CHAPTER 68 Bone Substitutes
Basic Science and Clinical Applications
Spinal fusion is a common treatment for various conditions including deformity, trauma, and degenerative disc disease with instability. Over the past century, autogenous bone grafting has improved our ability to direct bone formation required for spinal arthrodesis. Nonetheless, significant rates of pseudoarthrosis up to 26% have been reported in the literature.1 Even with modern bone graft techniques and advances in internal fixation, symptomatic pseudoarthrosis still occurs in 10% to 15% of cases.2–4 Nonunion may result in poor clinical outcomes and result in extensive medical expenditure. Bone formation is essential to arthrodesis of the spine, and numerous techniques over the past decades have evolved to achieve bone formation for spinal fusion. In addition, autograft bone has certain limitations including local morbidity and limited availability. These problems have led surgeons to devise new biologic strategies, search for substitutes for autogenous bone grafting, and apply these new approaches to stimulate bone fusion.
Bone formation requires three essential components: an osteogenic potential capable of directly providing cells to the newly forming bone, osteoinductive factors that are able to signal the osteoblastic differentiation of osteoprogenitor stem cells, and an osteoconductive scaffold that assists neovascularization and supports the ingrowth of bone. The ideal bone substitute possesses all of these three properties along with an optimal biologic reaction and without risk of disease transmission. Autogenous bone grafts share these properties and are therefore considered the standard against which bone substitutes may be measured. There are, however, disadvantages with autogenous bone grafting. Autogenous iliac crest bone harvesting is associated with considerable donor site morbidity, increased operative time, and increased blood loss. Up to 30% of all patients undergoing the harvesting of iliac crest bone graft will experience significant postoperative pain or complications including infection, hematoma, nerve or vascular injury, fracture, persistent pain, abdominal herniation, or pelvic instability.3–17 Prior studies report autologous bone graft harvest to be associated with major complications in 8.6% of patients and minor complications in 20.6%.14 In addition, the amount of autograft available may be insufficient for long fusions. Similarly, revision patients may have undergone prior bone grafting procedures with little or no additional useful iliac crest bone available. For these reasons, bone substitutes may be required to augment, expand, or substitute for autogenous bone graft.
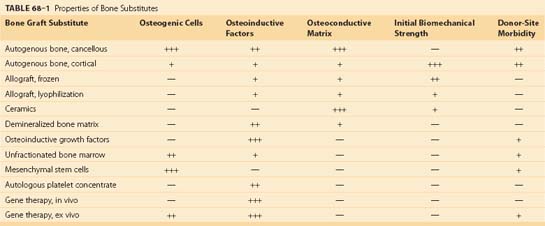
In order to avoid morbidity associated with harvesting autogenous graft and to optimize bone formation for fusion, several classes of bone substitutes have been developed. These include allografts, ceramics, demineralized bone matrices (DBMs), osteoinductive factors, autogenous platelet concentrate, mesenchymal stem cells, and gene therapy (Table 68–1). Although bone substitutes currently in clinical practice do not provide the same osteogenic, osteoinductive, and osteoconductive properties of autograft, various bone substitutes have demonstrated efficiency for bone formation in basic science and clinical studies. Advances in regional gene therapy, development of osteoinductive proteins, and production of new osteoconductive matrices herald a new era of bone biology for spine fusion.
Allografts
Allograft bone may be applied to the graft bed in a crushed particulate form or can be machined to create structural spacers. Cortical allografts offer substantial structural stability and are best suited for interbody arthrodesis. Corticocancellous allograft initially imparts little mechanical support to the fusion site but, because of its relatively large surface area, is integrated more rapidly than cortical bone.18,19 Genetic incompatibility between donor and recipient has been found to be associated with increased resorption of the allograft and histologic evidence of rejection.20
Allografts are prepared either by freezing or lyophilization (i.e., freeze-drying) in order to decrease their antigenicity and permit storage for extended periods of time.21 Frozen allografts may be stored for up to 1 year. Lyophilized allografts are dehydrated and vacuum packed, which allows for storage at room temperature. Freeze-drying reduces immunogenicity even more than freezing, but on rehydration these grafts may lose up to 50% of their mechanical strength.22 Other sterilization techniques such as ethylene oxide or radiation may compromise the material properties and osteoinductive capacity.
Despite aseptic techniques, allografts pose potential risks for bacterial contamination. In addition, there is concern for possible transmission of viral diseases such as those caused by the human immunodeficiency virus (HIV) and hepatitis virus. Nevertheless, there have been only two documented cases of human immunodeficiency virus transmission from allograft bone, both of which involved unprocessed grafts.23 The combination of rigorous donor screening and tissue processing has lowered the risk of infection to less than one per million transplants.24 Other complications observed after the implantation of structural allografts include nonunion and fracture of the graft.25 Compared with autograft, allograft incorporates slower and less completely with decreased vascularization and osteoconduction.26
Structural allografts have been used extensively for anterior interbody fusions in both the cervical and lumbar spine. Previous studies evaluating patients undergoing single-level fusions of the anterior cervical spine, with either allograft or autogenous bone, have demonstrated similar fusion rates.27,28 Structural allograft is less efficacious than autograft for promoting multilevel cervical fusions.29–31
In the posterior lumbar spine, autogenous bone graft appears to be superior to allograft for promoting posterolateral arthrodesis. In two prospective clinical trials comparing autograft with various allograft preparations, patients treated with autograft achieved solid posterolateral fusion more frequently than those receiving allograft.32,33
Jorgenson and colleagues32 conducted a prospective analysis of autografts versus allografts in posterolateral lumbar fusion in the same patient and concluded that an ethylene oxide–treated allograft is inferior to an autograft and should not be used for posterior lumbar fusions. Another prospective comparison of autografts and allografts for adult posterolateral lumbar spinal fusion reported that autografts resulted in significantly greater bone density, followed by a mixture of autografts and allografts, frozen allografts, and freeze-dried allografts.33 These reports indicate that allografts alone were not able to achieve a sufficient fusion rate for posterior spinal fusion in the adult patients. However, several studies have recommended the use of allografts as bone extenders in adolescent idiopathic scoliosis. Aurori and colleagues34 retrospectively compared the incidence of pseudoarthrosis in fusions for scoliosis supplemented with autografts and frozen allografts that were obtained from femoral heads and reported that the incidence of pseudoarthrosis was not significantly different. Dodd and colleagues35 conducted a case-control study on the use of autografts versus allografts that were from femoral heads in the surgery of idiopathic adolescent scoliosis. They reported that there was no difference, either in a radiographic assessment of bone graft mass or in the maintenance of the curve correction.
Additional studies have reported excellent outcomes with the use of femoral ring allografts in the anterior lumbar spine.36,37 In cases involving revision anterior lumbar fusions, one study found that the results obtained with tricortical allograft may be comparable with those obtained with autogenous bone graft taken from the iliac crest.38 Overall, these studies suggest that cortical allografts may be regarded as acceptable alternatives to autogenous bone graft in certain clinical situations requiring structural support and graft material such as anterior lumbar and single-level cervical fusions. Cancellous allograft may be efficacious in the adolescent patient with scoliosis undergoing fusion and may also be used to supplement a limited quantity of autograft for posterolateral arthrodesis.
Ceramics
Ceramics are bone substitutes designed to be osteoconductive to ingrowth of new bone.39 The most commonly used ceramic scaffolds for spinal fusion are calcium phosphates such as hydroxyapatite, tricalcium phosphate (TCP), and a combination of these materials. Ceramics are favorable bone substitutes because they are biodegradable, nontoxic, nonimmunogenic, easy to sterilize, and available in virtually unlimited supply without donor site morbidity or infection risk. Disadvantages of ceramic structures are their brittle structure and reduced shear strength and resistance to fracture. Because they offer minimal mechanical stability in the immediate postoperative period, ceramics are commonly used in conjunction with rigid internal fixation and must be protected from loading forces until they are incorporated into the surrounding bone.
In general, ceramic scaffolds can be used as bone graft extenders to expand an existing quantity of available local autograft bone chips for posterolateral spinal fusion. With rigid instrumentation, several studies have reported that ceramic scaffolds are efficient bone graft extenders in posterolateral spinal fusion.40–42 Although ceramic scaffolds appear to be suitable bone graft extenders, hydroxyapatite alone may be insufficient for intertransverse posterolateral fusion. An adequate vascularized bone surface such as decorticated lamina may be required for incorporation of coralline hydroxyapatite mixed with local bone and bone marrow. As such, iliac bone autografts remained the gold standard for achieving solid posterolateral fusion.43
On the other hand, successful results have been reported for the implantation of ceramic scaffolds for posterior spinal fusion in scoliosis cases, which require extensive bone graft.44,45 Ransford and colleagues46 conducted a prospective randomized study to evaluate the use of a synthetic porous ceramic as a bone graft substitute in posterior spinal fusion for idiopathic scoliosis; they concluded that porous ceramic is a safe and effective bone substitute. Tricalcium phosphate may be an alternative to allograft as an extender when large volumes of graft are necessary in posterior spinal fusion for scoliosis.47 For anterior spinal fusion, ceramic scaffolds need to be used with rigid internal fixation. Thalgott and colleagues48–49 reported a retrospective study to evaluate the efficacy of coralline hydroxyapatite as a bone replacement in anterior interbody fusion in both the cervical and lumbar spine. They concluded that the use of coralline hydroxyapatite with rigid anterior plating appeared to be a promising bone replacement in anterior fusion, but it was not recommended for stand-alone anterior interbody fusion.48–49
The bioresorbability of a ceramic is influenced by the shape, density, and chemical composition of the material. Hydroxyapatite is a relatively inert substance that is retained in vivo for prolonged periods of time, whereas the more porous tricalcium phosphate typically biodegrades in about 6 weeks.50 There is some concern that ceramic particles may provoke an inflammatory response that could eventually bring about the significant resorption of bone, similar to the osteolysis triggered by debris from total joint prostheses, but at this time there is little evidence substantiating this risk. Although the implantation of ceramics alone has been associated with successful outcomes after anterior cervical intervertebral fusion51 and posterior spinal fusion for adolescent idiopathic scoliosis,52,53 these osteoconductive scaffolds are usually coupled with other osteogenic or osteoinductive materials. When loaded with a source of osteogenic cells such as autogenous bone or bone marrow, ceramic scaffolds assist cellular adhesion, support vascular ingrowth, and promote new bone formation.54 Ceramic carriers may also function as effective vehicles for the delivery of osteoinductive growth factors.
Demineralized Bone Matrices
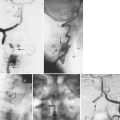
DBMs are derived from allograft bone by removing the mineralized component but preserving type I collagen and noncollagenous proteins including numerous growth factors. The osteoinductive properties of DBMs were first recognized by Urist55 in 1965, when he reported that the introduction of decalcified bone brought about the formation of heterotopic bone in rodents. The bone morphogenetic proteins (BMPs) represent less than 0.1% of all bone proteins by weight,56 but these growth factors are essential to the process of osteoinduction, initiating a cascade of cellular events leading to bone formation. Commercially available DBMs have demonstrated marked variability in osteoinductive potential that may reflect differences in their BMP content in rat spinal fusion models (Fig. 68–1).57–59
Posterolateral spinal fusion may be successful using DBMs alone or in conjunction with autograft in a rabbit and nonhuman primate model.60–63 Clinical studies also support the efficacy of DBMs as bone graft extenders for posterolateral spinal fusion.64,65 A composite consisting of DBM putty and aspirated bone marrow offers a similar performance as autograft in posterolateral spinal fusion. A multicenter prospective study compared the effectiveness of a Grafton (Osteotech; Eatontown, N.J.) DBM gel composite with iliac crest autograft in posterolateral spinal fusion; this demonstrated that Grafton DBM could extend a volume of autograft that was less than normally required to achieve a solid spinal fusion.66 DBM and bone marrow composite has been successful for posterior spinal fusion in scoliosis cases, and the fusion rates have been reported to be comparable with those of autograft.67 In a case series of anterior lumbar interbody fusions with DBM composites consisting of titanium mesh cages, coralline hydroxyapatite, and DBM, one study concluded that the DBM composite was effective for anterior interbody fusion of the lumbar spine when used as part of a rigidly instrumented circumferential fusion.68 On the other hand, An and colleagues69 prospectively analyzed the fusion rates of an allograft-DBM composite, as compared with autograft, in anterior cervical fusion and concluded that the allograft-DBM construct resulted in a higher rate of graft collapse and pseudarthrosis than autograft alone.
In a direct comparison of multiple formulations of the same DBM, the putty and flexible sheet forms enhanced spinal fusion to a greater extent than a gel, most likely because the former are fiber-based preparations and exhibit improved handling characteristics over the putty form.70 Because they do not contain viable cells, DBMs are most effective when implanted in environments that offer suitable vascularity and an adequate supply of osteoprogenitor cells. In an attempt to further augment bone formation, autogenous bone graft or bone marrow aspirates are often added to DBMs in order to increase the number of osteogenic cells able to respond to the osteoinductive growth factors. DBMs provide minimal structural support and are generally implanted into locations where they are not subjected to excessive biomechanical forces. The osteoinductive potential of DBMs has been tested in both intervertebral (interbody) and posterolateral spinal fusion models in a wide range of animals. DBMs have been found to promote successful arthrodesis of the spine when used alone or in conjunction with autograft, bone marrow, or ceramics.71–73
Osteoinductive Growth Factors
Numerous growth factors capable of signaling cell-surface receptors and directing cellular activities are involved in the regeneration of new bone tissue. In spinal surgery, growth factors critical to embryonic bone formation and fracture healing are powerful adjuvants to obtain fusion. In 1965 Marshal Urist55 first observed that DBM possessed osteoinductive ability; subsequently, the osteoinductive BMPs were isolated and characterized. BMPs are members of the transforming growth factor-beta (TGF-β) superfamily. By binding to specific receptors present on the surface of the osteogenic progenitor, intracellular cascades that recapitulate endochondral ossification are activated. BMPs stimulate mesenchymal stem cells to differentiate. Early BMP extracts were acquired in a partially purified form using techniques that called for large amounts of bone; with these inefficient methods, 10 kg of cortical bone yielded less than 20 g of osteoinductive protein.74 In addition, these crude preparations contained a heterogeneous collection of growth factors including several different types of BMPs, as well as other biologically inactive proteins.
Taking advantage of advances in molecular and cellular sciences, the genes encoding the BMP proteins were sequenced and subsequently cloned, allowing for the mass production of a single BMP including BMP-2 and BMP-7 (also known as osteogenic protein-1 [OP-1]).75 Because they are available in almost unlimited quantities, BMP-2 and BMP-7 have become the most widely used recombinant BMPs for animal studies and are the only BMPs currently being evaluated in human clinical trials. In contrast to purified extracts, recombinant growth factors are free of impurities and do not elicit a host immune response. Recombinant human BMPs (rhBMPs) are soluble factors that tend to diffuse away from the fusion site when used alone, resulting in attenuation of their osteoinductive capacity. For this reason, before implantation, these factors are combined with a carrier matrix that serves to restrict their movement, confine them to the location where they are needed, and allow them to release consistently over time. These substrates may also act as osteoconductive scaffolds that support new bone formation by promoting cellular adhesion and angiogenesis. Autogenous bone graft, DBMs, collagen, ceramics, and polylactic acid (PLA) have all been used to deliver rhBMPs, but at this time the ideal carrier has not been identified.75–87 It is likely that the most suitable method of delivery may be dependent on the specific clinical application being treated and the location into which the growth factors will be introduced. Once these growth factors have been distributed to the area of interest, osteogenic cells that are able to respond appropriately to these osteoinductive proteins must also be present for any significant bone production to occur. Multiple animal studies, many of which were performed in nonhuman primates, have established that the implantation of rhBMPs such as BMP-2 and BMP-7 in the posterior spine results in fusion rates equivalent or superior to those obtained with autogenous bone graft. Furthermore, they may generate fusion masses with improved biomechanical properties that may obviate the need for decortication of the posterior elements, a procedure that is normally required to provide endogenous growth factors that are essential for the successful arthrodesis of the posterolateral spine.88 Boden and colleagues89 conducted a prospective randomized clinical pilot study on the use of rhBMP-2 for posterolateral fusion in humans. In that study, the authors randomly divided the enrolled patients into three treatment groups as follows: autograft with instrumentation, rhBMP-2/ ceramic granules with instrumentation, and rhBMP-2/ceramic granules only without instrumentation. They reported that the fusion rate of the rhBMP-2/ceramic granules without instrumentation group was 100%, which was superior to the autograft with instrumentation group (40%).89 Following this pilot study, Dimar and colleagues90 conducted a prospective randomized study comparing the use of iliac crest bone graft to rhBMP-2 combined with a carrier consisting of bovine collagen and tricalcium/hydroxyapatite for single-level posterolateral fusions. Those authors also reported that the rhBMP-2 group demonstrated increased fusion rates as compared with the autograft group.90 In a prior study Boden and colleagues91 described the human pilot trial of the use of rhBMP-2/collagen inside lumbar interbody spinal fusion cages. Although the number of patients enrolled in that study was small, they reported at the 2-year follow-up that fusion occurred more reliably in patients treated with rhBMP-2-filled cages than in controls treated with autogenous bone graft.91 Burkus and colleagues also conducted a prospective study on the use of rhBMP-2/collagen sponge with allograft dowels or tapered cylindrical fusion devices in anterior lumbar interbody fusion and concluded that the use of those rhBMP-2 composites showed promise in assisting anterior intervertebral spinal fusion.92–94 Slosar and colleagues,95 in a prospective study on anterior lumbar interbody fusions, compared patients treated with allografts, either with or without the addition of rhBMP-2, with posterior instrumentation and demonstrated excellent results with the use of rhBMP-2. These reports supported the use of rhBMP-2 for anterior lumbar interbody fusion. On the other hand, there are reports that rhBMP-2 can cause aggressive resorption of an implanted graft before osteoinduction and interbody fusion occurs. McClellan and colleagues96 retrospectively investigated cases with a transforaminal lumbar interbody fusion with BMP; they reported a high rate of bone resorption defects and assumed that the osseous remodeling potential of rhBMP-2 may lead to bone resorption within the vertebral body. Pradhan and colleagues97 reported that the pseudarthrosis rate among patients who received femoral ring allografts with rhBMP-2 was higher than that in patients who received femoral ring allografts with autogenous iliac bone. They concluded that this appeared to be caused by the aggressive resorptive phase of allograft incorporation, which occurs before the osteoinduction phase.97 These results suggest that caution must be exercised in deciding between autograft and rhBMP-2 for anterior lumbar interbody fusion and that further clinical studies are warranted.
It appears that the osteoinductive activity of the BMPs may compensate for the inhibitory effects of nicotine and nonsteroidal anti-inflammatory drugs (NSAIDs), two agents implicated in hindering spinal fusion in humans.98–102 As noted previously, recombinant growth factors have been used in conjunction with intervertebral fusion devices in an attempt to achieve arthrodesis of the anterior spinal column. Titanium cages or cortical allograft dowels loaded with rhBMPs proved to be more efficacious than similar devices carrying autogenous bone for stimulating interbody fusion in a number of different animal models, a finding that was consistent whether these composite grafts were implanted in the cervical, thoracic, or lumbar spine.101–103 These encouraging results were corroborated by a prospective, randomized clinical trial in which patients with degenerative disc disease limited to a single level of the lumbar spine were treated with a threaded cylindric cage filled with either rhBMP-2 protein or autograft.104 After 24 months, all 11 patients who had received rhBMP-2 exhibited radiographic evidence of solid fusion, compared with only two of the three control patients who were implanted with interbody devices containing their own iliac crest bone. The rhBMP-2 group initially experienced a more rapid resolution of their original symptoms, although at 6 months both groups demonstrated similar levels of clinical improvement. In addition, no complications were reported with the use of rhBMP-2. These studies have confirmed that partially purified BMP extracts and recombinant growth factors are able to induce spinal fusion in animals and humans. In all of these studies, however, the concentration of BMP necessary to bring about adequate bone formation in this environment was several magnitudes of order greater than normal physiologic levels, an observation that raises potential safety concerns. In a canine lumbar spine fusion model, the placement of osteogenic protein-1 (OP-1) over a dural tear stimulated new bone formation in the subarachnoid space, resulting in mild spinal stenosis at the site of dural decompression.105 Before osteoinductive growth factors may be used clinically as a bone graft substitute, they must be subjected to further testing to ensure that the introduction of milligram doses of these proteins into patients is not associated with any significant immunogenicity, toxicity, or other adverse effects.
In contrast to anterior lumbar fusion, there are studies that caution against the use of high-dose rhBMP-2 for cervical anterior spinal fusion. Shields and colleagues107 reported a retrospective review of patients who underwent anterior cervical fusion using high-dose rhBMP-2/collagen sponge. The authors reported that 23.2% of patients suffered complications such as hematomas, dysphagia, and excessive edema. Vaidya and colleagues108 also reported that complications were associated with anterior cervical spinal fusion using rhBMP-2 including dysphagia that was shown to be significantly more frequent and more severe in patients in whom rhBMP-2 was used. As a consequence, in July 2009 the U.S. Food and Drug Administration issued a Public Health Notification citing serious adverse effects with use of BMP in the cervical spine. Therefore rhBMP-2 must be used cautiously for anterior cervical spinal fusions until more research is undertaken and these clinical issues are resolved. Because the administration of large amounts of BMP is expensive, economic analyses should be completed to determine the cost-effectiveness of using growth factor therapy as a substitute for autogenous bone graft in the spine. In order to minimize the quantities of BMP required for a successful fusion and outcome, it will be important to establish the appropriate dose for each spinal application and develop efficient carrier systems to deliver these osteoinductive factors. Finally, it is important to note that, in the spine, BMP is FDA approved only as an adjunct with a threaded lumbar interbody cage. Other uses are considered an off-label use.
New adjuvant agents such as bone morphogenetic binding peptide (BBP), which may enhance the efficiency of BMP, are under investigation in animal and in vitro studies. BBP is a BMP-specific binding protein that was isolated on the basis of the early work of Urist. BBP binds rhBMP-2 with an intermediate affinity, which makes it an ideal “slow release” agent.108 As such, BBP may reduce the time to fusion and more thoroughly control the distribution of bone healing in spinal fusion.
Autologous Platelet Concentrate
Platelet degranulation is part of the normal cascade of bone healing with release of several growth factors such as platelet-derived growth factor (PDGF) and TGF-β. These growth factors promote chemotaxis and proliferation of mesenchymal stem cells and osteoblasts and enhance bone healing.109–110 By concentrating these platelet factors as autologous growth factor concentrate (AGF), one may enhance the formation of new bone in lumbar spinal fusion when used in combination with autografts.111 In the autologous platelet gel systems currently in development, platelet-rich plasma is separated from a sample of the patient’s blood and concentrated in a fibrinogen matrix. This fibrinogen preparation is combined with thrombin, forming a fibrin clot that can be administered with an osteoconductive scaffold or a source of osteogenic cells to form a composite bone graft. Recently, several reports have addressed in detail the efficacy of AGF for spinal fusion. Weiner and colleagues115 retrospectively compared autograft alone with an autograft with AGF in posterolateral spinal fusion; the authors reported that the use of AGF resulted in inferior rates of fusion compared with those of autograft alone. Hee and colleagues116 conducted a prospective study on AGF in instrumented transforaminal lumbar interbody spinal fusion (TLIF) and concluded that the use of AGF in TLIF procedures did not increase the overall fusion rates, although it might promote a faster rate of fusion. Carreon and colleagues114–117 retrospectively investigated the effectiveness of platelet gel in instrumented posterolateral fusion and reported that platelet gel failed to enhance the fusion rate when added to autograft. In present systems, autologous platelet concentrate has not been conclusively shown to enhance fusion and further investigation may be warranted.
Mesenchymal Stem Cells
Mesenchymal stem cells (MSCs) are self-renewing and pluripotent cells that have attracted attention for possible clinical uses. MSCs have been identified in a variety of tissues including bone marrow,118 muscle,119,120 periosteum,121 and adipose tissue.122 Among these tissues, bone marrow has been well established as a source of MSCs. In a variety of animal models, bone marrow–derived MSCs have demonstrated an efficacy in spinal fusion. Minamide and colleagues123 cultured MSCs derived from bone marrow and implanted these cells onto the posterolateral lumbar transverse process with a hydroxyapatite-granule carrier in a rabbit model; five of seven rabbits in the high-number cultured cell group were deemed to be fused using manual palpation. The authors demonstrated that these cells acted as a substitute for the autograft in spinal fusion.120 Using a rhesus monkey model, Wang and colleagues124 expanded autologous MSCs derived from bone marrow in culture, stimulated them with osteogenic supplements, and constructed calcium phosphate ceramic composites with MSCs. They demonstrated that autologous MSC composites could enhance bone regeneration and achieve osseous spinal fusion in an anterior interbody fusion model.121 Clinically, Gan and colleagues125 used bone marrow–derived MSCs combined with porous beta-TCP for posterior spinal fusion and reported 95.1% spinal fusion.
Unfractionated bone marrow exhibits only moderate osteogenic potential because it possesses only a limited quantity of mesenchymal stem cells (MSCs) capable of differentiating into osteoblasts. The bone marrow of healthy adults contains only one MSC for every 50,000 nucleated cells, and this population is even further diminished in older patients and those with metabolic diseases such as osteoporosis.123–125 Moreover, as bone marrow is aspirated from the iliac crest, it undergoes extensive dilution with peripheral blood, further decreasing the concentration of MSCs. Amplification or concentration of bone marrow aspirate may improve the yield of osteogenic MSCs from bone marrow. The implantation of MSCs may serve to enhance bone production by augmenting the number of osteogenic cells available to participate in this regenerative process. Multiple studies have confirmed that the amplification of osteoprogenitor cells that occurs after the culture expansion of MSCs results in greater bone formation than the use of unfractionated bone marrow, which demonstrates a relative paucity of osteogenic cells.126,129 The introduction of culture-expanded MSCs has also been shown to be superior to bone marrow for eliciting the repair of critical-sized skeletal defects in an animal model.130 MSCs may prove to be an effective alternative to autogenous bone graft for stimulating spinal fusion. Because successful spinal fusion is largely mediated by endogenous osteoblasts, MSC therapy may be particularly beneficial for older patients and others with reduced cellular stores.
Gene Therapy
Gene therapy involves the transfer of a specific DNA sequence to target cells that subsequently express the therapeutic protein. The ongoing delivery of osteoinductive growth factors by genetically modified cells may be directed to stimulate fusion of the spinal column. Recombinant BMPs have been used successfully to stimulate fusion in several clinical trials.131–138 Gene therapy may provide a more potent osteoinductive signal than recombinant growth factors because these methods result in the sustained local release of osteogenic proteins at levels more closely resembling physiologic levels than the administration of a single large dose. Gene therapy is composed of three basic components: DNA encoding the protein of interest, target cells into which this sequence will be inserted, and a vector that assists the transfer of the gene into the cells. Both viral and nonviral vectors are available to deliver genetic material into target cells by a process known as transduction. Nonviral vectors are easier to produce and are more stable than viruses; because no infectious agents are administered to the patient, these constructs are also less antigenic and are theoretically safer than viral vectors. Examples of nonviral vectors include liposomes (DNA suspended in lipid vesicles that are able to bind to cell membranes) and gene-activated matrices (osteoconductive scaffolds loaded with genetic material). Nevertheless, viral vectors are often favored over nonviral strategies because of their superior transduction efficiencies. Viral vectors have been successful in animal experiments. Lu and colleagues139 tested a new osteoinductive factor, Nell-1 (Nel-like molecule-1), for in vivo gene therapy in a rat spinal fusion model and concluded that it may be a potent osteoinductive molecule. However, there are several potential limitations of using adenoviral vectors in a clinical setting. Although these vectors transfect both dividing and nondividing cells, they cannot integrate into the host genome; thus protein production by the transfected cells is limited to 3 weeks, even in an immunocompromised animal model.140 This is probably due to the episomal nature of the adenoviral DNA that makes it susceptible to degeneration by host nucleases. Furthermore, adenoviral vectors generally retain their ability to synthesize adenoviral proteins, which stimulate the host immune response.141–142 Host immunity destroys the transduced cells and reduces the effect of transgene expression. Recently, in order to compensate for the disadvantage of adenoviral vectors, various other viral vectors such as adeno-associated viral vector and lentiviral vector have been tested.143–145 Although viral-based gene therapy promises several advantages, there are major concerns regarding the safety of using viral vectors in clinical scenarios. Various improvements have been implemented to ensure such safety,145–147 and gene therapy has been validated as a safe technique in preclinical animal experiments.148–152 However, long-term results have not yet been elucidated and further studies are required before these vectors can be used in clinical practice.
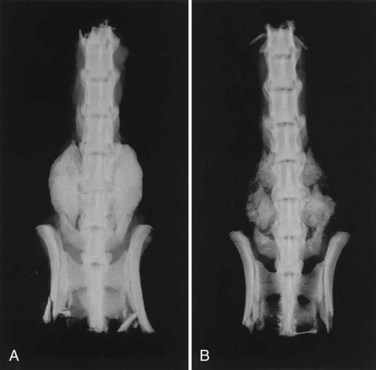
Using pluripotential cells as vehicles for gene therapy may prove to augment bone regeneration even more because this method contributes both osteoprogenitor cells and osteoinductive growth factors. These transduced stem cells not only have the capacity to induce the osteoblastic differentiation of surrounding cells but may also respond to their own osteogenic proteins. This composite bone grafting technique has been implemented in several animal models for spinal arthrodesis. Wang and colleagues153 employed ex vivo gene therapy to promote posterolateral spinal fusions in rats. Rat bone marrow cells transduced with the BMP-2 gene were combined with a guanidine-extracted DBM and implanted in the posterolateral spine. Treatment with BMP-2-producing marrow cells generated solid fusion masses comparable with those resulting from the use of recombinant BMP-2 protein (Fig. 68–2). Boden and colleagues154 achieved single-level posterior fusions of the lumbar and thoracic spines in rats by supplying bone marrow cells with the gene encoding the LIM mineralization protein (LMP-1), a signaling protein that stimulates the expression of multiple osteoinductive growth factors. Consistent fusions were obtained in all of the animals receiving bone marrow cells containing the LMP-1 DNA sequence, whereas no bone formation was observed in those implanted with cells carrying an inactive copy of the gene. In a related study, an attempt was made to induce similar fusions in rabbits using buffycoat cells derived from either bone marrow or peripheral blood.155 After being infected for only 10 minutes with an adenoviral vector bearing the LIM-1 gene, these cells were placed in the posterolateral spine in conjunction with an osteoconductive carrier. Once again, successful fusion was noted in all of the animals that had been treated with these transduced cells. Although gene therapy has been validated by preclinical studies as an effective technique for enhancing bone formation and may be a viable bone graft substitute for spinal fusion, significant concerns remain regarding its safety in humans, especially the potential risks related to the use of viruses. Viral vectors routinely elicit a substantial host inflammatory response, and their long-term systemic effects have not been well characterized. These viruses are unable to replicate because portions of their genome essential to this process are deleted and replaced with the DNA sequence of interest, yet it is still conceivable that these viruses may regain the ability to propagate and trigger an uncontrollable infection. Some viruses insert randomly into the DNA of target cells, raising the possibility of malignant transformation. In addition, the cost-effectiveness of gene transfer methods has not been definitively established. Regardless of its efficacy, these safety and economic issues may ultimately determine whether gene therapy is viewed as an acceptable alternative to autogenous bone graft for augmenting spinal fusion.
Conclusion
Key Points
1 Boden SD, Zdeblick TA, Sandhu HS, et al. The use of rhBMP-2 in interbody fusion cages. Definitive evidence of osteoinduction in humans: a preliminary report. Spine. 2000;25:376-381.
2 Cammisa FPJr, Lowery G, Garfin SR, et al. Two-year fusion rate equivalency between Grafton DBM gel and autograft in posterolateral spine fusion: a prospective controlled trial employing a side-by-side comparison in the same patient. Spine. 2004;29:660-666.
3 Muschik M, Ludwig R, Halbhubner S, et al. Beta-tricalcium phosphate as a bone substitute for dorsal spinal fusion in adolescent idiopathic scoliosis: preliminary results of a prospective clinical study. Eur Spine J. 2001;10(Suppl 2):S178-S184.
1 Steinmann JC, Herkowitz HN. Pseudarthrosis of the spine. Clin Orthop. 1992;284:80-90.
2 Hamer AJ, Strachan JR, Black MM, et al. Biomechanical properties of cortical allograft bone using a new method of bone strength measurement: a comparison of fresh, freshfrozen, and irradiated bone. J Bone Joint Surg Br. 1996;78:363-368.
3 Silcox DH, Boden SD, Schimandle JH, et al. Reversing the inhibitory effect of nicotine on spinal fusion using an osteoinductive protein extract. Spine. 1998;23:291-296.
4 Alden TD, Pittman DD, Beres EJ, et al. Percutaneous spinal fusion using bone morphogenetic protein-2 gene therapy. J Neurosurg. 1999;90:109-114.
5 Reid RL. Hernia through an iliac bone graft donor site. A case report. J Bone Joint Surg Am. 1968;50:757-760.
6 Cockin J. Autogenous bone graft complications and the donor site. J Bone Joint Surg Br. 1971;53:153.
7 Coventry MB, Tapper EM. Pelvic instability-a consequence of removing iliac crest for bone grafting. J Bone Joint Surg Am. 1972;54:83-101.
8 Challis JA, Lyttle JA, Stuart AE. Strangulated lumbar hernia and volvulus following removal of iliac crest bone graft. Acta Orthop Scand. 1975;46:230-233.
9 Escales F, DeWald RL. Combined traumatic arteriovenous fistula and urethral injury: a complication of bone grafting. J Bone Joint Surg Am. 1977;59:270-271.
10 Cowley SP, Anderson LD. Hernias through donor sites for iliac crest bone grafts. J Bone Joint Surg Am. 1983;65:1023-1025.
11 Kuhn DA, Moreland MS. Complications following iliac crest bone grafting. Clin Orthop. 1986;209:224-226.
12 Kurz LT, Garfin SR, Booth RE. Harvesting autogenous iliac bone grafts: a review of complications and techniques. Spine. 1989;14:1324-1331.
13 Summers BN, Eisenstein SM. Donor site pain from the ilium: a complication of lumbar spine fusion. J Bone Joint Surg Br. 1989;71:677-680.
14 Younger EM, Chapman MW. Morbidity at bone graft donor sites. J Orthop Trauma. 1989;3:192-194.
15 Catinella FP, Delaria GA, DeWald RL. False aneurysm of the superior gluteal artery-a complication of iliac crest bone grafting. Spine. 1990;15:1360-1362.
16 Fernyhough JC, Schimandle JJ, Weigel MC, et al. Chronic donor site pain complicating bone graft harvesting from the posterior iliac crest for spinal fusion. Spine. 1992;17:1474-1480.
17 Banwart JC, Asher MA, Hassanein RS. Iliac crest bone graft harvest donor site morbidity: a statistical evaluation. Spine. 1994;20:1055-1060.
18 Goulet JA, Senunas LE, DeSilva GL, et al. Autogenous iliac crest bone graft. Complications and functional assessment. Clin Orthop. 1997;339:76-81.
19 Stevenson S, Horowitz M. The response to bone allografts. J Bone Joint Surg Am. 1992;74:939-950.
20 Bauer TW, Muschler GF. Bone graft materials. An overview of the basic science. Clin Orthop. 2000;371:10-27.
21 Stevenson S, Emery SE, Goldberg VM. Factors affecting bone graft incorporation. Clin Orthop. 1996;324:66-74.
22 Hamer AJ, Strachan JR, Black MM, et al. Biomechanical properties of cortical allograft bone using a new method of bone strength measurement: a comparison of fresh, freshfrozen, and irradiated bone. J Bone Joint Surg Br. 1996;78:363-368.
23 Tomford WW. Transmission of disease through transplantation of musculoskeletal allografts. J Bone Joint Surg Am. 1995;77:1742-1754.
24 Asselmeier MA, Casperi RB, Bottenfeld S. A review of allograft processing and sterilization techniques and their role in transmission of the human immunodeficiency virus. Am J Sports Med. 1993;21:170-175.
25 Sim FH, Frassica FJ. Use of allografts following resection of tumors of the musculoskeletal system. Instr Course Lect. 1993;42:405-413.
26 Tsuang YH, Yang RS, Chen PQ, et al. Experimental allograft in spinal fusion in dogs. Taiwan Yi Xue Hui Za Zhi. 1989;88:989-994.
27 Cloward RB. The anterior approach for removal of ruptured cervical discs. J Neurosurg. 1958;15:602.
28 Brown MD, Malinin TI, Davis PB. A roentgenographic evaluation of frozen allografts versus autografts in anterior cervical spine fusions. Clin Orthop. 1976;119:231-236.
29 Young WF, Rossenwasser RH. An early comparative analysis of the use of fibular allograft versus autologous iliac crest graft for interbody fusion after anterior cervical discectomy. Spine. 1993;18:1123-1124.
30 Zdeblick TA, Ducker TB. The use of freeze-dried allograft bone for anterior cervical fusions. Spine. 1991;16:726-729.
31 Zhang ZH, Yin H, Yang K, et al. Anterior intervertebral disc excision and bone grafting in cervical spondylitic myelopathy. Spine. 1983;8:16-19.
32 Jorgenson SS, Lowe TG, France J, et al. A prospective analysis of autograft versus allograft in posterolateral lumbar fusion in the same patient. A minimum of 1-year follow-up in 144 patients. Spine. 1994;19:2048-2053.
33 An HS, Simpson JM, Glover JM, et al. Comparison between allograft plus demineralized bone matrix versus autograft in anterior cervical fusion. A prospective multi-center study. Spine. 1995;20:2211-2216.
34 Aurori BF, Weierman RJ, Lowell HA, et al. Pseudarthrosis after spinal fusion for scoliosis. A comparison of autogeneic and allogeneic bone grafts. Clin Orthop Relat Res. 1985;199:153-158.
35 Dodd CA, Fergusson CM, Freedman L, et al. Allograft versus autograft bone in scoliosis surgery. J Bone Joint Surg Br. 1988;70:431-434.
36 Kozak JA, Heilman AE, O’Brian JP. Anterior lumbar fusion options: techniques and graft materials. Clin Orthop. 1994;300:45-51.
37 Silcox DH. Laparoscopic bone dowel fusions of the lumbar spine. Orthop Clin North Am. 1998;29:655-663.
38 Butterman GR, Glazer PA, Hu SS, Bradford DS. Revision of failed lumbar fusions. A comparison of anterior autograft and allograft. Spine. 1997;22:2748-2755.
39 Tay BK, Patel VV, Bradford DS. Calcium sulfate and calcium phosphate- based bone substitutes. Mimickry of the moneral phase of bone. Orthop Clin North Am. 1999;30:615-623.
40 Chen WJ, Tsai TT, Chen LH, et al. The fusion rate of calcium sulfate with local autograft bone compared with autologous iliac bone graft for instrumented short-segment spinal fusion. Spine. 2005;30:2293-2297.
41 Epstein NE. A preliminary study of the efficacy of beta tricalcium phosphate as a bone expander for instrumented posterolateral lumbar fusions. J Spinal Disord Tech. 2006;19:424-429.
42 Xie Y, Chopin D, Morin C, et al. Evaluation of the osteogenesis and biodegradation of porous biphasic ceramic in the human spine. Biomaterials. 2006;27:2761-2767.
43 Korovessis P, Koureas G, Zacharatos S, et al. Correlative radiological, self-assessment and clinical analysis of evolution in instrumented dorsal and lateral fusion for degenerative lumbar spine disease. Autograft versus corallinemhydroxyapatite. Eur Spine J. 2005;14:630-638.
44 Passuti N, Daculsi G, Rogez JM, et al. Macroporous calcium phosphate ceramic performance in human spine fusion. Clin Orthop Relat Res. 1989;248:169-176.
45 Ransford AO, Morley T, Edgar MA, et al. Synthetic porous ceramic compared with autograft in scoliosis surgery. A prospective, randomized study of 341 patients. J Bone Joint Surg Br. 1998;80:13-18.
46 Ransford AO, Morley T, Edgar MA, et al. Synthetic porous ceramic compared with autograft in scoliosis surgery. A prospective, randomized study of 341 patients. J Bone Joint Surg Br. 1998;80:13-18.
47 Thalgott JS, Fritts K, Giuffre JM, et al. Anterior interbody fusion of the cervical spine with coralline hydroxyapatite. Spine. 2001;24:1295-1299.
48 Thalgott JS, Giuffre JM, Klezl Z, et al. Anterior lumbar interbody fusion with titanium mesh cages, coralline hydroxyapatite, and demineralized bone matrix as part of a circumferential fusion. Spine J. 2002;2:63-69.
49 Thalgott JS, Klezl Z, Timlin M, et al. Anterior lumbar interbody fusion with processed sea coral (coralline hydroxyapatite) as part of a circumferential fusion. Spine. 2002;27:E518-E525.
50 Jarcho M. Calcium phosphate ceramics as hard tissue prosthetics. Clin Orthop. 1981;157:259-278.
51 Thalgott JS, Fritts K, Giuffre JM, et al. Anterior interbody fusion of the cervical spine with coralline hydroxyapatite. Spine. 1999;24:1295-1299.
52 Passuti N, Daculsi G, Rogez JM, et al. Macroporous calcium phosphate ceramic performance in human spine fusion. Clin Orthop. 1989;248:169-176.
53 Ransford AO, Morley T, Edgar MA, et al. Synthetic porous ceramic compared with autograft in scoliosis surgery: a prospective, randomized study of 341 patients. J Bone Joint Surg Br. 1998;80:13-18.
54 Ohgushi H, Goldberg VM, Caplan AI. Heterotopic osteogenesis in porous ceramics induced by marrow cells. J Orthop Res. 1989;7:568-578.
55 Urist MR. Bone: formation by autoinduction. Science. 1965;150:893-899.
56 Sandhu HS, Grewal HS, Parvataneni H. Bone grafting for spinal fusion. Orthop Clin North Am. 1999;30:685-698.
57 Lee YP, Jo M, Luna M, et al. The efficacy of different commercially available demineralized bone matrix substances in an athymic rat model. J Spinal Disord Tech. 2005;18:439-444.
58 Peterson B, Whang PG, Iglesias R, et al. Osteoinductivity of commercially available demineralized bone matrix. Preparations in a spine fusion model. J Bone Joint Surg Am. 2004;86-A:2243-2250.
59 Wang JC, Alanay A, Mark D, et al. A comparison of commercially available demineralized bone matrix for spinal fusion. Eur Spine J. 2007;16:1233-1240.
60 Choi Y, Oldenburg FP, Sage L, et al. A bridging demineralized bone implant facilitates posterolateral lumbar fusion in New Zealand white rabbits. Spine. 2007;32:36-41.
61 Louis-Ugbo J, Murakami H, Kim HS, et al. Evidence of osteoinduction by Grafton demineralized bone matrix in nonhuman primate spinal fusion. Spine. 2004;29:360-366.
62 Martin GJJr, Boden SD, Titus L, et al. New formulations of demineralized bone matrix as a more effective graft alternative in experimental posterolateral lumbar spine arthrodesis. Spine. 1999;24:637-645.
63 Yee AJ, Bae HW, Friess D, et al. Augmentation of rabbit posterolateral spondylodesis using a novel demineralized bone matrix-hyaluronan putty. Spine. 2003;28:2435-2440.
64 Girardi FP, Cammisa FPJr. The effect of bone graft extenders to enhance the performance of iliac crest bone grafts in instrumented lumbar spine fusion. Orthopedics. 2003;26:s545-s548.
65 Sassard WR, Eidman DK, Gray PM, et al. Augmenting local bone with Grafton demineralized bone matrix for posterolateral lumbar spine fusion: avoiding second site autologous bone harvest. Orthopedics. 2000;23:1059-1064.
66 Cammisa FPJr, Lowery G, Garfin SR, et al. Two-year fusion rate equivalency between Grafton DBM gel and autograft in posterolateral spine fusion: a prospective controlled trial employing a side-by-side comparison in the same patient. Spine. 2004;29:660-666.
67 Vaccaro AR, Stubbs HA, Block JE. Demineralized bone matrix composite grafting for posterolateral spinal fusion. Orthopedics. 2007;30:567-570.
68 Thalgott JS, Giuffre JM, Klezl Z, et al. Anterior lumbar interbody fusion with titanium mesh cages, coralline hydroxyapatite, and demineralized bone matrix as part of a circumferential fusion. Spine J. 2002;2:63-69.
69 An HS, Simpson JM, Glover JM, et al. Comparison between allograft plus demineralized bone matrix versus autograft in anterior cervical fusion. A prospective multicenter study. Spine. 1995;20:2211-2216.
70 Martin GJ, Boden SD, Titus L, et al. New formulations of demineralized bone matrix as a more effective graft alternative in experimental posterolateral lumbar spine arthrodesis. Spine. 1999;24:637-645.
71 Bostrom MP, Yang X, Kennan M, et al. An unexpected outcome during testing of commercially available demineralized bone graft materials: how safe are the nonallograft components? Spine. 2001;26:1425-1428.
72 Edwards JT, Diegmann MH, Scarborough NL. Osteoinduction of human demineralized bone: characterization in a rat model. Clin Orthop. 1998;357:219-228.
73 Frenkel SR, Moskovich R, Spivak J, et al. Demineralized bone matrix: enchancement of spinal fusion. Spine. 1993;18:1634-1639.
74 Wang EA, Rosen V, D’Alessandro JS, et al. Recombinant human bone morphogenetic protein induces bone formation. Proc Natl Acad Sci U S A. 1990;87:2220-2224.
75 Boden SD, Schimandle JH, Hutton WC. 1995 Volvo award in basic sciences. The use of an osteoinductive growth factor for lumbar spinal fusion. Part II: study of dose, carrier, and species. Spine. 1995;20:2633-2644.
76 Silcox DH, Boden SD, Schimandle JH, et al. Reversing the inhibitory effect of nicotine on spinal fusion using an osteoinductive protein extract. Spine. 1998;23:291-296.
77 Martin GJ, Boden SD, Titus L. Recombinant human bone morphogenetic protein-2 overcomes the inhibitory effect of ketorolac, a nonsteroidal anti-inflammatory drug (NSAID), on posterolateral lumbar intertransverse spine fusion. Spine. 1999;24:2188-2193.
78 Cook SD, Dalton JE, Tan EH, et al. In vivo evaluation of recombinant human osteogenic protein (rhOP-1) implant as a bone graft substitute for spinal fusions. Spine. 1994;19:1655-1663.
79 Schimandle JH, Boden SD, Hutton WC. Experimental spinal fusion with recombinant human bone morphogenetic protein-2. Spine. 1995;20:1326-1337.
80 Boden SD, Moskovitz, Morone MA, et al. Video-assisted lateral intertransverse process arthrodesis. Validation of a new minimally invasive lumbar spinal fusion technique in the rabbit and nonhuman primate (rhesus) models. Spine. 1996;21:2689-2697.
81 Boden SD, Martin GJ, Morone M, et al. The use of coralline hydroxyapatite with bone marrow, autogenous bone graft, or osteoinductive bone protein extract for posterolateral lumbar spinal fusion. Spine. 1999;24:320-327.
82 Boden SD, Martin GJ, Morone M, et al. Posterolateral lumbar intertransverse process spine arthrodesis with recombinant human bone morphogenetic protein 2/hydroxyapatite-tricalcium phonsphate after laminectomy in the nonhuman primate. Spine. 1999;24:1179-1185.
83 Lovell TP, Dawson EG, Nilsson OS, et al. Augmentation of spinal fusion with bone morphogenetic protein in dogs. Clin Orthop. 1989;243:266-274.
84 Muschler GF, Hyodo A, Manning T, et al. Evaluation of human bone morphogenetic protein 2 in a canine spinal fusion model. Clin Orthop. 1994;308:229-240.
85 Sandu HS, Kanim LE, Kabo JM, et al. Evaluation of rhBMP-2 with an OPLA carrier in a canine posterolateral (transverse process) spinal fusion model. Spine. 1995;20:2669-2682.
86 Sandu HS, Kanim LE, Kabo JM, et al. Effective doses of recombinant human bone morphogenetic protein-2 in experimental spinal fusion. Spine. 1996;21:2115-2122.
87 Patel TC, Erulkar JS, Grauer JN, et al. Osteogenic protein-1 overcomes the inhibitory effect of nicotine on posterolateral lumbar fusion. Spine. 2001;26:1656-1661.
88 Peterson B, Whang PG, Iglesias R, et al. Osteoinductivity of commercially available demineralized bone matrix. Preparations in a spine fusion model. J Bone Joint Surg Am. 2004;86-A:2243-2250.
89 Boden SD, Kang J, Sandhu H, et al. Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: a prospective, randomized clinical pilot trial. Spine. 2002;27:2662-2673.
90 Dimar JR, Glassman SD, Burkus KJ, et al. Clinical outcomes and fusion success at 2 years of single-level instrumented posterolateral fusions with recombinant human bone morphogenetic protein-2/compression resistant matrix versus iliac crest bone graft. Spine. 2006;31:2534-2539.
91 Boden SD, Zdeblick TA, Sandhu HS, et al. The use of rhBMP-2 in interbody fusion cages. Definitive evidence of osteoinduction in humans: a preliminary report. Spine. 2000;25:376-381.
92 Burkus JK, Dorchak JD, Sanders DL. Radiographic assessment of interbody fusion using recombinant human bone morphogenetic protein type 2. Spine. 2003;28:372-377.
93 Burkus JK, Gornet MF, Dickman CA, et al. Anterior lumbar interbody fusion using rhBMP-2 with tapered interbody cages. J Spinal Disord Tech. 2002;15:337-349.
94 Burkus JK, Transfeldt EE, Kitchel SH, et al. Clinical and radiographic outcomes of anterior lumbar interbody fusion using recombinant human bone morphogenetic protein-2. Spine. 2002;27:2396-2408.
95 Slosar PJ, Josey R, Reynolds J. Accelerating lumbar fusions by combining rhBMP-2 with allograft bone: a prospective analysis of interbody fusion rates and clinical outcomes. Spine J. 2007;7:301-307.
96 McClellan JW, Mulconrey DS, Forbes RJ, et al. ertebral bone resorption after transforaminal lumbar interbody fusion with bone morphogenetic protein (rhBMP-2). J Spinal Disord Tech. 2006;19:483-486.
97 Pradhan BB, Bae HW, Dawson EG, et al. Graft resorption with the use of bone morphogenetic protein: lessons from anterior lumbar interbody fusion using femoral ring allografts and recombinant human bone morphogenetic protein-2. Spine. 2006;31:E277-E284.
98 Silcox DH, Boden SD, Schimandle JH, et al. Reversing the inhibitory effect of nicotine on spinal fusion using an osteoinductive protein extract. Spine. 1998;23:291-296.
99 Martin GJ, Boden SD, Titus L. Recombinant human bone morphogenetic protein-2 overcomes the inhibitory effect of ketorolac, a nonsteroidal anti-inflammatory drug (NSAID), on posterolateral lumbar intertransverse process spine fusion. Spine. 1999;24:2188-2193.
100 Reference deleted in proofs.
101 Zdeblick TA, Ghanayem AJ, Rapoff AJ, et al. Cervical interbody fusion cages. An animal model with and without bone morphogenetic protein. Spine. 1998;23:758-765.
102 Cunningham BW, Kanayama M, Parker LM, et al. Osteogenic protein versus autologous interbody arthrodesis in the sheep thoracic spine. A comparative endoscopic study using the Bagby and Kuslich interbody fusion device. Spine. 1999;24:509-518.
103 Boden SD, Nartin GJ, Horton WC, et al. Laparoscopic anterior spinal arthrodesis with rhBMP-2 in a titanium interbody threaded cage. J Spinal Disord. 1998;11:95-101.
104 Hecht BP, Fischgrund JS, Herkowitz HN, et al. The use of recombinant human bone morphogenetic protein 2 (rhBMP-2) to promote spinal fusion in a nonhuman primate anterior interbody fusion model. Spine. 1999;24:629-636.
105 Sandhu HS, Toth JM, Diwan AD, et al. Histologic evaluation of the efficacy of rhBMP-2 compared with autograft bone in sheep spinal anterior interbody fusion. Spine. 2002;27:567-575.
106 Paramore CG, Lauryssen C, Rauzzino MJ, et al. The safety of OP-1 for lumbar fusion with decompression-a canine study. Neurosurgery. 1999;44:1151-1155.
107 Shields LB, Raque GH, Glassman SD, et al. Adverse effects associated with high-dose recombinant human bone morphogenetic protein-2 use in anterior cervical spine fusion. Spine. 2006;31:542-547.
108 Vaidya R, Carp J, Sethi A, et al. Complications of anterior cervical discectomy and fusion using recombinant human bone morphogenetic protein-2. Eur Spine J. 2007;16:1257-1265.
109 Alanay A, Chen C, Lee S, et al. The adjunctive effect of a binding peptide on bone morphogenetic protein enhanced bone healing in a rodent model of spinal fusion. Spine. 2008;33:1709-1713.
110 Guizzardi S, Di Silvestre M, Scandroglio R, et al. Implants of heterologous demineralized bone matrix for induction of posterior spinal fusion in rats. Spine. 1992;17:701-707.
111 Lindholm TS, Ragni P, Lindholm TC. Response of bone marrow stroma cells to demineralized cortical bone matrix in experimental spinal fusion in rabbits. Clin Orthop. 1988;230:296-302.
112 Lindholm TS, Nilsson OS, Lindholm TC. Extraskeletal and intraskeletal new bone formation induced by demineralized bone matrix combined with bone marrow cells. Clin Orthop. 1982;171:251-255.
113 Ferrara N, Houck K, Jakeman L, et al. Molecular and biological properties of the vascular endothelial growth factor family of proteins. Endocr Rev. 1992;13:18-32.
114 Lowery GL, Kulkarni S, Pennisi AE. Use of autologous growth factors in lumbar spinal fusion. Bone. 1999;25:47S-50S.
115 Weiner BK, Walker M. Efficacy of autologous growth factors in lumbar intertransverse fusions. Spine. 2003;28:1968-1970.
116 Hee HT, Majd ME, Holt RT, et al. Do autologous growth factors enhance transforaminal lumbar interbody fusion? Eur Spine J. 2003;12:400-407.
117 Carreon LY, Glassman SD, Anekstein Y, et al. Platelet gel (AGF) fails to increase fusion rates in instrumented posterolateral fusions. Spine. 2005;30:E243-E246.
118 Caplan AI, Bruder SP. Mesenchymal stem cells: building blocks for molecular medicine in the 21st century. Trends Mol Med. 2001;7:259-264.
119 Jackson KA, Mi T, Goodell MA. Hematopoietic potential of stem cells isolated from murine skeletal muscle. Proc Natl Acad Sci U S A. 1999;96:14482-14486.
120 Williams JT, Southerland SS, Souza J, et al. Cells isolated from adult human skeletal muscle capable of differentiating into multiple mesodermal phenotypes. Am Surg. 1999;65:22-26.
121 Nakahara H, Dennis JE, Bruder SP, et al. In vitro differentiation of bone and hypertrophic cartilage from periosteal-derived cells. Exp Cell Res. 1991;195:492-503.
122 Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211-228.
123 Minamide A, Yoshida M, Kawakami M, et al. The use of cultured bone marrow cells in type I collagen gel and porous hydroxyapatite for posterolateral lumbar spine fusion. Spine. 2005;30:1134-1138.
124 Wang T, Dang G, Guo Z, et al. Evaluation of autologous bone marrow mesenchymal stem cell-calcium phosphate ceramic composite for lumbar fusion in rhesus monkey interbody fusion model. Tissue Eng. 2005;11:1159-1167.
125 Gan Y, Dai K, Zhang P, et al. The clinical use of enriched bone marrow stem cells combined with porous beta-tricalcium phosphate in posterior spinal fusion. Biomaterials. 2008;29:3973-3982.
126 Muschler GF, Boehm C, Easley KA. Aspiration to obtain osteoblast progenitor cells from human bone marrow: the influence of aspiration volume. J Bone Joint Surg Am. 1997;79:1699-1709.
127 Muschler GF, Nitto H, Boehm C, et al. Age- and genderrelated changes in the cellularity of human bone marrow and the prevalence of osteoblastic progenitors. J Orthop Res. 2001;19:117-125.
128 Inoue K, Ohgushi H, Yoshikawa T, et al. The effects of aging on bone formation in porous hydroxyapatite: biochemical and histologic analysis. J Bone Miner Res. 1997;12:989-994.
129 Kahn A, Gibbons R, Perkins S, et al. Age-related bone loss: a hypothesis and initial assessment in mice. Clin Orthop. 1995;313:69-75.
130 Kadiyala S, Jaiswal N, Bruder SP. Culture-expanded bone marrowderived mesenchymal stem cells can regenerate a critical-sized segmental bone defect. Tissue Eng. 1997;3:173-185.
131 Baskin DS, Ryan P, Sonntag V, et al. A prospective, randomized, controlled cervical fusion study using recombinant human bone morphogenetic protein-2 with the CORNERSTONE-SR allograft ring and the ATLANTIS anterior cervical plate. Spine. 2003;28:1219-1224.
132 Boden SD, Kang J, Sandhu H, Heller JG. Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: a prospective, randomized clinical pilot trial. Spine. 2002;27:2662-2673.
133 Boden SD, Zdeblick TA, Sandhu HS, et al. The use of rhBMP-2 in interbody fusion cages. Definitive evidence of osteoinduction in humans: a preliminary report. Spine. 2000;25:376-381.
134 Vaccaro AR, Whang PG, Patel T, et al. The safety and efficacy of OP-1 (rhBMP-7) as a replacement for iliac crest autograft for posterolateral lumbar arthrodesis: minimum 4-year follow-up of a pilot study. Spine J. 2007;8:457-465.
135 Vaccaro AR, Anderson DG, Patel T, et al. Comparison of OP-1 Putty (rhBMP-7) to iliac crest autograft for posterolateral lumbar arthrodesis: a minimum 2-year follow-up pilot study. Spine. 2005;30:2709-2716.
136 Vaccaro AR, Patel T, Fischgrund J, et al. A pilot safety and efficacy study of OP-1 putty (rhBMP-7) as an adjunct to iliac crest autograft in posterolateral lumbar fusions. Eur Spine J. 2003;12:495-500.
137 Vaccaro AR, Patel T, Fischgrund J, et al. A 2-year follow-up pilot study evaluating the safety and efficacy of op-1 putty (rhbmp-7) as an adjunct to iliac crest autograft in posterolateral lumbar fusions. Eur Spine J. 2005;14:623-629.
138 Vaccaro AR, Patel T, Fischgrund J, et al. A pilot study evaluating the safety and efficacy of OP-1 Putty (rhBMP-7) as a replacement for iliac crest autograft in posterolateral lumbar arthrodesis for degenerative spondylolisthesis. Spine. 2004;29:1885-1892.
139 Lu SS, Zhang X, Soo C, et al. The osteoinductive properties of Nell-1 in a rat spinal fusion model. Spine J. 2007;7:50-60.
140 Feeley BT, Conduah AH, Sugiyama O, et al. In vivo molecular imaging of adenoviral versus lentiviral gene therapy in two bone formation models. J Orthop Res. 2006;24:1709-1721.
141 Mack CA, Song WR, Carpenter H, et al. Circumvention of anti-adenovirus neutralizing immunity by administration of an adenoviral vector of an alternate serotype. Hum Gene Ther. 1997;8:99-109.
142 Yang Y, Nunes FA, Berencsi K, et al. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc Natl Acad Sci U S A. 1994;91:4407-4411.
143 Chen Y, Luk KD, Cheung KM, et al. Gene therapy for new bone formation using adeno-associated viral bone morphogenetic protein-2 vectors. Gene Ther. 2003;10:1345-1353.
144 Miyazaki M, Sugiyama O, Tow B, et al. The effects of lentiviral gene therapy with bone morphogenetic protein-2-producing bone marrow cells on spinal fusion in rats. J Spinal Disord Tech. 2008;21:372-379.
145 Sugiyama O, An DS, Kung SP, et al. Lentivirus-mediated gene transfer induces long-term transgene expression of BMP-2 in vitro and new bone formation in vivo. Mol Ther. 2005;11:390-398.
146 Stieger K, Le Meur G, Lasne F, et al. Long-term doxycycline-regulated transgene expression in the retina of nonhuman primates following subretinal injection of recombinant AAV vectors. Mol Ther. 2006;13:967-975.
147 Zufferey R, Dull T, Mandel RJ, et al. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J Virol. 1998;72:9873-9880.
148 Boden SD, Titus L, Hair G, et al. Lumbar spine fusion by local gene therapy with a cDNA encoding a novel osteoinductive protein (LMP-1). Spine. 1998;23:2486-2492.
149 Dumont RJ, Dayoub H, Li JZ, et al. Ex vivo bone morphogenetic protein- 9 gene therapy using human mesenchymal stem cells induces spinal fusion in rodents. Neurosurgery. 2002;51:1239-1244.
150 Hidaka C, Goshi K, Rawlins B, et al. Enhancement of spine fusion using combined gene therapy and tissue engineering BMP-7-expressing bone marrow cells and allograft bone. Spine. 2003;28:2049-2057.
151 Miyazaki M, Sugiyama O, Tow B, et al. The effects of lentiviral gene therapy with bone morphogenetic protein-2- producing bone marrow cells on spinal fusion in rats. J Spinal Disord Tech. 2008;21:372-379.
152 Peterson B, Iglesias R, Zhang J, et al. Genetically modified human derived bone marrow cells for posterolateral lumbar spine fusion in athymic rats: beyond conventional autologous bone grafting. Spine. 2005;30:283-289.
153 Wang JC, Kanim LE, Yoo S, et al. Effect of regional gene therapy with bone morphogenetic protein-2-producing bone marrow cells on spinal fusion in rats. J Bone Joint Surg Am. 2003;85-A:905-911.
154 Boden SD, Titus L, Hair G, et al. Lumbar spine fusion by local gene therapy with a cDNA encoding a novel osteoinductive protein (LMP-1). Spine. 1998;23:2486-2492.
155 Viggeswarapu M, Boden SD, Liu Y, et al. Adenoviral delivery of LIM mineralization protein-1 induces new-bone formation in vitro and in vivo. J Bone Joint Surg Am. 2001;83-A:364-376.