Chapter 21 Brain Stimulation in Epilepsy—An Old Technique with a New Promise?
Introduction
Neurostimulation is not a new technique. The earliest recorded human effort at neurostimulation may have been that of the Mesopotamian healer Largus, who applied electrical torpedo fish to the human body and evoked an immediate and residual numbness in an extremity. Following the development of the battery by Volta, Faraday and Franklin experimented with electricity, giving rise to devices that could transcutaneously affect nerves and representing the precursors of today’s TENS systems. In the early 20th century an “electreat” device was patented by Charles Kent to treat pain. In the 1950s efforts were made in a number of medical device manufacturing companies in collaboration with universities to miniaturize these devices and make them implantable. Around this time electrodes to insert into the brain and record or stimulate were also designed. Initially the major clinical indications for neurostimulation were psychiatric (e.g., treatment of schizophrenia) and refractory pain. More recent progress in biotechnology in combination with successes of neurostimulation in movement disorders has led to renewed interest to investigate neurostimulation as a therapeutic option in various neurological and psychiatric disorders.
Electrical stimulation of the tenth cranial nerve or vagus nerve stimulation (VNS) is an extracranial but invasive type of stimulation that was developed in the 1980s and is currently routinely available in epilepsy centers around the world. Through an implanted device and electrode, electrical pulses are administered to the afferent fibers of the left vagus nerve in the neck (Figure 21-1). It is indicated in patients with refractory epilepsy who are unsuitable candidates for epilepsy surgery or who have had insufficient benefit from such a treatment.1 As stimulation is applied to that part of the vagus nerve that passes through the neck, direct intracerebral manipulation is unnecessary. Other cranial nerves are being targeted to treat refractory seizures. Preliminary but promising results are available for noninvasive trigeminal nerve stimulation (TNS).2
Transcranial magnetic stimulation (TMS) and direct current stimulation (tDCS) represent different types of extracranial and noninvasive neurostimulation techniques.3 In TMS, a coil that transmits magnetic fields is held over the scalp and allows a noninvasive evaluation of separate excitatory and inhibitory functions of the cerebral cortex. In addition, repetitive TMS (rTMS) can modulate the excitability of cortical networks.4 This therapeutic form of TMS is currently being investigated as a treatment option for refractory epilepsy with varying results.5 tDCS uses sponge electrodes attached to the patient’s head to deliver electrical currents over longer periods of time (minutes) to achieve changes in cortical excitability that persist even after stimulation has ceased, hence with therapeutic potential in diseases characterized by a disturbed cortical excitability.3
Intracerebral neurostimulation requires accessing the intracranial nervous system as stimulation electrodes are inserted into intracerebral targets for “deep brain stimulation” (DBS) or placed over the cortical convexity for “cortical stimulation” (CS). These modalities of neurostimulation are not entirely new for neurological indications. Some have been extensively used (e.g., for movement disorders and pain).6,7 Moreover, several new indications such as obsessive compulsive behavior and cluster headaches are being investigated with promising results.8,9 In the past, DBS and CS of different brain structures such as the cerebellum, the locus coeruleus, and the thalamus have already been performed. This was done mostly in patients with spasticity or psychiatric disorders who also had epilepsy, but the technique was not fully explored or developed into an efficacious treatment option.9–13 The vast progress in biotechnology along with the experience in other neurological diseases in the past 10 years has led to a renewed interest in intracerebral stimulation for epilepsy. Several epilepsy centers around the world have recently reinitiated trials with DBS in different intracerebral structures such as the thalamus, the subthalamic nucleus, the caudate nucleus, and medial temporal lobe structures.14–19 Also, CS is being investigated in a multicenter trial and incorporated in a so-called closed-loop system (the responsive neurostimulator system, RNS).20
Vagus Nerve Stimulation
Electrical stimulation of the tenth cranial nerve or VNS was developed in the 1980s. In the past decade it has become a valuable option in the therapeutic armamentarium for patients with refractory epilepsy, and it is currently routinely available in epilepsy centers worldwide. Through an implanted device and electrode, electrical pulses are administered to the afferent fibers of the left vagus nerve in the neck. It is indicated in patients with refractory epilepsy who are unsuitable candidates for epilepsy surgery or who have had insufficient benefit from such a treatment.1
Anatomical Basis and Mechanism of Action
The vagus nerve is a mixed cranial nerve that consists of ∼ 80% afferent fibers originating from the heart, aorta, lungs, and gastrointestinal tract and of ∼ 20% efferent fibers that provide parasympathetic innervation of these structures and also innervate the voluntary striated muscles of the larynx and the pharynx.21–23 Somata of the efferent fibers are located in the dorsal motor nucleus and nucleus ambiguus, respectively. Afferent fibers that are targeted for therapeutic VNS have their origin in the nodose ganglion and primarily project to the nucleus of the solitary tract. At the cervical level, the vagus nerve mainly consists of small-diameter unmyelinated C-fibers (65 to 80%) and of a smaller portion of intermediate-diameter myelinated B-fibers and large-diameter myelinated A-fibers. The nucleus of the solitary tract connects to other brain stem nuclei and has widespread projections to numerous areas in the forebrain, including important areas for epilepsy such as the amygdala and the thalamus. The diffuse pathways of the vagus nerve mediate important visceral reflexes such as coughing, vomiting, swallowing, control of blood pressure, and heart rate. Heart rate is mostly influenced by the right vagus nerve that has dense projections primarily to the atria of the heart.24
Since the first human implant of the VNS therapy device in 1989, over 50,000 patients have been treated with VNS worldwide. As for many antiepileptic treatments, clinical application of VNS preceded the elucidation of its mechanism of action. Following a limited number of animal experiments in dogs and monkeys, investigating safety and efficacy, the first human trial was performed.25 The basic hypothesis on the mechanics of action was based on the knowledge that the tenth cranial nerve afferents have numerous projections within the central nervous system and that in this way, action potentials generated in vagal afferents have the potential to affect the entire organism.26 To date the precise mechanism of action of VNS and how it suppresses seizures remains to be elucidated.
Crucial questions with regard to the mechanism of VNS occur at different levels. Vagus nerve stimulation aims at inducing action potentials within the different types of fibers that constitute the nerve at the cervical level. The question remains, what fibers are responsible and/or necessary for its seizure-suppressing effect? Unidirectional stimulation, activating afferent vagal fibers, is preferred, as epilepsy is considered a disease with cortical origin, and efferent stimulation may cause side effects. The next step is to identify central nervous system structures located on the anatomical pathways from the cervical part of the vagus nerve up to the cortex that play a functional role in the mechanism of action (MOA) of VNS. These could be central gateway or pacemaker function structures such as the thalamus or more specific targets involved in the pathophysiology of epilepsy, such as the limbic system, or a combination of both. Another issue concerns the identification of the potential involvement of specific neurotransmitters. The intracranial effect of VNS may be based on local or regional GABA increases or glutamate and aspartate decreases or may involve other neurotransmitters that have been shown in the past to have a seizure threshold regulating role such as serotonin and norepinephrine.27
From the extensive body of research on the mechanism of action, it has become conceivable that effective stimulation in humans is primarily mediated by afferent vagal A- and B-fibers.28,29 Unilateral stimulation influences both cerebral hemispheres, as shown in several functional imaging studies.30,31 Crucial brainstem and intracranial structures have been identified and include the locus coeruleus, the nucleus of the solitary tract, the thalamus, and limbic structures.32–34 Neurotransmitters playing a role may involve the major inhibitory neurotransmitter GABA but also serotoninergic and adrenergic systems.35,36 An extensive overview on the mechanism of action of VNS can be found in Vonck et al.37
CLINICAL EFFICACY
The first descriptions of the implantable VNS Therapy System for electrical stimulation of the vagus nerve in humans appeared in the literature in the early 1990s.38,39 At the same time, initial results from two single-blinded pilot clinical trials (phase-1 trials EO1 and EO2) in a small group of patients with refractory complex partial seizures, who were implanted since November 1988 in three epilepsy centers in the United States, were reported.25,40–42 In nine of 14 patients, treated for 3 to 22 months, a reduction in seizure frequency of at least 50% was observed.39 One of the patients was seizure free for more than 7 months. Complex partial seizures, simple partial seizures, as well as secondary generalized seizures were affected.40 It was noticed that a reduction in frequency, duration, and intensity of seizures lagged 4 to 8 weeks after the initiation of treatment.25
In 1993, Uthman et al. reported on the long-term results from the EO1 and EO2 study.43 Fourteen patients had now been treated for 14 to 35 months. There was a mean reduction in seizure frequency of 46%. Five patients had a seizure reduction of at least 50%, of whom two experienced long-term seizure freedom. In none of the patients did VNS induce seizure exacerbation.
In the meantime, two prospective multicenter (n = 17) double-blind randomized studies (EO3 and EO5) were started including patients from centers in the United States (n = 12), Canada (n = 1), as well as in Europe (n = 4).44–48 In these two studies, patients over the age of 12 with partial seizures were randomized to a HIGH or LOW stimulation paradigm. The parameters in the HIGH stimulation group (output: gradual increase up to 3.5 mA, 30 Hz, 500 μs, 30 s on, 5 min off) were those believed to be efficacious based on animal data and the initial human pilot studies. Because patients can sense stimulation, the LOW stimulation parameters (output: single increase to point of patient perception, no further increase, 1 Hz, 130 μs, 30 s on, 3 hours off) were chosen to provide some sensation to the patient to protect the blinding of the study. LOW stimulation parameters were believed to be less efficacious, and the patients in this group represented an active control group. The results of EO3 in 113 patients were promising with a decrease in seizures of 24% in the HIGH stimulation group versus 6% in the LOW stimulation group after 3 months of treatment.45–47 The number of patients was insufficient to achieve Food and Drug Administration (FDA) approval leading to the EO5 study in the United States including 198 patients. Ninety-four patients in the HIGH stimulation group had a 28% decrease in seizure frequency versus 15% in patients in the LOW stimulation group.48
The controlled EO3 and EO5 studies had their primary efficacy endpoint after 12 weeks of VNS. Patients who ended the controlled trials were offered enrollment in a long-term (1 to 3 years of follow-up [FU]) prospective efficacy and safety study. Patients belonging to the LOW stimulation groups were crossed-over to HIGH stimulation parameters. In all published reports on these long-term results increased efficacy with longer treatment was found.49–53 In these open extension trials, the mean reduction in seizure frequency increased up to 35% at 1 year and up to 44% at 2 years of FU. After that improved seizure control reached a plateau.52
In the following years, other large prospective clinical trials were conducted in different epilepsy centers worldwide. In Sweden, long-term follow-up FU in the largest patient series (n = 67) in one center not belonging to the sponsored clinical trials at that time reported similar efficacy rates with a mean decrease in seizure frequency of 44% in patients treated up to 5 years.54 A joint study of two epilepsy centers in Belgium and the United States included 118 patients with a minimum FU duration of 6 months. They found a mean reduction in monthly seizure frequency of 55%.55 Only in a minority of patients (7%) long-term seizure freedom was achieved. In China a mean seizure reduction of 40% was found in 13 patients after 18 months of VNS.56 From a clinical point of view, prospective randomized trials investigating long-term efficacy in comparison to other therapeutic options for patients with refractory epilepsy are still lacking. An ongoing multicenter randomized trial called PulSE is currently recruiting patients worldwide and may shed light on the exact position of VNS. On the basis of currently available data the responder rate in patients treated with VNS is not substantially higher compared to recently marketed antiepileptic drugs.
Children
There are no controlled studies of VNS in children, but many epilepsy centers have reported safety and efficacy results in patients less than 18 years of age in a prospective way. All these studies report similar efficacy and safety profiles compared to findings in adults.57–60 Rare adverse events, unique to this age group, included drooling and increased hyperactivity.61 In children with epileptic encephalopathies, efficacy may become evident only after >12 months of treatment.62 A recent Korean multicenter study evaluated long-term efficacy in 28 children with intractable epilepsy. In half of the children there was a >50% seizure reduction after a FU of at least 12 months.63 In our own prospective analysis of 118 patients, 13 children with a mean age of 12 years (range: 4 to 17 years) were included with similar efficacy rates and without specific side effects.55
Elderly
A study of Sirven et al. included 45 patients who were 50 years of age and older. Thirty-one of 45 patients had a FU of 1 year, with a reported responder rate of 68%, good tolerance, and improvement of quality-of-life scores.64
Seizure Type and Syndrome
The open-label longitudinal multicenter EO4 study also included patients with generalized epilepsy (n = 24).65,66 In these patients, overall seizure frequency reduction was 46%. Generalized tonic seizures responded significantly better compared with generalized tonic-clonic seizures. Quintana et al.,67 Michael and Devinsky68 and Kostov et al.69 described in a retrospective way that primary generalized seizures and generalized epilepsy syndromes responded equally well to VNS, compared with partial epilepsy syndromes. A prospective study of Holmes et al. in 16 patients with generalized epilepsy syndromes and stable antiepileptic drug (AED) regimens showed an overall mean seizure frequency reduction of 43% after a FU of at least 12 months.70 Ben-Menachem et al. included nine patients with generalized seizures in a prospective long-term FU study. Especially important, the patients with absence epilepsy had a significant seizure reduction.54
A few studies are available specifically describing the use of VNS in patients diagnosed with Lennox-Gastaut syndrome (LGS). One prospective study in 16 patients with Lennox-Gastaut (FU = 6 months) found that one-quarter of patients had a >50% reduction in seizure frequency, which is comparable to the response rates in the controlled studies that included a few patients with LGS.71 Other prospective studies reported higher responder rates with a >50% seizure frequency reduction in half of the patients (n = 13, FU = 6 months),72 in six of seven patients (FU = 6 months)73 and in seven of nine patients (FU = 1 to 35 months).74 A retrospective multicenter study in 46 patients with LGS reported responder rates of 43%.75
There have been many reports on various other seizure types and syndromes, such as seizures in patients with hypothalamic hamartomas,76 tuberous sclerosis,77,78 progressive myoclonic epilepsy,79,80 Landau-Kleffner syndrome,81 Asperger syndrome,82 epileptic encefalopathies,76 and syndromes with developmental disability and mental retardation.83–86 All these studies reported good efficacy with regard to controlling seizures as well as other disease-related symptoms, such as cerebellar dysfunction, behavioral disturbances, and mood disturbances. One study in children with infantile spasms reported less favorable results with long-term benefit in only two to ten patients and with four patients who interrupted VNS due to behavioral problems.87 A recent report on the efficacy of VNS in five children with mitochondrial electron transport chain deficiencies described no significant seizure reduction in any of the children.88 Also, a study in patients with previous resective epilepsy surgery showed a limited seizure-suppressing effect of VNS,89 although another report described improved seizure control in this specific patient group.90
SAFETY, SIDE EFFECTS, AND TOLERABILITY
The most prominent and consistent sensation in patients when the vagus nerve is stimulated for the first time, even at low output current levels, is a tingling sensation in the throat and hoarseness of the voice. The tingling sensation may be due to secondary stimulation of the superior laryngeal nerve that branches off from the vagus nerve superior to the location of the implanted electrode but travels along the vagus nerve in the carotid sheath.91 The superior laryngeal nerve carries sensory fibers to the laryngeal mucosa. Stimulation of the recurrent laryngeal nerve that branches off distally from the location of the electrode and carries motor (Aα) fibers to the laryngeal muscles causes the stimulation-related hoarseness.92,93
With regard to side effects related to stimulation of vagal efferents, effect on heart rate and gastrointestinal digestion are of major concern. Stimulation of the efferent fibers may induce bradycardia and hypersecretion of gastric acid. The stimulation electrode is always implanted on the left vagus nerve, which is believed to contain fewer sinoatrial fibers than the right. It has been suggested that the electrode be implanted below the superior cardiac branch of the vagus nerve. In the initial pilot trials and controlled randomized trials, extensive internal investigations were performed, including continued monitoring in the long-term extension phases. With regard to potential central nervous system side effects related to stimulation of vagal afferents and their connections in the brainstem and cerebral hemispheres, some studies were performed to evaluate changes in EEG, sleep stages, balance, and cognition. In most studies, systematic AED plasma monitoring was performed. No systematic side effects on heart functioning or other internal organ or cerebral functions were found. There was no effect on AED serum levels.25,43 Side effects are almost always related to the stimulation on-time and consist of hoarseness and tingling sensation and coughing.
In the long-term extension trials, the most frequent side effects were hoarseness in 19% of patients and coughing in 5% of patients at 2-year follow-up and shortness of breath in 3% of patients at 3 years.52 There was a clear trend toward diminishing side effects over the 3-year stimulation period. Ninety-eight percent of the symptoms were rated mild or moderate by the patients and the investigators.94 Side effects can usually be resolved by decreasing stimulation parameters. Central nervous system side effects typically seen with AEDs were not reported. After 3 years of treatment, 72% of the patients were still on the treatment.52 The most frequent reason for discontinuation was lack of efficacy.
Initial studies on small patient groups treated for 6 months with VNS showed no negative effect on cognitive motor performance and balance.95–97 These findings were confirmed in larger patient groups with a FU of 2 years.98,99 Hoppe et al. showed no changes in extensive neuropsychological testing in 36 patients treated for 6 months with VNS.100
Cardiac Side Effects
Despite the fact that the initial studies showed no clinical effect on heart rate, occurrence of bradycardia and ventricular asystole during intraoperative testing of the device (stimulation parameters: 1 mA, 20 Hz, 500 μs, ∼17 sec) have been reported in a few patients. None of the reported patients had a history of cardiac dysfunction, nor did they show abnormal cardiac testing after surgery. Tatum et al. reported on four patients who experienced ventricular asystole intraoperatively during device testing.101 In three patients, the implantation procedure was aborted. In one patient a rechallenge of stimulation with incremental increases from 0.25 to 1 mA did not reveal a reappearance of bradycardia. Implantation was completed, and no further cardiac events were noticed after start of VNS. Asconape et al. reported on a single patient who developed asystole during intraoperative device testing. After removal of the device, the patient recovered completely.102 Ali et al. described three similar cases. Cardiac rhythm strips were available and showed a regular p-wave (atrial rhythm) with no ventricular activity indicating a complete AV nodal block. In two of these patients, the device was subsequently removed. In one patient, the device was left in place without any other adverse events after start of VNS.103 Andriola et al. reported on three patients who experienced an asystole during intraoperative lead testing and who were subsequently chronically stimulated.104 Ardesch et al. reported on three patients with intraoperative bradycardia and subsequent uneventful stimulation.105 Possible hypotheses with regard to the underlying cause are inadvertent placement of the electrode on one of the cervical branches of the vagus nerve or indirect stimulation of these branches; reversal of the polarities of the electrodes, which would lead to primary stimulation of efferents instead of afferents; indirect stimulation of cardiac branches; activation of afferent pathways affecting higher autonomic systems or of the parasympathetic pathway with an exaggerated effect on the atrioventricular node; technical malfunctioning of the device; or idiosyncratic reactions. The contributing role of specific AEDs should be further investigated. Suggested steps to be taken in the operating room in case of bradycardia or asystole during generator and lead impedance testing have been formulated by Asconape et al.102 Adverse cardiac complications at start or during ramping up of the stimulation intensity have not been observed.46 Very recently, one case report described a late onset bradyarrhythmia after 2 years of vagus nerve stimulation.106
Magnetic Resonance Imaging
Most patients with refractory epilepsy who are treated with VNS have previously undergone magnetic resonance imaging (MRI) during the presurgical evaluation. It is not uncommon for such patients to require MRI after VNS implantation to further monitor underlying neurological diseases (e.g., in case of unexplained increase in seizure frequency, FU of intracranial lesions, and also for MRI indications as in the general population). Based on laboratory testing using a phantom to simulate a human body, the VNS Therapy System device is labeled MRI compatible when used with a send-and-receive head coil.107 In addition to the safety issues, there was no significant image distortion.108 A retrospective analysis of 27 MRI scans performed in 25 patients at 12 different centers was performed to confirm the findings from the experimental setup in a clinical series. All patients were scanned on a 1.5 Tesla machine. On one occasion, a body coil was used. Three scans were performed with the stimulator in the on mode. One patient reported a mild voice change for several minutes; one child reported chest pain and claustrophobia. Twenty-three patients reported no discomfort around the lead or the generator. It was concluded that MRI is safe as long as guidelines stated in the physician’s manual of the implanted device are followed. In these guidelines, it is suggested to program the pulse generator output current to 0 mA. On one occasion, this has led to the occurrence of a generalized status epilepticus in a patient who was well controlled with an output current of 2 mA. The authors of the report recommend that intravenous access should be obtained and a benzodiazepine should be either available or preadministered in patients with a well-defined response who are undergoing elective MRI and in whom the generator is acutely programmed to 0 mA. Functional MRI (fMRI) is a recently developed technique that allows noninvasive evaluation of cerebral functions such as finger movements and language.109 It has been widely used for research but is currently increasingly applied to evaluate functional tissue in the neighborhood of lesions before resective surgery and also for assessing language dominance in the presurgical evaluation of epilepsy patients.110 When fMRI in patients with VNS is used for research purposes to evaluate VNS-induced changes in cerebral blood flow, scanning should be performed in the on mode. To prevent the device from being turned off during scanning, an adjustment in the surgical positioning of the device is necessary. The device should be positioned so that the electrode pins that are plugged into the generator are parallel instead of perpendicular to the long axis of the body.111 There have been several studies in patients treated with VNS for epilepsy as well as for depression showing that fMRI is safe and feasible.112–115 These studies were performed to elucidate the mechanism of action of VNS and will be discussed later in this study. The use of body coils may be indicated in patients requiring spinal MRI. When removal of electrodes is indicated (e.g., due to insufficient efficacy), complete removal is recommended over cutting the distal edges and leaving the electrode in place.116 Full removal of the electrodes allows potential future MRI with body coils. Heating of the electrodes is related to the lead length. If full removal of the electrodes is difficult, the leads should be cut to less than 10 cm.
Deep Brain Stimulation
Deep brain stimulation (DBS) is a more recently explored field in epilepsy. Compared to VNS, it is a more invasive treatment option. Parallel to VNS, the precise mechanism of action and the ideal candidates for this treatment option are currently unidentified. Moreover, it is unknown which intracerebral structures should be targeted to achieve optimal clinical efficacy. Two major strategies for structure targeting have been followed. One approach is to target crucial central nervous systems structures that are considered to have a pacemaker, triggering, or gating role in the epileptogenic networks that have been identified, such as the thalamus or the subthalamic nucleus.27 Another approach is to interfere with the ictal onset zone itself. This implies the identification of the ictal onset zone, a process that sometimes requires implantation with intracranial electrodes.
TARGETS
The earliest reports on intracranial neurostimulation involved stimulation of cerebellar structures. In most instances, electrical current was administered through electrodes bilaterally placed on the superior medial cerebellar cortex.117 Intermittent (1 to 8 min on, 1 to 8 min off) high-frequency (150 to 200 Hz) cerebellar stimulation was initially investigated for the treatment of spasticity due to cerebral palsy or stroke in several hundreds of patients with implantation duration times of up to 20 years. Some of these patients also had refractory seizures that were completely abolished in 60% of patients. Two controlled studies in small patient groups (n = 5, n = 12) did, however, not show significant effects.11,118 In view of this controversy and with the advent of fully implantable and programmable pulse generators, Velasco et al. performed a reevaluation double-blind study in five patients showing significant decreases in tonic-clonic seizures after 1 to 2 months of stimulation.119
The selection of other targets for DBS in humans partially resulted from the progress in the identification of epileptogenic networks.27 Although the cortex plays an essential role in seizure origin, increasing evidence shows that subcortical structures may be involved in the clinical expression, propagation, control, and sometimes initiation of seizures. Consequently, several subcortical nuclei have been targeted in pilot trials for different types of epilepsy. The suppressive effects of pharmacological or electrical inhibition of the subthalamic nucleus (STN) in different animal models for epilepsy and the extensive experience with STN DBS in patients with movement disorders led to a pilot trial with high-frequency (130 Hz) continuous STN DBS in five patients by the group from Grenoble.17,120 Three patients with symptomatic partial seizures had a >60% reduction in seizure frequency. Four other centers have reported STN DBS results. In one patient with LGS, generalized seizures were fully suppressed and myoclonic and absence seizures reduced by >75%.121 Loddenkemper et al. reported seizure frequency reductions of more than 60% in two of five patients treated with STN DBS.122 Handforth et al. reported on one patient with bitemporal seizures in whom half the seizures were suppressed and in on one patient with frontal lobe epilepsy who experienced a one-third reduction of seizures.123 Vesper et al. described a 50% reduction in myoclonic seizures in a patient with progressive myoclonic epilepsy in whom generalized seizures had been successfully treated with previous VNS.124
Thalamocortical interactions are known to play an important role in several types of seizures. Since 1984, Velasco et al. have investigated a large patient series (n = 57) with different seizure types who underwent DBS of the centromedian (CM) nucleus, a structure that can be stereotactically targeted fairly easily due to its relatively large size, its spherical shape, and its location on each side of the third ventricle.125,126 Intermittent (1 min on, 4 min off) high-frequency (60 to 130 Hz) stimulation that alternated between the left and right CM thalamic nucleus was most effective in children (n = 5) with epilepsia partialis continua in whom full seizure control was reached between 3 and 4 months after stimulation. Secondary generalized seizures in these children were the earliest to respond after 1 month of treatment. Atypical absences and generalized seizures (primary or secondary) responded significantly. Three of 22 patients with Lennox-Gastaut syndrome became seizure free. Complex partial seizures responded less successfully, although after long-term stimulation over 1 year, partial improvements were found, and patients tended to be satisfied with the treatment that significantly decreased or abolished secondary generalized convulsions. In a separate report, Velasco et al. reported on 11 patients with LGS with an overall seizure reduction of 80% and two patients rendered seizure free.127
In a double-blind cross-over protocol performed by Fisher et al., CM thalamic stimulation did not significantly improve generalized seizures in seven patients.14 Bilateral intermittent (1 min on, 5 min off during 2 h/d) high-frequency (60 Hz) stimulation was performed in blocks of 3 months alternating between on and off stimulation in a double-blinded manner. A reduction of 30% of tonic-clonic seizures had been observed during blocks with the stimulation on versus 8% in blocks with stimulation off. An open extension phase of the trial using 24-hour stimulation resulted in a 50% decrease in three of six of the patients. It has become clear, especially from the experience with VNS, but also from other studies, that increased efficacy may be observed after longer duration of stimulation, possibly on the basis of neuromodulatory changes that take time to develop.126,128
There is sufficient evidence to suggest an equally important role of the anterior nucleus (AN) of the thalamus in the pathogenesis of seizure generalization. Hodaie et al. performed bilateral AN thalamic DBS (1 min on, 5 min off, 100 Hz, alternating between right and left AN) in five patients and showed a seizure-frequency reduction between 24% and 89%.18 Andrade et al. reported on the long-term follow-up of six patients with AN DBS. After 7 years of FU, five patients showed a more than 50% reduction in seizure frequency.129 Changes in stimulation parameters over the years did not further improve seizure control. Kerrigan et al. reported that four of five patients who underwent high-frequency AN DBS showed significant decreases in seizure severity and in the frequency of secondarily generalized seizures. Moreover, there was an immediate seizure recurrence when DBS was stopped.130
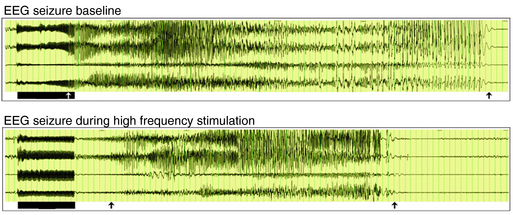
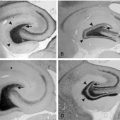
The choice of targeting the medial temporal lobe region for a pilot trial in humans at Ghent University Hospital was based on several considerations. This region often shows specific initial electroencephalographic epileptiform discharges as a reflection of seizure onset in human temporal lobe epilepsy. These findings have been recorded with implanted depth electrodes in patients with refractory epilepsy in whom the ictal onset zone could not be identified on the basis of noninvasive evaluations. Subsequent to the localization of the ictal onset zone, patients may undergo resective surgery to treat seizures. Temporal lobectomy and more specifically selective amygdalohippocampectomy are effective in reducing seizures with a well-defined mesiobasal limbic seizure onset.131 Basic research involving evoked potential excitability studies in humans and anatomical studies with tracer injections and single-unit recordings with histological studies in animals have also confirmed the involvement of the amygdala and the hippocampus in the epileptogenic network.132–134 Some studies have applied electrical fields to in vitro hippocampal slices with positive effects on epileptic activity.135,136 Also in vivo studies in rats have shown that high-frequency stimulation affects seizures in the kindling model (Figure 21-2).137 Bragin et al. described repeated stimulation of the hippocampal perforant path in rats showing spontaneous seizures 4 to 8 months after intrahippocampal kainate injection.138 During perforant path stimulation, spontaneous seizures were significantly reduced. In humans, preliminary short-term stimulation of hippocampal structures showed promising results on interictal epileptiform activity and seizure frequency.139 Not all patients with temporal lobe epilepsy who underwent resective epilepsy surgery remain seizure free in the long term. Moreover, temporal lobe resection, especially left-sided, may be associated with memory decline, and temporal lobe resection is contraindicated in patients with bilateral ictal onset. In an initial pilot trial at Ghent University Hospital, ten patients scheduled for invasive video-EEG monitoring of the medial temporal lobe were offered high-frequency medial temporal lobe DBS following ictal onset localization.19 Long-term follow-up in 10 of these patients showed that one in ten stimulated patients was seizure free (> 1 year), one in ten patients had a > 90% reduction in seizure frequency; five in ten patients had a seizure frequency reduction of ≥ 50%, two in ten patients had a seizure frequency reduction of 30% to 49%, and one in ten patients is a nonresponder. None of the patients reported side effects. In one patient, MRI showed asymptomatic intracranial hemorrhages along the trajectory of the DBS electrodes. None of the patients showed changes in clinical neurological testing. In the meantime, a controlled randomized multicenter study has been set up at Ghent University Hospital. In the CoRaStiR study (Controlled Randomized Stimulation versus Resection) patients with unilateral hippocampal sclerosis and TLE will be randomized to amygdalohippocampal resection or medial temporal lobe DBS.
Two other groups have reported on long-term hippocampal stimulation. In four patients with complex partial seizures based on left-sided hippocampal sclerosis, high-frequency stimulation was performed in a randomized, double-blind protocol with periods of 1 month off or on by Tellez-Zenteno et al. During the stimulation on periods, seizures decreased by 26% as compared to baseline.140 During the off periods, seizures increased by 49%. Neuropsychological testing revealed no difference between on or off periods, not even in one patient who was stimulated left-sided following previous right-sided temporal lobectomy.
Velasco et al. reported results in 11 patients after 18 months of hippocampal high-frequency stimulation (uni- or bilateral, with or without hippocampal sclerosis on MRI).141 Patients with normal MRIs showed optimal outcome, with four of them seizure free after 1 to 2 months of stimulation. None of the patients showed neuropsychological decline with a trend toward improvement.
An implanted responsive neurostimulator (the RNS system) is being evaluated for safety and efficacy in a multicenter trial. The device records cortical EEG signals by means of subdural electrodes and delivers responsive stimulation. Chabolla et al. reported on 18 adults with uni- or bilateral temporal lobe epilepsy who were treated with the RNS system and showed a 43% and 53% reduction in seizure frequency, respectively.20
Conclusion
For all types of neurostimulation currently being investigated, major issues remain unresolved. The ideal targets and stimulation parameters for a specific type of patient, seizure, or epilepsy syndrome are unknown. The characterization of the full and long-term side effects profile needs to be further investigated. The elucidation of the mechanism of action of different neurostimulation techniques requires more basic research to demonstrate its potential to achieve long-term changes and true neuromodulation.
1. Ben-Menachem E. Vagus-nerve stimulation for the treatment of epilepsy. Lancet Neurol. 2002;1:477-482.
2. DeGiorgio CM, Shewmon A, Murray D, Whitehurst T. Pilot study of trigeminal nerve stimulation (TNS) for epilepsy: a proof of concept trial. Epilepsia. 2006;47(7):1213-1215.
3. Sparing R, Mottaghy FM. Noninvasive brain stimulation with transcranial magnetic or direct current stimulation—from insights into human memory to therapy of its dysfunction. Methods. 2008;44:329-337.
4. Tassinari CA, Cincotta M, Zaccara G, Michelucci R. Transcranial magnetic stimulation and epilepsy. Clin Neurophysiol. 2003;114(5):777-798.
5. Fregni F. A randomized clinical trial of repetitive transcranial magnetic stimulation in patients with refractory epilepsy. Ann Neurol. 2006;60:447-455.
6. Pollak P, Fraix V, Krack P, et al. Treatment results: Parkinson’s disease. Mov Dis. 2002;17(S3):S75-S83.
7. Ngyuen JP, Lefaucher JP, Le Guerinel C, et al. Motor cortex stimulation in treatment of central and neuropathic pain. Arch Med Res. 2000;31(3):263-265.
8. Nuttin B, Cosyns P, Demeulemeester H, Gybels J, Meyerson B. Electrical stimulation in anterior limbs of internal capsules in patients with obsessive-compulsive disorder. Lancet. 1999;30(354):1526.
9. Leone M, Franzini A, Broggi G, Bussone G. Hypothalamic deep brain stimulation for intractable chronic cluster headache: a 3-year follow-up. Neurol Sci. 2003;24(2):143-145.
10. Cooper IS. Cerebellar Stimulation in Man. New York: Raven Press Books, 1978.
11. Wright GD, McLellan DL, Brice JG. A double-blind trial of chronic cerebellar stimulation in twelve patients with severe epilepsy. J Neurol Neurosurg Psychiatry. 1984;47:769-774.
12. Upton AR, Cooper IS, Springman M, Amin I. Suppression of seizures and psychosis of limbic system origin by chronic stimulation of anterior nucleus of the thalamus. Int J Neurol. 1985;19:223-230.
13. Feinstein B, Gleason CA, Libet B. Stimulation of locus coeruleus in man. Preliminary trials for spasticity and epilepsy. Stereot Funct Neurosurg. 1989;43:906.
14. Fisher RS, Uematsu S, Krauss GL, et al. Placebo-controlled pilot study of centromedian thalamic stimulation in treatment of intractable seizures. Epilepsia. 1992;33:841-851.
15. Velasco F, Velasco M, Velasco AL, Jimenez F, Marquez I, Rise M. Electrical stimulation of the centromedian thalamic nucleus in control of seizures: long-term studies. Epilepsia. 1995;36(1):63-71.
16. Chkhenkeli SA, Chkhenkeli IS. Effects of therapeutic stimulation of nucleus caudatus on epileptic electrical activity of brain in patients with intractable epilepsy. Stereot Funct Neurosurg. 1997;69:221-224.
17. Chabardes S, Kahane P, Minotti L, Koudsie A, Hirsch E, Benabid AL. Deep brain stimulation in epilepsy with particular reference to the subthalamic nucleus. Epil Disord. 2002;4(S3):83-93.
18. Hodaie M, Wennberg R, Dostrovsky JO, Lozano A. Chronic anterior thalamus stimulation for intractable epilepsy. Epilepsia. 2002;43(6):603-608.
19. Boon P, Vonck K, De Herdt V, et al. Deep brain stimulation in patients with refractory temporal lobe epilepsy. Epilepsia. 2007;48:1551-1560.
20. Morrell M. Brain stimulation for epilepsy: can scheduled or responsive neurostimulation stop seizures? Curr Opin Neurol. 2006;19:164-168.
21. Paintal AS. Vagal sensory receptors and their reflex effects. Physiol Rev. 1997;53:159-227.
22. Foley J, DuBois F. Quantitative studies of the vagus nerve in the cat. I. The ratio of sensory and motor fibers. J Compr Neurol. 1937;67:49-97.
23. Agostini E, Chinnock JE, Daly MD, Murray JG. Functional and histological studies of the vagus nerve and its branches to the heart, lungs and abdominal viscera in the cat. J Physiol. 1957;135:182-205.
24. Saper C, Kibbe M, Hurley K, et al. Brain natriuretic peptide-like immunoreactive innervation of the cardiovascular and cerebrovascular systems in the rat. Circul Res. 1990;67:1345-1354.
25. Uthman BM, Wilder BJ, Hammond EJ, Reid S. Efficacy and safety of vagus nerve stimulation in patients with complex partial seizures. Epilepsia. 1990;31:S44-S50.
26. Berthoud H, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci. 2000;85:1-17.
27. Proctor M, Gale K. Basal ganglia and brain stem anatomy and physiology. In: Engel J, Pedley TA, editors. Epilepsy, the Comprehensive CD-ROM. Philadelphia: Lippincott Williams and Wilkins, 1999.
28. Zagon A, Kemeny A. Slow hyperpolarization in cortical neurons: a possible mechanism behind vagus nerve stimulation therapy for refractory epilepsy? Epilepsia. 2000;41:1382-1389.
29. Evans MS, Verma-Ahuja S, Naritoku DK, Espinosa JA. Intraoperative human vagus nerve compound action potentials. Acta Neurol Scand. 2004;110:232-238.
30. Henry TR, Bakay RAE, Votaw JR, et al. Brain blood flow alterations induced by therapeutic vagus nerve stimulation in partial epilepsy: I. acute effects at high and low levels of stimulation. Epilepsia. 1998;39:983-990.
31. Van Laere K, Vonck K, Boon P, Versijpt J, Dierckx R. Perfusion SPECT changes after acute and chronic vagus nerve stimulation in relation to prestimulus condition and long-term clinical trial. J Nucl Med. 2002;43:733-744.
32. Naritoku D, Terry WJ, Helfert RH. Regional induction of fos immunoreactivity in the brain by anticonvulsant stimulation of the vagus nerve. Epil Res. 1995;22:53-62.
33. Krahl SE, Clark KB, Smith DC, Browning RA. Locus coeruleus lesions suppress the seizure-attenuating effects of vagus nerve stimulation. Epilepsia. 1998;39(7):709-714.
34. Osharina V, Bagaev V, Wallois F, Larnicol N. Autonomic response and fos expression in the NTS following intermittent vagal stimulation: importance of pulse frequency. Auton Neurosci. 2006;126:72-80.
35. Hammond EJ, Uthman BM, Wilder BJ, Ben-Menachem E, Hamberger A, Hedner T, Ekman R. Neurochemical effects of vagus nerve stimulation in humans. Brain Res. 1952;583:300-303.
36. Ben-Menachem E, Hamberger A, Hedner T, et al. Effects of vagus nerve stimulation on amino acids and other metabolites in the CSF of patients with partial seizures. Epi Res. 1995;20:221-227.
37. Vonck K, Boon P, Van Roost D. Anatomical and physiological basis and mechanism of action of neurostimulation for epilepsy. Acta Neurochir Suppl. 2007;97(2):321-328.
38. Terry R, Tarver WB, Zabara J. An implantable neurocybernetic prosthesis system. Epilepsia. 1990;31:S33-S37.
39. Terry RS, Tarver WB, Zabara J. The implantable neurocybernetic prosthesis system. Pac Clin Electrophysiol. 1991;14:86-93.
40. Penry KJ, Dean C. Prevention of intractable partial seizures by intermittent vagal stimulation in humans: preliminary results. Epilepsia. 1990;31:S40-S43.
41. Hammond EJ, Uthman BM, Reid SA, Wilder BJ, Ramsay RE. Vagus nerve stimulation in humans: neurophysiological studies and electrophysiological monitoring. Epilepsia. 1990;31:S51-S59.
42. Wilder BJ, Uthman BM, Hammond EJ. Vagal stimulation for control of complex partial seizures in medically refractory epileptic patients. Pac Clin Electrophysiol. 1991;14:108-115.
43. Uthman B, Wilder BJ, Penry JK, Dean C, Ramsay RE, Reis SA, Hammond EJ, Tarver WB, Wernicke JF. Treatment of epilepsy by stimulation of the vagus nerve. Neurol. 1993;43:1338-1345.
44. Holder LK, Wernicke JF, Tarver WB. Treatment of refractory partial seizures: preliminary results of a controlled study. Pac Clin Electrophyisol. 1992;15(10):1557-1571.
45. Ben-Menachem E, Manon-Espaillat R, Ristanovic R, et al. Vagus nerve stimulation for treatment of partial seizures: 1. A controlled study of effect on seizures. Epilepsia. 1994;35(3):616-626.
46. Ramsay RE, Uthman BM, Augustinsson LE, et al. Vagus nerve stimulation for treatment of partial seizures 2. Safety, side-effects and tolerability. Epilepsia. 1994;35:627-636.
47. The Vagus Nerve Stimulation Study Group. A randomized controlled trial of chronic vagus nerve stimulation for treatment of medically intractable seizures. Neurol. 1995;45:224-230.
48. Handforth A, DeGiorgio CM, Schachter SC, et al. Vagus nerve stimulation therapy for partial onset seizures. A randomized, active control trial. Neurol. 1998;51:48-55.
49. Holder L, Wernicke JF, Tarver WB. Long-term follow-up of 37 patients with refractory partial seizures treated with vagus nerve stimulation. J Epilepsy. 1993;6:206-214.
50. George R, Salinsky M, Kuzniecky R, et al. Vagus nerve stimulation for treatment of partial seizures: 3. Long-term follow-up on first 67 patients exiting a controlled study. Epilepsia. 1994;35(3):637-643.
51. Salinsky MC, Uthman BM, Ristanovic RK, Wernicke JF, Tarver WB. Vagus nerve stimulation for the treatment of medically intractable seizures. Results of a 1-year open-extension trial. Arch Neurol. 1996;53:1176-1180.
52. Morris GL, Mueller WM. Long-term treatment with vagus nerve stimulation in patients with refractory epilepsy. Neurol. 1999;53:1731-1735.
53. DeGiorgio CM, Schachter SC, Handforth A, et al. Prospective long-term study of vagus nerve stimulation for the treatment of refractory seizures. Epilepsia. 2000;41(9):1195-1200.
54. Ben-Menachem E, Hellstrom K, Waldton C, Augustinsson LE. Evaluation of refractory epilepsy treated with vagus nerve stimulation for up to 5 years. Neurol. 1999;52:1265-1267.
55. Vonck K, Thadani V, Gilbert K, et al. Vagus nerve stimulation for refractory epilepsy: a transatlantic experience. J Clin Neurophysiol. 2004;21:283-289.
56. Hui Che Fai A, Lam Man Kuen J, Ka Shing W, Kay R, Wai Sing P. Vagus nerve stimulation for refractory epilepsy: long-term efficacy and side-effects. Chin Med J. 2004;117(1):58-61.
57. Lundgren J, Amark P, Blennow G, Stromblad LG, Wallstedt L. Vagus nerve stimulation in 16 children with refractory epilepsy. Epilepsia. 1998;39:809-813.
58. Murphy JV, the pediatric VNS Study Group. Left vagal nerve stimulation in children with medically refractory epilepsy. J Ped. 1999;134:563-566.
59. Zamponi N, Rychicki F, Cardinali C, Luchetti A, Trignani R, Ducati A. Intermittent vagal nerve stimulation in paediatric patients: 1 year follow-up. Child Nerv Syst. 2002;18:61-66.
60. Buoni S, Mariottini A, Pieri S, et al. Vagus nerve stimulation for drug-resistant epilepsy in children and young adults. Brain Dev. 2004;26:158-163.
61. Helmers SL, Wheless JW, Frost M, et al. Vagus nerve stimulation therapy in pediatric patients with refractory epilepsy: retrospective study. J Child Neurol. 2001;16:843-848.
62. Parker APJ, Polkey CE, Binnie C, Madigan C, Ferrie C, Robinson O. Vagal nerve stimulation in epileptic encephalopathies. Ped. 1999;103:778-782.
63. You Si, Kang HC, Kim HD, et al. Vagus nerve stimulation in intractable childhood epilepsy: a Korean multicenter experience. J Korean Med Sci. 2007;22:442-445.
64. Sirven JI, Sperling M, Naritoku D, et al. Vagus nerve stimulation therapy for epilepsy in older adults. Neurol. 2000;54:1179-1182.
65. Salinsky M. Results from an open label safety study: the EO4 experience. Proceedings of the 1997 VNS Investigator Meeting and Symposium, Colorado Springs, Colorado. 33-34.
66. Labar D, Murphy J, Tecoma E. Vagus nerve stimulation for medication-resistant generalized epilepsy. Neurol. 1999;52:1510-1512.
67. Quintana C, Tecoma ES, Iragui VJ. Evidence that refractory partial onset and generalized epilepsy syndromes respond comparably to adjunctive vagus nerve stimulation therapy. Epilepsia. 2002;43(S7):344-345.
68. Michael NG, Devinsky O. Vagus nerve stimulation for refractory idiopathic generalised epilepsy. Seizure. 2004;13:176-178.
69. Kostov H, Larsson PG, Roste GK. Is vagus nerve stimulation a treatment option for patients with drug-resistant idiopathic generalized epilepsy? Acta Neurol Scan Suppl. 2007;187:55-58.
70. Holmes MD, Silbergeld DL, Drouhard D, Wilensky AJ, Ojemann LM. Effect of vagus nerve stimulation on adults with pharmacoresistant generalized epilepsy syndromes. Seizure. 2004;13:340-345.
71. Majoie HJM, Berfelo MW, Aldenkamp AP, Evers SMAA, Kessels AGH, Renier WO. Vagus nerve stimulation in children with therapy-resistant epilepsy diagnosed as Lennox-Gastaut syndrome. J Clin Neurophysiol. 2001;18:419-428.
72. Hosain S, Nikalov B, Harden C, Li M, Fraser R, Labar D. Vagus nerve stimulation treatment for Lennox-Gastaut syndrome. J Child Neurol. 2000;15:509-512.
73. Helmers S, Al-Jayyousi M, Madsen J. Adjunctive treatment in Lennox-Gastaut syndrome using vagal nerve stimulation. Epilepsia. 1998;39(S6):169.
74. Murphy J, Hornig G. Chronic intermittent stimulation of the left vagal nerve in nine children with Lennox-Gastaut syndrome. Epilepsia. 1998;39(S6):169.
75. Frost M, Gates J, Helmers S, et al. Vagus nerve stimulation in children with refractory seizures associated with Lennox-Gastaut syndrome. Epilepsia. 2001;42:1148-1152.
76. Murphy JV, Wheless JW, Schmoll CM. Left vagal nerve stimulation in six patients with hypothalamic hamartomas. Ped Neurol. 2000;23:167-168.
77. Parain D, Delangre T, Piniello MJ, Freger P. Vagal nerve stimulation in refractory epilepsy with tuberous sclerosis. Epilepsia. 1997;38(S8):109.
78. Parain D, Peniello M, Berquen P, Delangre T, Billard C, Murphy JV. Vagal nerve stimulation in tuberous sclerosis patients. Ped Neurol. 2001;25:213-216.
79. Smith B, Shatz R, Elisevich K, Bespalova IN, Burmeister M. Effects of vagus nerve stimulation on progressive myoclonus epilepsy of Unverricht-Lundborg Type. Epilepsia. 2000;41:1046-1048.
80. Silander HC, Runnerstam M, Ben-Menachem E. Use of vagus nerve stimulation in patients with Baltic myoclonic epilepsy (PME1). Epilepsia. 2000;41(S7):226.
81. Park YD. The effects of vagus nerve stimulation therapy on patients with intractable seizures and Landau-Kleffner syndrome or autism. Epi Behav. 2003;4:286-290.
82. Warwick TC, Griffith J, Reyes B, Legesse B, Evans M. Effects of vagus nerve stimulation in a patient with temporal lobe epilepsy and Asperger syndrome: case report and review of the literature. Epi Beh. 2007;10:344-347.
83. Andriola MR, Vitale SA. Vagus nerve stimulation in the developmentally disabled. Epi Behav. 2007;2:129-134.
84. Gates J, Huf R, Frost M. Vagus nerve stimulation for patients in residential treatment facilities. Epi Behav. 2001;2:563-567.
85. Huf RL, Mamelak A, Kneedy-Cayem K. Vagus nerve stimulation therapy: 2-year prospective open-label study of 40 subjects with refractory epilepsy and low IQ who are living in long-term care facilities. Epi Behav. 2005;6:417-423.
86. Penovich PE, Korby B. Vagus nerve stimulation use in patients with epilepsy and mental retardation. Paper prepared for AES 2002, Seattle, WA.
87. Fohlen M, Jalin C, Pinard JM, Delalande O. Results of vagus nerve stimulation in 10 children with refractory infantile spasms. Epilepsia. 1998;39(S6):170.
88. Arthur TM, Saneto RP, Sotero de Menezes M, et al. Vagus nerve stimulation in children with mitochondrial electron transport chain deficiencies. Mitochondrion. 2007;7:279-283.
89. Koutroumanidis M, Binnie CD, Henessy MJ, et al. VNS in patients with previous unsuccessful resective epilepsy surgery: antiepileptic and psychotropic effects. Acta Neurol Scand. 2003;107:117-121.
90. Frost MD, Hoskin C, Moriarty GL, Penovich PE, Ritter FJ, Gates J. Use of the vagus nerve stimulator in patients who have failed epilepsy surgery. Epilepsia. 1998;39(S6):192.
91. Claes J, Jaco P. The nervus vagus. Acta Oto-Rhino-Laryngol Belg. 1986;40:215-241.
92. Banzett RB, Guz A, Paydarfar D, Shea SA, Schachter SC, Lansing RW. Cardiorespiratory variables and sensation during stimulation of the left vagus in patients with epilepsy. Epi Res. 1999;35:1-11.
93. Charous SJ, Kempster G, Manders E, Ristanovic R. The effect of vagal nerve stimulation on voice. The Laryngoscope. 2001;111:2028-2031.
94. Ben Menachem E. Vagus nerve stimulation, side effects and long-term safety. J Clin Neurophysiol. 2001;18:415-418.
95. Clarke BM, Upton ARM, Griffin HM. Cognitive motor function after electrical stimulation of the vagus nerve. Pac Clin Electrophysiol. 1992;15:1603-1607.
96. Clarke BM, Upton ARM, Kamath M, Griffin HM. Electrostimulation effects of the vagus nerve on balance in epilepsy. Pac Clin Electrophysiol. 1992;15:1614-1630.
97. Clarke BM, Upton ARM, Kamath M, Griffin HM. Acute effects of high frequency vagal nerve stimulation on balance and cognitive motor performance in epilepsy: three case study reports. Pac Clin Electrophyisol. 1992;15:1608-1613.
98. Clarke BM, Upton ARM, Griffin H, Fitzpatric D, DeNardis M. Chronic stimulation of the left vagus nerve in epilepsy: balance effects. Can J Neurol Sci. 1997;24:230-234.
99. Clarke BM, Upton ARM, Griffin H, Fitzpatric D, DeNardis M. Chronic stimulation of the left vagus nerve in epilepsy: cognitive motor effects. Can J Neurol Sci. 1997;24:226-229.
100. Hoppe C, Helmstaedter C, Scherrmann J, Elger CE. No evidence for cognitive side effects after 6 months of vagus nerve stimulation in epilepsy patients. Epi Behav. 2001;2:351-356.
101. Tatum WO, Moore DB, Stecker MM. Ventricular asystole during vagus nerve stimulation for epilepsy in humans. Neurol. 2000;52:1267-1269.
102. Asconape JJ, Moore DD, Zipes DP, Hartman LM, Duffell WH. Bradycardia and asystole with the use of vagus nerve stimulation for the treatment of epilepsy: a rare complication of intraoperative device testing. Epilepsia. 1998;39:998-1000.
103. Ali I, Pirzada N, Kanjwal Y. Complete heart block with ventricular asystole during left vagus nerve stimulation for epilepsy. Epi Behav. 2004;5:768-771.
104. Andriola MR, Rosenweig T, Vlay S, Brook S. Vagus nerve stimulator (VNS): induction of asystole during implantations with subsequent successful stimulation. Epilepsia. 2000;41(s7):223.
105. Ardesch JJ, Buschman HP, van der Burgh PH, Wagener-Schimmel LJ, van der Aa HA, Hageman G. Cardiac responses of vagus nerve stimulation: intraoperative bradycardia and subsequent chronic stimulation. Clin Neurol Neurophysiol. 2007;109:849-852.
106. Amark P. Stodberg T, Wallstedt L. Late onset bradyarrhythmia during vagus nerve stimulation. Epilepsia. 2007;48(5):1023-1025.
107. Nyenhuis JA, Bourland JD, Foster KS, Graber GP, Terry RS, Adkins RA. Testing of MRI compatibility of the cyberonics model 100 NCP and model 300 series lead. Epilepsia. 1997;38(S8):140.
108. Benbadis SR, Nyhenhuis J, Tatum WO, Murtagh FR, Gieron M, Vale FL. MRI of the brain is safe in patients implanted with the vagus nerve stimulator. Seizure. 2001;10:512-515.
109. Duncan J. The current status of neuroimaging for epilepsy. Curr Opin Neurol. 2003;16:163-164.
110. Achten E, Jackson GD, Cameron JA, Abbott DF, Stella DL, Fabinyi GCA. Presurgical evaluation of the motor hand area with functional MR imaging in patients with tumors and dysplastic lesions. Radiology. 1999;210:529-538.
111. Maniker A, Liu WC, Marks D, Moser K, Kalnin A. Positioning of vagal nerve stimulators: technical note. Surg Neurol. 2000;53:178-181.
112. Sucholeiki R, Alsaadi TM, Morris GLIII, Ulmer JL, Biswal B, Mueller WM. fMRI in patients implanted with a vagal nerve stimulator. Seizure. 2002;11:157-162.
113. Bohning DE, Lomarev MP, Denslow S, Nahas Z, Shastri A, George MS. Feasibility of vagus nerve stimulation-synchronized blood oxygenation level-dependent functional MRI. Invest Radiol. 2001;36:470-479.
114. Narayanan JT, Watts R, Haddad N, Labar DR, Li PM, Filippi CG. Cerebral activation during vagus nerve stimulation: a functional MR study. Epilepsia. 2002;43:1509-1514.
115. Lomarev M, Denslow S, Nahas Z, Chae JH, George MS, Bohning DE. Vagus nerve stimulation (VNS) synchronized BOLD fMRI suggests that VNS in depressed adults has frequency/dose dependent effects. J Psychiatr Res. 2002;36:219-227.
116. Espinosa J, Aiello MT, Naritoku DK. Revision and removal of stimulating electrodes following long-term therapy with the vagus nerve stimulator. Surg Neurol. 1999;51:659-664.
117. Davis R. Cerebellar stimulation for cerebral palsy spasticity, function and seizures. Arch Med Res. 2000;31:290-299.
118. Van Buren JM, Wood JH, Oakley J, Hambrecht F. Preliminary evaluation of cerebellar stimulation by double-blind stimulation and biological criteria in the treatment of epilepsy. J Neurosurg. 1978;48:407-416.
119. Velasco F, Carrillo-Ruiz JD, Brito F, et al. Double-blind, randomized controlled pilot study of bilateral cerebellar stimulation for the treatment of intractable motor seizures. Epilepsia. 2005;46(7):1071-1081.
120. Benabid A, Minotti L, Koudsie A, de Saint, Martin A, Hirsch E. Antiepileptic effects of high-frequency stimulation of the subthalamic nucleus (corpus Luysi) in a case of medically intractable epilepsy caused by focal dysplasia: a 30-month follow-up: technical case report. Neurosurgery. 2002;50:1385.
121. Alaraj A, Commair Y, Mikati M, Wakim J, Louak E, Atweh S. Subthalamic nucleus deep brain stimulation: a novel method for the treatment of non-focal intractable epilepsy. Neuromodulation: defining the future. Poster presentation. Cleveland, Ohio; 2001.
122. Loddenkemper T, Pan A, Neme S, et al. Deep brain stimulation in epilepsy. J Clin Neurophysiol. 2001;18:514-532.
123. Handforth A, DeSalles A, Krahl SE. Deep brain stimulation of the subthalamic nucleus as adjunct treatment for refractory epilepsy. Epilepsia. 2006;47(7):1239-1241.
124. Vesper J, Steinhoff B, Rona S, et al. Chronic high-frequency deep brain stimulation of the STN/SNr for progressive myoclonic epilepsy. Epilepsia. 2007;48(10):1984-1989.
125. Velasco M, Velasco F, Velasco AL. Centromedian-thalamic and hippocampal electrical stimulation for the control of intractable epileptic seizures. J Clin Neurophysiol. 2001;18:495-513.
126. Velasco F, Velasco M, Jimenez F, Velasco AL, Rojas B, Perez ML. Centromedian nucleus stimulation for epilepsy. Clinical, electroencephalographic and behavioural observations. Thal Syst. 2002;1:387-398.
127. Velasco A, Velasco F, Jimenez F, et al. Neuromodulation of the centromedian thalamic nuclei in the treatment of generalized seizures and the improvement of the quality of life in patients with Lennox-Gastaut syndrome. Epilepsia. 2006;47(7):1203-1212.
128. Parain D, Blondeau C, Peudenier S, Delangre T. Vagus nerve stimulation in refractory childhood absence epilepsy. Epilepsia. 2003;44(suppl. 9):326.
129. Andrade DM, Zumsteg D, Hamani C. Long-term follow-up (up to 7 years) of patients with thalamic deep brain stimulation for epilepsy. Neurol. 2006;66:1571-1573.
130. Kerrigan JF, Litt B, Fisher RS, et al. Electrical stimulation of the anterior nucleus of the thalamus for the treatment of intractable seizures. Epilepsia. 2004;45:346-354.
131. Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal lobe epilepsy. N Eng J Med. 2001;345:311-318.
132. Bragin A, Wilson CL, Engel J. Chronic epileptogenesis requires development of a network of pathologically interconnected neuron clusters: a hypothesis. Epilepsia. 2000;41:S144-S152.
133. Kemppainen S, Jolkkonen E, Pitkanen A. Projections from the posterior cortical nucleus of the amygdala to the hippocampal formation and parahippocampal region in rat. Hippocampus. 2002;12(6):735-755.
134. Wilson CL, Engel J. Electrical stimulation of the human epileptic limbic cortex. In: Devinsky O, Beric A, Dogali M, editors. Electric and magnetic stimulation of the brain and spinal cord. New York: Raven Press; 1993:103-113.
135. Lian J, Bikson M, Sciortino C, Stacey WC, Durand DM. Local suppression of epileptiform activity by electrical stimulation in rat hippocampus in vitro. J Physiol. 2003;547:427-434.
136. Su Y, Radman T, Vaynshteyn J, Parra LC, Bikson M. Effects of high-frequency stimulation on epileptiform activity in vitro: on/off control paradigm. Epilepsia. 2008 (epub ahead of print).
137. Wyckhuys T, De Smedt T, Claeys P, et al. High frequency deep brain stimulation in the hippocampus modifies seizure characteristics in kindled rats. Epilepsia. 2007;48:1543-1550.
138. Bragin A, Wilson CL, Engel J. Increased afterdischarge threshold during kindling in epileptic rats. Exp Brain Res. 2002;144:30-37.
139. Velasco M, Velasco F, Velasco AL, et al. Subacute electrical stimulation of the hippocampus blocks intractable temporal lobe seizures and paroxysmal EEG activities. Epilepsia. 2000;41:158-169.
140. Tellez-Zenteno JF, McLachlan RS, Parrent A, Kubu CS, Wiebe S. Hippocampal electrical stimulation in mesial temporal lobe epilepsy. Neurol. 2006;66:1490-1494.
141. Velasco A, Velasco F, Velasco M, Trejo D, Castro G, Carrillo-Ruiz JD. Electrical stimulation of the hippocampal epileptic foci for seizure control: a double-blind, long-term follow-up study. Epilepsia. 2007;48(10):1895-1903.