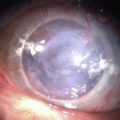
Boston Keratoprosthesis Complications
Introduction
Many of the complications associated with the Boston type 1 and type 2 keratoprosthesis (KPro) have been addressed successfully through design alterations, surgical technique modifications, and medical therapy. Despite this, KPro implantation remains a surgery of last resort, in part because the complications can result in permanent vision loss (Table 51.1). Although the use of a donor cornea improves the biocompatibility and ease of implantation, complications related to implant integration after Boston KPro surgery remain of significant concern, including implant extrusion, periprosthetic tissue necrosis, and infection. The ocular surface is the gateway to many of these complications. In this chapter, the authors describe the complications associated with the Boston keratoprosthesis, with special emphasis on the role of the ocular surface in the pathology.
Table 51.1
Postoperative Complications after Boston Type 1 Keratoprosthesis Implantation
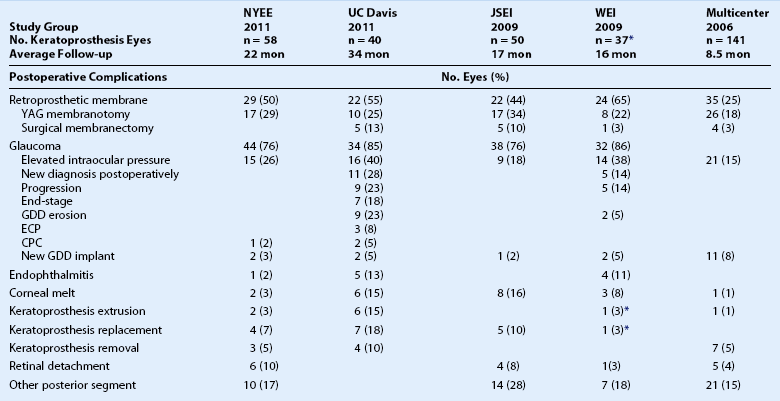
NYEE: New York Eye and Ear Infirmary. See manuscript text reference no. 8.
JSEI: Jules Stein Eye Institute. See manuscript text reference no. 2.
WEI: Wills Eye Institute. See manuscript text reference no. 6.
Multicenter: Boston Type 1 Keratoprosthesis Study Group. See manuscript text reference no. 7.
Epithelial Defects and Contact Lens Related Complications
Epithelial defects on the donor or recipient cornea can be problematic after Boston KPro implantation and, as expected, occur more frequently in dry eye, inflammatory, and stem cell deficiency states. Given the elevated profile of the KPro’s anterior plate relative to the donor cornea, the surrounding ocular surface is more vulnerable to evaporative forces and dellen formation,1 and the area adjacent to the anterior plate in particular has been associated with persistent epithelial defects.2 In addition to this mechanical disadvantage, chronic ocular surface dryness and an inhospitable ocular surface related to the preoperative diagnosis can also lead to epithelial defects. Frequently, one or more of the common mechanisms contributing to KPro related epithelial defects – diminished aqueous production from conjunctival scarring, evaporative dysfunction from meibomian gland scarring, exposure keratopathy from lagophthalmos, and trichiasis from cicatricial entropion – is associated with the pathology prompting KPro surgery. Occult microbial keratitis without corneal infiltrate can prevent epithelialization, and positive fungal and bacterial cultures of persistent epithelial defects with resolution after appropriate antimicrobial treatment have been documented.2 Regardless of the cause, epithelial defects place the donor cornea at risk for further tissue destruction from collagenases, as well as other enzymes released from polymorphonuclear leukocytes in the tear film.1 These defects can ultimately lead to keratolysis, implant extrusion, microbial keratitis, and endophthalmitis.
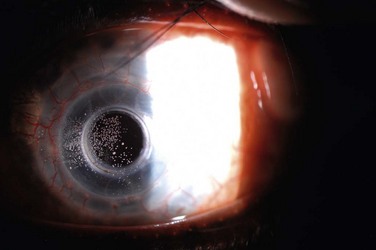
The routine use of a wide-diameter contact lens, typically a Kontur™ hydrogel SCL of 16-mm diameter or larger (Kontur Kontact Lens Co., Inc., Hercules, CA), helps prevent and treat epithelial defects and reduces the risk of corneal infection and melting. A suitable contact lens fit maintains a thin tear meniscus, especially around the anterior plate, and diminishes evaporative forces to achieve better hydration of the corneal surface. In the presence of an epithelial defect, the SCL promotes epithelialization by acting as a mechanical barrier protecting the vulnerable corneal surface from the shear forces of blinking and may limit access of polymorphonuclear leukocytes to the underlying tissue, thereby retarding stromal keratolysis.1 Postoperative use of a SCL may also provide improved comfort for patients bothered by the anterior plate edge, correction of spherical refractive error, and in the case of tinted lenses, diminished glare and photophobia with improved cosmesis.1 Typically, KPro surgeons replace the SCL on a periodic basis and/or whenever there is debris build-up on the lens surface, since the deposits can adversely affect vision and represent a nidus for attachment of microbes (Fig. 51.1).
SCL use after Boston KPro surgery has improved outcomes, but bandage contact lens use per se is not free of complications. Difficulty retaining the SCL can be problematic, and patients with multiple contact lens dislocations require particular attention. Replacement requires additional office visits and contributes significantly to the real cost of the prosthesis. As with any SCL, infection can occur and has been reported with the Kontur™ SCL used in conjunction with KPro surgery, although the majority of reported Kontur™ SCL-related infections have occurred in patients with a history of glaucoma surgery.1,3,4 Kontur™ SCLs have been associated, in particular, with glaucoma drainage tube erosion (Fig. 51.2).5,6 In the series reported by Li et al., one-third of patients with glaucoma drainage device (GDD) erosion incurred conjunctival breakdown at the edge of contact lens. In that series, the erosion event was implicated in the development of endophthalmitis and periprosthetic infection leading to extrusion in two eyes, despite daily prophylaxis with vancomycin. Presumably, the focal mechanical trauma of the contact lens edge on top of the GDD, in combination with a dysfunctional ocular surface, contributes to the erosion of these devices. Although an SCL is needed to protect the ocular surface, careful attention must be paid at each follow-up visit to the status of all hardware in KPro eyes and, in particular, to glaucoma surgical sites to detect erosion and infection.
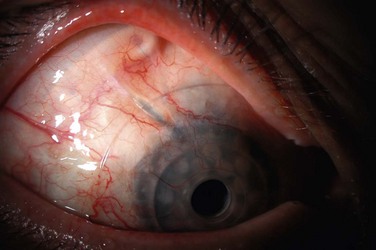
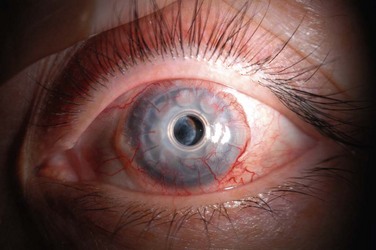
Corneal Infiltrates
Infiltrates can occur on the donor or recipient cornea, and although the location of the infiltrate relative to the implant is important for structural integrity, an etiology may not be presumed based on location alone. Donor corneal infiltrates may be inflammatory and sterile, but with bacterial and fungal isolates recovered in all of the major series in the KPro literature,2,3,6–8 the suspicion for microbial keratitis should be very high (Fig. 51.3). Risk factors for bacterial keratitis after KPro surgery include significant blepharitis, noncompliance with the postoperative chronic antibacterial eye drop regimen, lid abnormalities, and the presence of other surgical hardware in the eye, including GDDs.5 Risk factors for fungal keratitis after KPro surgery include use of a contact lens and topical vancomycin,9 and similar to PK, topical steroid administration. Corneal infiltrates may be diagnosed clinically as infectious, but a corneal scraping for Gram stain and bacterial and fungal cultures typically is performed. Persistent epithelial defects may also be considered for culture, given the possibility of occult microbial keratitis.2 Although the ocular surface is commonly colonized with Gram-positive organisms, it is important to ensure that antibacterial treatment of a corneal infiltrate covers both Gram-negative and Gram-positive microbes, since most patients will already have been receiving daily vancomycin prophylaxis, and a selection pressure is placed on an eye with administration of topical vancomycin alone.3
Fungal keratitis is of particular concern after KPro surgery for its morbidity and increased incidence in this era of routine SCL and topical vancomycin use (Fig. 51.4). As Barnes and colleagues reported,9 Candida species colonize and can cause devastating infections after KPro surgery. However, since none of the eyes in which surveillance cultures were performed developed fungal keratitis or endophthalmitis, surveillance cultures do not appear to be useful predictors of fungal infection. It is also imperative to consider treatment with topical and possibly oral antifungal agents when suspicion for fungal infiltrates is high.
Corneal Melts and Implant Extrusion
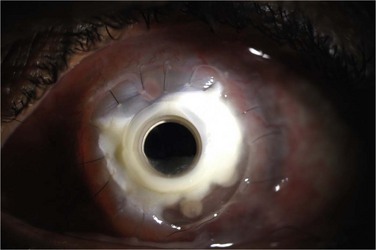
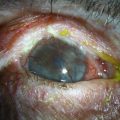
Corneal melting may result from desiccation of the ocular surface and erosion into the donor stroma, immune-related inflammation, keratolysis, and infection. More frequently, corneal melts are an inflammatory rather than an infectious complication. Preoperative diagnoses associated with corneal melting include Stevens–Johnson syndrome (SJS), ocular cicatricial pemphigoid (OCP), and aniridia, all of which predispose the prosthesis to an inhospitable, poorly hydrated and stem cell-deficient ocular surface (Fig. 51.5).10 When assessing the status of the implant at postoperative examination, it is of particular importance to examine the area of cornea adjacent to the anterior plate carefully because of its vulnerability to chronic desiccation, persistent epithelial defects, and consequent melting. Seidel testing should be performed routinely to look for aqueous leaks, with special attention paid to areas adjacent to hardware and any regions of thinning and erosion. Regardless of etiology, progression of an erosion through Bowman’s layer into stroma – and through the stroma – can occur quickly, and extrusion of implant hardware may occur spontaneously (Fig. 51.6).
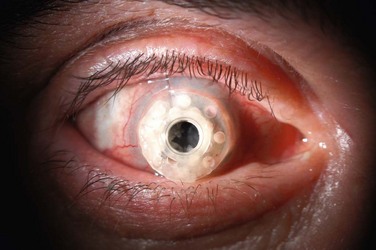
Figure 51.6 Extrusion of Boston keratoprosthesis implant.
Although corneal melting typically leads to further morbidity and intervention, it does not always lead directly to vision loss – even in high-risk patients with as SJS11 and rheumatoid arthritis.3 Treatment of corneal melts can extend the life of the implant and maintain useful ambulatory vision. Initial treatment of a corneal melt is fundamentally similar to treatment of a persistent epithelial defect. Copious lubrication, SCL placement, and early consideration of tarsorrhaphy are cornerstones of the approach to this difficult problem. Additionally, patients may be treated with oral doxycycline to help inhibit matrix metalloproteinase activity. In cases of aqueous leak, anatomic stability needs to be achieved either with cyanoacrylate glue application to temporize, followed by wound revision, KPro replacement if warranted, or explantation with PK.
Endophthalmitis
Although endophthalmitis after KPro surgery can be bacterial or fungal in origin, infection typically occurs with Gram-positive bacteria, reflecting the central role of commensal organisms of the ocular surface in this devastating complication (Fig. 51.7). In their landmark study of bacterial endophthalmitis prevention and the Boston KPro, Durand and Dohlman12 found that the addition of vancomycin eye drops (14 mg/mL) to a fluoroquinolone eye drop was effective in preventing bacterial endophthalmitis in KPro eyes. That study included 255 eyes with type I and type II Boston KPros, noted a reduction in the rate of endophthalmitis from 4.13% without vancomycin to 0.35% with vancomycin per patient-year, and has influenced the practice of most KPro surgeons. However, it is imperative to pay particular attention to the possibility of Gram-negative and fungal infections, due to selection pressure from inclusion of vancomycin in the antibiotic regimen. Of the five eyes that developed endophthalmitis at UC Davis, all were using vancomycin eye drops at the time of infection; three developed Gram-negative infections and one developed a fungal infection. Clearly, there is a need for long-term treatment with antibiotics that cover for Gram-negative in addition to Gram-positive organisms in the postoperative treatment regimen.
Several risk factors have been associated with endophthalmitis after KPro surgery. Most significantly, prior glaucoma surgery appears to be a risk factor, with reports of endophthalmitis developing in eyes with prior trabeculectomy and GDD implants.1,3,4 Given that the conjunctiva has been violated surgically, and glaucoma drainage hardware is often present, there may be a greater risk for infection with virulent organisms that do not typically grow on the ocular surface in these eyes. Other risk factors include preoperative SJS, OCP, and chemical burns, although a substantial number of endophthalmitis cases have occurred in eyes with prior graft failures. Treatment noncompliance, keratitis, aqueous leak, and hardware exposure are also clear risk factors for endophthalmitis.
Sterile Vitritis
Sterile vitritis may occur after KPro surgery, with clinical features that can masquerade as endophthalmitis. An incidence of approximately 5% was documented in the definitive study of this complication by Nouri et al.13 Hallmark characteristics – and distinguishing findings that separate this entity from endophthalmitis – include sudden-onset vision loss with little to no discomfort or conjunctival injection, massive ‘snowflake’ vitritis, and anterior chamber reaction. The etiology is unclear, and no clear associations with preoperative conditions have been discerned, due to its low incidence, but it is generally thought to represent an immune-related uveitis. Although the diagnosis is clinical, the work-up typically involves vitreous aspiration or vitrectomy for culture to rule out infection. Treatment generally includes injection of broad-spectrum antibiotics, as well as corticosteroids. Unlike in endophthalmitis, visual acuity typically recovers to pre-episode levels within weeks with appropriate treatment.
Retroprosthetic Membranes
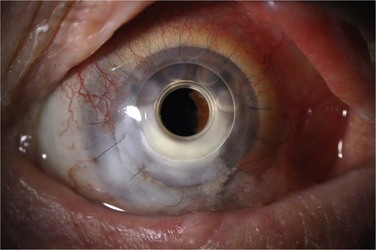
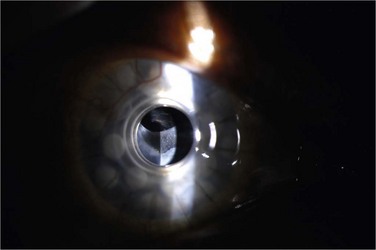
Retroprosthetic membrane (RPM) formation is the most common complication after Boston KPro implantation. These membranes develop in the early postoperative period,6 can be refractory to initial treatment with yttrium–aluminum–garnet (YAG) laser capsulotomy, and can limit vision significantly (Fig. 51.8). The histopathology of membranes refractory to initial YAG capsulotomy reveal compact, fibrous, multilayered membranes comprising host stroma admixed with multiple cellular and extracellular components, including iris stroma, metastatic lens epithelium, lens capsule, myofibroblasts and collagen.14 In addition to stromal downgrowth from the host cornea, epithelial downgrowth from the ocular surface has been implicated in RPM formation.15 Factors contributing to RPM formation include inflammation, as well as breaks in Descemet’s membrane induced by contact with the back plate, which allows a pathway for migration of stroma.14 The use of a back plate constructed from titanium rather than polymethyl methacrylate may reduce the frequency of RPM formation,16 a difference that appears to be significant both statistically and clinically. However, widespread incorporation of the titanium back plate has not yet occurred at the time of writing, and the clinical impact of this design modification remains to be seen.
Although YAG laser membranotomy is successful in many cases, early treatment is warranted because of the progressive nature of these membranes. RPMs that recur after laser present a substantial problem to patients and physicians and generally require surgical intervention, via either a pars plana membranectomy or possibly replacement of the KPro (Fig. 51.9). One concern with YAG laser membranotomy is the risk of pitting or cracking of the PMMA optic within the KPro device during the laser procedure. This occurrence may ultimately affect visual acuity regardless of complete membrane removal, and care must be taken to avoid this complication, if possible.
Glaucoma
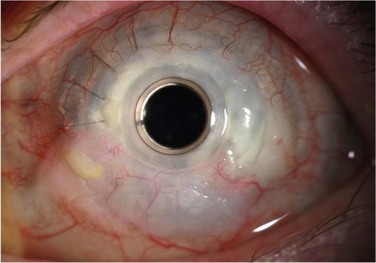
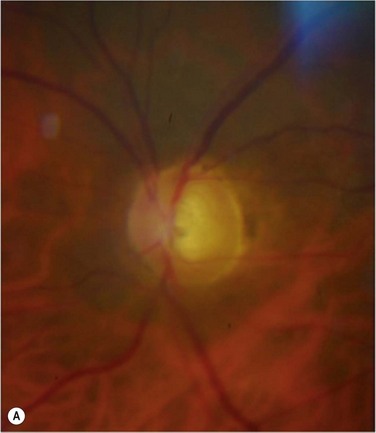
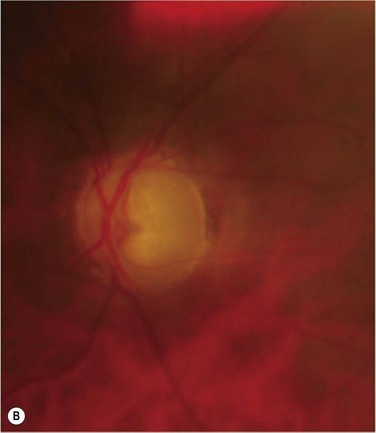
Glaucoma represents the most significant complication of Boston KPro surgery. Glaucoma after KPro is often resistant to treatment and may lead to permanent vision loss. It is frequently present prior to KPro surgery and, as with PK, may develop or worsen after KPro surgery, due to progressive angle closure from peripheral anterior synechiae or anterior segment crowding, trabecular meshwork collapse, forward rotation of a residual iris stump, or steroid response. The current method of monitoring intraocular pressure (IOP) palpation is inaccurate and can lead to a loss of IOP control, although promising developments in tonometry technology are on the horizon. Humphrey and Goldmann visual fields and retinal nerve fiber layer analysis of the optic nerve head are useful for monitoring the health of the optic nerve, but a compromised view in to the posterior pole may not allow for adequate perimetry, optic nerve examination or imaging. Moreover, the small optical entrance of the KPro can produce generalized constriction of the visual field. Together, limitations in both tonometry and optic nerve imaging/assessment complicate postoperative monitoring and increase the probability of glaucoma progression (Fig. 51.10). Progressive nerve dysfunction and end-stage glaucomatous optic neuropathy can occur despite close monitoring.3,6 Awareness of other glaucoma-related events that cause vision loss, including branch retinal vein occlusion and loss of fixation, should be high.
Given that a significant number of patients are diagnosed preoperatively with glaucoma and postoperatively with glaucoma progression, some authors have advocated for implantation of GDDs in eyes with marginal IOP control. Although these devices can achieve IOP control and prevent glaucoma progression, they have been associated with complications that can cause vision loss. Li and colleagues recently evaluated the long-term safety and efficacy of GDDs, including Ahmed glaucoma valves and Baerveldt glaucoma implants, in this patient population.5 The authors found that glaucoma drainage tube erosions occurred in 10 of 40 eyes and led to endophthalmitis in at least two cases. Risk factors for GDD erosion include GDD implantation prior to KPro surgery (older tubes are more likely to erode), localized trauma of the contact lens edge on top of the tube, and the presence of hardware known to be associated with erosions, such as Hoffman elbows. In addition to avoiding hardware known to be associated with erosions, their study recommended considering an approach to GDD implantation that minimizes contact between the tube and contact lens and maximizes coverage of the tube with tissue and/or graft reinforcement. Alternatives, such as trans-scleral or endoscopic cyclophotocoagulation should be considered carefully in glaucoma management with the Boston KPro. The authors also concluded that pre-surgical evaluation for glaucoma and involvement of the glaucoma specialist in the pre-surgical assessment of all KPro patients is mandatory. The UC Davis group commonly places a stage I Baerveldt GDD at the time of the initial KPro surgery for hook-up at a later date if necessary.
Retinal Detachment
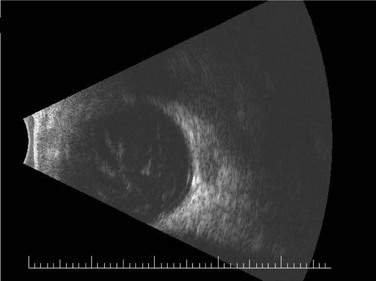
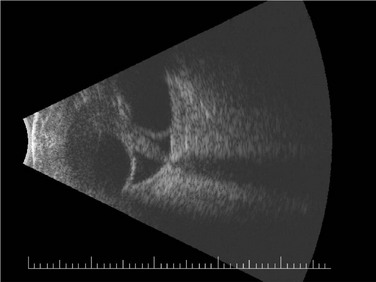
The incidence of retinal detachment (RD) was reported at 12% in the largest study of posterior segment complications after KPro surgery (Fig. 51.11).17 In that series, RDs that occurred in isolation or with treatable vitreous opacities (including RPM and hemorrhage) had some recovery in vision, but RDs that were associated with other retinal pathology (including proliferative vitreoretinopathy and subretinal fibrosis) had no improvement or a decline in vision after surgery. Due to the presence of foreshortened anterior segment anatomy, modifications to standard transconjunctival pars plana vitrectomy approaches are required, including placement of incisions as anteriorly as possible. Retinal and choroidal detachments occur more frequently after type II Boston KPro implantation, which has been speculated to result from chronic inflammation causing vitreoretinal traction and subsequent RD in this population enriched with SJS and OCP patients.18 Retinal surgeons are hampered by limitations in visibility of the retinal periphery when faced with the need for performing pars plana vitrectomy or RD repair in the KPro patient. In patients who are at high risk for post-surgical retinal pathology, collaborative evaluation of the patient by the retina surgeons preoperatively is important.
References
1. Dohlman, CH, Dudenhoefer, EJ, Khan, BF, et al. Protection of the ocular surface after keratoprosthesis surgery: the role of soft contact lenses. CLAO J. 2002;28:72–74.
2. Aldave, AJ, Kamal, KM, Vo, RC, et al. The Boston type I keratoprosthesis: improving outcomes and expanding indications. Ophthalmology. 2009;116:640–651.
3. Greiner, MA, Li, JY, Mannis, MJ. Longer-term vision outcomes and complications with the Boston type 1 keratoprosthesis at the University of California, Davis. Ophthalmology. 2011;118:1543–1550.
4. Tsui, I, Uslan, DZ, Hubschman, JP, et al. Nocardia farcinica infection of a Baerveldt implant and endophthalmitis in a patient with a Boston type 1 keratoprosthesis. J Glaucoma. 2010;19:339–340.
5. Li, JY, Greiner, MA, Brandt, JD, et al. Long-term complications associated with glaucoma drainage devices and Boston keratoprosthesis. Am J Ophthalmol. 2011;152:209–218.
6. Chew, HF, Ayres, BD, Hammersmith, KM, et al. Boston keratoprosthesis outcomes and complications. Cornea. 2009;28:989–996.
7. Zerbe, BL, Belin, MW, Ciolino, JB. Boston Type 1 Keratoprosthesis Study Group. Results from the multicenter Boston Type 1 Keratoprosthesis Study. Ophthalmology. 2006;113:1779.e1-e7.
8. Patel, AP, Wu, EI, Ritterband, DC, et al. Boston type 1 keratoprosthesis: the New York Eye and Ear experience. Eye. 2011;26:418–425.
9. Barnes, SD, Dohlman, CH, Durand, ML. Fungal colonization and infection in Boston keratoprosthesis. Cornea. 2007;26:9–15.
10. Yaghouti, F, Nouri, M, Abad, JC, et al. Keratoprosthesis: preoperative prognostic categories. Cornea. 2001;20:19–23.
11. Sayegh, RR, Ang, LP, Foster, CS, et al. The Boston keratoprosthesis in Stevens–Johnson syndrome. Am J Ophthalmol. 2008;145:438–444.
12. Durand, ML, Dohlman, CH. Successful prevention of bacterial endophthalmitis in eyes with the Boston keratoprosthesis. Cornea. 2009;28:896–901.
13. Nouri, M, Durand, ML, Dohlman, CH. Sudden reversible vitritis after keratoprosthesis: an immune phenomenon? Cornea. 2005;24:915–919.
14. Stacy, RC, Jakobiec, FA, Michaud, NA, et al. Characterization of retrokeratoprosthetic membranes in the Boston type 1 keratoprosthesis. Arch Ophthalmol. 2011;129:310–316.
15. Dudenhoefer, EJ, Nouri, M, Gipson, IK, et al. Histopathology of explanted collar button keratoprostheses: a clinicopathologic correlation. Cornea. 2003;22:424–428.
16. Todani, A, Ciolino, JB, Ament, JD, et al. Titanium back plate for a PMMA keratoprosthesis: clinical outcomes. Graefes Arch Clin Exp Ophthalmol. 2011;249:1515–1518.
17. Ray, S, Khan, BF, Dohlman, CH, et al. Management of vitreoretinal complications in eyes with permanent keratoprosthesis. Arch Ophthalmol. 2002;120:559–566.
18. Pujari, S, Siddique, SS, Dohlman, CH, et al. The Boston keratoprosthesis type II: the Massachusetts Eye and Ear Infirmary experience. Cornea. 2011;30:1298–1303.