5-FU, capecitabine, and gemcitabine, induce apoptosis through their effects on DNA replication.87,88 Alkylating agents, such as cyclophosphamide, or platinum salts, such as cisplatin and carboplatin, induce apoptosis by cross-linking DNA strands.
Table 27-4 Chemotherapy Agents Commonly Used to Treat Breast Cancer and Associated Toxicities
| DRUGS | SINGLE-AGENT RESPONSE RATE | TOXICITIES |
|---|---|---|
| Anthracyclines | ||
| Doxorubicin101,102 | 10-50% | Myelosuppression, nausea/vomiting, alopecia, fatigue, damage to myocardium/congestive heart failure, diarrhea, mucositis |
| Epirubicin103 | 13-48% | Myelosuppression, nausea/vomiting, alopecia, fatigue, damage to myocardium/congestive heart failure, diarrhea, mucositis |
| Liposomal doxorubicin104,105 | 10-50% | Myelosuppression, nausea/vomiting, fatigue, damage to myocardium/congestive heart failure (less than seen with other anthracyclines), diarrhea, mucositis |
| Agents Affecting Microtubules | ||
| Docetaxel106,107 | 18-68% | Myelosuppression, neuropathy, hypersensitivity reactions, alopecia, fatigue, nausea, diarrhea |
| Paclitaxel108 | 16-62% | Myelosuppression, neuropathy, hypersensitivity reactions, alopecia, fatigue, nausea, diarrhea |
| Nab-paclitaxel108 | 33-48% | Myelosuppression, neuropathy, alopecia, fatigue, nausea |
| Ixabepilone109,110 | 12-57% | Myelosuppression, neuropathy, alopecia, fatigue, nausea, diarrhea |
| Vinorelbine111 | 25-50% | Myelosuppression, neuropathy, fatigue, nausea |
| Nucleoside Analogues | ||
| Capecitabine112 | 20-35% | Myelosuppression, fatigue, diarrhea, hand foot syndrome |
| Gemcitabine113 | 12-37% | Myelosuppression, fatigue, nausea |
| Platinum Salts | ||
| Cisplatin/carboplatin114 | 9-51% | Myelosuppression, neuropathy, fatigue, nausea/vomiting, hypersensitivity reactions, renal insufficiency/failure |
Common toxicities associated with chemotherapy drugs used to treat breast cancer are listed in Table 27-4. Anthracyclines and/or taxanes are the most frequently used neoadjuvant and adjuvant chemotherapy regimens and may also be used to treat patients with metastatic disease. Taxanes, nucleoside analogues, vinca alkaloids, and platinum salts are commonly combined with targeted agents, such as trastuzumab, bevacizumab, or lapatinib, to enhance their cytotoxic effects.
Radiation Therapy
Adjuvant radiation therapy is indicated after a lumpectomy for virtually all patients; this recommendation is based on data from a phase III randomized trial.89–91 Women older than 70 years with small, low-grade, ER-negative invasive tumors who take tamoxifen have been reported to have sufficiently low risk of recurrence without radiation therapy, indicating that observation may be considered.92 Women with early-stage breast cancer who undergo segmental mastectomy now have several adjuvant radiation therapy options, including a shorter course of radiation therapy to the whole breast (more dose per day than the standard 5- to 6-wk regimens)93,94 and accelerated partial breast irradiation (treats only the tissue adjacent to the lumpectomy cavity with high dose per fraction twice a day for 1 wk). Selection of patients and techniques for the latter approach are still under investigation and should be carefully considered.95 Adjuvant radiation therapy in the postmastectomy setting reduces the risk of locoregional recurrence by two thirds and should be offered to all patients with stage III breast cancer and high-risk node-positive patients with stage II breast cancer.89 Common toxicities are summarized in Table 27-5. Adjuvant radiation therapy for patients who receive neoadjuvant systemic therapy has not been studied extensively but is routinely given to patients who presented with stage III disease, regardless of response, and to stage II patients with residual disease, particularly in the lymph nodes.96,97 Adjuvant radiation therapy has been shown to improve survival by preventing locoregional recurrences.89 A 21-gene expression signature has recently been shown to predict for locoregional recurrence, and in the future, it may be possible to better select patients who are at the highest risk of locoregional recurrence.38
| RADIATION THERAPY | TOXICITIES |
|---|---|
| Short Term | |
| During treatment 1 to 2 months after treatment |
Fatigue Radiation dermatitis |
| Long Term | Soft tissue fibrosis Soft tissue contracture Telangiectasia Pneumonitis Cardiovascular disease. |
Key Points Treatment
Therapies
• Neoadjuvant chemotherapy: to reduce tumor size and possibly enable breast conservation.
• Surgery: to remove the primary breast tumor and nodes; breast reconstruction.
• Postoperative chemotherapy: to decrease risk of metastases; to treat palliative symptoms.
• Radiation therapy: to improve overall survival after breast-conservation surgery.
Surveillance
Monitoring Tumor Response
Increasingly, neoadjuvant chemotherapy is used in the management of locally advanced breast cancer. Early initiation of systemic therapy can improve overall and disease-free survival for patients with locally advanced breast cancer or inflammatory cancer or for patients at high risk of recurrence. By downstaging the primary tumor, neoadjuvant chemotherapy can make surgery or breast-conservation therapy an option for patients whose cancer was initially considered inoperable. Nodal disease can be eradicated in 40% of patients who have node-positive disease at initial presentation,98 and the eradication of nodal disease is likely to increase with the improvement in targeted therapies, such as trastuzumab in combination with chemotherapy.99 Therefore, it is important to determine early in the course of the disease which patients are most likely to respond to neoadjuvant chemotherapy.
Physical examination, mammography, and ultrasonography have all been used to assess the response to neoadjuvant chemotherapy. The principal value of these techniques is in measuring the size of the residual lesion based on morphologic changes in the tumor. Unfortunately, treatment-induced fibrosis or inflammation can result in an overestimation or underestimation of the extent of residual disease, meaning the true response to therapy is not reflected in the findings from physical examination, mammography, or ultrasonography. MRI with intravenous contrast and advanced MRI techniques provide new opportunities for assessing tumor morphologic changes, tumor vascularity, tumor cellularity, and tumor metabolic features. MRI has been shown to be more reliable than the conventional methods of physical examination, mammography, and sonography in the assessment of tumor size and vascularity changes during and after chemotherapy.100 The addition of advanced imaging techniques such as diffusion-weighted imaging or proton MRS to further characterize tumor cellularity and metabolic features appears promising. However, there is still no consensus on the role of MRI for assessing response to neoadjuvant chemotherapy or on standardized MRI studies in patients receiving neoadjuvant chemotherapy. More investigations are needed to determine the potential of MRI in predicting those patients who would respond to neoadjuvant chemotherapy.
Detection of Recurrence
After mastectomy or breast-conserving surgery for breast cancer, disease can reappear locally, regionally, or at distant sites. Locoregional recurrent disease refers to the reappearance of the original breast cancer in the treated breast within the skin flaps, mastectomy scars, or ipsilateral chest wall. Regional recurrent disease refers to the breast cancer recurring in the regional lymphatic system of the treated breast such as the ipsilateral axillary, infraclavicular, supraclavicular, or internal mammary lymph nodes. The recurrent disease may present as a small solitary tumor or a palpable lesion or the recurrent disease may be more diffuse, involving the entire chest wall or regional lymph nodes. The majority of recurrences occur in the first decade after the diagnosis of the primary breast cancer with peak incidence at 2 years and a second peak incidence at 5 years after initial diagnosis.115 Therefore, the goal of an intensive surveillance program after treatment is to detect potential curable recurrences or secondary primary tumors.
The modes of surveillance recommended by the American Society for Clinical Oncology 2006 clinical guidelines116 are as follows: a monthly breast self-examination, a complete history and physical examination every 3 to 6 months for the first 3 years followed by every 6 to 12 months for years 4 and 5, and regular screening mammography. A mammogram is recommended 1 year after the initial mammogram that led to the diagnosis of breast cancer or 6 months after definitive radiation therapy. However, diagnostic tests, including serum markers or other imaging studies (such as breast MRI, radionuclide scans, CT scans, or PET scans), are not routinely recommended. When there is a clinical suspicion of locoregional recurrent disease, a biopsy is required to confirm the diagnosis and to rule out common benign processes associated with post-treatment changes such as fat necrosis, granulomas, or foreign body reactions. Locoregional recurrent disease, with or without simultaneous distant metastasis, occurs in up to 49% of cases during 18 years of follow-up.117 At least half of the patients with locoregional recurrent disease develop distant metastases.118–119 Some prognostic factors associated with an increased likelihood of locoregional recurrence include a tumor greater than 5 cm in diameter, disease-positive lymph nodes, and initial disease involving the skin or fascia. Isolated locoregional recurrent disease detected on imaging or before the manifestation of symptoms has better rates of survival than locoregional recurrences detected late or with symptomatology.120 More than half of the patients with locoregional recurrent disease will develop distant metastases within 5 years, and approximately two-thirds of these patients will develop distant metastases within 10 years.121
1. American Cancer Society. Cancer Facts & Figures. Atlanta: American Cancer Society; 2009:9-31. 2008.
2. Jemal A., Siegel R., Ward E., et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225-249.
3. Anderson B.O., Yip C.H., Smith R.A., et al. Guideline implementation for breast healthcare in low-income and middle-income countries: overview of the Breast Health Global Initiative Global Summit 2007. Cancer. 2008;113(Suppl 8):2221-2243.
4. Coleman M.P., Quaresma M., Berrino F., et al. Cancer survival in five continents: a worldwide population-based study (CONCORD). Working Group. Lancet Oncol. 2008;9:730-756.
5. Shapiro S. Evidence on screening for breast cancer from a randomized trial. Cancer. 1977;39:2772-2782.
6. Nystrîm L., Rutqvist L.E., Wall S., et al. Breast cancer screening with mammography: overview of Swedish randomized trials. Lancet. 1993;341:973-978.
7. Hetzel L., Smith A.. A. The 65 Years and Over Population: 2000. Washington, DC, Census 2000 Brief, C2KBR/01–10, United States Census Bureau. Available at www.census.gov/prod/2001pubs/c2kbr01-10.pdf (accessed March 25, 2010).
8. Ravdin P.M., Cronin K.A., Howlader N., et al. The decrease in breast-cancer incidence in 2003 in the United States. N Engl J Med. 2007;356:1670-1674.
9. Krieger N., Chen J.T., Waterman P.D. Decline in US breast cancer rates after the Women’s Health Initiative: socioeconomic and racial/ethnic differentials. Am J Public Health. 2001;100:S132-S139.
10. Ziegler R.G., Hoover R.N., Pike M.C., et al. Migration patterns and breast cancer risk in Asian-American women. J Natl Cancer Inst. 1993;85:1819-1827.
11. von Eschenbach A.C. NCI remains committed to current mammography guidelines. Oncologist. 2002;7:170-171.
12. Howe H.L., Wingo P.A., Thun M.J., et al. Annual report to the nation on the status of cancer (1973 through 1998), featuring cancers with recent increasing trends. J Natl Cancer Inst. 2001;93:824-842.
13. Tabár L., Fagerberg C.J., Gad A., et al. Reduction in mortality from breast cancer after mass screening with mammography: randomised trial from the Breast Cancer Screening Working Group of the Swedish National Board of Health and Welfare. Lancet. 1985;1:829-832.
14. Smart C.R., Hendrick R.E., Rutledge J.H.3rd, Smith R.A. Benefit of mammography screening in women ages 40–49 years. Current evidence from randomized controlled trials. Cancer. 1995;75:1619-1626.
15. Thurfjell E.L., Lindgren J.A. Breast cancer survival rates with mammographic screening: similar favorable survival rates for women younger and those older than 50 years. Radiology. 1996;201:421-426.
16. American College of Radiology (ACR). ACR-RADS®—Mammography. 4th Edition. In:. ACR Breast Imaging Reporting and Data System, Breast Imaging Atlas. Reston, VA: American College of Radiology; 2003.
17. Schulz-Wendtland R., Fuchsjäger M., Wacker T., Hermann K. Digital mammography: an update. Eur J Radiol. 2009;72:258-265.
18. Berg W.A., Blume J.D., Cormack J.B., et al. Combined screening with ultrasound and mammography versus mammography alone in women at elevated risk of breast cancer. JAMA. 2008;299:2151-2163.
19. Kuhl C.K., Kuhn W., Schild H. Management of women at high risk for breast cancer: new imaging beyond mammography. Breast. 2005;14:480-486.
20. Saslow D., Boetes C., Burke W., et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89.
21. Lehman C.D., Blume J.D., Weatherall P., et al. Screening women at high risk for breast cancer with mammography and magnetic resonance imaging. Cancer. 2005;103:1898-1905.
22. Morris E.A., Liberman L., Ballon D.J., et al. MRI of occult breast carcinoma in a high-risk population. AJR Am J Roentgenol. 2003;181:519-525.
23. Lehman C.D., Blume J.D., Thickman D., et al. Added cancer yield of MRI in screening the contralateral breast of women recently diagnosed with breast cancer: results from the International Breast Magnetic Resonance Consortium (IBMC) trial. J Surg Oncol. 2005;92:9-15.
24. Warner E., Plewes D.B., Shumak R.S., et al. Comparison of breast magnetic resonance imaging, mammography, and ultrasound for surveillance of women at high risk for hereditary breast cancer. J Clin Oncol. 2001;19:3524-3531.
25. Stoutjesdijk M.J., Boetes C., Jager G.J., et al. Magnetic resonance imaging and mammography in women with a hereditary risk of breast cancer. J Natl Cancer Inst. 2001;18(93):1095-1102.
26. Lehman C.D., Isaacs C., Schnall M.D., et al. Cancer yield of mammography, MR, and US in high-risk women: prospective multi-institution breast cancer screening study. Radiology. 2007;244:381-388.
27. Schrading S., Kuhl C.K. Mammographic, US, and MR imaging phenotypes of familial breast cancer. Radiology. 2008;246:58-70.
28. Kuhl C.K., Schrading S., Leutner C.C., et al. Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J Clin Oncol. 2005;20(23):8469-8476.
29. Cowie A.T. Overview of mammary gland. J Invest Dermatol. 1974;63:2-9.
30. Wellings S.R., Jensen H.M. On the origin and progression of ductal carcinoma in the human breast. J Natl Cancer Inst. 1973;50:1111-1118.
31. Diab S.G., Clark G., Osborne C.K., et al. Tumor characteristics and clinical outcome of tubular and mucinous carcinomas. J Clin Oncol. 1999;17:1442-1449.
32. Rubens J.R., Lewandrowski K.B., Kopans D.B., et al. Medullary carcinoma of the breast: Overdiagnosis of a prognostically favorable neoplasm. Arch Surg. 1990;125:601-604.
33. Fisher B., Wolmark N., Redmond C., et al. Findings from NSABP Protocol No. B-04: comparison of radical mastectomy with alternative treatments. II. The clinical and biologic significance of medial-central breast cancers. Cancer. 1981;48:1863-1872.
34. Haupt B., Ro J.Y., Schwartz M.R. Basal-like breast carcinoma: a phenotypically distinct entity. Arch Pathol Lab Med. 2010;134:130-133.
35. Anders C.K., Carey L.A. Biology, metastatic patterns, and treatment of patients with triple-negative breast cancer. Clin Breast Cancer. 2009;9(suppl 2):S73-S81.
36. Osteen R.T., Cady B., Chmiel J.S., et al. 1991 survey of carcinoma of the breast by the Commission on Cancer. J Am Coll Surg. 1994;178:213-219.
37. Mathis K.L., Hoskin T.L., Boughey J.C., et al. Palpable presentation of breast cancer persists in the era of screening mammography. J Am Coll Surg. 2010;210:314-331.
38. Edge S.B., Byrd D.R., Compton C.C., et al. Breast. In: AJCC Cancer Staging Manual, 7th ed. New York: Springer; 2010. 347–369
39. Kennecke H., Yerushalmi R., Woods R., et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28:3271-3277.
40. Thomas J.M., Redding W.H., Sloane J.P. The spread of breast cancer: importance of the intrathoracic lymphatic route and its relevance to treatment. Br J Cancer. 1979;40:540-547.
41. Tingberg A. X-ray tomosynthesis: a review of its use for breast and chest imaging. Radiat Prot Dosimetry. 2010;139:100-107.
42. Stavros A.T. Breast ultrasound technique. In: In Breast Ultrasound. Philadelphia: Lippincott Williams & Wilkins; 2004:42-55.
43. Le-Petross H., Stafford R. The need for MRI before breast conserving surgery. Curr Breast Cancer Rep. 2009;1:98-103.
44. Houssami N., Ciatto S., Macaskill P., et al. Accuracy and surgical impact of magnetic resonance imaging in breast cancer staging: systematic review and meta-analysis in detection of multifocal and multicentric cancer. J Clin Oncol. 2008;26:3248-3258.
45. Brennan M.E., Houssami N., Lord S., et al. Accuracy and surgical impact of magnetic resonance imaging in breast cancer staging: systematic review and meta-analysis in detection of multifocal and multicentric cancer. J Clin Oncol. 2009;27:5640-5649.
46. Turnbull L., Brown S., Harvey I., et al. Comparative effectiveness of MRI in breast cancer (COMICE) trial: a randomised controlled trial. Lancet. 2010;375:563-571.
47. Mann R.M. The effectiveness of MR imaging in the assessment of invasive lobular carcinoma of the breast. Magn Reson Imaging Clin North Am. 2010;18:259-276.
48. Le-Petross C.H., Bidaut L., Yang W.T. Evolving role of imaging modalities in inflammatory breast cancer [review]. Semin Oncol. 2008;35:51-63.
49. Kuhl C. The current status of breast MR imaging. Part I. Choice of technique, image interpretation, diagnostic accuracy, and transfer to clinical practice. Radiology. 2007;244:356-378.
50. Kuerer H., Albarracin C., Yang W., et al. Ductal carcinoma in situ: state of the science and roadmap to advance the field. J Clin Oncol. 2009;27:279-288.
51. Kuhl C.K., Schrading S., Bieling H.B., et al. MRI for diagnosis of pure ductal carcinoma in situ: a prospective observational study. Lancet. 2007;370:485-492.
52. Ernster V., Ballard-Barbash R., Barlow W., et al. Detection of ductal carcinoma in situ in women undergoing screening mammography. J Natl Cancer Inst. 2002;94:1546-1554.
53. Kumar A., Chen D., Au A., et al. Biologic significance of false-positive magnetic resonance imaging enhancement in the setting of ductal carcinoma in situ. Am J Surg. 2006;192:520-524.
54. Berg W.A., Weinberg I.N., Narayanan D., et al. High resolution FDG positron emission tomography with compression positron emission mammography is highly accurate in depicting primary breast cancer. Breast J. 2006;12:309-323.
55. Bartella L., Morris E.A., Dershaw D.D., et al. Proton MR spectroscopy with choline peak as malignancy marker improves positive predictive value for breast cancer diagnosis: preliminary study. Radiology. 2006;239:686-692.
56. Tse G.M., Cheung H.S., Pang L.M., et al. Characterization of lesions of the breast with proton MR spectroscopy: comparison of carcinomas, benign lesions, and phyllodes tumors. AJR Am J Roentgenol. 2003;181:1267-1272.
57. Boone J.M., Kwan A.L., Yang K., et al. Computed tomography for imaging the breast. J Mammary Gland Biol Neoplasia. 2006;11:103-111.
58. Estourgie S.H., Tanis P.J., Nieweg O.E., et al. Should the hunt for internal mammary chain sentinel nodes begin? An evaluation of 150 breast cancer patients. Ann Surg Oncol. 2003;10:935-941.
59. American College of Surgeons Oncology Group. Z1071. A Phase II Study Evaluating the Role of Sentinel Lymph Node Surgery and Axillary Lymph Node Dissection Following Preoperative Chemotherapy in Women with Node Positive Breast Cancer (T1–4, N1-2, M0) at Initial Diagnosis. Available at https://www.acosog.org/ (accessed May 23, 2010).
60. Alvarez S.A., Añorbe E., Alcorta P. Role of sonography in the diagnosis of axillary lymph node metastases in breast cancer: a systematic review. AJR Am J Roentgenol. 2006;186:1342-1348.
61. Krishnamurthy S. Current applications and future prospects of fine-needle aspiration biopsy of locoregional lymph nodes in the management of breast cancer. Cancer. 2009;117:451-462.
62. Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomized trials. Lancet. 2005;365:1687-1717.
63. Hanrahan E.O., Broglio K.R., Buzdar A.U., et al. Combined-modality treatment for isolated recurrences of breast carcinoma: update on 30 years of experience at the University of Texas M.D. Anderson Cancer Center and assessment of prognostic factors. Cancer. 2005;104:1158-1171.
64. Mueller G.C., Hussein H.K., Carlos R.C., et al. Effectiveness of MR imaging in characterizing small hepatic lesions: routine versus expert interpretation. AJR Am J Roentgenol. 2003;180:673-680.
65. Hoe A.L., Royle G.T., Taylor I. Breast liver metastases—incidence, diagnosis and outcome. J R Soc Med. 1991;84:714-716.
66. Sheu J.C., Sung S.L., Chen D.S., et al. Ultrasonography of small hepatic tumors using high-resolution linear-array real time instruments. Radiology. 1984;150:797-802.
67. Saphner T., Triest-Robertson S., Li H., Holzman P. The association of nonalcoholic steatohepatitis and tamoxifen in patients with breast cancer. Cancer. 2009;115:3189-3195.
68. Brookes M., MacVicar D., Husband J. Metastatic carcinoma of the breast: the appearances of metastatic spread to the abdomen and pelvis as demonstrated by CT. Br J Radiol. 2007;80:284-292.
69. Eichler A.F., Loeffler J.S. Multidisciplinary management of brain metastases. Oncologist. 2007;12:884-898.
70. Fisher E.R., Anderson S., Redmond C., Fisher B. Pathologic findings from the National Surgical Adjuvant Breast Project protocol B-06. 10-year pathologic and clinical prognostic discriminants. Cancer. 1993;71:2507-2514.
71. Park C., Mitsumori M., Nixon A., et al. Outcome at 8 years after breast conserving surgery and radiation therapy for invasive breast cancer: influence of margin status and systemic therapy on local recurrence. J Clin Oncol. 2000;18:1668-1675.
72. Clarke M., Collins R., Darby S., et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087-2106.
73. Giulano A.E., Kirgan D.M., Guenther J.M., Morton D.M. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg. 1994;220:391-401.
74. Chu K., Turner R., Hansen N., et al. Do all patients with sentinel node metastasis from breast carcinoma need complete axillary node dissection? Ann Surg. 1999;229:536-541.
75. Naik A.M., Fey J., Gemignani M., et al. The risk of axillary relapse after sentinel lymph node biopsy for breast cancer is comparable with that of axillary lymph node dissection: a follow-up study of 4008 procedures. Ann Surg. 2004;240:462-468. discussion 468–471
76. Bilimoria K.Y., Bentrem D.J., Hansen N.M., et al. Comparison of sentinel lymph node biopsy alone and completion axillary lymph node dissection for node-positive breast cancer. J Clin Oncol. 2009;27:2946-2953.
77. Wasif N., Ye X., Giuliano A.E. Survey of ASCO members on management of sentinel node micrometastases in breast cancer: variation in treatment recommendations according to specialty. Ann Surg Oncol. 2009;16:2442.
78. Elliott L.F. Breast reconstruction. In: Thorne C.H, Beasley R.W., Aston S.J., et al. Grabb & Smith’s Plastic Surgery. Philadelphia: Lippincott Williams & Wilkins; 2007:648-656.
79. Blondeel P.N., Morrison C.M. Deep inferior epigastric artery perforator flap. In: Blondeel P.N., Morris S.F., Hallock G.G., Neligan P.C., et al. Perforator Flaps: Anatomy, Technique, & Clinical Applications. St. Louis: Quality Medical Publishing; 2006:385-403.
80. Early Breast Cancer Trialists’ Collaborative Group. Polychemotherapy for early breast cancer: an overview of the randomised trials. Lancet. 1998;352:930-942.
81. Early Breast Cancer Trialists’ Collaborative Group. Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet. 1998;351:1451-1467.
82. Walker J.V., Nitiss J.L. DNA topoisomerase II as a target for cancer chemotherapy. Cancer Invest. 2002;20:570-589.
83. Bull J.M., Tormey D.C., Li S.H., et al. A randomized comparative trial of adriamycin versus methotrexate in combination drug therapy. Cancer. 1978;41:1649-1657.
84. Schiff P.B., Fant J., Horwitz S.B. Promotion of microtubule assembly in vitro by taxol. Nature. 1979;277:665-667.
85. Bollag D.M., McQueney P.A., Zhu J., et al. Epothilones, a new class of microtubule-stabilizing agents with a taxol-like mechanism of action. Cancer Res. 1995;55:2325-2333.
86. Abeloff M.D. Vinorelbine (Navelbine) in the treatment of breast cancer: a summary. Semin Oncol. 1995;22:1-4. discussion 41–44
87. Galmarini C.M., Jordheim L., Dumontet C. Pyrimidine nucleoside analogs in cancer treatment. Expert Rev Anticancer Ther. 2003;3:717-728.
88. Adema A.D., Bijnsdorp I.V., Sandvold M.L., et al. Innovations and opportunities to improve conventional (deoxy)nucleoside and fluoropyrimidine analogs in cancer. Curr Med Chem. 2009;16:4632-4643.
89. Early Breast Cancer Trialists’ Collaborative Group. Radiotherapy for early breast cancer. Cochrane Database Syst Rev. 2002;2:CD003647.
90. Bijker N., Meijnen P., Peterse J.L., et al. Breast-conserving treatment with or without radiotherapy in ductal carcinoma-in-situ: ten-year results of European Organisation for Research and Treatment of Cancer randomized phase III trial 10853—a study by the EORTC Breast Cancer Cooperative Group and EORTC Radiotherapy Group. J Clin Oncol. 2006;24:3381-3387.
91. Hughes K.S., Schnaper L.A., Berry D., et al. Lumpectomy plus tamoxifen with or without irradiation in women 70 years of age or older with early breast cancer. N Engl J Med. 2004;351:971-977.
92. Bentzen S.M., Agrawal R.K., Aird E.G., et al. The UK Standardisation of Breast Radiotherapy (START) Trial A of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet Oncol. 2008;9:331-341.
93. Whelan T.J., Pignol J.P., Levine M.N., et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362:513-520.
94. Smith B.D., Arthur D.W., Buchholz T.A., et al. Accelerated partial breast irradiation consensus statement from the American Society for Radiation Oncology (ASTRO). J Am Coll Surg. 2009;209:269-277.
95. Buchholz T.A., Lehman C.D., Harris J.R., et al. Statement of the science concerning locoregional treatments after preoperative chemotherapy for breast cancer: a National Cancer Institute conference. J Clin Oncol. 2008;26:791-797.
96. Buchholz T.A., Tucker S.L., Masullo L., et al. Predictors of local-regional recurrence after neoadjuvant chemotherapy and mastectomy without radiation. J Clin Oncol. 2002;20:17-23.
97. Mamounas E.P., Tang G., Fisher B., et al. Association between the 21-gene recurrence score assay and risk of locoregional recurrence in node-negative, estrogen receptor–positive breast cancer: results from NSABP B-14 and NSABP B-20. J Clin Oncol. 2010;28:1677-1683.
98. Kuerer H.M., Sahin A.A., Hunt K.K., et al. Incidence and impact of documented eradication of breast cancer axillary lymph node metastases before surgery in patients treated with preoperative chemotherapy. Ann Surg. 1999;230:72-78.
99. Buzdar A.U., Ibrahim N.K., Francis D., et al. Significantly higher pathologic complete remission rate after preoperative therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23:3676-3685.
100. Le-Petross H., Hylton N. Role of breast MR imaging in neoadjuvant chemotherapy. Magn Reson Imaging Clin North Am. 2010;18:249-258. viii-ix
101. Sledge G.W., Neuberg D., Bernardo P., et al. Phase III trial of doxorubicin, paclitaxel, and the combination of doxorubicin and paclitaxel as front-line chemotherapy for metastatic breast cancer: an intergroup trial (E1193). J Clin Oncol. 2003;21:588-592.
102. Norris B., Pritchard K.I., James K., et al. Phase III comparative study of vinorelbine combined with doxorubicin versus doxorubicin alone in disseminated metastatic/recurrent breast cancer: National Cancer Institute of Canada Clinical Trials Group Study MA8. J Clin Oncol. 2000;18:2385-2394.
103. Hortobagyi G.N., Yap H.Y., Kau S.W., et al. A comparative study of doxorubicin and epirubicin in patients with metastatic breast cancer. Am J Clin Oncol. 1989;12:57-62.
104. Keller A.M., Mennel R.G., Georgoulias V.A., et al. Randomized phase III trial of pegylated liposomal doxorubicin versus vinorelbine or mitomycin C plus vinblastine in women with taxane-refractory advanced breast cancer. J Clin Oncol. 2004;22:3893-3901.
105. O’Brien M.E., Wigler N., Inbar M., et al. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol. 2004;15:440-449.
106. Nabholtz J.M., Senn H.J., Bezwoda W.R., et al. Prospective randomized trial of docetaxel versus mitomycin plus vinblastine in patients with metastatic breast cancer progressing despite previous anthracycline-containing chemotherapy. 304 Study Group. J Clin Oncol. 1999;17:1413-1424.
107. Jones S.E., Erban J., Overmoyer B., et al. Randomized phase III study of docetaxel compared with paclitaxel in metastatic breast cancer. J Clin Oncol. 2005;23:5542-5551.
108. Gradishar W.J., Tjulandin S., Davidson N., et al. Superior efficacy of albumin-bound paclitaxel, ABI-007, compared with polyethylated castor oil-based paclitaxel in women with metastatic breast cancer: results of a phase III trial. J Clin Oncol. 2005;23:7794-7803.
109. Moulder S., Li H., Wang M., et al. A phase II trial of trastuzumab plus weekly ixabepilone and carboplatin in patients with HER2-positive metastatic breast cancer: an Eastern Cooperative Oncology Group Trial. Breast Cancer Res Treat. 2010;119:663-671.
110. Perez E.A., Lerzo G., Pivot X., et al. Efficacy and safety of ixabepilone (BMS-247550) in a phase II study of patients with advanced breast cancer resistant to an anthracycline, a taxane, and capecitabine. J Clin Oncol. 2007;25:3407-3414.
111. Seidman A.D., O’Shaughnessy J., Misset J.L. Single-agent capecitabine: a reference treatment for taxane-pretreated metastatic breast cancer? Oncologist. 2002;7(suppl 6):20-28.
112. Silvestris N., D’Aprile M., Andreola G., et al. Rationale for the use of gemcitabine in breast cancer [review]. Int J Oncol. 2004;24:389-398.
113. Decatris M.P., Sundar S., O’Byrne K.J. Platinum-based chemotherapy in metastatic breast cancer: current status. Cancer Treat Rev. 2004;30:53-81.
114. Clarke M., Collins R., Darby S., et al. Early Breast Cancer Trialists’ Collaborative Group. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087-2106.
115. Jatoi I., Tsimelzon A., Weiss H., et al. Hazard rates of recurrence following diagnosis of primary breast cancer. Breast Cancer Res Treat. 2005;89:173-178.
116. Khatcheressian J.L., Wolff A.C., Smith T.J., et al. American Society of Clinical Oncology 2006 update of the breast cancer follow-up and management guidelines in the adjuvant setting. J Clin Oncol. 2006;24:5091-5097.
117. Nielsen H.M., Overgaard M., Grau C., et al. Loco-regional recurrence after mastectomy in high-risk breast cancer—risk and prognosis. An analysis of patients from the DBCG 82 b&c randomization trials. Radiother Oncol. 2006;79:147-155.
118. Ragaz J., Olivotto I.A., Spinelli J.J., et al. Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy: 20-year results of the British Columbia randomized trial. J Natl Cancer Inst. 2005;97:116-126.
119. Tennvall-Nittby L., Tenegrup I., Landberg T. The total incidence of loco-regional recurrence in a randomized trial of breast cancer TNM stage II: the South Sweden Breast Cancer Trial. Acta Oncol. 1993;32:641-646.
120. Lu W.L., Jasen L., Post W.J., et al. Impact on survival of early detection of isolated breast recurrences after the primary treatment for breast cancer: a meta-analysis. Breast Cancer Res Treat. 2009;114:403-412.
121. J. Willner, I.C. Kiricuta, O. Kolbl. Locoregional recurrence of breast cancer following mastectomy: always a fatal event? Results of univariate and multivariate analysis. Int J Radiat Oncol Biol Phys.. 1997;37:853-863.