148 Botulism
Botulism is the neuroparalytic disorder resulting from intoxication with exotoxins produced by Clostridium botulinum and several other strains of clostridia. C. botulinum are spore-forming obligate anaerobic bacilli1 whose heat-resistant spores are widely distributed in soil and marine sediment throughout the world.2 The term botulism is derived from the Latin word for “sausage,” botulus. Botulism initially was recognized as sausage poisoning in Europe in the early 19th century. Kerner, a German health official, characterized the relationship between sausage ingestion and paralysis in 230 people in 1820.3 The toxin and the bacterium were initially demonstrated by Van Ermengem4 in his study of an epidemic of foodborne botulism following raw ham consumption at a Belgian funeral music festival in 1895. In addition to foodborne botulism after ingestion of preformed toxin, forms of botulism owing to in vivo toxin production subsequently were recognized, including wound botulism in 1943,5 infant botulism in 1976,6,7 and adult intestinal botulism in 1986.8 Inhalational botulism has been identified only in a single outbreak in humans9 but has received more recent attention related to the potential for aerosolized toxin used as a biological weapon.10
 Toxin Characteristics
Toxin Characteristics
Seven distinct serotypes of botulinum toxin, A through G, are defined by the absence of cross-neutralization with antitoxin.11 Human disease is produced by types A, B, E, and rarely F toxin, whereas types C and D produce disease in birds and mammals.12 Type G has been implicated in human disease only rarely.12,13 Neurotoxigenic strains of Clostridium baratii may produce type F toxin,14,15 and some strains of Clostridium butyricum may produce type E toxin.16
Botulinum toxins are 150-kD polypeptides that are converted during bacterial lysis by proteases into an active form consisting of a 50-kD light chain and a 100-kD heavy chain joined by a disulfide bond.17 After absorption into the systemic circulation, the carboxy-terminal domain of the heavy chain facilitates binding of the toxin to polysialoganglioside receptors on neuronal membranes, whereas the amino-terminal domain of the heavy chain mediates translocation of the toxin into motor or autonomic neurons.18,19 The light chain is a zinc endopeptidase that cleaves a toxin-specific location of one or more of the SNARE (soluble N-ethylmaleimide-sensitive fusion associated protein receptor) proteins mediating the docking and fusion of acetylcholine vesicles with the presynaptic membrane at the neuromuscular junction, in autonomic ganglia, and in parasympathetic nerve terminals.20 SNARE proteins, SNAP-25 (synaptosomal-associated protein of 25 kD) and syntaxin, are associated with the presynaptic membrane, whereas synaptobrevin or vesicle-associated membrane protein (VAMP) is located on the synaptic vesicle membrane. SNAP-25 is cleaved by types A, C, and E toxins, and syntaxin is cleaved by type C toxin.21–23 Synaptobrevin is cleaved by types B, D, F, and G and tetanus toxins.24–26 After cleavage of SNARE proteins by botulinum toxin, the release of acetylcholine is permanently halted at affected synapses. Recovery from botulism occurs when the presynaptic neuron sprouts another nerve terminal to reform the cholinergic synapse.27–29 The original synapse remains intact, however, and over a period of months becomes functional again. After this occurs, the new synapse is pruned.
Botulinum toxin is considered to be the most toxic substance by weight,30 with a lethal dose in humans estimated to be approximately 1 ng/kg of type A toxin.31 By extrapolation from primate studies,32 the lethal dose of type A toxin for a 70-kg man is estimated to be 70 µg by mouth, 0.70 to 0.90 µg by inhalation, and 0.09 to 0.15 µg by intramuscular or intravenous routes.33 In contrast to the heat-resistant spores of C. botulinum, the toxins are heat labile and are inactivated by heating to 85°C for at least 5 minutes.34
 Forms of Human Botulism
Forms of Human Botulism
Foodborne Botulism
Ingestion of contaminated food, with absorption of toxin from the duodenum and jejunum, causes foodborne botulism. Because several individuals may be exposed to a single contaminated food source, foodborne botulism often presents in outbreaks. The average annual number of foodborne botulism cases in the United States between 1973 and 1998 was 24 (range 14-94),35 with an average of 9.4 outbreaks a year between 1950 and 1996.12 The most frequently implicated foods include home-canned vegetables, fruits, and fish.12 Failure to use a proper combination of heat, pressure, and time to kill spores during home canning, particularly with low-acid (pH > 5) foods, may permit survival and germination of spores.36 Although restaurant and commercially prepared foods are responsible for fewer outbreaks (7% from 1950-1996),12 nearly half of foodborne cases may arise from these sources.37 Fish preparation using fermentation among Alaskan natives is responsible for a large fraction of the total cases (29% from 1973-1998).12
Foodborne botulism due to type A toxin is most common in the United States, constituting 45% of outbreaks compared with 36% of outbreaks due to type E and 13% due to type B toxin during the period 1990 to 1996. Type F foodborne outbreaks are rare in the United States.12 The geographic distribution of foodborne botulism outbreaks mirrors the type of spores residing in soil. Type A spores predominate in the western United States, and type B spores predominate in the northeastern and central United States.38,39 Type E spores are found in marine life and sediments.40,41 In a corresponding fashion, during the period 1950 to 1996, 86% of the type A outbreaks occurred west of the Mississippi River, whereas 61% of the type B outbreaks were from eastern states. Marine products have been implicated in 91% of type E outbreaks.12
Signs and symptoms of foodborne botulism generally develop within 12 to 36 hours of ingestion of contaminated food, with the acuity and severity of illness related to the amount of toxin absorbed. In general, a symmetrical, descending paralysis with multiple cranial neuropathies evolves rapidly in the absence of fever or altered sensorium. In foodborne botulism, initial symptoms are often gastrointestinal (GI) and include nausea, vomiting, diarrhea, and abdominal cramping, which may be due to ingestion of other bacterial metabolites along with botulinum toxin in contaminated food.42 Parasympathetic dysfunction may present early with dry mouth and blurred vision associated with dilated, poorly reactive pupils. Diplopia often develops secondary to extraocular muscle weakness with paretic, disconjugate eye movements. With paralysis of bulbar muscles, patients may exhibit flaccid dysarthria, chewing difficulty, and dysphagia. The upper extremities, trunk, and lower extremities may become paretic in a descending fashion. Autonomic dysfunction may manifest as GI dysmotility, orthostatic hypotension, altered resting pulse, urinary retention, or hypothermia.43
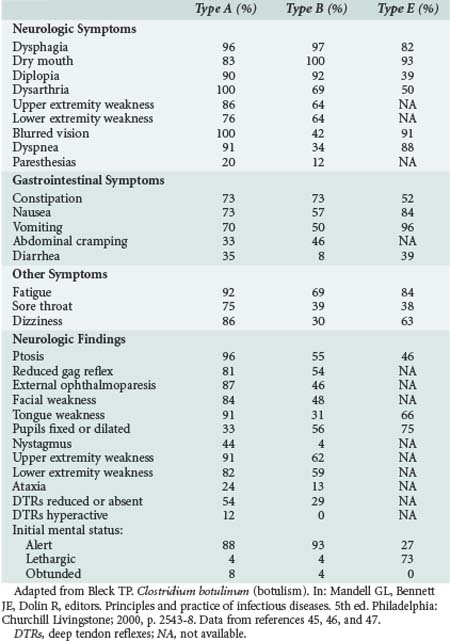
Respiratory compromise may occur secondary to a combination of upper airway obstruction from weak oropharyngeal muscles and diaphragmatic weakness. Requirements for mechanical ventilation are more prolonged for patients with type A disease (mean 58 days) compared with patients with type B disease (mean 26 days).44 The clinical findings related to intoxication with various types of botulinum toxin are varied (Table 148-1), with type A disease causing more frequent extraocular and bulbar muscle weakness, and type B and E disease causing relatively more pupillary and autonomic dysfunction.45–47
With improvements in respiratory care, the case-fatality rate has diminished from 60% during 1899 to 1949 to 12.5% during 1950 to 1996.12 The fatality risk for the index case in an outbreak is 25%, with a 4% fatality risk for subsequent cases after recognition of an outbreak.48 Because of the potential for exposure of other individuals to a contaminated food source and for additional cases accumulating from previous exposure, every case of suspected foodborne botulism should be reported to local and state public health authorities.
Wound Botulism
Wound botulism results from in vivo toxin production in abscessed and devitalized wounds.49 In the event of contamination by spores, these wounds provide an ideal anaerobic environment for spore germination and local colonization by C. botulinum with absorption of toxin into systemic circulation. In contrast to the rapid onset of botulism in foodborne disease with ingestion of preformed toxin, the incubation period for wound botulism is 7 days (range 4-14 days).50 Single cases occur in isolation with a case-fatality rate of approximately 15%.51 Before 1980, wound botulism was a rare disorder generally associated with deep wounds containing avascular areas. Between 1943 and 1985, 33 cases were reported in the United States.12 During the period 1986 through 1996, 78 cases of wound botulism were reported in the United States, most related to subcutaneous injection or “skin popping” of black tar heroin.12,52 Wound botulism due to sinusitis after repeated cocaine inhalation also has been observed.53 The neurologic signs and symptoms are virtually identical to foodborne disease except for the absence of prodromal GI symptoms.12 When present, fever is related to the wound infection.54 The diagnosis should be suspected in patients with a drug-injection history and without known exposure to a contaminated food source.55
Intestinal Botulism
Infant Intestinal Botulism
Infant and adult intestinal botulism result from the ingestion of C. botulinum spores that germinate, colonize the large intestine, and produce botulinum toxin in vivo.6 Infant intestinal botulism is now recognized as the most common form of botulism in the United States, with approximately 100 cases reported annually. About half of the cases relate to type A toxin, and the other half to type B intoxication.12 Most individual cases occur sporadically, although rare unexplained clusters are reported.56–58 Since the recognition of infant intestinal botulism in 1976, nearly half of the reported cases have occurred in California. The geographic distribution of infant botulism is unexplained, with the highest incidence rates observed in Delaware, Hawaii, Utah, and California.12 The average age of onset is 13 weeks, and most cases occur before 6 months, although some cases have occurred at 15 months of age.12
Ingestion of ambient C. botulinum spores, distributed widely in soils and dust, is thought to represent the primary route of exposure.56 Honey is also a source of spores and has been implicated as a significant risk for infant intestinal botulism.59–61 In an animal model of infant intestinal botulism, mice between 7 and 13 days old proved susceptible to intestinal colonization with C. botulinum after intragastric injection of spores.62 Epidemiologic studies suggest a parallel peak human susceptibility to intestinal colonization by C. botulinum between 2 and 4 months of age.63 This susceptibility appears related to the intestinal flora in the immature infant GI tract. The resident flora are influenced by an infant’s food sources,64 although the potential significance of breastfeeding versus formula feeding as a risk for infant intestinal botulism is unresolved.65
A clinical spectrum of disease exists, with some infants exhibiting relatively mild and limited disease involving several days of constipation, poor feeding, and lethargy, and other infants developing acute tetraparesis and respiratory failure.65 In classic cases, constipation is often the initial symptom, followed by lethargy, poor feeding, and weak cry. Examination reveals hypotonia with head lag, ptosis, reduced facial expression, and reduced gag, suck, and swallow reflexes. Deep tendon reflexes are reduced or absent. Extraocular movements are often paretic, and pupils may be large and poorly reactive. In one series, more than half of the patients were intubated and mechanically ventilated, usually following loss of protective upper airway reflexes.66 Although the course is variable, most hospitalized infants reach maximal paralysis at approximately 1 to 2 weeks after hospitalization and begin to improve after 1 to 3 weeks.65 In California between 1976 and 1991, the average length of hospitalization was 4.9 weeks. A longer length of stay was documented for type A cases (5.7 weeks) compared with type B cases (3.6 weeks), suggesting that type A intoxication causes more severe disease.65 The case-fatality rate is less than 1% in hospitalized patients in the United States.56
Adult Intestinal Botulism
Children and adults also may be susceptible to intestinal colonization and in vivo toxin production by C. botulinum, C. baratii, or C. butyricum when the gastric barrier is compromised and the intestinal flora are altered.8,15,67,68 Previously classified by the Centers for Disease Control and Prevention (CDC) as “botulism of undetermined origin,” adult intestinal botulism has occurred in the setting of intestinal surgery, gastric achlorhydria, broad-spectrum antibiotic treatment, and inflammatory bowel disease.69–71 Although adult intestinal botulism is uncommon (10 cases reported between 1986 and 1996),71 it is probably underdiagnosed.
Inhalational Botulism
Inhalational botulism does not occur in nature but is the result of an attempt to use the toxin in aerosolized form as a bioweapon.10 The three documented human cases were reported from Germany in 1962, when botulinum toxin type A became accidentally reaerosolized during disposal of laboratory animals.9 These patients initially developed dysphagia on day 3 after exposure and exhibited tonic pupils, paretic eye movements, dysarthria, and diffuse weakness by day 4. In animal experiments, monkeys became symptomatic 12 to 18 hours after exposure to aerosolized toxin, with descending paralysis and death in some animals.72 Aerosolized botulinum toxin was released by the Japanese religious cult, Aum Shinrikyo, on several occasions in the 1990s in Japan, although the attacks were not known to have produced human illness.10 By the time of the 1991 Persian Gulf War, the state of Iraq had produced large quantities of concentrated botulinum toxin which were loaded onto weapons for military use but never deployed.73
Release of aerosolized toxin has the potential to produce a botulism outbreak. The features of such an outbreak that might suggest a deliberate release of toxin10 include: numerous cases within an outbreak (the mean number of cases in foodborne outbreaks has averaged 2.5 for many years)12; toxin types within an outbreak that rarely cause natural disease (type C, D, F, G, or E not related to marine sources); outbreaks with a common geographic factor without a common dietary exposure; and multiple simultaneous outbreaks.74
Iatrogenic or Inadvertent Botulism
The therapeutic use of botulinum toxin for dystonia, spasticity, hyperhidrosis, sialorrhea, and other conditions occasionally has resulted in inadvertent paresis of nearby noninjected muscles, such as dysphagia in neck muscle injections for cervical dystonia75 and jaw dislocation after parotid injections for sialorrhea in amyotrophic lateral sclerosis.76 Although there are rare reports of paretic muscles distant to the site of injection,77,78 it has been estimated that healthy patients would require a 10-fold toxin overdosing to develop systemic symptoms.79 Nevertheless, single-fiber electromyography studies showed abnormal neuromuscular transmission in muscles distant to botulinum toxin injections.80–82 Patients with underlying neuromuscular disorders seem to be predisposed to developing generalized weakness after therapeutic intramuscular botulinum toxin injections.83–85 Systemic autonomic dysfunction also has been noted after therapeutic injections of type B toxin.86
 Diagnosis
Diagnosis
The diagnosis of botulism is primarily clinical, with the use of laboratory techniques initially to exclude other diagnoses. Currently the confirmation of a diagnosis of botulism and the identification of the toxin type takes several days. Recommendations for specimen acquisition and diagnostic testing change over time; the most current recommendations are Available at: http://www.bt.cdc.gov/agent/botulism/lab-testing.asp.
Electrodiagnostic Studies
Electrodiagnostic studies may confirm the presence of a presynaptic neuromuscular junctional disorder and strongly suggest the diagnosis of botulism or may support an alternative diagnosis. In botulism, sensory nerve conduction studies should be normal, and no evidence for segmental demyelination on motor nerve conduction studies (e.g., prolonged F latencies, conduction block, temporal dispersion) should be observed to suggest Guillain-Barré syndrome. Compound muscle action potential amplitudes commonly are reduced in clinically affected muscles.87 The more affected muscles are often proximal ones, however, and routine motor nerve conduction studies recorded in intrinsic hand or foot muscles may fail to detect this nonspecific abnormality. In contrast with myasthenia gravis88 and Lambert-Eaton myasthenic syndrome,89 low-frequency (2-3 Hz) repetitive nerve stimulation studies in botulism rarely show a decremental response in terms of reduced amplitude and area of the compound muscle action potential elicited by stimulating a motor nerve.87
The characteristic finding in a presynaptic neuromuscular junctional disorder such as botulism is postexercise facilitation or posttetanic facilitation with high-frequency (20-50 Hz) repetitive nerve stimulation. Although Lambert-Eaton myasthenic syndrome and botulism share this finding, posttetanic facilitation may be less prominent90 and more sustained91 in botulism. Proximal muscles also may exhibit a comparatively greater degree of facilitation in botulism.90 Posttetanic facilitation may be more common in type B botulism compared with type A disease.92
Needle electromyography may reveal low-amplitude, short-duration motor unit potentials with an unstable firing pattern, although this nonspecific finding may be observed in many motor unit disorders.90 Positive sharp waves and fibrillation potentials also are observed in about half of cases.93
Single-fiber electromyography uses statistical analysis of muscle fiber action potentials generated by the same motor neuron to evaluate neuromuscular transmission. It is the most sensitive diagnostic test for detecting abnormal neuromuscular transmission, although it cannot distinguish reliably between presynaptic neuromuscular disorders. In botulism, it is more sensitive than repetitive nerve stimulation studies for showing neuromuscular junctional pathology.94–96
Mouse Bioassay
Confirmatory testing for botulism currently involves a mouse bioassay, which is available only through the CDC and several state laboratories. In the mouse bioassay, mice are inoculated with type-specific antisera and patient serum or extracts from samples of body fluids or suspicious foodstuffs. In a positive mouse bioassay, all the mice die except those receiving the antisera matching the botulinum toxin type present in the patient or food specimens. Test results are generally available within 1 or 2 days after inoculation.10 Details regarding specimen preparation and handling are available online.12 Specimen samples in suspected cases of foodborne botulism should include serum, feces, gastric aspirates, vomitus, and foods suspected to be contaminated. In wound botulism, serum, feces, exudate, débrided tissue, and wound swab samples should be examined. In intestinal botulism, serum and feces should be sampled.12 To obtain an adequate fecal sample, particularly in infant botulism where constipation is common, an enema using sterile, non-bacteriostatic water may be necessary. In addition to administering extracts to mice, the samples are anaerobically cultured, and the culture isolates are evaluated using the mouse bioassay.
Mass spectroscopy for detection and characterization of botulinum toxins is emerging as a valuable diagnostic technique.97 Molecular diagnostic techniques are under development and should eventually replace older techniques.
 Management
Management
All cases of suspected botulism should be reported to the hospital epidemiologist or infection control officer and to local or state health departments and the CDC for cases in the United States. This reporting is essential to coordinate laboratory testing and shipment of antitoxin and to initiate investigation of the toxin source.10 Patients with suspected or confirmed botulism should be monitored carefully in an intensive care unit (ICU), with particular attention to their ability to protect the upper airway. Many patients require intubation and mechanical ventilation. Purgatives or activated charcoal may be useful if there is suspicion of residual contaminated food in the GI tract.54 The use of antibiotics that impair neuromuscular transmission should be avoided, particularly aminoglycosides and macrolides.98,99
Botulinum antitoxins may reduce the duration and severity of neurologic dysfunction associated with botulism if administered early in the course of disease. The effect of antitoxin is limited to circulating toxin, and the paralytic effects of previously bound and internalized toxin are not reversed by antitoxin.48,65 Four types of antitoxin currently are available in the United States: (1) a licensed bivalent (A, B) human antiserum for infant botulism, (2) a licensed bivalent (A, B) equine antiserum, (3) an investigational monovalent (E) equine antiserum, and (4) an investigational heptavalent (A, B, C, D, E, F, G) antiserum.100
Botulism Immune Globulin Intravenous (Human)
Botulism immune globulin intravenous (BIG-IV) is a licensed, bivalent human antiserum (type A, B) available for treatment of infant botulism. A 5-year randomized, double-blinded, placebo-controlled treatment trial demonstrated the safety and efficacy of botulism immune globulin intravenous in infant botulism. The mean length of hospital stay was significantly reduced in patients receiving BIG-IV (from 5.5 weeks to 2.5 weeks).65 BIG-IV was officially licensed by the U.S. Food and Drug Administration (FDA) in October 2003 for treatment of infant botulism types A and B under the proprietary name of BabyBIG. BIG-IV is available through the California Department of Health Services (24-hour telephone number: 510-231-7600). Current information on infant botulism treatment is Available at: http://www.infantbotulism.org.
Serotherapy
A heptavalent despeciated equine antitoxin (H-BAT) is available from the U.S. Centers for Disease Control and Prevention, and is essentially devoid of serum sickness risk. H-BAT is available through state health departments; more information is Available at: http://www.cdc.gov/laboratory/drugservice/formulary.html#ia. and through http://www.infantbotulism.org/general/babybig.php.
Pentavalent Toxoid
An investigational pentavalent toxoid (type A, B, C, D, E) for preexposure prophylaxis is available for military personnel and laboratory workers. A primary series of immunizations is given at 0, 2, and 12 weeks, followed by a 1-year booster.100,101 Because it induces immunity over several months, the toxoid is not appropriate for postexposure prophylaxis.10
Key Points
Arnon SS. Infant botulism. In: Feigin RD, Cherry JD, editors. Textbook of pediatric infectious diseases. 5th ed. Philadelphia: Saunders; 2004:1758-1766.
Arnon SS, Schechter R, Inglesby TV, et al. Botulinum toxin as a biological weapon: medical and public health management. JAMA. 2001;285:1059-1070.
Centers for Disease Control and Prevention. Botulism in the United States, 1899-1996: handbook for epidemiologists, clinicians, and laboratory workers. Atlanta: Centers for Disease Control and Prevention; 1998.
Available at: http://www.cdc.gov/ncidod/dbmd/diseaseinfo/botulism.pdf. This comprehensive work provides contemporary epidemiologic information relating to all forms of human botulism and detailed information relating to laboratory confirmation and specimen preparation and handling.
Hughes JM, Blumenthal JR, Merson MH, et al. Clinical features of types A and B foodborne botulism. Ann Intern Med. 1981;95:442-445.
1 Cato EP, George WL, Finegold SM. Genus Clostridium praemozski 1880, 23AL. In: Smeath PHA, Mair NS, Sharpe ME, Holt JG, editors. Bergey’s Manual of Systematic Bacteriology, vol. 2. Baltimore: Williams & Wilkins; 1986:1141-1200.
2 Hauschild AHW. Clostridium botulinum. In: Doyle MP, editor. Foodborne Bacterial Pathogens. New York: Marcel Dekker; 1989:112-189.
3 Kerner J. Neue Beobachtungen über die in Würtemburg so haüfig vorfallen Vergiftung durch den Genuss gerauchter Würst. Tubingen. 1820. Quoted in Damon SR: Food Infections and Food Intoxications. Baltimore: Williams & Wilkins; 1928. p. 67
4 Van Ermengem E. Üeber einen neuen anaeroben Bacillus und seine Beziehungen zum Botulismus. Z Hyg Infekt. 1897;26:1-56.
5 Davis JB, Mattman LH, Wiley M. Clostridium botulinum in a fatal wound infection. JAMA. 1951;146:646-648.
6 Midura TF, Arnon SS. Identification of Clostridium botulinum and its toxins in feces. Lancet. 1976;2:934-936.
7 Pickett J, Berg B, Chaplin E, et al. Syndrome of botulism in infancy: Clinical and electrophysiologic study. N Engl J Med. 1976;295:770-772.
8 Chia JK, Clark JB, Ryan CA, Pollack M. Botulism in an adult associated with food-borne intestinal infection with Clostridium botulinum. N Engl J Med. 1986;315:239-241.
9 Holzer VE. Botulism from inhalation [in German]. Med Klin. 1962;57:1735-1738.
10 Arnon SS, Schechter R, Inglesby TV, et al. Botulinum toxin as a biological weapon: Medical and public health management. JAMA. 2001;285:1059-1070.
11 Hatheway CL. Bacterial sources of clostridial neurotoxins. In: Simpson LL, editor. Botulinum Neurotoxin and Tetanus Toxin. San Diego: Academic Press; 1989:14-25.
12 Centers for Disease Control and Prevention. Botulism in the United States, 1899-1996: Handbook for Epidemiologists, Clinicians, and Laboratory Workers. Atlanta: Centers for Disease Control and Prevention; 1998. Available at: http://www.cdc.gov/ncidod/dbmd/diseaseinfo/botulism.pdf
13 Sonnabend O, Sonnabend W, Heinzle R, et al. Isolation of Clostridium botulinum type G and identification of type G botulinal toxin in humans: Report of five sudden unexpected deaths. J Infect Dis. 1981;143:22-27.
14 Hall JD, McCroskey LM, Pincomb BJ, Hatheway CL. Isolation of an organism resembling Clostridium baratii which produces type F botulinal toxin from an infant with botulism. J Clin Microbiol. 1985;21:654-655.
15 McCroskey LM, Hatheway CL, Woodruff BA, et al. Type F botulism due to neurotoxigenic Clostridium baratii from an unknown source in an adult. J Clin Microbiol. 1991;29:2618-2620.
16 Aureli P, Fenicia L, Pasolini B, et al. Two cases of type E infant botulism caused by neurotoxigenic Clostridium butyricum in Italy. J Infect Dis. 1986;154:207-211.
17 DasGupta BR. The structure of botulinum neurotoxin. In: Simpson LL, editor. Botulinum Neurotoxin and Tetanus Toxin. San Diego: Academic Press; 1989:53-67.
18 Simpson LL. The origin, structure, and pharmacological activity of botulinum toxin. Pharmacol Rev. 1981;33:155-188.
19 Schiavo G, Montecucco C. The structure and mode of botulinum and tetanus toxins. In: Rood J, McClane BA, Songer JG, Titball RW, editors. The Clostridia: Molecular Biology and Pathogenesis. San Diego: Academic Press; 1997:295-322.
20 Schiavo G, Rossetto O, Tonello F, Montecucco C. Intracellular targets and metalloprotease activity of tetanus and botulism neurotoxins. Curr Top Microbiol Immunol. 1995;195:257-274.
21 Blasi J, Chapman ER, Link E, et al. Botulinum neurotoxin A selectively cleaves the synaptic protein SNAP-25. Nature. 1993;365:160-163.
22 Blasi J, Chapman ER, Yamasaki S, et al. Botulinum neurotoxin C1 blocks neurotransmitter release by means of cleaving HPC-1/syntaxin. EMBO J. 1993;12:4821-4828.
23 Foran P, Lawrence GW, Shone CC, et al. Botulinum neurotoxin C1 cleaves both syntaxin and SNAP-25 in intact and permeabilized chromaffin cells: Correlation with its blockade of catecholamine release. Biochemistry. 1996;25:2630-2636.
24 Schiavo G, Benfenati F, Poulain B, et al. Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature. 1992;359:832-835.
25 Pellizzari R, Rossetto O, Lozzi L, et al. Structural determinants of the specificity for synaptic vesicle-associated membrane protein/synaptobrevin of tetanus and botulinum type B and G neurotoxins. J Biol Chem. 1996;271:20353-20358.
26 Pellizzari R, Mason S, Shone CC, Montecucco C. The interaction of synaptic vesicle-associated membrane protein/synaptobrevin with botulinum neurotoxins D and F. FEBS Lett. 1997;409:339-342.
27 Duchen LW. An electron microscopic study of the changes induced by botulism toxin in the motor end-plates of slow and fast skeletal muscle fibres of the mouse. J Neurol Sci. 1971;14:47-60.
28 Duchen LW. Motor nerve growth induced by botulinum toxin as a regenerative phenomenon. Proc R Soc Med. 1972;65:196-197.
29 De Pavia A, Meunier FA, Molgo J, et al. Functional repair of motor end-plates after botulinum neurotoxin type A poisoning: Biphasic switch of synaptic activity between nerve sprouts and their parent terminals. Proc Natl Acad Sci U S A. 1999;96:3200-3205.
30 Lamanna C. The most poisonous poison. Science. 1959;130:763-772.
31 Gill MD. Bacterial toxins: A table of lethal amounts. Microbiol Rev. 1982;46:86-94.
32 Schantz EJ, Johnson EA. Properties and use of botulinum toxin and other microbial neurotoxins in medicine. Microbiol Rev. 1992;56:80-99.
33 Scott AB, Suzuki D. Systemic toxicity of botulinum toxin by intramuscular injection in the monkey. Mov Disord. 1988;3:333-335.
34 Siegel LS. Destruction of botulinum toxin in food and water. In: Hauschild AH, Dodds KL, editors. Clostridium botulinum: Ecology and Control in Foods. New York: Marcel Dekker; 1993:93-101.
35 Centers for Disease Control and Prevention. Outbreak of botulism type E associated with eating a beached whale—Western Alaska, July 2002. MMWR Morb Mortal Wkly Rep. 2003;52:24-26.
36 Benenson AS. Botulism/infant botulism. editor. In: Control of Communicable Diseases Manual. Washington: American Public Health Association; 1995:66-69.
37 MacDonald KL, Cohen ML, Blake PA. The changing epidemiology of adult botulism in the United States. Am J Epidemiol. 1986;124:794-799.
38 Meyer KF, Dubovsky BJ. The distribution of the spores of B. botulinus in the United States. J Infect Dis. 1922;31:559-594.
39 Smith LDS. The occurrence of Clostridium botulinum and Clostridium tetani in the soil of the United States. Health Lab Sci. 1978;15:74-80.
40 Ward BQ, Carroll BJ, Garrett ES, et al. Survey of the U.S. Gulf Coast for the presence of Clostridium botulinum. Appl Microbiol. 1967;15:629-639.
41 Bott TL, Deffner JS, McCoy E, et al. Clostridium botulinum type E in fish from the Great Lakes. J Bacteriol. 1966;91:919-924.
42 Smith LDS. Botulism: The Organism, Its Toxins, the Disease. Springfield, IL: Charles C Thomas; 1977.
43 Vita G, Girlanda P, Puglisi RM, et al. Cardiovascular-reflex testing and single-fiber electromyography in botulism: A longitudinal study. Arch Neurol. 1987;44:202-206.
44 Hughes JM, Blumenthal JR, Merson MH, et al. Clinical features of types A and B foodborne botulism. Ann Intern Med. 1981;95:442-445.
45 Hughes JM, Hatheway CL, Ostroff SM. Botulism. In: Scheld WM, Whitley RJ, Durack DT, editors. Infections of the Central Nervous System. 2nd ed. Philadelphia: Lippincott-Raven; 1997:615-628.
46 Weber JT, Hibbs RG, Darwish A, et al. A massive outbreak of type E botulism associated with traditional salted fish in Cairo. J Infect Dis. 1993;167:451-454.
47 Tacket CO, Rogawski MA. Botulism. In: Simpson LL, editor. Botulinum Neurotoxin and Tetanus Toxin. San Diego: Academic Press; 1989:351-378.
48 Tacket CO, Shandera WX, Mann JM, et al. Equine antitoxin use and other factors that predict outcome in type A foodborne botulism. Am J Med. 1984;76:794-798.
49 Weber JT, Goodpasture HC, Alexander H, et al. Wound botulism in a patient with a tooth abscess: Case report and literature review. Clin Infect Dis. 1993;16:635-639.
50 Merson MH, Dowell VR. Epidemiologic, clinical and laboratory aspects of wound botulism. N Engl J Med. 1973;289:1105-1110.
51 Hatheway CL. Botulism: The present status of the disease. Curr Top Microbiol Immunol. 1995;195:55-75.
52 Passaro DJ, Werner SB, McGee J, et al. Wound botulism associated with black tar heroin among injecting drug users. JAMA. 1998;279:859-863.
53 Kudrow DB, Henry DA, Haake DA, et al. Botulism associated with Clostridium botulinum sinusitis after intranasal cocaine abuse. Ann Intern Med. 1988;109:984-985.
54 Reddy P, Bleck TP. Clostridium botulinum. In: Mandell GM, Bennett JE, Dolin R, editors. Principles and practice of infectious diseases. 7th ed. New York: Churchill, Livingstone, El-sevier; 2010:3097-3102.
55 Centers for Disease Control and Prevention. Wound botulism—California, 1995. MMWR Morb Mortal Wkly Rep. 1995;44:889-892.
56 Centers for Disease Control and Prevention. Infant botulism—New York City, 2001-2002. MMWR Morb Mortal Wkly Rep. 2003;52:21-24.
57 Istre G, Compton R, Novotny T, et al. Infant botulism: Three cases in a small town. Am J Dis Child. 1986;140:1013-1014.
58 Long SS. Epidemiologic study of infant botulism in Pennsylvania: Report of the Infant Botulism Study Group. Pediatrics. 1985;75:928-934.
59 Arnon SS, Midura TF, Damus K, et al. Honey and other environmental risk factors for infant botulism. J Pediatr. 1979;94:331-336.
60 Midura TF, Snowden S, Wood RM, Arnon SS. Isolation of Clostridium botulinum from honey. J Clin Microbiol. 1979;9:282-283.
61 Spika JS, Shaffer N, Hargrett-Bean N, et al. Risk factors for infant botulism in the United States. Am J Dis Child. 1989;143:828-832.
62 Sugiyama H, Mills DC. Intraintestinal toxin in infant mice challenged intragastrically with Clostridium botulinum spores. Infect Immun. 1978;21:59-63.
63 Arnon SS, Damus K, Chin J. Infant botulism: Epidemiology and relation to sudden infant death syndrome. Epidemiol Rev. 1981;3:45-66.
64 Stark PL, Lee A. The microbial ecology of the large bowel of breast-fed and formula-fed infants during the first year of life. J Med Microbiol. 1982;15:189-203.
65 Arnon SS. Infant botulism. In: Feigin RD, Cherry JD, editors. Textbook of Pediatric Infectious Diseases. 5th ed. Philadelphia: WB Saunders; 2004:1758-1766.
66 Schreiner MS, Field E, Ruddy R. Infant botulism: A review of 12 years’ experience at the Children’s Hospital of Philadelphia. Pediatrics. 1991;87:159-165.
67 McCroskey LM, Hatheway CL. Laboratory findings in four cases of adult botulism suggest colonization of the intestinal tract. J Clin Microbiol. 1988;26:1052-1054.
68 Fenecia L, Franciosa G, Pourshaben M, Aureli P. Intestinal toxemia botulism in two young people caused by Clostridium butyricum type E. Clin Infect Dis. 1999;29:1381-1387.
69 Li LYJ, Kelkar P, Exconde RE, et al. Adult-onset “infant” botulism: An unusual case of weakness in the intensive care unit. Neurology. 1999;53:891.
70 Freedman M, Armstrong RM, Killian JM, et al. Botulism in a patient with jejunoileal bypass. Ann Neurol. 1986;20:641-643.
71 Griffin PM, Hatheway CL, Rosenbaum RB, Sokolow R. Endogenous antibody production to botulinum toxin in an adult with intestinal colonization botulism and underlying Crohn’s disease. J Infect Dis. 1997;175:633-637.
72 Franz DR, Pitt LM, Claton MA, et al. Efficacy of prophylactic and therapeutic administration of antitoxin for inhalation botulism. In: DasGupta BR, editor. Botulinum and Tetanus Neurotoxins: Neurotransmission and Biomedical Aspects. New York: Plenum Press; 1993:473-476.
73 Zilinskas RA. Iraq’s biological weapons: The past as future? JAMA. 1997;278:418-424.
74 Bleck TP. Botulism as a potential agent of bioterrorism. In: Fong IW, editor. Bioterrorism and infectious agents: a new dilemma for the 21st century. New York: Springer; 2005:193-204.
75 Comella CL, Tanner CM, DeFoor-Hill L, Smith C. Dysphagia after botulinum toxin injections for spasmodic torticollis: Clinical and radiologic findings. Neurology. 1992;42:1307-1310.
76 Tan EK, Lo YL, Seah A, Auchus AP. Recurrent jaw dislocation after botulinum toxin treatment for sialorrhoea in amyotrophic lateral sclerosis. J Neurol Sci. 2001;190:95-97.
77 Bhatia KP, Munchau A, Thompson PD, et al. Generalised muscular weakness after botulinum toxin injections for dystonia: A report of three cases. J Neurol Neurosurg Psychiatry. 1999;67:90-93.
78 Bakheit AM, Ward CD, McLellan DL. Generalised botulism-like syndrome after intramuscular injections of botulinum toxin type A: A report of two cases. J Neurol Neurosurg Psychiatry. 1997;62:198.
79 Brin MF. Botulinum toxin: Chemistry, pharmacology, toxicity, and immunology. Muscle Nerve. 1997;S6:146-168.
80 Sanders DB, Massey EW, Buckley EC. Botulinum toxin for blepharospasm: Single fiber EMG studies. Neurology. 1986;36:545-547.
81 Lange DJ, Brin MF, Warner CL, et al. Distant effects of local infection of botulinum toxin. Muscle Nerve. 1987;10:552-555.
82 Olney RK, Aminoff MJ, Gelb DJ, Lowenstein DH. Neuromuscular effects distant from the site of botulinum neurotoxin injection. Neurology. 1988;38:1780-1783.
83 Erbguth F, Claus D, Engelhardt A, Dressler D. Systemic effect of local botulinum toxin injections unmasks subclinical Lambert-Eaton myasthenic syndrome. J Neurol Neurosurg Psychiatry. 1993;56:1235-1236.
84 Mezaki T, Kaji R, Kohara N, Kimura J. Development of general weakness in a patient with amyotrophic lateral sclerosis after focal botulinum toxin injection. Neurology. 1996;46:845-846.
85 Borodic G. Myasthenic crisis after botulinum toxin. Lancet. 1998;352:1832.
86 Dressler D, Benecke R. Autonomic side effects of botulinum toxin type B treatment of cervical dystonia and hyperhidrosis. Eur Neurol. 2003;49:34-38.
87 Cherington M. Clinical spectrum of botulism. Muscle Nerve. 1998;21:701-710.
88 Maselli RA. Electrodiagnosis of disorders of neuromuscular transmission: Myasthenia gravis and related diseases. Ann N Y Acad Sci. 1998;841:696-711.
89 Tim RW, Massey JM, Sanders DB. Lambert-Eaton myasthenic syndrome (LEMS) clinical and electrodiagnostic features and response to therapy in 59 patients. Ann N Y Acad Sci. 1998;841:823-826.
90 Maselli RA, Bakshi N. Botulism. Muscle Nerve. 2000;23:1137-1144.
91 Fakadej AV, Gutmann L. Prolonged post-tetanic facilitation in infant botulism. Muscle Nerve. 1982;5:727-729.
92 Cherington M. Electrophysiologic methods as an aid in the diagnosis of botulism: A review. Muscle Nerve. 1982;5(Suppl):S28-S29.
93 Cornblath DR, Sladky JT, Sumner AJ. Clinical electrophysiology of infantile botulism. Muscle Nerve. 1983;6:448-452.
94 Padua L, Aprile I, Lo Monaco M, et al. Neurophysiological assessment in the diagnosis of botulism: usefulness of single-fiber EMG. Muscle Nerve. 1999;22:1388-1392.
95 Chaudhry V, Crawford TO. Stimulation single-fiber EMG in infant botulism. Muscle Nerve. 1999;22:1698-1703.
96 Mandler RN, Maselli RA. Stimulated single-fiber electromyography in wound botulism. Muscle Nerve. 1996;19:1171-1173.
97 Barr JR, Moura H, Boyer AE, et al. Botulinum neurotoxin detection and differentiation by mass spectrometry. Emerg Infect Dis. 2005;11:1578-1583.
98 Santos JI, Swensen P, Glasgow LA. Potentiation of Clostridium botulinum toxin by aminoglycoside antibiotics: Clinical and laboratory observations. Pediatrics. 1981;68:50-54.
99 Schulze J, Toepfer M, Schroff K-C, et al. Clindamycin and nicotinic neuromuscular transmission. Lancet. 1999;354:1792-1793.
100 U.S. Army Medical Research Institute of Infectious Diseases. Botulinum. In USAMRIID’S Medical Management of Biological Casualties Handbook, 5th ed, Fort Detrick, MD: USAMRIID; 2004:81-87. Available at: http://www.usamriid.army.mil/education/blueboohhtm
101 Siegel LS. Human immune response to botulinum pentavalent (ABCDE) toxoid determined by a neutralization test and by an enzyme-linked immunosorbent assay. J Clin Microbiol. 1988;26:2351-2356.