CHAPTER 19 Brain stem
The brain stem consists of the medulla oblongata, pons and midbrain. It is situated in the posterior cranial fossa, and its ventral surface lies on the clivus. It contains numerous intrinsic neuronal cell bodies and their processes, some of which are the brain stem homologues of spinal cell groups. Some brain stem cell groups are the nuclei of cranial nerves III–XII: they are concerned with the sensory, motor and autonomic innervation of the head and neck. Other autonomic fibres that arise from the brain stem are distributed more widely via the vagus nerve. The brain stem also contains a complex and sometimes ill-defined network of neurones, the reticular formation, that extends throughout its length, and is continuous caudally with its spinal counterpart. Some reticular nuclei are referred to as vital centres since they are concerned with regulation of cardiac and respiratory activities; other parts of the reticular formation are essential for cerebral cortical arousal and the maintenance of consciousness, or are involved in the regulation of muscle tone, posture and reflex activities. The brain stem is the site of termination of numerous ascending and descending fibres and is traversed by many others. The spinothalamic tract (spinal lemniscus), medial lemniscus (see Fig. 18.10) and the trigeminothalamic tracts (see Fig. 19.16) all ascend through the brain stem to reach the thalamus Prominent corticospinal projections descend through the brain stem (see Fig. 18.17) and corticobulbar projections end within it.
OVERVIEW OF CRANIAL NERVES AND CRANIAL NERVE NUCLEI
The cranial nerves are the routes by which the brain receives information directly from, and controls the functions of, structures which are located mainly, although not exclusively, within the head and neck. All but two of the twelve pairs of cranial nerves attach to the brain stem. Below is a brief overview of the cranial nerves (consult also Table 15.1), their associated cranial nerve nuclei, and some of the brain stem reflexes to which they contribute. The cranial nerves are described in detail on a regional basis in appropriate chapters.
The cranial nerves are individually named and numbered (using Roman numerals) in a rostro-caudal sequence, reflecting their order of attachment to the brain. The first cranial nerve (olfactory) terminates directly in cortical and subcortical areas of the frontal and temporal lobes. It is closely associated functionally with the limbic system and is described in that context (Ch. 23). The fibres of the second cranial nerve (optic) pass into the optic chiasma where the centrally located fibres decussate; all of the fibres emerge as the optic tract, which terminates in the lateral geniculate nucleus of the thalamus. Cranial nerves III (oculomotor) and IV (trochlear) attach to the midbrain. Cranial nerve V (trigeminal) attaches to the pons, medial to the middle cerebellar peduncle. Cranial nerves VI (abducens), VII (facial) and VIII (vestibulocochlear) attach to the brain stem at, or close to, the junction of the pons with the medulla. Cranial nerves IX (glossopharyngeal), X (vagus), the cranial part of XI (accessory) and XII (hypoglossal) all attach to the medulla.
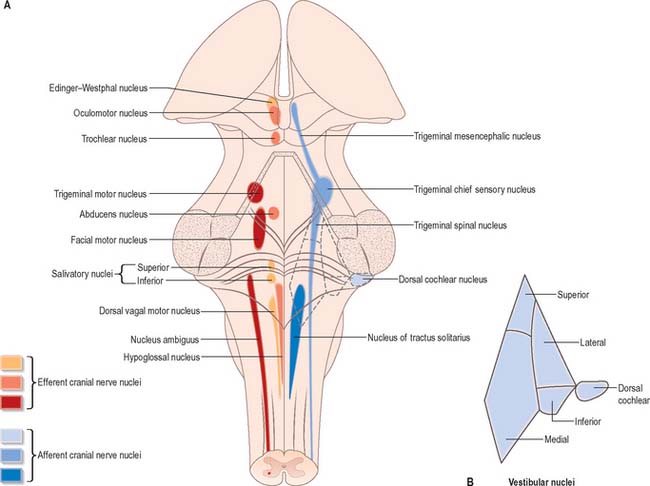
Cranial nerves III–XII, which attach to the brain stem, are associated with the brain stem cell groupings referred to collectively as the cranial nerve nuclei (Fig. 19.1). The nuclei are either the origin of efferent cranial nerve fibres or the site of termination of cranial nerve afferents. They are considered to be organized into six discontinuous longitudinal cell columns that correspond to columns that may be identified in the embryo (see Fig. 15.3). Three columns are ‘sensory’ and three are ‘motor’ in function.
MEDULLA OBLONGATA
EXTERNAL FEATURES AND RELATIONS
The medulla oblongata extends from just above the first pair of cervical spinal nerves to the lower border of the pons (Fig. 28.11). It is approximately 3 cm in length and 2 cm in diameter at its widest. The ventral surface of the medulla is separated from the basilar part of the occipital bone and apex of the dens by the meninges and occipito-axial ligaments. Caudally, the dorsal surface of the medulla occupies the midline notch between the cerebellar hemispheres.
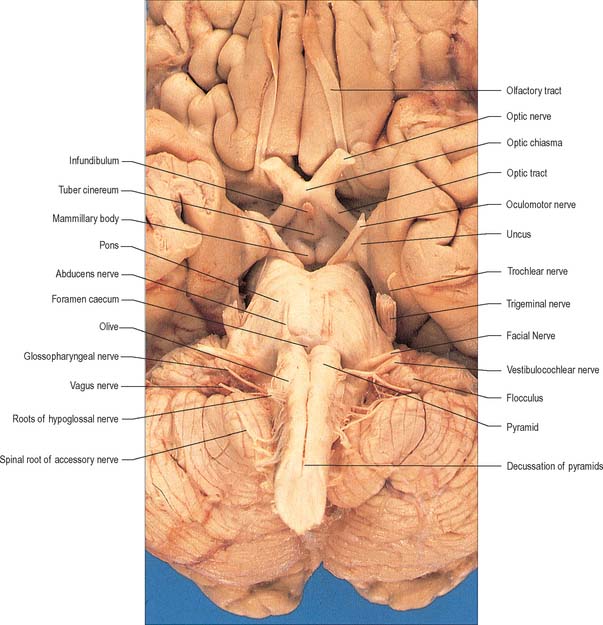
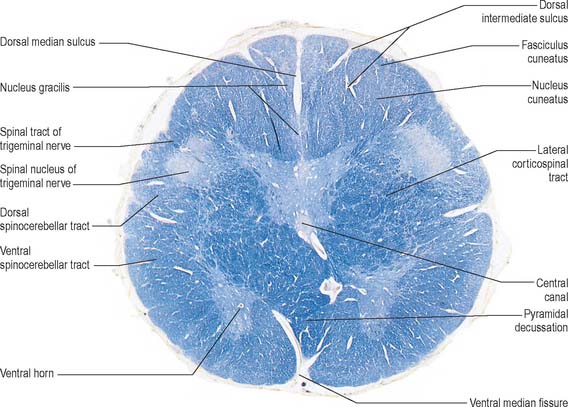
The ventral and dorsal surfaces of the medulla (Fig. 19.2, Fig. 19.3) possess a longitudinal median fissure and sulcus, respectively, which are continuous with their spinal counterparts. Caudally, the ventral median fissure is interrupted by the obliquely crossing fascicles of the pyramidal decussation. Rostrally, it ends at the pontine border in a diminutive depression, the foramen caecum. Immediately lateral to the ventral median fissure there is a prominent elongated ridge, the pyramid, which contains descending pyramidal, or corticospinal, axons. The lateral margin of the pyramid is indicated by a shallow ventrolateral sulcus. From this emerges, in line with the ventral spinal nerve roots, a linear series of rootlets which constitute the hypoglossal nerve. The abducens nerve emerges at the slightly narrowed rostral end of the pyramid, where it adjoins the pons. Caudally the pyramid tapers into the spinal ventral funiculus. Lateral to the pyramid and the ventrolateral sulcus there is an oval prominence, the olive (Fig. 19.2), which contains the inferior olivary nucleus. Lateral to the olive is the posterolateral sulcus. The glossopharyngeal, vagus and accessory nerves join the brain stem along the line of this sulcus, in line with the dorsal spinal nerve roots.
The spinal central canal extends into the caudal half of the medulla, migrating progressively more dorsally until it opens out into the lumen of the fourth ventricle. This divides the medulla into a closed part, which contains the central canal, and an open part, which contains the caudal half of the fourth ventricle (Fig. 19.3).
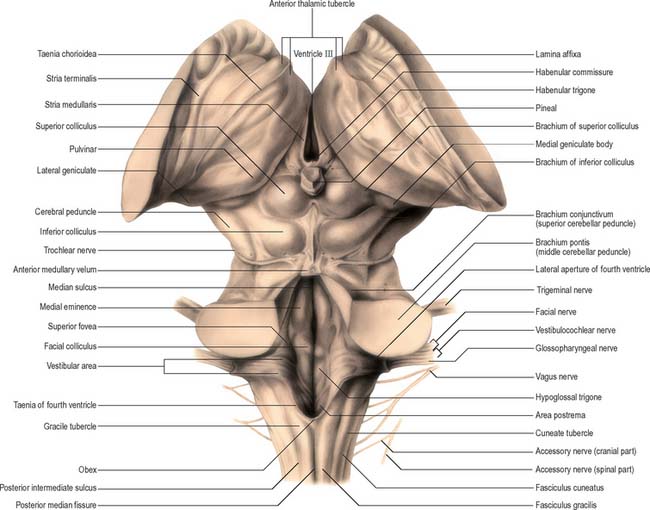
In the closed part of the medulla, a shallow dorsal intermediate sulcus, on either side of the dorsal median sulcus and continuous with its cervical spinal counterpart, indicates the location of the ascending dorsal columns (fasciculus gracilis and fasciculus cuneatus). The ascending fasciculi are at first parallel to each other, but at the caudal end of the fourth ventricle they diverge, and each develops an elongated swelling, the gracile and cuneate tubercles, produced by the subjacent nuclei gracilis and cuneatus respectively (Fig. 19.3, Fig. 19.4, Fig. 19.5). Most fibres in the fasciculi synapse with neurones in their respective nuclei, and these project to the contralateral thalamus, which, in turn, projects to the primary somaesthetic cortex (see Fig. 18.10). The inferior cerebellar peduncle forms a rounded ridge between the caudal part of the fourth ventricle and the glossopharyngeal and vagal rootlets. The peduncles of the two sides diverge and incline to enter the cerebellar hemispheres, where they are crossed by the striae medullares which run to the dorsal median sulcus of the ventricular floor (Fig. 19.3). Here also the peduncles form the anterior and rostral boundaries of the lateral recess of the fourth ventricle. This becomes continuous with the subarachnoid space through the lateral apertures of the fourth ventricle, or foramina of Luschka. A tuft of choroid plexus, continuous with that of the fourth ventricle, protrudes from the foramina on either side.
INTERNAL STRUCTURE
Transverse section of the medulla at the level of the pyramidal decussation
A transverse section across the lower medulla oblongata (Fig. 19.4) intersects the dorsal, lateral and ventral funiculi, which are continuous with their counterparts in the spinal cord. The ventral funiculi are separated from the central grey matter by corticospinal fibres, which cross in the pyramidal decussation to reach the contralateral lateral funiculi (see Fig. 19.8). The decussation displaces the central grey matter and central canal dorsally. Continuity between the ventral grey column and central grey matter, which is maintained throughout the spinal cord, is lost. The column subdivides into the supraspinal nucleus (continuous above with that of the hypoglossal nerve), which is the efferent source of the first cervical nerve, and the spinal nucleus of the accessory nerve, which provides some spinal accessory fibres and merges rostrally with the nucleus ambiguus.
Transverse section of the medulla at the level of the decussation of the medial lemniscus
The medullary white matter is rearranged above the level of the pyramidal decussation (Fig. 19.5). The pyramids form two large ventral bundles flanking the ventral median fissure on the ventral surface of the medulla.
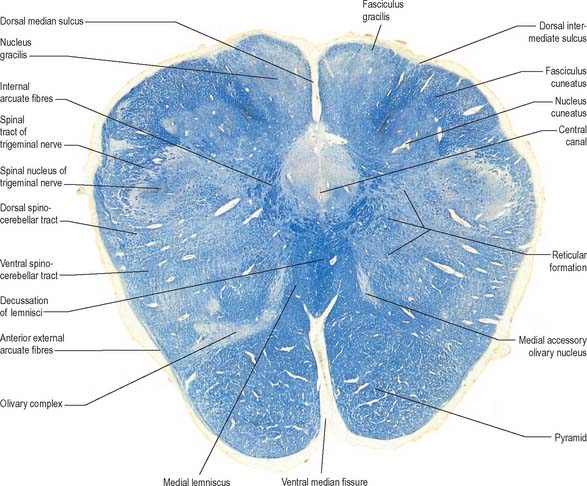
They contain corticospinal fibres of ipsilateral origin. The nucleus gracilis is prominent on the dorsal aspect, with diminishing numbers of fibres of the fasciculus gracilis located on its dorsal, medial and lateral margins. The nucleus cuneatus is well developed. Both nuclei retain continuity with the central grey matter at this level, but this is lost more rostrally. First-order afferent fibres contained within the fasciculi gracilis and cuneatus synapse upon neurones in their respective nuclei. Second-order axons emerge from the nuclei as internal arcuate fibres, at first curving ventrolaterally around the central grey matter and then ventromedially between the spinal tract of the trigeminal nerve and the central grey matter. The fibres decussate in the midline thereafter forming the medial lemniscus which ascends to the thalamus. The decussation of internal arcuate fibres is located dorsal to the pyramids and ventral to the central grey matter, which is therefore more dorsally displaced than in the previous section.
Transverse section of the medulla at the caudal end of the fourth ventricle
A transverse section at the lower end of the fourth ventricle (Fig. 19.6) shows some new features together with most of those already described. The total area of grey matter is increased by the presence of the large olivary nuclear complex and nuclei of the vestibulocochlear, glossopharyngeal, vagus and accessory nerves.
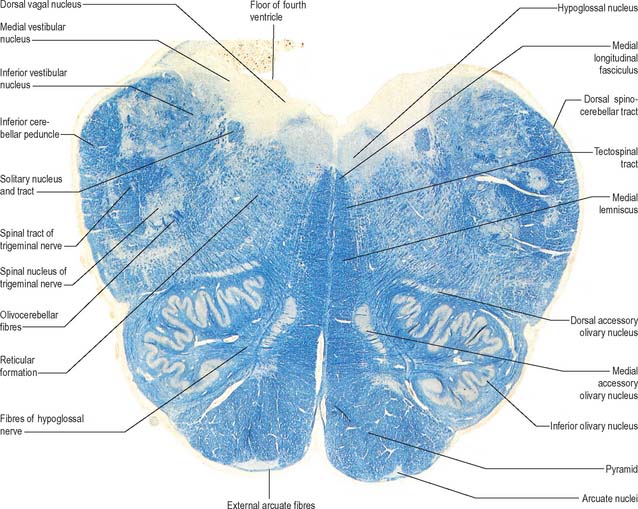
Fig. 19.6 Transverse section through the medulla oblongata at the caudal end of the fourth ventricle.
A smooth, oval elevation, the olive, lies between the ventrolateral and dorsolateral sulci of the medulla. It is formed by the underlying inferior olivary complex of nuclei, and lies lateral to the pyramid, separated from it by the ventrolateral sulcus and emerging hypoglossal nerve fibres. The roots of the facial nerve emerge between its rostral end and the lower pontine border, in the cerebellopontine angle. The arcuate nuclei are curved, interrupted bands, ventral to the pyramids, and are said to be displaced pontine nuclei. Anterior external arcuate fibres and those of the striae medullares are derived from them. They project mainly to the contralateral cerebellum through the inferior cerebellar peduncle (Fig. 19.7).
The inferior olivary nucleus is an irregularly crenated mass of grey matter with a medially directed hilum, through which numerous fibres enter and leave the nucleus. It has prominent connections with the cerebellum and is described more fully in Chapter 20.
The spinocerebellar, spinotectal, vestibulospinal, rubrospinal and lateral spinothalamic (spinal lemniscal) tracts all lie in the ventrolateral area of the medulla at this level. The tracts are limited dorsally by the nucleus of the spinal tract of the trigeminal and ventrally by the pyramid.
Pyramidal tract
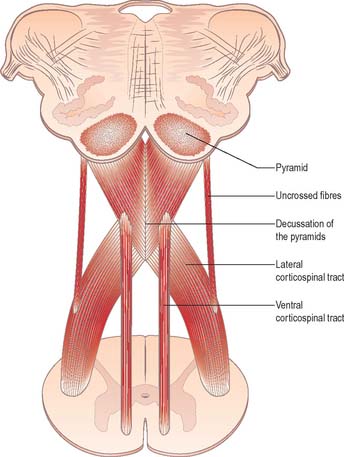
Each pyramid contains descending corticospinal fibres, derived from the ipsilateral cerebral cortex, which have traversed the internal capsule, midbrain and pons (Fig. 19.8). Approximately 70–90% of the axons leave the pyramids in successive bundles, crossing in and deep to the ventral median fissure as the pyramidal decussation. In the rostral medulla fibres cross by inclining ventromedially, whereas more caudally they pass dorsally, decussating ventral to the central grey matter. The decussation is orderly, such that fibres destined to end in the cervical segments cross first. Fibres continue to pass dorsally as they descend, and reach the contralateral spinal lateral funiculus as the crossed lateral corticospinal tract. Most uncrossed corticospinal fibres descend ventromedially in the ipsilateral ventral funiculus, as the ventral corticospinal tract. A minority run dorsolaterally to join the lateral corticospinal tracts as a small uncrossed component. The corticospinal tracts display somatotopy at almost all levels. In the pyramids the arrangement is like that at higher levels, in that the most lateral fibres subserve the most medial arm and neck movements. Similar somatotopy is ascribed to the lateral corticospinal tracts within the spinal cord.
Dorsal column nuclei
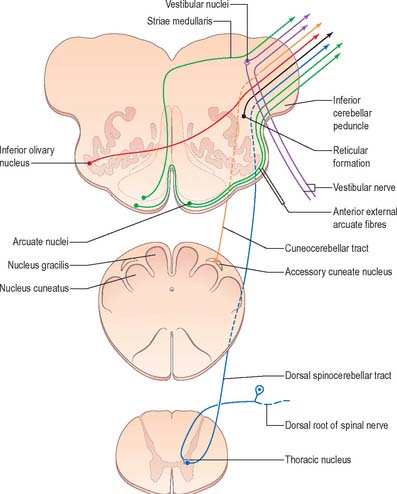
The accessory cuneate nucleus, dorsolateral to the cuneate nucleus, is part of the spinocerebellar system of precerebellar nuclei (Fig. 19.7). It contains large neurones like those in the spinal thoracic nucleus and receives the lateral fibres of the fasciculus cuneatus, which carry proprioceptive impulses from the upper limb that enter the cervical spinal cord rostral to the thoracic nucleus. The accessory cuneate neurones give rise to the posterior external arcuate fibres that enter the cerebellum by the ipsilateral inferior cerebellar peduncle in the cuneocerebellar tract. A group of neurones, nucleus Z, identified in animals between the upper pole of the nucleus gracilis and the inferior vestibular nucleus, is said to be present in the human medulla. Its input is probably from the dorsal spinocerebellar tract, which carries proprioceptive information from the ipsilateral lower limb, and it projects through internal arcuate fibres to the contralateral medial lemniscus.
Trigeminal sensory nucleus
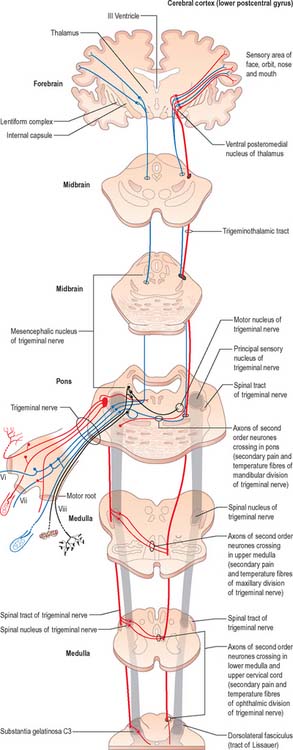
On entering the pons, the fibres of the sensory root of the trigeminal nerve run dorsomedially towards the principal sensory nucleus, which is situated at this level (Fig. 19.9). Before reaching the nucleus approximately 50% of the fibres divide into ascending and descending branches – the others ascend or descend without division. The descending fibres, of which 90% are less than 4 μm in diameter, form the spinal tract of the trigeminal nerve, which reaches the upper cervical spinal cord. The tract embraces the nucleus of the spinal tract of the trigeminal (spinal trigeminal nucleus; Fig. 19.4, Fig. 19.5, Fig. 19.6, Fig. 19.10, Fig. 19.11). There is a precise somatotopic organization in the tract. Fibres from the ophthalmic division of the trigeminal lie ventrolaterally, those from the mandibular division lie dorsomedially, and the maxillary fibres lie between them. The tract is completed on its dorsal rim by fibres from the sensory roots of the facial, glossopharyngeal and vagus nerves. All of these fibres synapse in the nucleus caudalis.
Fibres of the glossopharyngeal, vagus and facial nerves subserving common sensation (general visceral afferent) form a column dorsally within the spinal tract of the trigeminal nerve and synapse with cells in the lowest part of the spinal trigeminal nucleus. Consequently, operative section of the dorsal part of the spinal tract results in analgesia that extends to the mucosa of the tonsillar sinus, the posterior third of the tongue and adjoining parts of the pharyngeal wall (glossopharyngeal nerve), and the cutaneous area supplied by the auricular branch of the vagus.
Vagal nucleus
The vagal nucleus (also known as the dorsal motor nucleus of the vagus) lies dorsolateral to the hypoglossal nucleus, from which it is separated by the nucleus intercalatus. It extends caudally to the first cervical spinal segment and rostrally to the open part of the medulla under the vagal triangle of the floor of the fourth ventricle (Fig. 19.6).
Hypoglossal nucleus
The prominent hypoglossal nucleus lies near the midline in the dorsal medullary grey matter. It is approximately 2 cm long. Its rostral part lies beneath the hypoglossal triangle in the floor of the fourth ventricle (Fig. 19.3) and its caudal part extends into the closed part of the medulla.
Several smaller groups of cells lie near the hypoglossal nucleus (perihypoglossal nuclei), but none is known for certainty to be connected with the hypoglossal nerve or nucleus. They include the nucleus intercalatus, sublingual nucleus, nucleus prepositus hypoglossi and nucleus paramedianus dorsalis (reticularis). Gustatory and visceral connections are attributed to the nucleus intercalatus.
Inferior olivary nucleus
The olivary nuclear complex consists of the large inferior olivary nucleus and the much smaller medial accessory and dorsal accessory olivary nuclei (Fig. 19.6). They are the so-called precerebellar nuclei, a group that also includes the pontine, arcuate, vestibular, reticulocerebellar and spinocerebellar nuclei, all of which receive afferents from specific sources and project to the cerebellum. The inferior olivary nucleus contains small neurones, most of which form the olivocerebellar tract, which emerges either from the hilum or through the adjacent grey matter, to run medially and intersect the medial lemniscus (Fig. 19.6). Its fibres cross the midline, and sweep either dorsal to, or through, the opposite olivary nucleus. They intersect the lateral spinothalamic and rubrospinal tracts and the spinal trigeminal nucleus, and enter the contralateral inferior cerebellar peduncle, where they constitute its major component. Fibres from the contralateral inferior olivary complex terminate on Purkinje cells in the cerebellum as climbing fibres – there is a one-to-one relationship between Purkinje cells and neurones in the complex. Afferent connections to the inferior olivary nucleus are both ascending and descending. Ascending fibres, mainly crossed, arrive from all spinal levels in the spino-olivary tracts and via the dorsal columns. Descending ipsilateral fibres come from the cerebral cortex, thalamus, red nucleus and central grey of the midbrain. In part the two latter projections make up the central tegmental tract (fasciculus).
The medial accessory olivary nucleus is a curved grey lamina, concave laterally, between the medial lemniscus and pyramid and the ventromedial aspect of the inferior olivary nucleus. The dorsal accessory olivary nucleus is a similar lamina, dorsomedial to the inferior olivary nucleus. Both nuclei are connected to the cerebellum. The accessory olivary nuclei are phylogenetically older than the inferior olivary nucleus, and they are connected with the paleocerebellum. In all connections, cerebral, spinal and cerebellar, the olivary nuclei display specific topographical organization (Ch. 20).
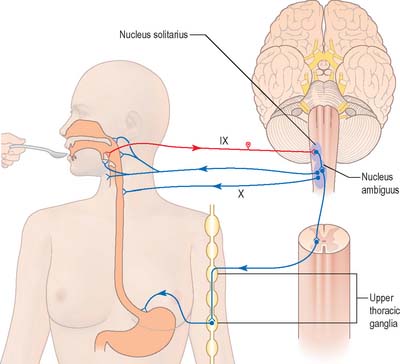
Nucleus solitarius
Swallowing and gag reflexes
During the normal processes of eating and drinking, passage of material to the rear of the mouth stimulates branches of the glossopharyngeal nerve in the oropharynx (Fig. 19.12). This information is relayed via the nucleus solitarius to the nucleus ambiguus, which contains the motor neurones innervating the muscles of the palate, pharynx, and larynx. The nasopharynx is closed off from the oropharynx by elevation of the soft palate. The larynx is raised, its entrance narrowed and the glottis is closed. Peristaltic activity down the oesophagus to the stomach is mediated through the pharyngeal plexus.
Nucleus ambiguus
The nucleus ambiguus is a group of large motor neurones, situated deep in the medullary reticular formation (see Fig. 19.10). It extends rostrally as far as the upper end of the vagal nucleus while caudally it is continuous with the nucleus of the spinal accessory nerve. Fibres emerging from it pass dorsomedially, then curve laterally. Rostral fibres join the glossopharyngeal nerve. Caudal fibres join the vagus and cranial accessory nerves and are distributed to the pharyngeal constrictors, intrinsic laryngeal muscles and striated muscles of the palate and upper oesophagus.
The nucleus ambiguus contains several cellular subgroups, and some topographical representation of the muscles innervated has been established. Individual laryngeal muscles are innervated by relatively discrete groups of cells in more caudal zones. Neurones that innervate the pharynx lie in the intermediate area, and neurones that innervate the oesophagus and soft palate are rostral.
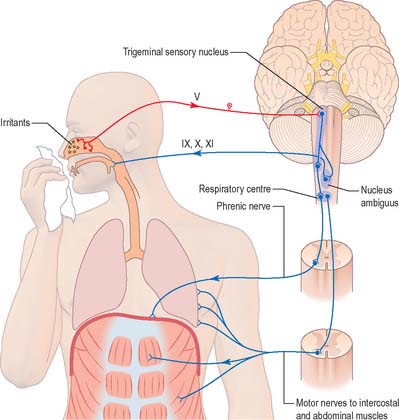
Cough and sneeze reflexes
A similar mechanism underlies sneezing (Fig. 19.13) except that the stimulus arises from the nasal mucosa and afferent impulses are conveyed by the ophthalmic or maxillary divisions of the trigeminal nerve to the trigeminal sensory nucleus. After sharp inhalation, explosive exhalation occurs with closure of the oropharyngeal isthmus by the action of palatoglossus, which diverts air through the nasal cavity.
PONS
EXTERNAL FEATURES AND RELATIONS
The dorsal surface of the pons is hidden by the cerebellum which covers the rostral half of the rhomboid fossa, into which the aqueduct of the midbrain empties. The roof of the fossa is formed by a thin sheet of tissue, the superior (anterior) medullary velum, and is overlain by the lingula of the vermis of the cerebellum. The velum is attached on each side to the superior cerebellar peduncles and is enclosed by pia mater above and ependyma below (Fig. 19.3). The trochlear nerves decussate in the velum.
INTERNAL STRUCTURE
Transverse sections of the pons
Transverse sections (Fig. 19.9, Fig. 19.11) reveal that the pons consists of a dorsal tegmentum, which is a continuation of the medulla (excluding the pyramids), and a ventral (basilar) part. The latter contains bundles of longitudinal descending fibres, some of which continue into the pyramids, while others end in the many pontine or medullary nuclei. It also contains numerous transverse fibres and scattered pontine nuclei.
Ventral pons
The pontine nuclei (Fig. 19.11) include all the neurones (in man, some 20 million) that are scattered throughout the ventral pons. They are probably all glutamatergic, and most project to the contralateral cerebellar cortex, with some input to the deep cerebellar nuclei. Efferent axons from the pontine nuclei constitute the transverse pontine (pontocerebellar) fibres which decussate and continue as the major constituent of the contralateral middle cerebellar peduncle. All pontocerebellar fibres end as mossy fibres in the cerebellar cortex, and a degree of somatotopy is maintained in these connections.
Pontine tegmentum
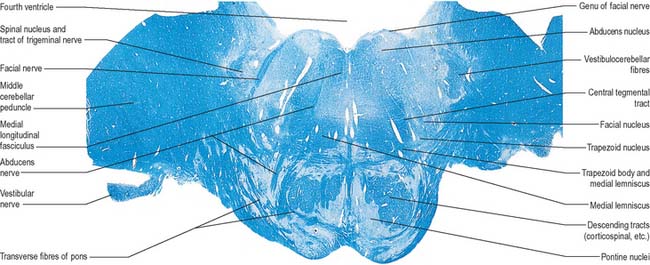
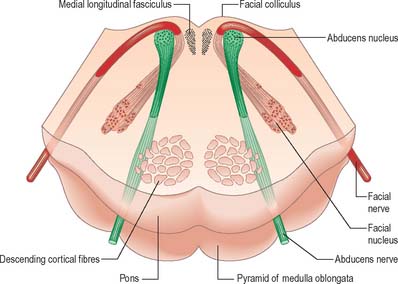
The pontine tegmentum varies in cytoarchitecture at different levels. A transverse section through the lower pontine tegmentum transects the facial colliculus in the floor of the fourth ventricle (Fig. 19.11, Fig. 19.14). The colliculus contains the nucleus of the abducens nerve and the overlying fibres of the facial nerve. More deeply placed are the facial nuclei, the nearby vestibular and cochlear nuclei and other isolated neuronal groups. The vestibular nuclei are laterally placed in the rhomboid fossa of the fourth ventricle, subjacent to the vestibular area, which spans the rostral medulla and caudal pons (Fig. 19.1, Fig. 19.3).
The striae medullares of the fourth ventricle (Fig. 19.10) are an aberrant cerebropontocerebellar connection, in which the arcuate nuclei and external arcuate fibres are involved. Axons from arcuate nuclei spread round the medulla, above and below the inferior olive, where they are superficially visible as the circumolivary fasciculus. All these fibres, which collectively are known as the external arcuate fibres, enter the inferior cerebellar peduncle (Fig. 19.7). Some fibres from arcuate nuclei pass dorsally through the medulla near its midline, decussate near the floor of the fourth ventricle, then turn laterally under the ependyma and enter the cerebellum through the inferior peduncle.
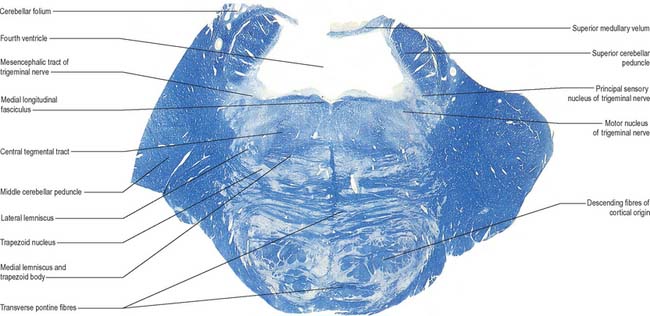
In addition to the tracts already noted at lower levels, the lower pontine tegmentum contains the trapezoid body, lateral lemniscus and emerging fibres of the abducens and facial nerves. The medial lemniscus is ventral, its transverse outline now a flat oval. It extends laterally from the median raphe (Fig. 19.9, Fig. 19.11) and is laterally related to the lateral spinothalamic tract and trigeminal lemniscus. The fibres of the latter originate from neurones of the contralateral spinal nucleus, serving pain and thermal sensibility in facial skin and mucosae of the conjunctiva, tongue, mouth, nose, etc. Here the lemnisci together form a transverse band which is composed, in lateral order from the midline, of the medial and trigeminal lemnisci, the lateral spinothalamic tract and the lateral lemniscus.
The trapezoid body contains cochlear fibres, mainly from the ventral cochlear and trapezoid nuclei. They ascend transversely in the ventral tegmentum, pass either through or ventral to the vertical medial lemniscal fibres, and decussate with the contralateral fibres in the median raphe. Below the emerging facial axons, the trapezoid fibres turn up into the lateral lemniscus. As the lateral lemniscus ascends it lies near the dorsolateral surface of the brain stem. Above, its fibres enter the inferior colliculus and medial geniculate body. The ascending auditory pathway is described in detail in Chapter 37.
A transverse section at an upper pontine tegmental level contains trigeminal elements (Fig. 19.9), but otherwise shows little notable alteration from a section through a lower pontine tegmental level. Its dorsolateral parts are invaded by the superior cerebellar peduncles. The small nucleus of the lateral lemniscus, a relay in the auditory pathway, lies medial to its tract in the upper pons, and receives some lemniscal terminals. Some of its efferent fibres enter the medial longitudinal fasciculus, others return to the lemniscus.
The medial lemniscus (Fig. 19.9, Fig. 19.11) retains its paramedian position in the ventral pontine tegmentum, where it lies a little lateral to the median raphe, and is joined medially by fibres from the principal trigeminal sensory nucleus. The trigeminal lemniscus, lateral spinothalamic tract, and the lateral lemniscus and its nucleus all lie dorsolaterally.
Cochlear nuclei
Cochlear nerve fibres, which are derived from neuronal somata in the spiral ganglion, bifurcate on entering the brain stem and terminate in both dorsal and ventral cochlear nuclei (see Fig. 37.20).
Though the cellular origins are not precisely known, axons of most neuronal types in the cochlear nuclei leave to end at pontine levels in the superior olivary, trapezoid and lateral lemniscal nuclei (Fig. 19.9). They leave the cochlear nuclei by three routes. The largest group of axons lies ventrally and decussates as the trapezoid body, level with the pontomedullary junction (Fig. 19.9, Fig. 19.11). Most of these axons ascend slightly, decussate and relay in the contralateral nuclei. A few do not cross, and synapse in the ipsilateral superior olivary nuclei. From both nuclei, the next order axons ascend in the corresponding lateral lemniscus. Occasional decussating fibres traverse the contralateral superior olive and enter the lateral lemniscus to relay in lemniscal nuclei.
The nucleus of the lateral lemniscus consists of small groups of neurones (counts of 18,000 to 24,000 have been recorded in humans) that lie among the fibres of the lateral lemniscus. Dorsal, ventral and intermediate groups probably receive afferent axons from both cochlear nuclei. Their efferents enter the midbrain along the lateral lemniscus and terminate in the inferior colliculi.
Vestibular nuclei
The vestibular nuclei project extensively to the cerebellum and also receive axons from the cerebellar cortex and the fastigial nuclei. Vestibulocerebellar fibres travel via the inferior cerebellar peduncle mainly to the flocculus and nodule. Cerebellovestibular fibres pass to the nuclei, also through the inferior cerebellar peduncle. They arise mainly in the flocculus and nodule (posterior lobe), but some fibres are derived from the anterior lobe and fastigial nucleus. The vestibular nuclear complex projects to the pontine reticular nuclei and to motor nuclei of the oculogyric muscles in the medial longitudinal fasciculus. The complex also projects to the spinal cord via the vestibulospinal tracts and the medial longitudinal fasciculus (see Fig. 18.19).
Abducens nucleus
The abducens nucleus is a group of approximately 6500 neurones, occupying a paramedian position in the central grey matter, in line with the trochlear, oculomotor and hypoglossal nuclei, with which it forms a somatic motor column (Fig. 19.1). It lies ventromedial to the medial longitudinal fasciculus, which is the means by which vestibular, cochlear and other cranial nerve nuclei, especially the oculomotor, connect with the abducens. The abducens nucleus contains large motor neurones and small multipolar interneurones, which are intermixed, although the latter are most heavily concentrated in its lateral and ventral aspects. Axons from the motor neurones cross the midline at the level of the nucleus and ascend in the medial longitudinal fasciculus to all three medial rectus subnuclei of the oculomotor nucleus.
Efferent abducens axons pass ventrally, descend through the reticular formation, trapezoid body and medial lemniscus, and traverse the ventral pons to emerge at its inferior border (Fig. 19.14): they innervate the lateral rectus muscle (see Ch. 39).
Facial nucleus
Efferent fibres of the large motor neurones of the facial nucleus form the motor root of the facial nerve. The motor nucleus represents the branchial efferent column, but it lies much more deeply in the pons than might be expected, and its axons have a most unusual course (Fig. 19.14). At first they incline dorsomedially towards the fourth ventricle, below the abducens nucleus, and ascend medial to it, near the medial longitudinal fasciculus. They then curve round the upper pole of the abducens nucleus and descend ventrolaterally through the reticular formation. Finally, they pass between their own nucleus medially and the spinal trigeminal nucleus. They emerge between the olive and the inferior cerebellar peduncle at the cerebellopontine angle.
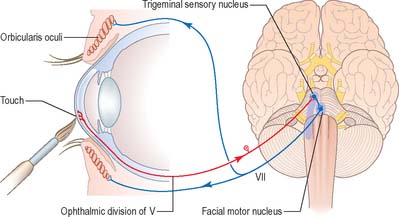
Corneal reflex
Touching the cornea or shining a bright light into the eye elicits reflex closure of both eyes. The former action stimulates nasociliary branches of the ophthalmic nerve, and the latter stimulates the retina and optic pathway. In both cases, afferent impulses enter the central nervous system and spread via interneurones to activate neurones in the facial motor nucleus in the pons (Fig. 19.15). Efferent impulses travel in the facial nerve to activate the palpebral component of orbicularis oculi, which contracts, producing a ‘blink’. The sweep of the eyelids carries lacrimal secretions across the eye helping to remove any irritating particles.
Trigeminal sensory nucleus
On entering the pons, the fibres of the sensory root of the trigeminal nerve run dorsomedially towards the principal sensory nucleus (Fig. 19.9). About 50% of the fibres divide into ascending and descending branches; the others ascend or descend without division. The descending fibres form the spinal tract of the trigeminal, which terminates in the subjacent nucleus of the spinal tract (spinal nucleus) of the trigeminal nerve.
Some ascending trigeminal fibres, many of them heavily myelinated, synapse around the small neurones in the principal sensory nucleus (Fig. 19.16), which lies lateral to the motor nucleus and medial to the middle cerebellar peduncle, and is continuous inferiorly with the spinal nucleus. The principal nucleus is considered to be mainly concerned with tactile stimuli.
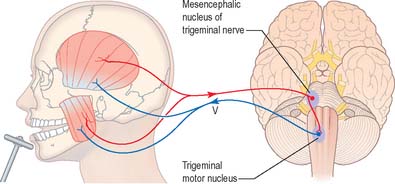
Jaw jerk reflex
Rapid stretching of the muscles that close the jaw (masseter, temporalis, medial pterygoid) activates muscle spindle afferents, which travel via the mandibular division of the trigeminal nerve to the brain stem (Fig. 19.17). The cell bodies of these primary afferent neurones are located in the mesencephalic nucleus of the trigeminal. Collaterals project monosynaptically to the motor nucleus of the trigeminal nerve in the pons. From here motor axons of the mandibular nerve innervate the muscles that close the jaw.
Trigeminal motor nucleus
The trigeminal motor nucleus is ovoid in outline and lies in the upper pontine tegmentum, under the lateral part of the floor of the fourth ventricle (Fig. 19.1). It lies medial to the principal sensory nucleus, and is separated from it by fibres of the trigeminal nerve. It occupies the position of the branchial (special visceral) efferent column.
Tensor tympani and stapedius reflex
Loud sound elicits reflex contraction of tensor tympani and stapedius, which attenuates movement of the tympanic membrane and middle ear ossicles (see Ch. 36). Afferent impulses travel in the cochlear nerve to the cochlear nuclei in the brain stem. Efferent fibres to tensor tympani arise in the trigeminal motor nucleus and travel in the mandibular division of the trigeminal nerve. Efferent fibres to stapedius originate in the facial nucleus and travel in the facial nerve.
MIDBRAIN
EXTERNAL FEATURES AND RELATIONS
The crura cerebri are superficially corrugated and emerge from the cerebral hemispheres. They converge as they descend and meet as they enter the pons, where they form the caudolateral boundaries of the interpeduncular fossa (Fig. 19.18, Fig. 19.19). At the level of the tentorial incisure, the basilar artery divides in the interpeduncular fossa into the right and left posterior cerebral arteries. The superior cerebellar arteries branch from the basilar artery immediately before this bifurcation. The posterior cerebral and superior cerebellar arteries both run laterally around the ventral surface of the crus, the former passing above the tentorium cerebelli, the latter passing below. The oculomotor and trochlear nerves lie between the two arteries. The roots of the oculomotor nerve emerge from the medial aspect of the crus (Fig. 15.7). The posterior communicating artery joins the posterior cerebral artery on the medial surface of the peduncle in the interpeduncular fossa. The median caudal part of the interpeduncular fossa constitutes the posterior perforated substance, which is pierced by central (perforating) branches of the posterior cerebral arteries. The optic tract winds dorsolaterally around the crus near where the latter emerges from the cerebral hemispheres.
The superior and inferior colliculi are two paired eminences on the dorsal surface of the midbrain (Fig. 19.3). They lie rostral to the superior medullary velum, inferior to the pineal gland and caudal to the posterior commissure. Below the splenium of the corpus callosum, they are partly overlain by the pulvinar of the thalamus. The superior and inferior colliculi are separated by a cruciform sulcus. The upper limit of the sulcus expands into a depression for the pineal gland, and a median frenulum veli is prolonged from its caudal end down over the superior medullary velum. The trochlear nerves emerge lateral to the frenulum, pass ventrally over the lateral aspects of the cerebral peduncles and traverse the interpeduncular cistern to the petrosal end of the cavernous sinus. The superior colliculi, larger than the inferior, are centres for visual reflex responses. The inferior colliculi are a part of the ascending auditory pathway.
A brachium passes ventrolaterally from the lateral aspect of each colliculus (Fig. 19.3). The brachium of the superior colliculus (superior quadrigeminal brachium) passes below the pulvinar, partly overlapping the medial geniculate body, and conveys fibres from the retina to the superior colliculus. The brachium of the inferior colliculus (inferior quadrigeminal brachium) ascends ventrally and conveys fibres from the lateral lemniscus and inferior colliculus to the medial geniculate body.
INTERNAL STRUCTURE
Transverse sections of the midbrain
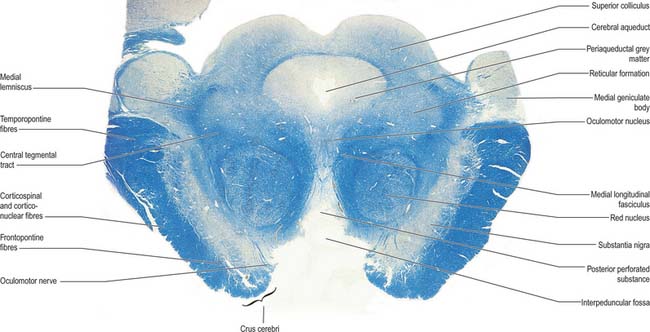
In transverse section, the cerebral peduncles are seen to be composed of dorsal and ventral regions separated by the substantia nigra (Fig. 19.18, Fig. 19.19). On each side, the dorsal region is the tegmentum and the ventral part is the crus cerebri. The tegmenti are continuous across the midline but the crura are separated.
Crus cerebri
The crus cerebri is approximately semilunar in section, being convex externally and concave internally. It contains corticobulbar and corticospinal fibres. Corticobulbar fibres terminate at various brainstem levels, some in association with the cranial nerve nuclei (corticonuclear fibres). Corticospinal fibres pass uninterrupted through the brainstem to enter the medullary pyramid and, thereafter, form the corticospinal tracts of the spinal cord (Fig. 18.18). Corticonuclear and corticospinal fibres occupy the middle two-thirds of the crus cerebri. Corticopontine fibres arise in the cerebral cortex and form two groups, both of which end in the pontine nuclei. Frontopontine fibres from the frontal lobe, principally areas 6 and 4, traverse the internal capsule and then occupy the medial sixth of the ipsilateral crus cerebri. Temporopontine fibres, which originate largely from the posterior region of the temporal lobe, traverse the internal capsule and then run in the lateral sixth of the ipsilateral crus. Parietopontine and occipitopontine fibres are also described in the crus, lying medial to the temporopontine fibres. There are few fibres from the primary sensory cortex in corticopontine projections.
Mesencephalic tegmentum
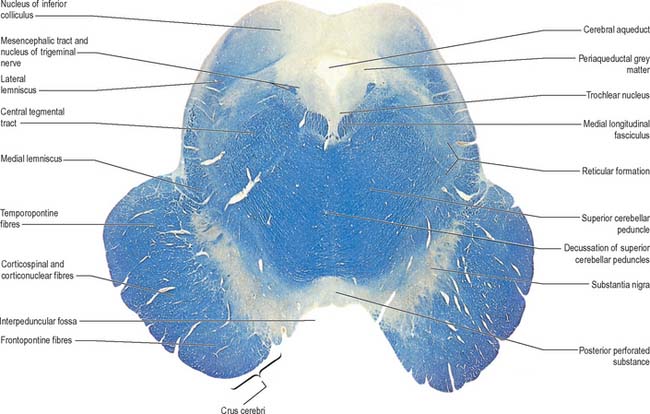
The mesencephalic nucleus of the trigeminal nerve occupies a lateral position in the central grey matter (see Fig. 19.2). It ascends from the main trigeminal sensory nucleus in the pons to the level of the superior colliculus in the midbrain. Its large ovoid neurones are unipolar, like those in peripheral sensory ganglia. They are arranged in many small groups which extend as curved laminae on the lateral margins of the periaqueductal grey matter: neurones are most numerous caudally.
The white matter of the mesencephalic tegmentum contains the majority of the tracts present in the pontine tegmentum. The decussation of the superior cerebellar peduncles is particularly prominent. Numerous ascending fibres enter the mesencephalic tegmentum from the cerebellum and pass ventromedially, decussating in the midline (Fig. 19.18). Thereafter, ascending fibres encapsulate and penetrate the red nucleus, in which some fibres, or their collaterals, terminate (Fig. 19.19). However, the majority of fibres ascend to terminate in the nucleus ventralis lateralis of the thalamus. A relatively small number of uncrossed fibres are believed to end in the periaqueductal grey matter and reticular formation, the interstitial nucleus and the nucleus of the posterior commissure (nucleus of Darkschewitsch). The latter nucleus may send efferent fibres to the medial longitudinal fasciculus and posterior commissure. Descending fascicles from the superior cerebellar peduncles also apparently end in the pontine and medullary reticular formation, the olivary complex and, possibly, motor cranial nerve nuclei.
The medial longitudinal fasciculus adjoins the somatic efferent cell column, dorsal to the decussating superior cerebellar peduncles (Fig. 19.18). The medial, trigeminal, lateral and spinal lemnisci form a curved band dorsolateral to the substantia nigra. Fibres in the medial, trigeminal and spinal lemnisci continue a rostral course to synapse with neurones in the lateral and medial parts of the ventral posterior nucleus of the thalamus. Some fibres of the lateral lemniscus end in the nucleus of the inferior colliculus, or send collaterals to it. The remaining lateral lemniscal fibres join with fibres originating in the inferior colliculus and enter the inferior quadrigeminal brachium, which carries them to the medial geniculate body.
At the level of the superior colliculus, the tegmentum contains the red nucleus (Fig. 19.19), which extends rostrally into the subthalamic region. The ventromedial central grey matter around the aqueduct contains the elongated oculomotor nucleus, which is adjacent ventrolaterally to the medial longitudinal fasciculus, and caudally reaches the trochlear nucleus. The oculomotor nucleus is divisible into neuronal groups which may be partially correlated with the motor distribution of the oculomotor nerve. A group of preganglionic parasympathetic neurones which control the activity of smooth muscle within the eyeball, the Edinger–Westphal nucleus, lies dorsal to the oculomotor nucleus.
Substantia nigra
The substantia nigra is a large nucleus that extends through the whole length of the midbrain (Fig. 19.18, Fig. 19.19). It has major connections with the basal ganglia and is central to the pathology of Parkinson’s disease (Ch. 22).
The pars compacta consists of darkly pigmented neurones, which contain neuromelanin granules. Their arrangement is irregular and they partially penetrate the subjacent pars reticulata. Pigmentation increases with age, is most abundant in primates, maximal in man, and present even in albinos. The pigmented pars compacta neurones synthesize dopamine as their neurotransmitter and project predominantly to the corpus striatum of the basal ganglia as well as to other sites. The pars compacta corresponds to the dopaminergic cell group A9 (Fig. 22.10). Two other dopaminergic cell groups are found in the ventral tegmentum: cell group A10 in the rostromedial region, which corresponds to the ventral tegmental area (of Tsai), and cell group A8 in the dorsolateral reticular area, which forms the nucleus parabrachialis pigmentosus. The whole ventral tegmental system of dopaminergic neurones appears to act as an integrative centre for adaptive behaviour. It projects via a number of pathways, mainly through ascending fibres in the ipsilateral medial forebrain bundle. These pathways are a mesodiencephalic system, which terminates in thalamic and hypothalamic nuclei; a mesostriatal projection to the caudate nucleus and putamen; a mesolimbic pathway to the nucleus accumbens, olfactory tubercle, lateral septum, interstitial nucleus of the stria terminalis, amygdala and entorhinal cortex; and mesocortical fibres that project to most cortical areas, particularly the prefrontal, orbitofrontal and cingulate cortex.
The pars compacta projects heavily to the caudate nucleus and putamen in a topographically organized fashion (nigrostriatal fibres). Lesser projections end in the globus pallidus and subthalamic nucleus. In Parkinson’s disease, the levels of dopamine in the substantia nigra and striatum decrease dramatically as a result of the degeneration of pars compacta neurones (Ch. 22).
There are reciprocal connections between the substantia nigra and the basal ganglia. Efferent fibres from the basal ganglia terminate largely, but not exclusively, in the pars reticulata. Topographically organized striatonigral fibres originate from the caudate nucleus and putamen and project to the pars reticulata. The head of the caudate nucleus projects to the rostral third of the substantia nigra, while the putamen projects to all parts. The fibres end in axodendritic synapses. A small number of GABAergic pallidonigral fibres from the lateral (external) segment of the globus pallidus end mostly in the pars reticulata. The subthalamic nucleus sends an important glutamatergic projection to the pars reticulata and to the globus pallidus. Subthalamonigral and subthalamopallidal projections are important in the pathophysiology of movement disorders such as Parkinson’s disease and dyskinesias (Ch. 22).
Red nucleus
The red nucleus is a pink, ovoid mass, approximately 5 mm in diameter and lying dorsomedial to the substantia nigra (Fig. 19.19). The tint appears only in fresh material and is caused by a ferric iron pigment in its multipolar neurones. The latter are of varying size and their relative proportions and arrangement vary between species. In primates the magnocellular element is decreased, and there is a reciprocal increase in the size of the parvocellular component. Small multipolar neurones occur in all parts of the nucleus. In man, the larger neurones are restricted to the caudal part of the nucleus and have been estimated to be as few as 200 in number. The magnocellular element is considered phylogenetically old, which accords with the parvocellular predominance in primates. Rostrally, the red nucleus is poorly demarcated, and it blends into the reticular formation and caudal pole of the interstitial nucleus. It is traversed and surrounded by fascicles of nerve fibres, including many from the oculomotor nucleus.
The largest group of efferents from the red nucleus in man is a descending component of the ipsilateral, central tegmental tract. Initially, this tract lies lateral to the medial longitudinal fasciculus and dorsolateral to both the red nucleus and the decussation of the superior cerebellar peduncles (Fig. 19.9, Fig. 19.10, Fig. 19.11, Fig. 19.18). Most fibres arise from the parvocellular part of the red nucleus and pass to the ipsilateral inferior olivary nucleus in the medulla. Some tract fibres terminate in the brain stem reticular nuclei. Ascending and descending axons from the brain stem reticular formation also run in the central tegmental tract. These axons include dorsal and ventral ascending noradrenergic bundles, a ventral ascending serotoninergic bundle, and some fibres of dorsal and ventral ascending cholinergic bundles.
As mentioned elsewhere (p. 269), lesions of the corticospinal system in man result in permanent paresis or paralysis. In monkeys, although initially complete, the paralysis disappears and good recovery ensues. The explanation for this interprimate variability in recovery from corticospinal lesions appears to lie in the differential capacity of the rubrospinal system to compensate for loss of corticospinal drive. Monkeys never fully recover from combined lesions of both the corticospinal and rubrospinal tracts, which suggests that the two systems are functionally interrelated in the control of movement. Both encode force, velocity and direction parameters, but the rubrospinal system primarily directs activity both during the terminal phase of a movement and preceding a movement. There is, thus, overlap of activity in the two systems for all parameters during movements of limbs and even of individual digits. The corticospinal system is most active during the learning of new movements, whereas the rubrospinal system is most active during the execution of learnt automated movements.
Oculomotor nucleus
The nuclear complex from which the efferent fibres of the oculomotor nerve arise consists of several groups of large motor neurones (collectively, the oculomotor nucleus; Fig. 19.19) and smaller preganglionic parasympathetic neurones (the Edinger–Westphal nucleus). The motor neurone groups of the oculomotor nucleus innervate, in dorsoventral order, the ipsilateral inferior rectus, inferior oblique and medial rectus. A medially placed column of cells innervates the contralateral superior rectus: the axons from this subnucleus decussate in its caudal part. The medial rectus subnucleus consists of three anatomically distinct subpopulations. The ventral portion, which contains the largest number of motor neurones, occupies the rostral two-thirds; a subpopulation of smaller motor neurones lies dorsally throughout the rostral two-thirds of the nucleus and innervates the small orbital fibres of the medial rectus; and another subpopulation lies dorsolaterally in the caudal two-thirds of the nucleus.
The Edinger–Westphal nucleus, composed of small, multipolar, preganglionic parasympathetic neurones, lies dorsal to the main oculomotor nucleus. Its neurones give rise to axons that travel in the oculomotor nerve and synapse with postganglionic neurones in the ciliary ganglion.
Trochlear nucleus
The trochlear nucleus lies in the grey matter in the floor of the cerebral aqueduct, level with the upper part of the inferior colliculus (Fig. 19.1, Fig. 19.18). It is in line with the ventromedial part of the oculomotor nucleus, in the position of the somatic efferent column. The medial longitudinal fasciculus is ventral and lateral to it. The oculomotor and trochlear nuclei often overlap slightly but can be distinguished by the smaller size of the trochlear neurones. The afferent inputs to the trochlear nucleus are similar to those described for the oculomotor nucleus. Trochlear efferent fibres pass laterodorsally round the central grey matter, then descend medial to the trigeminal mesencephalic nucleus to reach the upper end of the superior medullary velum, where they decussate and emerge lateral to the frenulum. A few fibres remain ipsilateral.
Medial longitudinal fasciculus
The medial longitudinal fasciculus (Fig. 19.9, Fig. 19.18, Fig. 39.12) is a heavily myelinated composite tract, lying near the midline, ventral to the periaqueductal grey matter. It ascends to the interstitial nucleus of Cajal, which lies in the lateral wall of the third ventricle, just above the cerebral aqueduct. The fasciculus retains its position relative to the central grey matter through the midbrain, pons and upper medulla, but is displaced ventrally by successive decussations of the medial lemnisci and lateral corticospinal tracts. At spinal levels it is synonymous with the medial vestibulospinal tract.
The medial longitudinal fasciculus interconnects the oculomotor, trochlear, abducens, Edinger–Westphal, vestibular, reticular and spinal accessory nuclei, coordinating conjugate eye movements and associated movements of the head and neck. Lesions cause internuclear ophthalmoplegia. All four vestibular nuclei contribute ascending fibres. Those from the superior nucleus remain uncrossed, while the others are partly crossed. Some fibres reach the interstitial and posterior commissural nuclei, and some decussate to the contralateral nuclei. Descending axons, from the medial vestibular nuclei and perhaps the lateral and inferior nuclei, partially decussate and descend in the fasciculus to form the medial vestibulospinal tract (Fig. 18.19). Fibres join the fasciculus from the dorsal trapezoid, lateral lemniscal and posterior commissural nuclei, which means that both the cochlear and vestibular components of the vestibulocochlear nerve may influence movements of the eyes and head via the medial longitudinal fasciculus. Some vestibular fibres may ascend in the medial longitudinal fasciculus as far as the thalamus.
Tectum
Inferior colliculus
The inferior colliculus (Fig. 19.18) is part of the ascending auditory pathway (Ch. 37) and is the principal site of termination of the lateral lemniscus. It has a central, ovoid, main nucleus, which is continuous with the periaqueductal grey matter. It is surrounded by a lamina of nerve fibres, many from the lateral lemniscus, which terminate in it. The central nucleus has dorsomedial and ventrolateral zones, which are covered by a dorsal cortex. In humans, the cortex has four cytoarchitectonic layers: layer I contains small neurones with flattened radial dendritic fields; layer II, medium-sized neurones with ovoid dendritic fields aligned parallel with the collicular surface; layer III, medium-sized neurones with spherical dendritic fields; and layer IV, large neurones with variably shaped dendritic fields. The central nucleus is laminated. Bands of cells with disc-shaped or stellate dendritic fields orthogonally span the fibre layers in which the terminals of lateral lemniscal fibres ramify. The neurones are sharply tuned to frequency, and the laminae may represent the structural basis of tonal discrimination. Experimental studies have found cells driven by low frequencies in the dorsal laminae, and others driven by high frequencies in the ventral laminae. Neurones are broadly frequency-tuned in the dorsal cortex and lateral nucleus.
Superior colliculus
The superior colliculus receives afferents from many sources including the retina, spinal cord, inferior colliculus and occipital and temporal cortices. The first three of these pathways convey visual, tactile and probably thermal, pain and auditory impulses. Collicular efferents pass to the retina, lateral geniculate nucleus, pretectum, parabigeminal nucleus, the inferior, medial and lateral pulvinar, and to numerous sites in the brain stem and spinal cord. Fibres passing from the pulvinar are relayed to primary and secondary visual cortices and form an extrageniculate retinocortical pathway for visual orientation and attention.
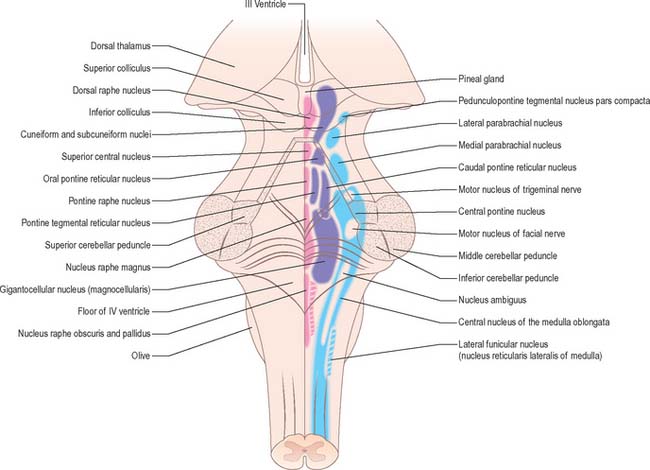
BRAIN STEM RETICULAR FORMATION
In general terms, the reticular formation is a continuous core that traverses the whole brain stem, and is continuous below with the reticular intermediate spinal grey laminae. It is divisible, on the basis of cytoarchitectonic, chemoarchitectonic and functional criteria, into three bilateral longitudinal columns: median; medial, containing mostly large reticular neurones; and lateral, containing mostly small to intermediate neurones (Fig. 19.20).
MEDIAN COLUMN OF RETICULAR NUCLEI
Raphe spinal serotoninergic axons originate mainly from neurones in the raphe magnus, pallidus and obscurus nuclei. They project as ventral, dorsal and intermediate spinal tracts in the ventral and lateral funiculi, and terminate respectively in the ventral horns and laminae I, II and V of the dorsal horns of all segments, and in the thoracolumbar intermediolateral sympathetic and sacral parasympathetic preganglionic cell columns. The dorsal raphe spinal projections function as a pain control pathway that descends from a mesencephalic pain control centre located in the periaqueductal grey matter, dorsal raphe and cuneiform nuclei (Ch. 18). The intermediate raphe-spinal projection is inhibitory, and, in part, modulates central sympathetic control of cardiovascular function. The ventral raphe spinal system excites ventral horn cells and could function to enhance motor responses to nociceptive stimuli.
Principally, the mesencephalic serotoninergic raphe system is reciprocally interconnected rostrally with the limbic system, septum, prefrontal cortex and hypothalamus. Efferents ascend and form a large ventral and a diminutive dorsal pathway. Both originate from neurones in the dorsal and central superior raphe nuclei. The raphe magnus nucleus also contributes to the dorsal ascending serotoninergic pathway, which is at first incorporated into the dorsal longitudinal fasciculus (of Schütz). A few fibres terminate in the central mesencephalic grey matter and posterior hypothalamus, but most continue into the medial forebrain bundle and merge with the axons of the ventral pathway, which are distributed to the same targets. The fibres of the ventral ascending serotoninergic pathway exit the ventral aspect of the mesencephalic raphe nuclei, and then course rostrally through the ventral tegmentum from where fibres pass to the ventral tegmental area, substantia nigra and interpeduncular nucleus. A large number of fibres then enter the habenulointerpeduncular tract and run rostrally to innervate the habenular nucleus, intralaminar, midline, anterior, ventral and lateral dorsal thalamic nuclei, and the lateral geniculate body. The ventral ascending serotoninergic pathway enters the medial forebrain bundle in the lateral hypothalamic area and splits to pass medially and laterally. The fibres in the medial tract terminate in the mammillary body, dorsomedial, ventromedial, infundibular, anterior and lateral hypothalamic, medial and lateral preoptic and suprachiasmatic nuclei. Those in the lateral tract take the ansa peduncularis–ventral amygdalofugal path to the amygdala, striatum and caudal neocortex. The medial forebrain bundle carries the remaining ventral ascending serotoninergic axons into the medullary stria, stria terminalis, fornix, diagonal band, external capsule, cingulate fasciculus and medial olfactory stria, to terminate in all the structures that these systems interconnect.
LATERAL COLUMN OF RETICULAR NUCLEI
Bilateral projections from the micturition centres travel in the lateral spinal funiculus. They terminate on preganglionic parasympathetic neurones in the sacral cord (which innervate the detrusor muscle in the urinary bladder), and on neurones in the nucleus of Onuf (which innervate the musculature of the pelvic floor and the anal and urethral sphincters) (Ch. 75).
The A1, A2, A5 and A7 noradrenergic cell groups project rostrally, mainly through the central tegmental tract. Their axons constitute a major longitudinal catecholamine pathway that continues through the medial forebrain bundle and ends in the amygdala, lateral septal nucleus, bed nucleus of the stria terminalis, nucleus of the diagonal band and the hypothalamus. The ascending dorsal periventricular pathway contains a few non-coerulean noradrenergic fibres, which terminate in the periventricular region of the thalamus.
BRAIN STEM LESIONS
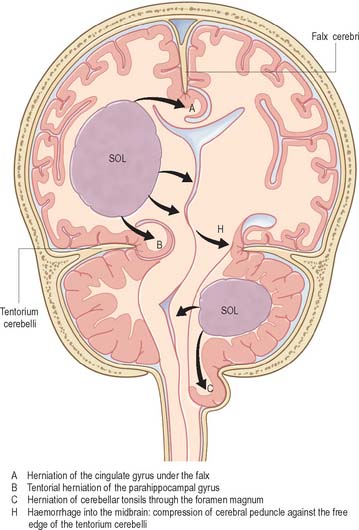
Unilateral brain stem lesions (Fig. 19.21, Fig. 19.22) may arise as a result of extrinsic compression of the brain stem by space occupying tumours (e.g. meningioma, acoustic neuroma or metastatic carcinoma) or may be caused by intrinsic disease (e.g. glioma, demyelination or stroke). The clinical syndrome is determined by the neuroanatomical site of the lesion. At the segmental level, an ipsilateral cranial nerve palsy occurs. Below the level of the lesion, there is a contralateral loss of power and sensation in the limbs (corresponding to dysfunction of the decussating corticospinal and ascending sensory pathways), and ipsilateral incoordination of the limbs (as a result of the interruption of efferent and afferent cerebellar connections).
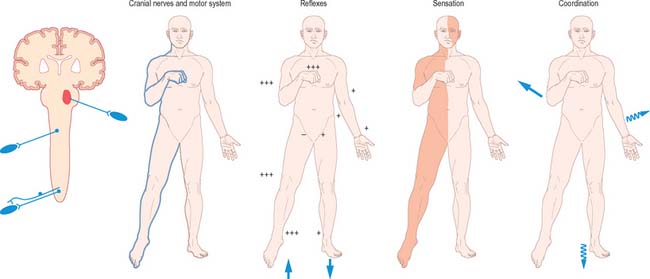
Fig. 19.21 Brain stem lesions.
(By permission from Crossman AR, Neary D 2000 Neuroanatomy, 2nd edn. Edinburgh: Churchill Livingstone.)
Bilateral destructive lesions of the brain stem are fatal if untreated, because of damage to ‘centres’ in the medulla that control respiration, heart rate and blood pressure. Impairment of the reticular activating system in the core of the brain stem leads to progressive impairment of consciousness, followed by stupor and coma. In this state of ‘brain stem death’, life can only be supported artificially. This is the fate of all untreated expanding space-occupying lesions in the cranium (e.g. haematoma, abscess, tumour, whether extrinsic or intrinsic to the brain, and cerebral oedema). A space-occupying lesion within the unyielding skull raises the intracranial pressure directly and also indirectly by obstruction of CSF flow, which causes headache and papilloedema. The brain is distorted and displaced downward (rostro-caudally) within the skull and meningeal framework. The brain stem is vulnerable to compression at two critical sites, which are determined by the neuroanatomical relationship of the meningeal tentorium and foramen magnum to the cerebral hemisphere (supratentorial) and brain stem (infratentorial). The downward displacement of the cerebral hemisphere leads to herniation of the ipsilateral medial temporal lobe (uncus) through the tentorial notch. There may be direct ipsilateral compression of the midbrain and emergent oculomotor and trochlear cranial nerves or contralateral compression of the upper brain stem by the abutting sharp edge of the tentorium. The ipsilateral posterior cerebral artery is vulnerable to compression at this site. Unilateral herniation is heralded by a progressive oculomotor nerve palsy (ophthalmoplegia, pupillary dilatation and ptosis), contralateral limb weakness, falling level of consciousness, and, if treatment is long delayed, a contralateral homonymous hemianopia. Compression of the contralateral brain stem by the tentorium leads to ipsilateral ‘false localizing’ signs.
Brodal P, Bjaalie JG. Organization of the pontine nuclei. Neurosci Res. 1992;13:83-118.
Ciriello J. Brainstem projections of aortic baroreceptor afferent fibres in the rat. Neurosci Lett. 1983;36:37-42.
Dahlström A, Fuxe K. Evidence for the existence of monamine-containing neurones in the central nervous system. Acta Physiol Scand Suppl. 1964;232:1-55.
Dahlström A, Fuxe K. Evidence for the existence of monoamine neurones in the central nervous system. II. Experimentally induced changes in the intraneuronal amine levels of bulbospinal neurone systems. Acta Physiol Scand Suppl. 1965;247:1-36.
Hamilton RB, Norgren R. Central projections of gustatory nerves in the rat. J Comp Neurol. 1984;222:560-577.
Johnston JB. The morphology of the forebrain vesicle in vertebrates. J Comp Neurol. 1909;19:458-539.
Millar J, Basbaum AI. Topography of the projection of the body surface of the cat to cuneate and gracile nuclei. Exp Neurol. 1975;49(1 pt 1):281-290.
Nathan PW, Smith MC. The rubrospinal and central tegmental tracts in man. Brain. 1982;105:223-269.
Nieuwenhuys R, Voogd J, van Huijzen C. The Human Central Nervous System. A Synopsis and Atlas. 3rd edn.. Berlin: Springer Verlag; 1988.
Olszewski J. On the anatomical and functional organization of the spinal trigeminal nucleus. J Comp Neurol. 1950;92:401-409.