chapter 23 Bones
INTRODUCTION AND OVERVIEW
Bones are complex organs with many important functions, the most obvious being structural. They provide support for the body and the means by which muscles can insert into fixed structures in order to allow movement. They are also important in hearing, through the transduction of sound via the ear’s ossicles, and they protect other soft organs that are easily damaged, such as the brain, eyes, kidneys, lungs and spleen. Bone marrow, which is largely within the medulla of the long bones, is the centre for production of blood cells (haematopoesis) and an important site for storage of fatty acids. Bones also have important metabolic functions, including:
This chapter describes the following conditions affecting bones:
OSTEOPOROSIS
DEFINITION
In 1991, osteoporosis was defined as a metabolic bone disease characterised by ‘low bone mass, microarchitectural deterioration of bone tissue leading to enhanced bone fragility and a consequent increase in fracture risk’.1 However, the current conception of osteoporosis is as a disease of compromised bone strength that predisposes an individual to an increased risk of fracture.2 In this new definition, osteoporosis is a dynamic, rather than an anatomical, condition. The disease is thus low bone mass and deteriorated bone quality, and fracture is the clinical consequence of the disease.
Osteoporosis is highly prevalent in the general population. Using the operational definition of osteoporosis (based on measurement of bone mineral density (BMD)), the prevalence of osteoporosis in Australian men and women aged 60 years and above was estimated to be 11% and 27% respectively.3 As expected, the prevalence of osteoporosis increases with advancing age, from approximately 5% (men) and 13% (women) at 60 years of age to over 28% (men) and 63% (women) at age 80 or above.4 With a rapidly ageing population, it is expected that osteoporosis will be an even greater problem in the next 20 years or so.
The outcome of osteoporosis is fragility fracture. Fractures are a major public health problem because of their high incidence rate in the population, and because individuals with fracture are at increased risk of mortality and morbidity and reduced quality of life, and incur significant healthcare costs. In several population-based epidemiological studies, all major fractures were associated with increased mortality, especially in men.5 Even in healthy older women, clinical vertebral fractures, commonly manifested as asymptomatic fracture, and hip fractures, are substantially associated with increased mortality.6,7 A more recent study demonstrated that asymptomatic vertebral deformity is a major risk factor for subsequent fracture and mortality.8
The increase in fracture cases will result in a significant increase in healthcare costs. The annual cost of treatment of fractures and associated sequelae has been estimated at $10–20 billion in the United States and £3 billion in England and Wales. Within the next 50 years the cost of hip fracture alone in the United States may exceed $240 billion.9 In Canada, the annual cost of hip fracture is estimated at $650 million and is expected to rise to $2.4 billion by 2041.10 In Australia, the direct and indirect costs of fracture have been estimated at $7.5 billion.11
EPIDEMIOLOGY OF FRACTURES
Theoretically, any fracture related to low bone density may be considered an osteoporotic fracture. Fractures of the spine (vertebrae), hip and wrist (distal forearm) have long been regarded as typical osteoporotic fractures.12–16 Most types of fracture occur more often in patients with low BMD,17,18 and therefore most types of age-related fractures are osteoporotic in nature. According to this definition, the following fracture types are considered osteoporotic:
In both women and men, fractures occurring at the skull and face, hands and fingers, feet and toes, ankle and patella are classified as not due to osteoporosis.19
Because fragility or osteoporotic fracture is defined as fracture-related with minimal trauma (i.e. a fall from standing height or less),20,21 in the research setting, fractures clearly due to major trauma (such as motor vehicle accidents) or due to underlying diseases (such as cancer or bone-related diseases) were excluded from analysis.5,16,18,22–26
Overall, the incidence of any fracture in women and men increases with advancing age, and is greater in women than in men. The incidence of fracture also varies according to geographic variations between and within countries. Fracture rates in women aged 60 or older are six times higher than those in women aged 35–59 years; in men aged 60 or older the rate is 1.4-fold greater than in men less than 60 years of age.27
Overall fracture rates are greater in urban than in rural areas.28,29 The difference is more pronounced in those aged 60 years or over, suggesting that environmental factors have an impact on bone health.
The magnitude of osteoporosis-related fracture can be quantified by the lifetime risk of fracture. In developed countries it is estimated that the residual lifetime risk of any fracture for men and women from age 60 is approximately a quarter and just under a half respectively.16 For individuals with osteoporosis, the mortality-adjusted lifetime risk of any fracture is probably over 40% for men and 65% for women.3 The three most common sites are hip, vertebral and Colles’ fracture.
TYPES OF FRACTURE
Hip fracture
Hip fractures include femoral neck, intertrochanteric or subtrochanteric fractures. Hip fracture is the most serious consequence of osteoporosis because it incurs many subsequent complications, including pain and disability.30
It is estimated that, from the age of 60 years, 11 out of 100 women will sustain a hip fracture during their remaining lifetime. This risk is higher than the risk of being diagnosed with breast cancer (around 10%). Approximately 17% of women and 30% of men who have sustained a fracture will die within 12 months after the event.31 However, the exact causes of death among these individuals are not clear. Among women who survive the fracture, over 20% require long-term care and over a third are unable to return to their prior work,32 and have a significantly reduced quality of life.33
Vertebral fracture
Osteoporosis has sometimes been referred to as a ‘silent disease’ because individuals often do not have apparent symptoms and pain until a fracture occurs. Asymptomatic vertebral fracture can be considered a silent disease, because patients do not realise that they have a fracture and do not seek medical attention. Currently, there is no ‘gold standard’ for the identification of vertebral fracture34,35; estimates of its overall prevalence and incidence rate in elderly women and men depends in part on the definition used.36
Asymptomatic vertebral fracture can be detected by conventional radiology, but it is not an attractive means of large-scale screening, because of cost and radiation exposure. In a large-scale study,37 vertebral fracture was present in 12% of women and men. As expected, the prevalence of vertebral fracture increases with age, more sharply in women.38 The use of corticosteroid therapy doubles the risk.39 Studies have found that about 33% of vertebral fractures or deformities are symptomatic40 and that only 23% of vertebral deformities in women were diagnosed clinically.41 In other words, there are many ‘silent’ vertebral fractures that produce no obvious symptoms. Vertebral deformities, whether clinically recognised or not, are related to an increase in chronic back pain and disability,42,43 and to low health-related quality of life44 and an increase in mortality.8,45
Distal forearm fracture
A fracture of the distal forearm is one that occurs through the distal third of the radius and/or ulna. It is frequently and typically seen in women.46,47 Although its consequence is less serious than that of a hip fracture, it is associated with significant pain and may be associated with severe and long-term complications.48,49 The incidence increases rapidly with advancing age in women (but less so with men), for up to 10 years following menopause, and tends to slow thereafter.50
CLINICAL ASSESSMENT OF OSTEOPOROSIS
The clinical assessment of osteoporosis and fracture risk is based on assessment of:
Risk factors
Risk factors can be broadly classified into modifiable and non-modifiable risk factors (listed in Box 23.1). Of these, the four key risk factors are:
A prior fragility fracture substantially elevates an individual’s risk of future fracture.5,8,51,52 The elevated risk is 1.5- to 9.5-fold depending on age at assessment, number of prior fractures and the site of the incident fracture. A pre-existing asymptomatic vertebral fracture increases the risk of a second vertebral fracture and non-vertebral fracture at least four-fold.8 A study of a placebo group showed that 20% of those who experienced a vertebral fracture during the period of observation had a second vertebral fracture within 1 year. Patients with a hip fracture are at increased risk of a second hip fracture. The risk of subsequent fracture among those with a prior fracture at any site is 2.2 times that of people without a prior fragility fracture.52 A family history of osteoporotic fracture is also a major risk factor for fracture.
Bone strength
At present there is no direct method for reliably measuring bone strength. However, BMD, measured by dual-energy X-ray absorptiometry (DXA), provides a benchmark for bone strength. BMD measurement could account for up to 70–75% of variance in bone strength.53 BMD is often standardised by expressing it as a T-score, i.e. the number of standard deviations (SD) from the young normal mean, taken as aged between 20 and 30 years.
There is a strong, continuous and consistent relationship between BMD and fracture risk, such that each SD lowering in BMD is associated with a 1.6-fold increase in fracture risk in both men and women.54 In Chinese women, the relative risk of fracture for each SD lowering in femoral neck BMD was two-fold.55 There is evidence that the magnitude of association between BMD and hip fracture risk (with relative risk being 2.256 to 3.657) is equivalent to or even stronger than the association between serum cholesterol and cardiovascular disease. Measurement of BMD is therefore considered the gold standard for diagnosis of osteoporosis in elderly men and postmenopausal women.
An operational definition of osteoporosis, by which a postmenopausal woman is considered to have osteoporosis, is if her femoral neck BMD (which is more reliable than lumbar spine measurements) is decreased by at least 2.5 SD compared with the mean value in young adults.58 The WHO classification also includes definitions of osteopenia and normal BMD (Table 23.1). The operational criteria of osteoporosis for women has also been adopted for men.59 These classifications do not apply to children.
TABLE 23.1 WHO diagnostic categories for BMD in postmenopausal women
| Category | Projected proportion of population* |
|---|---|
| Normal | BMD not more than 1 SD below peak BMD in young adult mean (T-score > −1) |
| Osteopenia | BMD 1–2.5 SD below young adult mean (T-score −1 to −2.5) |
| Osteoporosis | BMD 2.5 SD or more below young adult mean (T-score at or below −2.5) |
| Established osteoporosis | BMD 2.5 SD or more below young adult mean, and the presence of one or more fragility fractures |
* Urban, 2020 (%). SD: standard deviation. BMD: bone mineral density.
Source: Nolla et al. 200260
Who should have a BMD measurement?
Mass screening using DXA scanning is not recommended or feasible without some selection of the target population. One important and difficult question is how to decide which women should undergo BMD measurement and further evaluation based on the results of BMD scan. It is suggested that a case-finding strategy be adopted, to identify individuals ideally eligible for a BMD scan61—that is:
In the absence of BMD measurement, a number of clinical prediction rules, including the Osteoporosis Self-assessment Tool for Asians (OSTA), have been developed,62,63 to identify ‘candidates’ for a BMD scan. Most of these scores, based only on age and body weight, are a simple prediction rule that can potentially be useful in identifying women at high risk of osteoporosis. These scoring systems64,65 can be useful in ruling out osteoporosis.
Bone turnover markers
Bone turnover rate in postmenopausal women correlates negatively with BMD, and markers of bone resorption are associated with fracture risk.66 A reduction in biochemical markers appears to be correlated with a decrease in vertebral fracture incidence67 in some studies, but is not necessarily always predictive of response to therapies. Nevertheless, the predictive value of biomarkers in assessing an individual patient can be limited by their high variability within individuals.68
Assessment of absolute fracture risk
At any given level of BMD, fracture risk varies widely in relation to the burden of other risk factors56 (some modifiable), such as:
Thus, for any one individual, the likelihood of fracture depends onavv a combination of risk factors. This means that two individuals, both with osteoporosis, can have different risks of fracture because they have different non-BMD risk profiles. Likewise, an osteoporotic individual can have the same risk of fracture as a non-osteoporotic individual. A logical and appropriate assessment of fracture risk for an individual should therefore take into account the individual’s risk profile, including BMD and a history of fracture. A multivariable-based nomogram such as the one developed by Nguyen and colleagues69,70 and the WHO’s FRAX model71 can be used to estimate an individual’s absolute risk of fracture, and help select patients suitable for intervention.
The critical question of who should be treated can only be answered by a complete evaluation of an individual’s risk profile. Treatment is cost-effective if an individual’s 10-year risk of hip fracture is between 1.2% and 9.0%, depending on age.72 To facilitate the estimation of this threshold, a nomogram (Figure 23.1) is used to estimate the probability of fracture based on age, BMD and history of fractures and falls.
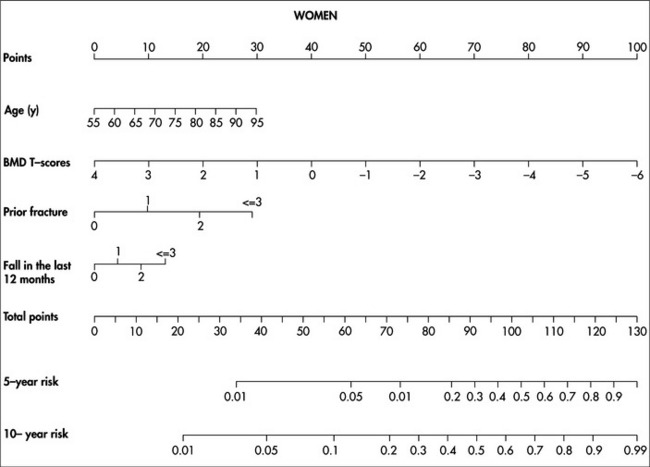
FIGURE 23.1 Nomogram for predicting 5-year and 10-year probability of hip fracture.69 To use, mark the age of an individual on the ‘Age’ axis and take a vertical line up to the ‘Points’ axis. Repeat this process for each additional risk factor, then sum the points. Locate the final sum on the ‘Total points’ axis and take a vertical line down to the 5-year and 10-year risk lines to determine the individual’s probability of sustaining a hip fracture within these timeframes
TREATMENT
Lifestyle advice
Nutrition and supplements
Nutrition
Higher dietary protein intake is associated with a lower rate of age-related bone loss,73 and fruit and vegetable intake is positively associated with bone density in men and women.74
Calcium and vitamin D
A combination of calcium and vitamin D reduces the incidence of hip and non-vertebral fractures.75–77 Elderly women (aged 69+ years) taking calcium (1200 mg/day) and vitamin D (800 IU) for 18 months had their risk of hip fracture reduced by 43% and non-vertebral fracture by 32%, although in a recent meta-analysis it was shown that the use of calcium or calcium plus vitamin D reduced fracture risk by 12%.55 In summary, calcium and vitamin D appear to be efficacious in reducing the risk of hip fracture and non-vertebral fractures. There is some evidence, however, that calcium supplements can lead to a moderately increased risk of myocardial infarction. Regular moderate sun exposure should also be remembered as a valuable way of maintaining adequate vitamin D levels. If vitamin D levels are very low (< 50 nmol/L2), a large loading dose of vitamin D of up to 50,000 IU per month until the level is at least > 75 nmol/L2 and then a maintenance dose of 2000 IU daily with 3-monthly monitoring.78 Some advocate a larger loading dose of 100,000 IU as being helpful in bringing up the levels more quickly, followed by a supplement of 1000–5000 IU daily, monitored at 3-monthly intervals until normal range is achieved. Then maintenance of 1000 IU daily if sun exposure has not increased.
Pharmacological
Hormone therapy
Hormone therapy (HT) has been used in treating osteoporosis for some time, despite there having been no randomised controlled trial or large-scale prospective studies of the efficacy of HT in postmenopausal women with osteoporosis or a pre-existing fracture. The Women’s Health Initiatives (WHI) study found that HT reduced the risk of hip fracture by an average of 34%, vertebral fracture by 34% and all fractures by 24%.79–81 However, women on HT in the HERS study (designed to evaluate the effect of oestrogen plus progestin on the risk of coronary heart disease) found no significant reduction in hip fracture risk and any fracture.80 Still, a recent meta-analysis of women who had been treated with HT for 12–120 months showed that HT could reduce non-vertebral fracture risk by 28% and vertebral fracture risk.82 Although these data collectively suggest that HT may reduce fracture risk with a modest efficacy, its anti-fracture benefit must be weighed against other adverse effects such as increased risk of breast cancer, thromboembolic disease and stroke.
Selective oestrogen receptor modulators
In postmenopausal women suffering from osteoporosis, raloxifene (at the dose of 60 mg/day) has been shown to significantly reduce the risk of vertebral fracture by as much as 50%.83,84 However, its effects on hip and non-vertebral fractures have not been consistent. Raloxifene could also reduce the risk of non-vertebral fractures in women with severe osteoporosis. The drug is mostly used in younger postmenopausal women who have bone loss predominantly at the spine, not the hip.
Calcitonin
Calcitonin (generally given as a nasal spray), due to its antiresorptive properties, has been used in the treatment of osteoporosis for many years, with significant beneficial effects in reducing bone loss and fracture risk, possibly up to 33% when combined with calcium (1000 mg/day) and vitamin D (400 IU per day).85 Similar risk reduction was also observed in those with a pre-existing vertebral fracture.85 A meta-analysis including four studies conducted between 1966 and 2000 found that calcitonin reduced vertebral fracture risk by 54%. However, its effect on non-vertebral fractures has not been shown. Calcitonin is also associated with pain relief in patients following acute osteoporotic vertebral fractures.
Bisphosphonates
Parathyroid hormone
Parathyroid hormone (PTH) is an anabolic agent97 and is FDA-approved for the treatment of postmenopausal osteoporosis. Although it has been found to reduce the incidence of fractures in men and women,98 there is concern regarding osteosarcomas in long-term use. PTH also has a beneficial effect on bone health in men99,100 and in women with glucocorticoid-induced osteoporosis.101,102
Strontium ranelate
Strontium ranelate (SR) is a trace element that stimulates bone formation and suppresses bone resorption.103,104 Two grams per day has been shown to reduce the incidence of vertebral fractures by 41% over 3 years.
Fluoride
Sodium fluoride is a potent stimulator of bone formation105 and gained popularity in the 1970s and 1980s.106 There was, however, concern about the high incidence of side effects, particularly with some formulations, and sodium fluoride therapy has not been shown to be effective in preventing fractures in postmenopausal osteoporosis. There have been no studies in premenopausal women.
Duration of treatment
The best duration of treatment is not known yet. Most randomised clinical trials followed patients for 3 years, with the longest study running for 7 years.102 Therefore, from the principle of evidence-based medicine, it seems that 3-year duration is the logical answer. However, stopping treatment could result in increased bone remodelling, bone loss, structural damage and increased fracture risk. It has been suggested that if treatment restores BMD to the normal range, it may be reasonable to consider stopping treatment and monitoring bone turnover markers and rates of bone loss.108
Alternative or adjunct therapies
Vitamin K and ipriflavone (a synthetic phyto-oestrogen) are the only alternative therapies for which sufficient data on BMD and fracture outcomes are available for evaluation. Phyto-oestrogens are weak oestrogen-like chemicals produced by plants, which have been shown to have oestrogen agonist and antagonist properties. There are three major groups of naturally occurring phyto-oestrogens: isoflavones (found principally in soybeans and other legumes), lignans (found principally in flax seed, fruits and vegetables) and coumestans (found in bean sprouts and fodder crops). Ecological studies have shown that communities with a high phyto-oestrogen intake (such as Asians living in Asia) have lower rates of hip fracture than North Americans.109 However, direct epidemiological and clinical evidence for a protective effect of natural phyto-oestrogens in humans is not yet conclusive. Although vitamin K may be efficacious in slowing bone loss in postmenopausal women with osteoporosis, it has not been shown to be superior to calcium and vitamin D. It is not efficacious in preventing bone loss associated with medication-induced ovarian failure.110 In summary, the data available to date suggest that vitamin K may be efficacious in the treatment of postmenopausal women with severe osteoporosis, but has not been shown to be superior to calcium and vitamin D.111
DHEA
Low dehydroepiandrosterone (DHEA) levels have been associated with decreased bone mineral density112 and an increased risk of fracture. DHEA supplementation is indicated where there is demonstrated to be a low serum DHEA level. Dose begins at 10 mg orally per day, reviewed monthly. The aim is to achieve normal serum level. Excessive levels should be avoided because of potential for androgenic side effects.
OSTEOPOROSIS IN MEN
Because of its oestrogen-related mechanism, osteoporosis has been commonly perceived as a disease of postmenopausal women. However, emerging evidence in recent years suggests that this perception is an oversight, because men are also susceptible to osteoporosis. Indeed, approximately one-third of all fracture cases in the general population occur in men.113 Furthermore, the lifetime risk of fracture in men is 1 in 4.3,114 Outcomes of fracture in men are often worse than in women. For instance, men who have sustained a fracture have an increased risk of mortality compared to women.5 Men are twice as likely as women to die in hospital after a hip fracture.115 However, most studies of osteoporosis have largely been conducted in women and, as a result, osteoporosis in men has not been well documented.
Men have, on average, higher BMD and lower rate of bone loss than women4,116 and they tend have a lower risk of fracture at a later age than women.117 Major risk factors for osteoporosis in men include:
Although reduced testosterone is a risk factor for fracture in men,70 lower level of oestrogen is also a risk factor for low bone mass and fracture.120–122 Low BMD is a major risk factor for fracture;18,56,123 therefore, evaluation of BMD by DEXA and radiograph can be used to make a diagnosis of osteoporosis in men. It has been estimated that approximately 10% of men aged 60 years or above have osteoporosis.4 Prognostic models that take into account various simple risk factors can be used to estimate the risk of fracture for a man.69 In randomised controlled clinical trials, bisphosphonates89,124 (alendronate and risedronate) and PTH125 (teriparatide, recombinant parathyhroid hormone) have been shown to be effective in reducing fracture risk in men. Calcium and vitamin D supplementation can also be useful in preventing osteoporosis and fracture in men.118
PREVENTION
Calcium
Calcium is an important component of fracture prevention. Although calcium alone may not prevent osteoporosis, having adequate calcium, whether through food or supplementation, is an important and practical way to maintain bone health. Low dietary calcium intake is associated with lower BMD and increased fracture risk.4 Up to 75% of women may have a calcium intake (including supplemental calcium) less than the recommended daily intake.126 Although a lower calcium intake is associated with greater risk of hip fracture,127 calcium supplementation appears to improve BMD.128,129 Despite this limited evidence, the public health message is that people should have adequate dietary calcium intake throughout life (Table 23.2). More recently, concerns have arisen regarding a potentially increased risk of myocardial infarction among people taking calcium supplements over the long term.
| Age/gender | Recommended daily calcium intake (mg) |
|---|---|
| < 6 months | 300 |
| 6 months − 1 year | 500 |
| 1–3 years | 700 |
| 4–7 years | 900 |
| Girls: | |
| 8–11 years | 900 |
| 12–15 years | 1000 |
| 16–18 years | 800 |
| Women: | |
| 19–54 years | 800 |
| > 54 years | 1000 |
| pregnant | 1100–1200 |
| Boys: | |
| 8–11 years | 800 |
| 12–15 years | 1200 |
| 16–18 years | 1000 |
| Men: | |
| 19–64 years | 800 |
| > 64 years | 800 |
* Australia, National Health and Medical Research Council
Source: NHMRC 1991130
Vitamin D
Although most calcium is deposited in bone, bone is not just calcium, and calcium does not function in isolation but in interaction with vitamin D—adequate vitamin D is important for bone health, as it regulates the efficiency of calcium absorption. Vitamin D here includes cholecalciferol (vitamin D3) and ergocalciferol (vitamin D2). Despite the common perception of Australia as ‘sunshine country’, there is a high prevalence of vitamin D deficiency among elderly people. For example, a study of institutionalised or housebound individuals found that up to 80% of women and 70% of men were deficient in vitamin D and, of these, 97% had a blood level of vitamin D below the median value of the healthy reference range.131,132
People with vitamin D deficiency may need a loading dose, depending on the vitamin D levels, and supplementation with 3000–5000 IU vitamin D3 per day for 12 weeks, before repeating the test o fvitamin D levels and reviewing.133
Lifestyle and dietary factors
Lifestyle and dietary factors, including caffeine, alcohol, smoking and salt intake, have been shown to be associated with fracture risk.134–138 In Caucasian populations, heavy caffeine ingestion (> 4 cups coffee/day) has been shown to be associated with hip fracture in men and women.139,140 The effect of sodium on bone health remains unclear; however, studies have shown a significant negative effect141 when daily intake exceeds 2100 mg (90 mmol). Cigarette smoking is a significant risk factor for hip fracture.134 Modification of lifestyle, including reducing or stopping smoking, and reducing alcohol, caffeine and salt intake, is recommended as a practical way to slow the progression of osteoporosis or to reduce the risk of fracture. Other factors such as dietary magnesium and protein are also important.
Physical activity
Adequate physical activity is an important factor in reducing the risk of fracture and falls142; however, excessive physical activity can be harmful to bone health.143 It is recommended that older adults maintain regular physical activity such as jogging, field and racquet sports and swimming to reduce bone loss and fracture risk. Weight-bearing exercise is the most useful in osteoporosis prevention and management. Appropriate resistance exercise is also recommended.
Fall prevention
In the elderly, approximately one-third of women and 20% of men fall each year.144 About half of falls occur indoors, mainly in the living room (30%), bathroom/toilet (23%), dining room (14.4%) and bedroom (12.3%). Falls are an important risk factor for fracture.56 Over 90% of hip fractures are a result of a fall145 but only 1–2% of all falls of the elderly lead to a hip fracture.146,147 Therefore, fall prevention is an important approach, to reduce the risk of fracture in the elderly.
POST-FRACTURE MANAGEMENT
An existing fracture increases the risk of subsequent fractures.8,148,149 Individuals with a personal history fracture—with or without osteoporosis—should be considered for treatment. However, several studies have suggested a very low uptake of treatment. In a study of 502 hip-fracture patients in a hospital setting, only 14% underwent BMD scan, 13% received calcium/vitamin D, and only 18% received HRT, calcitonin or bisphosphonates.150 Other studies have reported that only 5% of patients with recent hip fractures left the hospital with a new medication prescribed to reduce the risk of subsequent fractures.151–153 Most women attending primary care physicians who reported a fracture after menopause are not on any specific anti-osteoporotic therapy.153 Thus, despite both the magnitude of the problem and the introduction of osteoporosis treatment guidelines, most high-risk individuals (possibly 80%) are still not identified, and therefore not treated. An information-based intervention could increase the uptake of anti-fracture treatment among high-risk patients.154 Other strategies for improving treatment uptake include fracture discharge pathways, increased nurse contact and patient education.
RICKETS
Rickets was a once common bone disease of children and it is still common in poorer countries where nutrition is inadequate. Although far less common until recently in developed countries, it is now making a comeback in many places around the world, probably due to inadequate sun exposure leading to inadequate vitamin D levels. This rise in the incidence of rickets can be contributed to by a predominance of indoor activities, physical inactivity and an over-aggressive approach to sun protection. The breastfeeding children of mothers with inadequate vitamin D levels are also more likely to develop rickets. Dark-skinned children, especially those living in countries with relatively low levels of sunlight, are at greater risk, as are children who are not consuming milk. Vitamin D deficiency can also result in hypocalcaemia and hyperexcitability of muscles.
SYMPTOMS
DIAGNOSIS
Rickets is classically diagnosed on the clinical picture in association with the following tests.
MANAGEMENT
Clinically evident rickets requires co-management with a paediatric endocrinologist.
Neonates and infants under 3 months:
PAGET’S DISEASE
SYMPTOMS
MANAGEMENT
General measures as outlined in the chapter on pain management (Ch 38) will be useful for those with more severe and chronic pain. The mainstays of medical management are aimed at reducing pain and minimising deformities that can lead to complications such as hearing loss. Drugs commonly used for Paget’s disease (see the section above on osteoporosis) are:
BONE CANCER
As with other tumours, the management approaches for bone cancer are primarily:
The chapter on cancer (Ch 24) outlines these various therapies in more detail.
1 Anonymous. Consensus development conference: prophylaxis and treatment of osteoporosis. Am J Med. 1991;90(1):107-110.
2 NIH Consensus Development Panel on Osteoporosis. Osteoporosis prevention, diagnosis and therapy. JAMA. 2001;285:785-795.
3 Nguyen ND, Ahlborg HG, Center JR, et al. Residual lifetime risk of fractures in women and men. J Bone Miner Res. 2007;22(6):781-788.
4 Nguyen TV, Center JR, Eisman JA. Osteoporosis in elderly men and women: effects of dietary calcium, physical activity, and body mass index. J Bone Miner Res. 2000;15(2):322-331.
5 Center JR, Nguyen TV, Schneider D, et al. Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet. 1999;353(9156):878-882.
6 Cauley JA, Thompson DE, Ensrud KC, et al. Risk of mortality following clinical fractures. Osteoporos Int. 2000;11(7):556-561.
7 Cooper C, Atkinson EJ, Jacobsen SJ, et al. Population-based study of survival after osteoporotic fractures. Am J Epidemiol. 1993;137(9):1001-1005.
8 Pongchaiyakul C, Nguyen ND, Jones G, et al. Asymptomatic vertebral deformity as a major risk factor for subsequent fractures and mortality: a long-term prospective study. J Bone Miner Res. 2005;20(8):1349-1355.
9 Lindsay R. The burden of osteoporosis: cost. Am J Med. 1995;98(2A):9S-11S.
10 Wiktorowicz ME, Goeree R, Papaioannou A, et al. Economic implications of hip fracture: health service use, institutional care and cost in Canada. Osteoporos Int. 2001;12(4):271-278.
11 Access Economics Pty Ltd. The burden of brittle bones: costing osteoporosis in Australia. Canberra, ACT: Access Economics Pty Ltd, 2001.
12 Cooper C, Shah S, Hand DJ, et al. Screening for vertebral osteoporosis using individual risk factors. The Multicentre Vertebral Fracture Study Group. Osteoporos Int. 1991;2(1):48-53.
13 Cooper C. Bone mass, muscle function and fracture of the proximal femur. Br J Hosp Med. 1989;42(4):277-280.
14 Cumming RG, Klineberg RJ. Epidemiological study of the relation between arthritis of the hip and hip fractures. Ann Rheum Dis. 1993;52(10):707-710.
15 Cummings SR, Kelsey JL, Nevitt MC, et al. Epidemiology of osteoporosis and osteoporotic fractures. Epidemiol Rev. 1985;7:178-208.
16 Nguyen T, Sambrook P, Kelly P, et al. Prediction of osteoporotic fractures by postural instability and bone density. BMJ. 1993;307(6912):1111-1115.
17 Seeley DG, Kelsey J, Jergas M, et al. Predictors of ankle and foot fractures in older women. The Study of Osteoporotic Fractures Research Group. J Bone Miner Res. 1996;11(9):1347-1355.
18 Nguyen TV, Center JR, Sambrook PN, et al. Risk factors for proximal humerus, forearm, and wrist fractures in elderly men and women: the Dubbo Osteoporosis Epidemiology Study. Am J Epidemiol. 2001;153(6):587-595.
19 Kanis JA, Oden A, Johnell O, et al. The burden of osteoporotic fractures: a method for setting intervention thresholds. Osteoporos Int. 2001;12(5):417-427.
20 Compston JE, Cooper C, Kanis JA. Bone densitometry in clinical practice. BMJ. 1995;310(6993):1507-1510.
21 Kanis JA, Devogelaer JP, Gennari C. Practical guide for the use of bone mineral measurements in the assessment of treatment of osteoporosis: a position paper of the European foundation for osteoporosis and bone disease. The Scientific Advisory Board and the Board of National Societies. Osteoporos Int. 1996;6(3):256-261.
22 LeBoff MS, Bermas B, Ginsburg E, et al. Osteoporosis. Guide to prevention, diagnosis, and treatment. Boston, MA: Brigham and Women’s Hospital, 2001.
23 Melton LJIII, Atkinson EJ, O’Connor MK, et al. Fracture prediction by BMD in men versus women. J Bone Miner Res. 1997;12(Suppl 1):S362.
24 Nguyen TV, Eisman JA, Kelly PJ, et al. Risk factors for osteoporotic fractures in elderly men. Am J Epidemiol. 1996;144(3):255-263.
25 Keen RW, Hart DJ, Arden NK, et al. Family history of appendicular fracture and risk of osteoporosis: a population-based study. Osteoporos Int. 1999;10(2):161-166.
26 Hu R, Mustard CA, Burns C. Epidemiology of incident spinal fracture in a complete population. Spine. 1996;21(4):492-499.
27 Sanders KM, Seeman E, Ugoni AM, et al. Age- and gender-specific rate of fractures in Australia: a population-based study. Osteoporos Int. 1999;10(3):240-247.
28 Johnell O, Melton LJIII, Nilsson JA. Are annual fluctuations in hip fracture incidence dependent upon the underlying mortality rate? Osteoporos Int. 1998;8(2):192-195.
29 Melton LJIII, Crowson CS, O’Fallon WM. Fracture incidence in Olmsted County, Minnesota: comparison of urban with rural rates and changes in urban rates over time. Osteoporos Int. 1999;9(1):29-37.
30 Keene GS, Parker MJ, Pryor GA. Mortality and morbidity after hip fractures. BMJ. 1993;307(6914):1248-1250.
31 Wehren LE, Orwig DL, Hebel JR, et al. Gender differences in mortality after hip fracture: the role of infection. J Bone Miner Res. 2003;18(12):2231-2237.
32 Goeree R, O’Brien B, Pettit D, et al. An assessment of the burden of illness due to osteoporosis in Canada. J Soc Obstet Gynaecol Can. 1996;18:15-24.
33 Randell AG, Nguyen TV, Bhalerao N, et al. Deterioration in quality of life following hip fracture: a prospective study. Osteoporos Int. 2000;11(5):460-466.
34 Delmas PD, van de Langerijt L, Watts NB, et al. Underdiagnosis of vertebral fractures is a worldwide problem: the IMPACT study. J Bone Miner Res. 2005;20(4):557-563.
35 Genant HK, Jergas M. Assessment of prevalent and incident vertebral fractures in osteoporosis research. Osteoporos Int. 2003;14(Suppl 3):S43-S55.
36 Black DM, Cummings SR, Stone K, et al. A new approach to defining normal vertebral dimensions. J Bone Miner Res. 1991;6(8):883-892.
37 McCloskey EV, Spector TD, Eyres KS, et al. The assessment of vertebral deformity: a method for use in population studies and clinical trials. Osteoporos Int. 1993;3(3):138-147.
38 O’Neill TW, Felsenberg D, Varlow J, et al. The prevalence of vertebral deformity in european men and women: the European Vertebral Osteoporosis Study. J Bone Miner Res. 1996;11(7):1010-1018.
39 de Nijs RN, Bijlsma JW, Lems WF, et al. Osteoporosis Working Group, Dutch Society for Rheumatology. Prevalence of vertebral deformities and symptomatic vertebral fractures in corticosteroid treated patients with rheumatoid arthritis. Rheumatology (Oxford). 2001;40(12):1375-1385.
40 Jones G, White C, Nguyen T, et al. Prevalent vertebral deformities: relationship to bone mineral density and spinal osteophytosis in elderly men and women. Osteoporos Int. 1996;6(3):233-239.
41 Kanis JA, Johnell O, Oden A, et al. The risk and burden of vertebral fractures in Sweden. Osteoporos Int. 2004;15(1):20-26.
42 Nevitt MC, Ettinger B, Black DM, et al. The association of radiographically detected vertebral fractures with back pain and function: a prospective study. Ann Intern Med. 1998;128(10):793-800.
43 O’Neill TW, Cockerill W, Matthis C, et al. Back pain, disability, and radiographic vertebral fracture in European women: a prospective study. Osteoporos Int. 2004;15(9):760-765.
44 Cockerill W, Lunt M, Silman AJ, et al. Health-related quality of life and radiographic vertebral fracture. Osteoporos Int. 2004;15(2):113-119.
45 Kado DM, Duong T, Stone KL, et al. Incident vertebral fractures and mortality in older women: a prospective study. Osteoporos Int. 2003;14(7):589-594.
46 Eastell R. Forearm fracture. Bone. 1996;18(Suppl 3):203S-207S.
47 Cuddihy MT, Gabriel SE, Crowson CS, et al. Forearm fractures as predictors of subsequent osteoporotic fractures. Osteoporos Int. 1999;9(6):469-475.
48 de Bruijn HP. The Colles’ fracture, review and literature. Acta Orthop Scand. 1987;58(Suppl 23):7-25.
49 Cooney WPIII, Dobyns JH, Linscheid RL. Complications of Colles’ fractures. J Bone Joint Surg Am. 1980;62(4):613-619.
50 Melton LJIII, Amadio PC, Crowson CS, et al. Long-term trends in the incidence of distal forearm fractures. Osteoporos Int. 1998;8(4):341-348.
51 Ismail AA, Cockerill W, Cooper C, et al. Prevalent vertebral deformity predicts incident hip though not distal forearm fracture: results from the European Prospective Osteoporosis Study. Osteoporos Int. 2001;12(2):85-90.
52 Klotzbuecher CM, Ross PD, Landsman PB, et al. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res. 2000;15(4):721-739.
53 Ammann P, Rizzoli R. Bone strength and its determinants. Osteoporos Int. 2003;14(Suppl 3):S13-S18.
54 Johnell O, Kanis JA, Oden A, et al. Predictive value of BMD for hip and other fractures. J Bone Miner Res. 2005;20(7):1185-1194.
55 Kung AW, Lee KK, Ho AK, et al. Ten-year risk of osteoporotic fractures in postmenopausal Chinese women according to clinical risk factors and BMD T-scores: a prospective study. J Bone Miner Res. 2007;22(7):1080-1087.
56 Nguyen ND, Pongchaiyakul C, Center JR, et al. Identification of high-risk individuals for hip fracture: a 14-year prospective study. J Bone Miner Res. 2005;20(11):1921-1928.
57 Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312(7041):1254-1259.
58 Kanis JA, Melton LJIII, Christiansen C, et al. The diagnosis of osteoporosis. J Bone Miner Res. 1994;9(8):1137-1141.
59 Writing Group for the ISCD Position Development Conference. Diagnosis of osteoporosis in men, premenopausal women, and children. J Clin Densitom. 2004;7(1):17-26.
60 Nolla JM, Gómez-Vaquero C, Fiter J, et al. Usefulness of bone densitometry in postmenopausal women with clinically diagnosed vertebral fractures. Ann Rheum Dis. 2002;61:73-75.
61 Delmas PD, Rizzoli R, Cooper C, et al. Treatment of patients with postmenopausal osteoporosis is worthwhile. The position of the International Osteoporosis Foundation. Osteoporos Int. 2005;16(1):1-5.
62 Koh LK, Sedrine WB, Torralba TP, et al. A simple tool to identify Asian women at increased risk of osteoporosis. Osteoporos Int. 2001;12(8):699-705.
63 Pongchaiyakul C, Nguyen ND, Nguyen TV. Development and validation of a new clinical risk index for prediction of osteoporosis in Thai women. J Med Assoc Thai. 2004;87(8):910-916.
64 Nguyen TV. Individualization of osteoporosis risk. Osteoporos Int. 2007;18(9):1153-1156.
65 Hillier TA, Stone KL, Bauer DC, et al. Evaluating the value of repeat bone mineral density measurement and prediction of fractures in older women: the study of osteoporotic fractures. Arch Intern Med. 2007;167(2):155-160.
66 van Daele PL, Seibel MJ, Burger H, et al. Case-control analysis of bone resorption markers, disability, and hip fracture risk: the Rotterdam study. BMJ. 1996;312(7029):482-483.
67 Eastell R, Barton I, Hannon RA, et al. Relationship of early changes in bone resorption to the reduction in fracture risk with risedronate. J Bone Miner Res. 2003;18(6):1051-1056.
68 Nguyen TV, Meier C, Center JR, et al. Bone turnover in elderly men: relationships to change in bone mineral density. BMC Musculoskelet Disord. 2007;8(1):13.
69 Nguyen ND, Frost SA, Center JR, et al. Development of prognostic nomograms for individualizing 5-year and 10-year fracture risks. Osteoporos Int. 2008;19(10):1431-1444.
70 Meier C, Nguyen TV, Handelsman DJ, et al. Endogenous sex hormones and incident fracture risk in older men: the Dubbo Osteoporosis Epidemiology Study. Arch Intern Med. 2008;168(1):47-54.
71 Kanis JA, Johnell O, Oden A, et al. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008;19(4):385-397.
72 Kanis JA, Borgstrom F, Zethraeus N, et al. Intervention thresholds for osteoporosis in the UK. Bone. 2005;36(1):22-32.
73 Hannan MT, Tucker KL, Dawson-Hughes B, et al. Effect of dietary protein on bone loss in elderly men and women: the Framingham Osteoporosis Study. J Bone Miner Res. 2000;15:2504.
74 Tucker KL, Hannan MT, Chen H, et al. Potassium, magnesium, and fruit and vegetable intakes are associated with greater bone mineral density in elderly men and women. Am J Clin Nutr. 1999;69:727.
75 Chapuy MC, Arlot ME, Duboeuf F, et al. Vitamin D3 and calcium to prevent hip fractures in the elderly woman. N Engl J Med. 1992;327(23):1637-1642.
76 Chapuy MC, Pamphile R, Paris E, et al. Combined calcium and vitamin D3 supplementation in elderly women: confirmation of reversal of secondary hyperparathyroidism and hip fracture risk: the Decalyos II study. Osteoporos Int. 2002;13(3):257-264.
77 Dawson-Hughes B, Harris SS, Krall EA, et al. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med. 1997;337(10):670-676.
78 Victorian Government (Australia), Department of Health. Low vitamin D in Victoria: key health promotion messages for community health workers; December 2009. Online. Available: http://www.health.vic.gov.au/chiefhealthofficer/downloads/vitamin_d_comm.pdf.
79 Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291(14):1701-1712.
80 Wedick NM, Barrett-Connor E, Knoke JD, et al. The relationship between weight loss and all-cause mortality in older men and women with and without diabetes mellitus: the Rancho Bernardo Study. J Am Geriatr Soc. 2002;50(11):1810-1815.
81 Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321-333.
82 Torgerson DJ, Bell-Syer SE. Hormone replacement therapy and prevention of nonvertebral fractures: a meta-analysis of randomized trials. JAMA. 2001;285(22):2891-2897.
83 Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA. 1999;282(7):637-645.
84 Delmas PD, Ensrud KE, Adachi JD, et al. Efficacy of raloxifene on vertebral fracture risk reduction in postmenopausal women with osteoporosis: four-year results from a randomized clinical trial. J Clin Endocrinol Metab. 2002;87(8):3609-3617.
85 Chesnut CHIII, Silverman S, Andriano K, et al. A randomized trial of nasal spray salmon calcitonin in postmenopausal women with established osteoporosis: the prevent recurrence of osteoporotic fractures study. PROOF Study Group. Am J Med. 2000;109(4):267-276.
86 Liberman UA, Weiss SR, Broll J, et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med. 1995;333(22):1437-1443.
87 Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348(9041):1535-1541.
88 Saag KG, Emkey R, Schnitzer TJ, et al. Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. Glucocorticoid-Induced Osteoporosis Intervention Study Group. N Engl J Med. 1998;339(5):292-299.
89 Adachi JD, Saag KG, Delmas PD, et al. Two-year effects of alendronate on bone mineral density and vertebral fracture in patients receiving glucocorticoids: a randomized, double-blind, placebo-controlled extension trial. Arthritis Rheum. 2001;44(1):202-211.
90 Orwoll E, Ettinger M, Weiss S, et al. Alendronate for the treatment of osteoporosis in men. N Engl J Med. 2000;343(9):604-610.
91 Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA. 1999;282(14):1344-1352.
92 Reginster J, Minne HW, Sorensen OH, et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int. 2000;11(1):83-91.
93 McClung MR, Geusens P, Miller PD, et al. Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med. 2001;344(5):333-340.
94 Cranney A, Guyatt G, Krolicki N, et al. A meta-analysis of etidronate for the treatment of postmenopausal osteoporosis. Osteoporos Int. 2001;12(2):140-151.
95 Chesnut IC, Skag A, Christiansen C, et al. Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19(8):1241-1249.
96 Black DM, Delmas PD, Eastell R, et al. Once–yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356(18):1809-1822.
97 Reeve J, Meunier PJ, Parsons JA, et al. Anabolic effect of human parathyroid hormone fragment on trabecular bone in involutional osteoporosis: a multicentre trial. BMJ. 1980;280(6228):1340-1344.
98 Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344(19):1434-1441.
99 Slovik DM, Adams JS, Neer RM, et al. Deficient production of 1,25–dihydroxyvitamin D in elderly osteoporotic patients. N Engl J Med. 1981;305(7):372-374.
100 Kurland ES, Cosman F, McMahon DJ, et al. Parathyroid hormone as a therapy for idiopathic osteoporosis in men: effects on bone mineral density and bone markers. J Clin Endocrinol Metab. 2000;85(9):3069-3076.
101 Lane JM, Russell L, Khan SN. Osteoporosis. Clin Orthop. 2000;372:139-150.
102 Lane NE, Sanchez S, Modin GW, et al. Parathyroid hormone treatment can reverse corticosteroid-induced osteoporosis. Results of a randomized controlled clinical trial. J Clin Invest. 1998;102(8):1627-1633.
103 Delmas PD. Clinical effects of strontium ranelate in women with postmenopausal osteoporosis. Osteoporos Int. 2005;16(Suppl 1):S16-S19.
104 Meunier PJ, Roux C, Seeman E, et al. The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N Engl J Med. 2004;350(5):459-468.
105 Rich C, Ensinck J, Ivanovich P. The effects of sodium floride on calcium metabolism of subjects with metabolic bone diseases. J Clin Invest. 1964;43:545-555.
106 Riggs BL, Arnaud CD, Jowsey J, et al. Parathyroid function in primary osteoporosis. J Clin Invest. 1973;52(1):181-184.
107 Sambrook PN, Seeman E, Phillips SR, et al. Preventing osteoporosis: outcomes of the Australian Fracture Prevention Summit. Med J Aust. 2002;176(Suppl):S1-S16.
108 Tonino RP, Meunier PJ, Emkey R, et al. Skeletal benefits of alendronate: 7-year treatment of postmenopausal osteoporotic women. Phase III Osteoporosis Treatment Study Group. J Clin Endocrinol Metab. 2000;85(9):3109-3115.
109 Scheiber MD, Rebar RW. Isoflavones and postmenopausal bone health: a viable alternative to estrogen therapy? Menopause. 1999;6(3):233-241.
110 Somekawa Y, Chigughi M, Harada M, et al. Use of vitamin K2 (menatetrenone) and 1,25-dihydroxyvitamin D3 in the prevention of bone loss induced by leuprolide. J Clin Endocrinol Metab. 1999;84(8):2700-2704.
111 Shiraki M, Shiraki Y, Aoki C, et al. Vitamin K2 (menatetrenone) effectively prevents fractures and sustains lumbar bone mineral density in osteoporosis. J Bone Miner Res. 2000;15(3):515-521.
112 Osmanagaoglu MA, Okumus B, Osmanagaoglu T, et al. The relationship between serum dehydroepiandrosterone sulfate concentration and bone mineral density, lipids, and hormone replacement therapy in premenopausal and postmenopausal women. J Womens Health (Larchmt). 2004;13(9):993-999.
113 Jones G, Nguyen T, Sambrook PN, et al. Symptomatic fracture incidence in elderly men and women: the Dubbo Osteoporosis Epidemiology Study (DOES). Osteoporos Int. 1994;4(5):277-282.
114 Narayan KM, Boyle JP, Thompson TJ, et al. Lifetime risk for diabetes mellitus in the United States. JAMA. 2003;290(14):1884-1890.
115 Myers AH, Robinson EG, van Natta ML, et al. Hip fractures among the elderly: factors associated with in-hospital mortality. Am J Epidemiol. 1991;134(10):1128-1137.
116 Nguyen ND, Center JR, Eisman JA, et al. Bone loss, weight loss, and weight fluctuation predict mortality risk in elderly men and women. J Bone Miner Res. 2007;22(8):1147-1154.
117 Nguyen TV, Eisman JA. Risk factors for low bone mass in men. In: Orwoll ES, editor. Osteoporosis in men. San Diego, Ca: Academic Press; 1999:335-361.
118 Amin S, Felson DT. Osteoporosis in men. Rheum Dis Clin North Am. 2001;27(1):19-47.
119 Orwoll ES. Osteoporosis in men. Endocrinol Metab Clin North Am. 1998;27(2):349-367.
120 Khosla S, Melton LJIII, Robb RA, et al. Relationship of volumetric BMD and structural parameters at different skeletal sites to sex steroid levels in men. J Bone Miner Res. 2005;20(5):730-740.
121 Gennari L, Merlotti D, Martini G, et al. Longitudinal association between sex hormone levels, bone loss, and bone turnover in elderly men. J Clin Endocrinol Metab. 2003;88(11):5327-5333.
122 Khosla S, Melton LJIII, Atkinson EJ, et al. Relationship of serum sex steroid levels to longitudinal changes in bone density in young versus elderly men. J Clin Endocrinol Metab. 2001;86(8):3555-3561.
123 Nguyen ND, Eisman JA, Center JR, et al. Risk factors for fracture in nonosteoporotic men and women. J Clin Endocrinol Metab. 2007;92(3):955-962.
124 Wallach S, Cohen S, Reid DM, et al. Effects of risedronate treatment on bone density and vertebral fracture in patients on corticosteroid therapy. Calcif Tissue Int. 2000;67(4):277-285.
125 Orwoll ES, Scheele WH, Paul S, et al. The effect of teriparatide [human parathyroid hormone (1–34)] therapy on bone density in men with osteoporosis. J Bone Miner Res. 2003;18(1):9-17.
126 Pasco JA, Sanders KM, Henry MJ, et al. Calcium intakes among Australian women: Geelong Osteoporosis Study. Aust NZ J Med. 2000;30(1):21-27.
127 Lau E, Donnan S, Barker DJ, et al. Physical activity and calcium intake in fracture of the proximal femur in Hong Kong. BMJ. 1988;297(6661):1441-1443.
128 Lau EM, Woo J, Leung PC, et al. The effects of calcium supplementation and exercise on bone density in elderly Chinese women. Osteoporos Int. 1992;2(4):168-173.
129 Shea B, Wells G, Cranney A, et al. Meta-analyses of therapies for postmenopausal osteoporosis. VII. Meta-analysis of calcium supplementation for the prevention of postmenopausal osteoporosis. Endocr Rev. 2002;23(4):552-559.
130 National Health and Medical Research Council. Recommended dietary intakes for use in Australia. Canberra: AGPS; 1991. Online. Available: http://www.nhmrc.gov.au/publications/synopses/n6syn.htm.
131 Flicker L, Mead K, MacInnis RJ, et al. Serum vitamin D and falls in older women in residential care in Australia. J Am Geriatr Soc. 2003;51(11):1533-1538.
132 Sambrook PN, Cameron ID, Cumming RG, et al. Vitamin D deficiency is common in frail institutionalised older people in northern Sydney. Med J Aust. 2002;176(11):560.
133 Working Group of the Australian and New Zealand Bone and Mineral Society. Vitamin D and adult bone health in Australia and New Zealand: a position statement. Med J Aust. 2005;182(6):281-285.
134 Kanis JA, Johnell O, Oden A, et al. Smoking and fracture risk: a meta-analysis. Osteoporos Int. 2005;16(2):155-162.
135 Hallstrom H, Wolk A, Glynn A, et al. Coffee, tea and caffeine consumption in relation to osteoporotic fracture risk in a cohort of Swedish women. Osteoporos Int. 2006;17(7):1055-1064.
136 Heaney RP. Role of dietary sodium in osteoporosis. J Am Coll Nutr. 2006;25(Suppl 3):271S-276S.
137 Morris RCJr, Schmidlin O, Frassetto LA, et al. Relationship and interaction between sodium and potassium. J Am Coll Nutr. 2006;25(Suppl 3):262S-270S.
138 Kanis JA, Johansson H, Johnell O, et al. Alcohol intake as a risk factor for fracture. Osteoporos Int. 2005;16(7):737-742.
139 Kiel DP, Felson DT, Hannan MT, et al. Caffeine and the risk of hip fracture: the Framingham Study. Am J Epidemiol. 1990;132(4):675-684.
140 Prince R, Devine A, Dick I, et al. The effects of calcium supplementation (milk powder or tablets) and exercise on bone density in postmenopausal women. J Bone Miner Res. 1995;10(7):1068-1075.
141 Yano K, Heilbrun LK, Wasnich RD, et al. The relationship between diet and bone mineral content of multiple skeletal sites in elderly Japanese-American men and women living in Hawaii. Am J Clin Nutr. 1985;42(5):877-888.
142 Sinaki M. The role of physical activity in bone health: a new hypothesis to reduce risk of vertebral fracture. Phys Med Rehabil Clin N Am. 2007;18(3):593-608. xi–xii.
143 National Osteoporosis Foundation. Physician’s guide to prevention and treatment of osteoporosis. Washington DC: National Osteoporosis Foundation, 2003.
144 Lord SR, Sambrook PN, Gilbert C, et al. Postural stability, falls and fractures in the elderly: results from the Dubbo Osteoporosis Epidemiology Study. Med J Aust. 1994;160(11):684-685. 688–691.
145 Grisso JA, Kelsey JL, Strom BL, et al. Risk factors for falls as a cause of hip fracture in women. The Northeast Hip Fracture Study Group. N Engl J Med. 1991;324(19):1326-1331.
146 Nevitt MC, Cummings SR, Kidd S, et al. Risk factors for recurrent nonsyncopal falls. A prospective study. JAMA. 1989;261(18):2663-2668.
147 Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988;319(26):1701-1707.
148 Center JR, Bliuc D, Nguyen TV, et al. Risk of subsequent fracture after low-trauma fracture in men and women. JAMA. 2007;297(4):387-394.
149 Kanis JA, Johansson H, Oden A, et al. A family history of fracture and fracture risk: a meta-analysis. Bone. 2004;35(5):1029-1037.
150 Harrington JT, Broy SB, Derosa AM, et al. Hip fracture patients are not treated for osteoporosis: a call to action. Arthritis Rheum. 2002;47(6):651-654.
151 Kamel HK, Hussain MS, Tariq S, et al. Failure to diagnose and treat osteoporosis in elderly patients hospitalized with hip fracture. Am J Med. 2000;109(4):326-328.
152 Bauer DC. Osteoporotic fractures: ignorance is bliss? Am J Med. 2000;109(4):338-339.
153 Eisman J, Clapham S, Kehoe L. Osteoporosis prevalence and levels of treatment in primary care: the Australian Bone Care Study. J Bone Miner Res. 2004;19(12):1969-1975.
154 Bliuc D, Eisman JA, Center JR. A randomized study of two different information-based interventions on the management of osteoporosis in minimal and moderate trauma fractures. Osteoporos Int. 2006;17(9):1309-1317.