21 Botulinum Toxin
Treatment of facial lines and wrinkles (also called rhytids) with botulinum toxin has become the most frequently performed cosmetic procedure in the United States, according to statistics from the American Society for Aesthetic Plastic Surgery.1 It is also one of the most common entry procedures for health care professionals seeking to incorporate aesthetic procedures into their practice.2
Botulinum toxin is a potent neurotoxin protein derived from the Clostridium botulinum bacterium. It reduces muscular contraction through inhibiting release of acetylcholine at the neuromuscular junction. Injection of small quantities of botulinum toxin into specifically targeted muscles causes localized, temporary chemical denervation with resultant muscle relaxation.3
Botulinum toxin has been used for more than 20 years to treat a variety of clinical conditions such as blepharospasm, strabismus, cervical dystonia, hyperhidrosis, migraines, and muscle spasticity.4–6 Botulinum toxin is used in numerous facial aesthetic areas. However, treatment of the glabellar complex,7 frontalis8 and orbicularis oculi muscles,9 which contribute to formation of frown lines, horizontal forehead lines, and crow’s feet, respectively, offers the most predictable results, greatest efficacy, and fewest side effects.10,11 Botulinum toxin treatment of hyperdynamic muscles in the upper one-third of the face are a core aesthetic procedure for providers interested in incorporating aesthetic care into office practice.12
Cosmetic Indications
Alternative Therapies
Botulinum toxin is the only FDA-approved treatment for dynamic wrinkles. Static wrinkles can also be treated with chemical peels, microdermabrasion, topical products such as retinoids, nonablative lasers for soft-tissue coagulation and tightening using infrared and radio-frequency, nonablative lasers for collagen remodeling using 1320 and 1540 nm Q-switched lasers and others, and ablative and fractional ablative lasers using erbium and carbon dioxide. Limited data suggest that an acetylcholine blocker found in topical skin care products, acetyl hexapeptide-8, may reduce wrinkles.13 However, additional studies are necessary to support this finding.
Contraindications
Anatomy
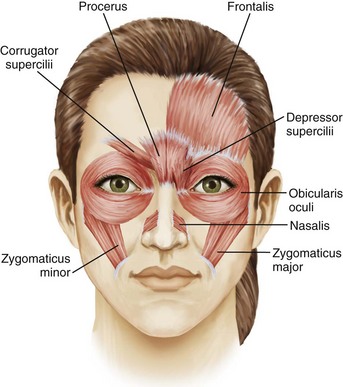
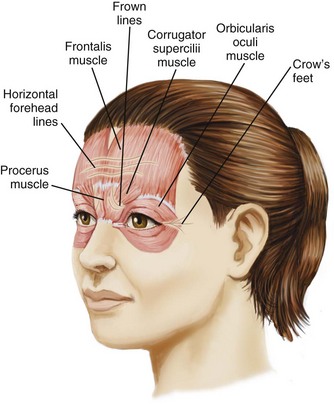
A thorough understanding of facial anatomy in the treatment areas is essential prior to performing botulinum toxin procedures (Figure 21-1). The muscles of facial expression are unique because, unlike most muscles, which have bony attachments, they have soft tissue attachments to the skin through the superficial muscular aponeurotic system. When a muscle contracts, the overlying skin moves with it and wrinkles are formed perpendicular to the direction of the muscle contraction. Figure 21-2 shows lines and wrinkles in the upper one-third of the face and the corresponding underlying musculature.
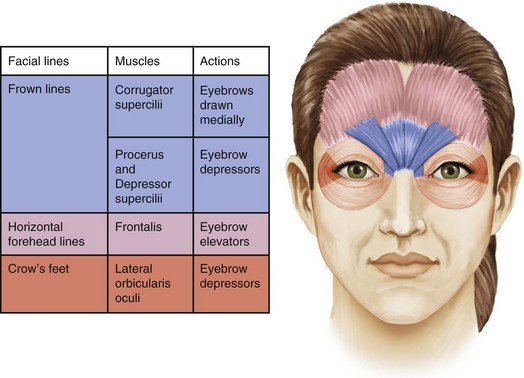
Glabellar wrinkles, or frown lines, are vertical lines that occur between the medial aspects of the eyebrows. The muscles that contribute to formation of frown lines are the glabellar complex depressor muscles, which pull the brows medially and inferiorly and include the corrugator supercilii, procerus, depressor supercilii, and medial orbicularis oculi (Figures 21-1, 21-2 and 21-3).
Horizontal forehead lines result from contraction of the broad frontalis muscle, which spans the forehead between the temporal fusion lines (see Figures 21-1, 21-2 and 21-3). The muscle fibers are vertically oriented and contraction of this levator muscle raises the eyebrows. The inferior 2-cm portion has the most marked effect on eyebrow height and shape. The goal of treatment in this area is to partially inhibit activity of the frontalis muscle to reduce horizontal forehead lines while maintaining a desirable eyebrow shape and height.
Periorbital wrinkles, commonly known as crow’s feet, result from contraction of the lateral portion of the orbicularis oculi, a thin, superficial muscle that encircles the eye (see Figures 21-1, 21-2 and 21-3). Contraction of the palpebral portion of the orbicularis oculi muscle results in closure of the eyelid. The goal of treatment in this area is to focally inhibit the lateral orbicularis oculi to reduce crow’s feet without excessive orbicularis oculi muscle relaxation.
Many of the muscles of facial expression interdigitate with one another and, while providing treatment with botulinum toxin to one area in isolation will often provide adequate wrinkle reduction results, in some cases an adjacent muscle group may require concomitant treatment to achieve the desired results. For example, the glabellar complex muscles interdigitate to a greater or lesser degree with the frontalis muscle, and treatment of the frontalis in addition to treatment of the glabellar complex muscles may be required to smooth frown lines in some cases14.
Reconstitution
Injectors reconstitute Botox with differing amounts of saline and there is no standardized reconstitution protocol. Botox efficacy is based on the number of units injected rather than the dilution. However, diffusion and the risk of complications are related to greater dilution volumes.15 The author’s reconstitution technique is outlined below.
Handling and Storage
Botox is shipped frozen, on dry ice. Prior to and after reconstitution it should be stored in the refrigerator at a temperature of 2 to 8°C (35.6 to 46.4°F). Prior to reconstitution it may be stored for 24 months. While the manufacturer recommends using Botox within 24 hours of reconstitution, the American Society for Plastic Surgery Botox Consensus Panel recommends using Botox within 6 weeks and notes no loss of potency during that time.3,16
Procedure Preparation
Botulinum Toxin: Steps and Principles
Frown Lines
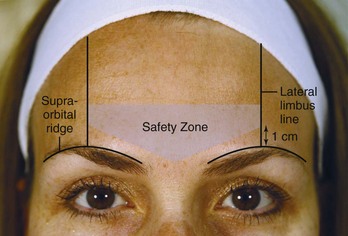
Botulinum toxin is placed within the Glabellar Complex Safety Zone. This region is bounded by two vertical lines at the lateral limbus that extend superiorly from the supraorbital ridge to the hair line. The lateral-most injection point for corrugator muscle injections should be 1 cm or above the supraorbital ridge at the lateral limbus line (Figure 21-4) to minimize the risk of blepharoptosis (droopy upper eyelid).
Most patients desire little to no movement of the glabellar complex muscles, however, this should be discussed with the patient prior to treatment. An overview of botulinum toxin injection points and starting doses for treatment of frown lines is shown in Figure 21-5. The starting dose for women is 20 units (0.8 mL of Botox reconstituted to 100 units/4 mL) and for men 25 units (1.0 mL of Botox reconstituted to 100 units/4 mL).
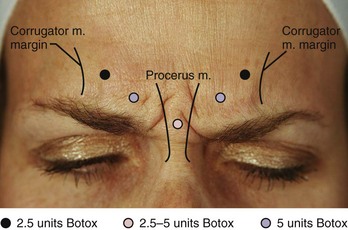
FIGURE 21-5 Glabellar complex botulinum toxin injection points and doses.
(Copyright Rebecca Small, MD.)
Performing Botulinum Toxin Injections for Frown Lines
Horizontal Forehead Lines
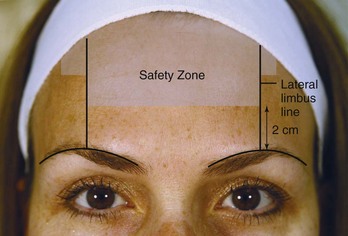
Botulinum toxin is placed within the Frontalis Safety Zone to minimize the risk of eyebrow ptosis and preserve eyebrow shape and height. The Frontalis Safety Zone is bounded by two vertical lines at the lateral limbus, and includes the area 2 cm above the supraorbital ridge to the hair line, as well as a small area lateral to the vertical lines approximately 2 cm inferior to the hair line (Figure 21-6).
An overview of botulinum toxin injection points and starting doses for treatment of horizontal forehead lines is shown in Figure 21-7. The starting dose for women is 15 to 22.5 units (0.6 to 0.9 mL of Botox reconstituted to 100 units/4 mL) and for men 20 to 25 units (0.8 to 1.0 mL of Botox reconstituted to 100 units/4 mL).
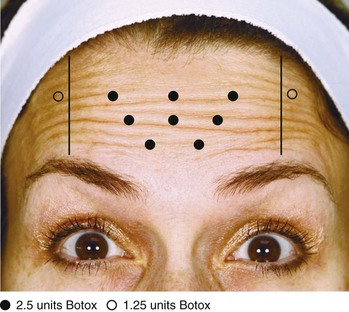
FIGURE 21-7 Frontalis muscle botulinum toxin injection points and doses.
(Copyright Rebecca Small, MD.)
Performing Botulinum Toxin Injections for Horizontal Forehead Lines
Crow’s Feet
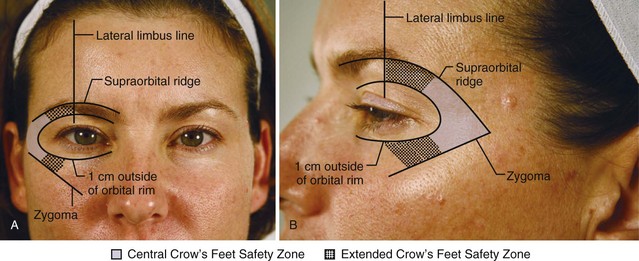
Botulinum toxin is placed within the Crow’s Feet Safety Zone to minimize the risk of globe trauma and upper lip ptosis. The Crow’s Feet Safety Zone is 1 cm outside of the orbital rim, above the level of the zygoma; it extends under the eyebrow to the lateral limbus line (Figures 21-8A and B). Botulinum toxin is concentrated within the Central Crow’s Feet Safety Zone, but injections may be placed in the Extended Safety Zone according to the patient’s anatomy.
The goal of treatment in the lateral orbicularis oculi muscle is to reduce crow’s feet lines and elevate the lateral eyebrow. An overview of botulinum toxin injection points and doses for treatment of crow’s feet is shown in Figure 21-9. The total (bilateral) starting dose for women is 15 to 20 units (0.6 to 0.8 mL of Botox reconstituted to 100 units/4 mL) and for men 20 to 25 units (0.8 to 1.0 mL of Botox reconstituted to 100 units/4 mL).
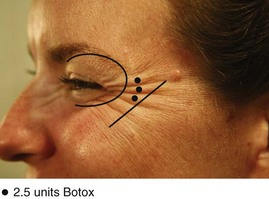
FIGURE 21-9 Orbicularis oculi muscle botulinum toxin injection points and doses.
(Copyright Rebecca Small, MD.)
Performing Botulinum Toxin Injections for Crow’s Feet
Results and Follow-Up
Return of muscle function in the treated area is gradual, typically 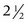 to 4 months after treatment, based on the area treated and botulinum toxin dose. Patients should follow up for subsequent treatments when muscles in the treated areas begin to contract, prior to facial lines returning to their pretreatment appearance.17
to 4 months after treatment, based on the area treated and botulinum toxin dose. Patients should follow up for subsequent treatments when muscles in the treated areas begin to contract, prior to facial lines returning to their pretreatment appearance.17
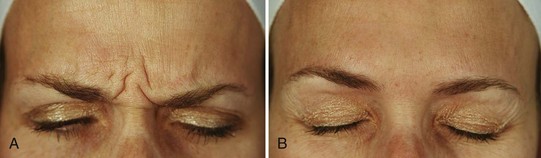
Treatment of the glabellar complex muscles with botulinum toxin results in a dramatic improvement of dynamic frown lines, as well as marked elevation in medial eyebrow position post-treatment due to the lack of depressor muscle function (Figures 21-10A and B). The duration of botulinum toxin effects in the glabellar complex for frown lines is typically 3 to 4 months.
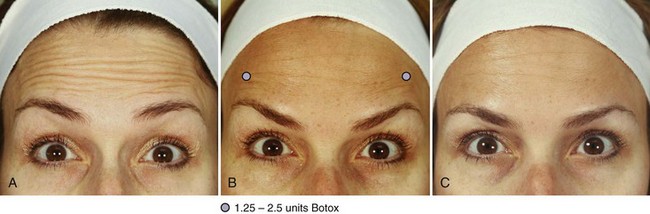
Patients receiving botulinum toxin treatments for horizontal forehead lines are usually seen in follow-up 2 weeks after treatment to evaluate eyebrow symmetry and shape at rest and with active elevation. Figure 21-11A shows a patient with dynamic horizontal forehead lines prior to treatment, and Figure 21-11B shows the patient 1 week after receiving 22.5 units of botulinum toxin in the frontalis muscle with mildly peaked eyebrows. A peaked eyebrow shape, or “quizzical brow” is treated with 1.25 to 2.5 units of botulinum toxin just inferior to the lateral Frontalis Safety Zone, in line with the most peaked portion of the eyebrow. Figure 21-11C shows the same patient 3 weeks after receiving 1.25 units of botulinum toxin above each peaked eyebrow and represents her final result. The duration of botulinum toxin effects in the frontalis muscle for reduction of horizontal forehead lines is typically 3 to 4 months.
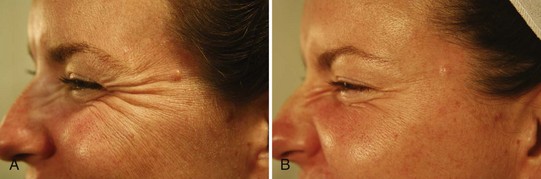
Treatment of the lateral orbicularis oculi muscle with botulinum toxin results in reduction of crow’s feet and elevates the lateral eyebrow (Figures 21-12A and B). Patients receiving botulinum toxin treatment for crow’s feet should be seen in follow-up 2 weeks after treatment to evaluate for compensatory contraction of the orbicularis oculi muscle adjacent to the treated area. Adjacent orbicularis oculi contraction is treated with 1.25 to 2.5 units of botulinum toxin in the region of orbicularis oculi muscle showing excessive activity, within the Crow’s Feet Safety Zone. The duration of botulinum toxin effects in the orbicularis oculi muscles for reduction of crow’s feet is typically 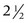 to 3 months.
to 3 months.
Complications
Temporary blepharoptosis can occur as a complication with treatment of the glabellar complex muscles for frown lines, particularly if botulinum toxin is injected inferior to the Glabellar Complex Safety Zone, too close to the supraorbital ridge at the lateral limbus. Figure 21-13 shows a patient 3 weeks after botulinum toxin treatment (not by the author) in the glabellar complex muscles with a profound right-sided blepharoptosis and mild right eyebrow ptosis. Blepharoptosis is infrequent (1% to 5%)7 and is almost always unilateral. It results from migration of botulinum toxin through the orbital septum fascia to the upper eyelid levator muscle, levator palpebrae superioris. At the bony supraorbital margin in the mid-eyebrow, some of the levator palpebrae superioris muscle fibers pass up through the orbital septum, and botulinum toxin can more easily migrate into and relax the levator palpebrae superioris from this site. Blepharoptosis is typically seen as a 2- to 3-mm lowering of the affected eyelid, which is most marked at the end of the day with muscle fatigue. It usually resolves spontaneously within 6 weeks.
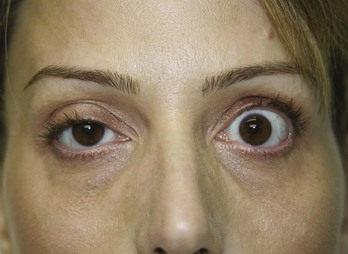
FIGURE 21-13 Right-sided blepharoptosis 3 weeks after botulinum toxin treatment for frown lines (not performed by the author).
(Copyright Rebecca Small, MD.)
Blepharoptosis can be treated using over-the-counter alpha-adrenergic eye drops such as Naphcon-A, 1 drop four times per day in the affected eye, or prescription Iopidine (apraclonidine), 0.5% solution, 1 to 2 drops three times per day. Both of these medications cause contraction of Mueller’s muscle, an adrenergic levator muscle of the upper eyelid, which results in elevation of the upper eyelid. Iopidine should be used with caution because it can exacerbate or unmask underlying glaucoma and is reserved for refractory cases.19
Severe complications resulting from distant spread of botulinum toxin from the site of injection such as generalized muscle weakness, ptosis, dysphagia, dysarthria, urinary incontinence, respiratory difficulties, and death due to respiratory compromise have been reported in patients receiving very large doses of botulinum toxin (e.g., 300 units in the calves20) and have not been reported with cosmetic use of botulinum toxin at the labeled dose of 20 units (for glabellar lines) or 100 units (for severe primary axillary hyperhidrosis). These symptoms have been reported hours to weeks after injection. The risk of symptoms is presumed to be greatest in children treated for clinical conditions such as spasticity, particularly in patients with compromised baseline respiratory function.
Current Developments
AbobotulinumtoxinA (trade name Dysport®, see the Resources section below) was FDA approved in 2009 for cosmetic use to treat glabellar frown lines. It is estimated that Dysport requires 2.5 to 3 times the number of units to achieve effects equivalent to Botox,21 but may have a more rapid onset of action.22
A physician-applied topical botulinum toxin by ReVance Therapeutics shows promise for the treatment of axillary hyperhidrosis23 and is under investigation for cosmetic applications including treatment of crow’s feet. The company claims to have a novel method of delivery of topical products (TransMTS™) that allows for macromolecules such as botulinum toxin to penetrate beneath the skin and reach the neuromuscular junction.
1. Cosmetic Surgery National Data Bank 2008 Statistics. American Society for Aesthetic Plastic Surgery; 2008.
2. Small R. Aesthetic procedures in office practice. Am Fam Physician. 2009;80(11):1231-1237.
3. Carruthers JD, Fagien S, Matarasso SL. Consensus recommendations on the use of botulinum toxin type a in facial aesthetics. Plast Reconstr Surg. 2004;114(6 Suppl):1S-22S.
4. Training guidelines for the use of botulinum toxin for the treatment of neurologic disorders. Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 1994;44:2401-2403.
5. Naumann MK, Lowe NJ. Botulinum toxin type A in treatment of bilateral primary axillary hyperhidrosis: randomised, parallel group, double blind, placebo controlled trial. Br Med J. 2001;323(7313):596-599.
6. Silberstein S, Matthew N, Saper J, et al. Botulinum toxin type A as a migraine preventive treatment. Headache. 2000;40(6):445-450.
7. Carruthers JA, Lowe NJ, Menter MA, et al. A multicenter, double-blind, randomized, placebo-controlled study of the efficacy and safety of botulinum toxin type A in the treatment of glabellar lines. J Am Acad Dermatol. 2002;46(6):840-849.
8. Carruthers A, Carruthers J, Cohen JL. A prospective, double-blind, randomized, parallel-group, dose-ranging study of botulinum toxin type a in female subjects with horizontal forehead rhytides. Derm Surg. 2003;29(5):461-467.
9. Lowe NJ, Lask GP, Yamauchi PS, et al. Bilateral, double-blind, randomized comparison of 3 doses of botulinum toxin type A and placebo in patients with crow’s feet. J Am Acad Dermatol. 2002;47(6):834-840.
10. Maas CS. Botulinum neurotoxins and injectable fillers: minimally invasive management of the aging upper face. Facial Plast Surg Clin North Am. 2006;14(3):241-245.
11. Carruthers JD, Carruthers A. The use of botulinum toxin type A in the upper face. Facial Plast Surg Clin North Am. 2006;14(3):253-260.
12. Small R. Aesthetic procedures introduction. In: Mayeaux E, editor. The Essential Guide to Primary Care Procedures. Philadelphia: Lippincott Williams & Wilkins; 2009:195-199.
13. Fields K, Falla TJ, Rodan K, et al. Bioactive peptides: signaling the future. J Cosm Derm. 2009;8:8-13.
14. Zimmerman EM. Botulinum toxin. In: Pfenninger J, Fowler G, editors. Pfenninger and Fowler’s Procedures for Primary Care. Philadelphia: Mosby/Elsevier, 2010.
15. Carruthers A, Bogle M, Carruthers JD, et al. A randomized, evaluator-blinded, two-center study of the safety and effect of volume on the diffusion and efficacy of botulinum toxin type A in the treatment of lateral orbital rhytides. Dermatol Surg. 2007;33(5):567-571.
16. Hexsel DM, De Almeida AT, Rutowitsch M, et al. Multicenter, double-blind study of the efficacy of injections with botulinum toxin type A reconstituted up to six consecutive weeks before application. Derm Surg. 2003;29(5):523-529.
17. Small R. Botulinum toxin type A for facial rejuvenation. In: Mayeaux E, editor. The Essential Guide to Primary Care Procedures. Philadelphia: Lippincott Williams & Wilkins; 2009:200-213.
18. Allergan Inc. Botox cosmetic (onabotulinumtoxin A) purified neurotoxin complex package insert. Irvine, CA: Allergan Inc.; 2010.
19. Fagien S. Temporary management of upper lid ptosis, lid malposition, and eyelid fissure asymmetry with botulinum toxin type A. Plast Reconstr Surg. 2004;114(7):1892-1902.
20. Nong LB, He WQ, Xu YH, et al. [Severe respiratory failure after injection of botulinum toxin: case report and review of the literature]. Chinese J Tuberculosis Resp Dis. 2008;31(5):369-371.
21. Monheit GD, Carruthers A, Brandt FS, et al. A randomized, double-blind, placebo-controlled study of botulinum toxin type A for the treatment of glabellar lines: determination of optimal dose. Derm Surg. 2007;33(1 Spec No.):S51-S59.
22. Lowe P, Patnaik R, Lowe NJ. Comparison of two formulations of botulinum toxin type A for the treatment of glabellar lines: a double-blind, randomized study. J Am Acad Dermatol. 2006;55(6):975-980.
23. Glogau RG. Topically applied botulinum toxin type A for the treatment of primary axillary hyperhidrosis: results of a randomized, blinded, vehicle-controlled study. Derm Surg. 2007;33(1):S76-S80.
Small R, Hoang D. Botulinum toxin introduction and foundation concepts. In: Small R, Hoang D, editors. A Practical Guide to Botulinum Toxin Procedures. Philadelphia: Lippincott Williams & Wilkins, 2011.
Small R, Hoang D. Crow’s feet. In: Small R, Hoang D, editors. A Practical Guide to Botulinum Toxin Procedures. Philadelphia: Lippincott Williams & Wilkins, 2011.
Small R, Hoang D. Frown lines. In: Small R, Hoang D, editors. A Practical Guide to Botulinum Toxin Procedures. Philadelphia: Lippincott Williams & Wilkins, 2011.
Small R, Hoang D. Horizontal forehead lines. In: Small R, Hoang D, editors. A Practical Guide to Botulinum Toxin Procedures. Philadelphia: Lippincott Williams & Wilkins, 2011.