Chapter 33 Bone Metastases
Introduction
Bone metastases are common in patients with advanced malignancies. Autopsy series have shown an incidence of approximately 70% in breast and prostate cancer and 35% in lung cancer.1,2 Osseous metastases can profoundly influence quality of life and prognosis. Early and accurate detection is important for therapeutic planning, and many imaging modalities can be used for this purpose. X-ray–based technologies such as radiography and computed tomography (CT) image bone calcium, skeletal scintigraphy (SS; bone scan) detects bone formation, magnetic resonance imaging (MRI) images the soft tissue of the marrow cavity, and positron-emission tomography (PET) reflects the glucose metabolism of the lesions. The therapeutic response of bony metastases can be assessed through means of response criteria that are also used to determine the clinical efficacy of cancer therapy. Response criteria developed at the M. D. Anderson Cancer Center (MDA criteria) are useful for interpreting the behavior of bone metastases.3,4 Successful new therapies are prolonging the lives of cancer patients with a concomitant increase in skeletal metastasis–related complications such as pain, pathologic fractures, and spinal cord compression.2,5 Patients with multiple painful bone metastases can be treated with systemic therapy such as bisphosphonates or radiopharmaceuticals. When symptomatic lesions are limited in number, they can be treated with radiation therapy, surgery, or percutaneous techniques such as vertebroplasty or radiofrequency ablation (RFA). This chapter discusses the role of imaging in the detection and therapeutic response of bony metastases with a discussion of treatment including topics such as medical therapy, radiation, surgery, and percutaneous procedures.
Distribution and Pathophysiology
Cortical bone is composed of compact, lamellar bone that provides structural support and forms a heavily calcified, well-defined boundary for the marrow cavity. The marrow contains delicate trabecular bone and either red, hematopoietic or yellow, fatty marrow. The majority of bone metastases are hematogenous and may spread by arterial or venous routes. Venous backflow to the spine and pelvis can occur through a relatively valveless network of blood vessels called Batson’s vertebral plexus following Valsalva episodes of increased abdominopelvic or intrathoracic pressure.6,7 Skip metastases are uncommon, occurring most frequently in osteosarcoma and rarely in patients with Ewing’s sarcoma.8,9 They are defined as lesions that are anatomically separate from a primary bone malignancy but arise either in the same bone or directly across an adjacent joint in the absence of other bony metastases. Enneking and Kagan10 proposed that skip metastases could arise in the same bone through invasion of the venous sinusoids of the marrow cavity and that transarticular spread could occur through venous backflow, similar to the mechanism involving Batson’s plexus.
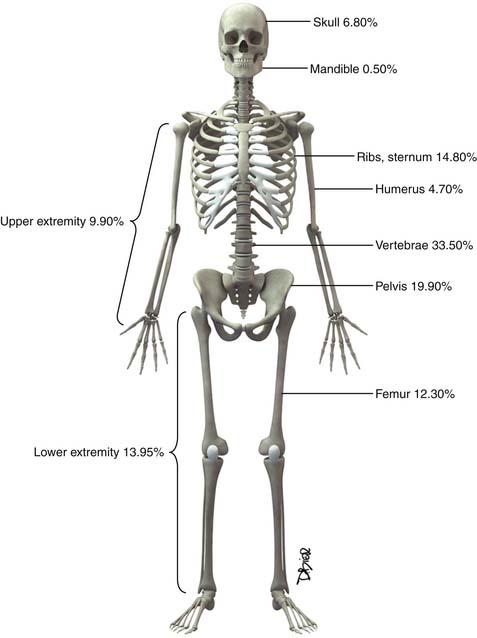
Following the distribution of highly vascular red marrow, osseous metastases occur most commonly in the medullary cavity of the bones of the axial skeleton. In a study of 4105 bone metastases in 2001 patients, Clain11 reported the highest incidence of lesions in the vertebrae (33.5%), pelvis (19.9%), ribs (12.2%), femora (12.3%), skull (6.8%), and humeri (4.7%) (Figure 33-1).
Bone metastases can be classified as lytic, blastic, or mixed depending on the activity of tumor-stimulated host osteoclasts and osteoblasts.12 Osteoclasts are large, multinucleated cells with a specialized cell membrane (the “ruffled border”) that resorb bone, and osteoblasts are smaller, mononucleated cells that form new bone.13 These cells produce lytic or blastic lesions, respectively, and a combination of the two processes results in mixed lesions. Microscopically, the tumor cells responsible for their activation do not need to be in the immediate vicinity of the osteoclasts and osteoblasts. The complex biochemical processes involved in the formation of bone metastases have been well reviewed in other sources14 and constitute an area of rapidly evolving cancer research that is beyond the scope of this chapter.
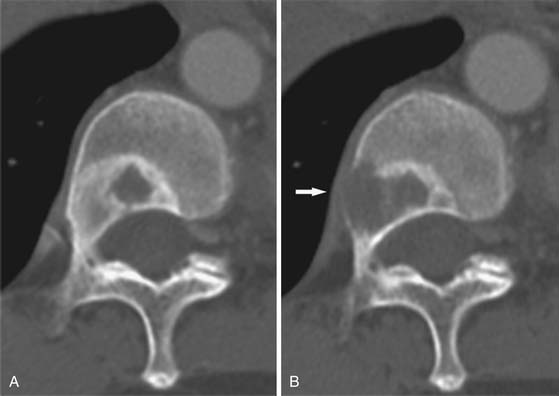
The appearance of untreated bony metastases can be predicted by the histology of the primary tumor. Of the most common primaries to metastasize to bone,2 prostate carcinoma most frequently produces blastic metastases; breast and lung cancer are predominantly lytic but can be mixed or blastic; and tumors such as renal cell carcinoma and thyroid carcinoma produce lytic metastases. Lesion margins can be well-defined, ill-defined, or expansile (Figure 33-2). Unlike primary bone tumors that typically demonstrate nonaggressive margins when benign and aggressive margins when malignant,15 virtually all bone metastases are malignant regardless of the appearance of the margins. Metastases from solid malignancies and myeloma are the most common bone tumors to arise in patients older than 40 years.
Detection
Conventional Radiography
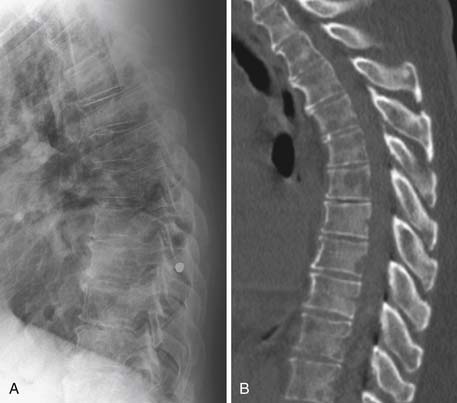
Radiography, CT, bone scan, single-photon emission tomography with integrated CT (SPECT/CT), MRI, and PET with integrated CT (PET/CT) are methods used to detect bone metastases and evaluate their response to treatment. Radiography, like all x-ray–based technologies, primarily images bone calcium and the majority of the calcium is found in the cortex. Radiography is specific but not sensitive for the detection of bony metastases.16 A minimum of 30% to 50% bone loss must occur for most lytic metastases to be detectable on radiographs (XRs),17,18 and metastases in the axial skeleton are often obscured by overlapping anatomic structures, such as bowel content or other bones (Figure 33-3). Nevertheless, the high specificity and low cost of radiography make it an ideal initial imaging modality for the detection of bony metastases in areas of focal bone pain.3 Radiography is also the optimal imaging modality to assess for pathologic fracture in the appendicular skeleton where no overlapping structures obscure the lesions.19
Computed Tomography
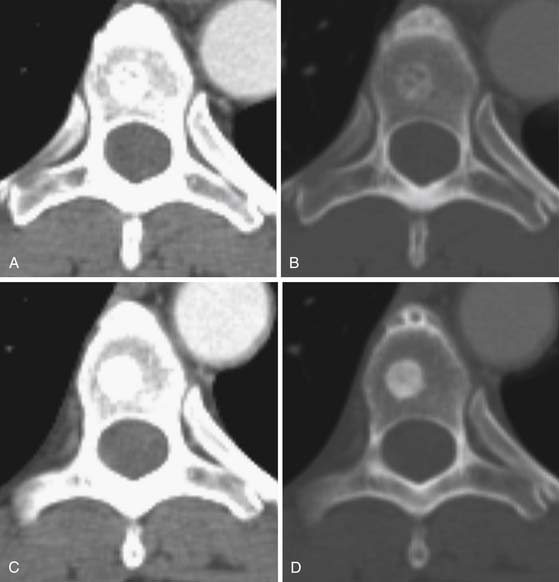
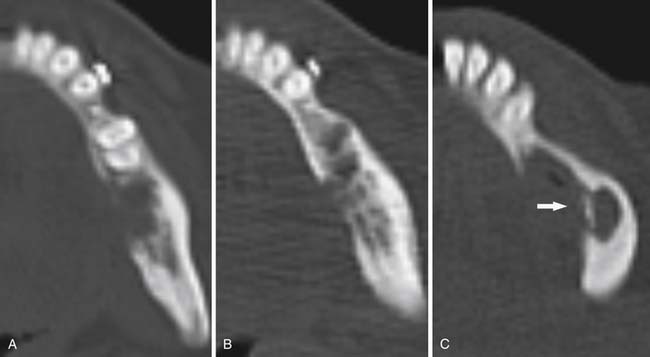
CT is an excellent modality for detailed imaging of mineralized structures such as bone or calcifications.20,21 CT is useful for determining the extent of cortical bone destruction in irregular bones such as the vertebrae, and the cross-sectional nature of CT eliminates the effect of overlapping structures when assessing the bony detail of the axial skeleton (see Figure 33-3). Owing to limited soft tissue contrast resolution, this modality is not the best choice for imaging the marrow cavity. Whereas radiography is the optimal method for evaluating the structural integrity of the long bones of the appendicular skeleton, CT is often the best choice for making the same determinations in the axial skeleton. For example, the cortical detail seen on CT of the spine (with sagittal and coronal re-formations) can provide an estimate of spinal stability (Figure 33-4). Bone windowing displays a greater degree of bony detail than soft tissue windowing and is recommended for the detection and follow-up of bone lesions (Figure 33-5).
Nuclear Medicine
Technetium-99m methylene diphosphonate (MDP) is one of the most commonly used tracers for SS and its mechanism of action involves a complex interaction of bone repair and blood flow. SS targets the bony cortex, binding to the hydroxyapetite produced when the bone attempts to repair damage caused by metastases.22 As a whole body imaging modality, bone scan is currently the method of choice for detecting asymptomatic osseous metastases during initial staging or restaging of patients with malignancies that produce blastic or mixed bone metastases, such as prostate, breast, and lung cancer.19 Lytic metastases may not allow sufficient reparative bone formation for detection on SS (see Figure 33-4). Therefore, the radiographic skeletal survey can be used in place of or in addition to bone scan for staging malignancies that produce purely lytic metastases such as renal cell and thyroid carcinoma. Myeloma lesions suppress osteoblast activity, producing false-negative results to the extent that bone scan is not recommended for staging these patients.23–25
Bone scans are more sensitive than specific for the detection of osseous metastases.16 A wide range of benign findings (e.g., arthritis, healing fractures, benign bone tumors such as enchondromas and fibrous dysplasia) can induce tracer uptake and mimic metastatic disease.26 Alternatively, metastases that heal with sclerosis can subsequently accumulate more radionuclide than was present during disease progression, resulting in a deceptive “flare” phenomenon.27,28 Because bone scans provide limited anatomic detail, these pitfalls cannot be resolved by SS alone. Therefore, the sensitivity of bone scan is often combined with the specificity of radiography to provide a powerful and relatively inexpensive means of diagnosing indeterminate findings on bone scan.3,19,26
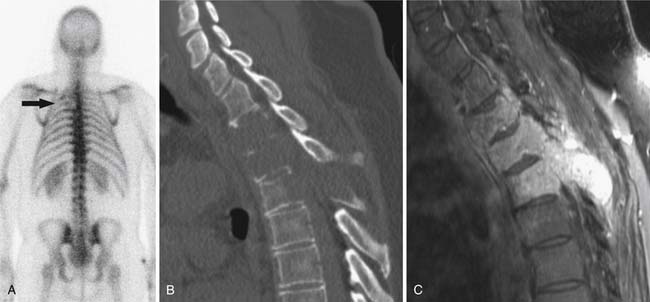
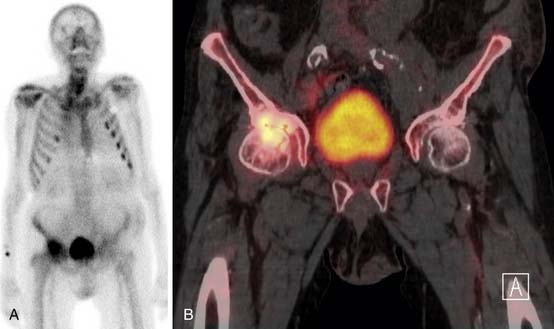
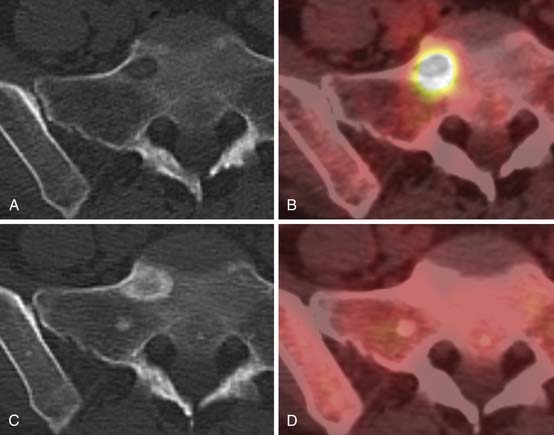
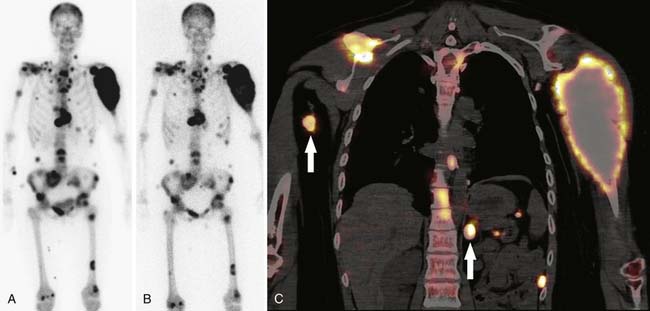
SPECT/CT is a dual-modality imaging technique that acquires scintigraphic and CT data sets on a single, hybrid scanner. Traditional bone scanning is a planar modality that produces single anterior and posterior whole body images with optional oblique or spot views. SPECT is used to perform bone scans in a cross-sectional manner. The axial images increase contrast resolution, resulting in greater sensitivity29 and specificity30 for detection of bony metastases than those achieved with planar scans. Nevertheless, anatomic detail remains limited and specificity of SPECT is further increased when fused with CT.31 Modern SPECT/CT scanners are multifunctional machines that can perform planar bone scan, SPECT, and CT on the same hybrid scanner. SPECT/CT is most commonly used to determine the etiology of indeterminate uptake on planar bone scan (Figure 33-6), which can be accomplished in the same imaging session and with the same dose of tracer on the hybrid scanner.
[18F]-fluoro-2-deoxy-D-glucose (FDG) PET is a functional imaging modality that reflects cellular glucose metabolism. FDG, the most commonly used PET tracer for oncologic indications, is a radiolabeled glucose molecule. Numerous malignancies have high glucose metabolism. Two important factors regarding the uptake and intracellular accumulation of FDG involve cell membrane glucose transport proteins (GLUTs) and the intracellular enzyme hexokinase. Of the GLUT family of transport proteins, GLUT 1 is both ubiquitous and up-regulated in many neoplasms. Once FDG enters the cell, it is phosphorylated by hexokinase into FDG-6-phosphate.32 This molecule is not suitable for further metabolism; the negative charge imputed by the phosphate moiety makes it impermeable to the cell membrane and dephosphorylation is slow. Therefore, FDG accumulates in the cells. The tracer emits positrons that, following an interaction with an electron, results in the near-simultaneous emission of a pair of 511-keV photons (nearly 180 degrees apart) that are detected by scintillation crystals embedded in the PET scanners, thereby localizing the decay event within the body.33
FDG uptake is not limited to tumor cells; other conditions result in FDG accumulation such as inflammatory (e.g., arthritis, infection) or physiologic (e.g., brain and liver function) processes. Despite these limitations, PET alone (no CT) was found to be more specific for detection of bony metastases than planar SS34–36 owing to the higher spatial resolution of PET.34,37,38 Evaluation of the morphology of the bone metastases has shown that PET reveals more lytic than blastic lesions.39,40 Lytic bone metastases typically heal with sclerosis41 and prior treatment likely influenced this conclusion in some studies. Otherwise, it has been suggested that blastic metastases may not be as easily detected by PET owing to relatively lower cellularity42 in comparison with more highly cellular lytic lesions. Current technology employs fused PET/CT data sets obtained on hybrid PET/CT scanners. A review that compared the reported sensitivity and specificity data of 10 studies that used PET alone with 2 studies that used PET/CT to detect bony metastases found the median sensitivity of PET/CT to be higher than PET (95.2%, and 83.9%, respectively) whereas the specificity was similar (94.6% and 95.8%, respectively).43 More research is needed to evaluate the influence of fused CT on the sensitivity and specificity of PET for the detection of bone metastases in general and blastic lesions in particular. Whereas PET scans are expensive and accessibility can be limited, the combination of functional and anatomic imaging makes FDG-PET/CT a powerful modality for whole body tumor assessment.
Magnetic Resonance Imaging
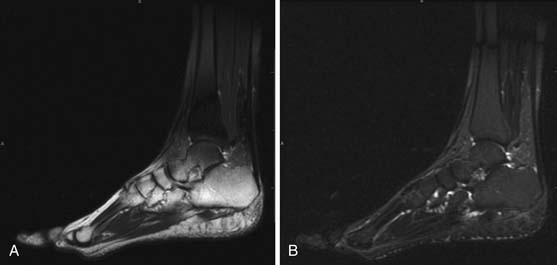
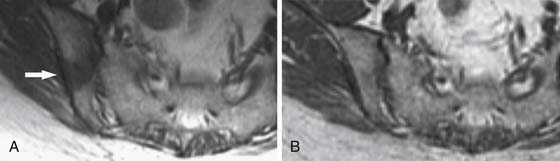
MRI is capable of providing the greatest soft tissue resolution of all imaging modalities. The optimal MRI pulse sequences for imaging the musculoskeletal system may differ from those used in body imaging. Bone movement occurs through the conscious contraction of muscles rather than through the autonomic nervous system. The majority of patients are able to remain still throughout a typical examination (~45 min), allowing the routine use of several relatively lengthy pulse sequences that permit exquisite soft tissue contrast resolution. The following pulse sequences are recommended for conventional MRI of musculoskeletal oncology: fast spin echo (FSE) T1-weighted, FSE fat-saturated (FS) T2-weighted, and FSE FS T1-weighted following intravenous gadolinium contrast. Short tau inversion recovery (STIR) can be substituted for FSE FS T2 in situations that predispose to uneven fat saturation such as when scanning irregular anatomy (foot, ankle [Figure 33-7]) or structures that are offset from the isocenter of the magnet (humeri). STIR is not used routinely owing to comparatively lower signal-to-noise ratio and resultant suboptimal image quality compared with FS T2. The metal from bony reconstructions can produce significant artifacts that can be minimized by excluding fat saturation from the contrasted sequences and replacing FS T2 with STIR.44
Most lytic and mixed bony metastases demonstrate low or intermediate T1 signal and high T2 signal and enhance. Sclerotic metastases may have a similar appearance or demonstrate low signal on all pulse sequences. Because the vast majority of skeletal metastases arise in the soft tissue of the marrow cavity, MRI allows early lesion detection by imaging the tumor while confined to the marrow.45 Conversely, radiography and CT depend primarily on the interaction of the tumor with cortex, and metastases are often visible only after the tumor has grown large enough to extend from the marrow into the cortex. MRI is also the optimal imaging modality for determining the extent of tumor in the medullary cavity21,46 and can be crucial for planning surgery or radiation therapy (Figure 33-8). Another advantage of MRI is that excellent soft tissue resolution allows detection of impending emergencies involving adjacent structures, such as major nerves, blood vessels, or the spinal cord.21,46–48 However, a disadvantage of MRI is that it cannot accurately depict cortical bone structure owing to a paucity of free protons, resulting in the black signal void typical of normal cortex. Therefore, radiography and CT are preferred for evaluating fine cortical detail. Whereas MRI has traditionally been used for limited anatomic coverage, whole body MRI is becoming increasingly feasible in the typical 1-hour patient scheduling allotment49 and may supplant bone scan and skeletal surveys as the preferred whole body screening modality for bone metastases in the future.
Future Imaging
Whole body MRI is an emerging technology that allows MRI of the entire body to be performed in a single imaging session. Conventional MRI is limited in anatomic coverage owing to a combination of the long acquisition times of traditional pulse sequences and field inhomogeneity artifacts that increase with large coverage areas. Whole body MRI has been repeatedly shown to detect more bony metastases than skeletal scintigraphy.50–54b
Considering the challenges of time limitation and artifact suppression, investigators of whole body MRI have used a variety of rapidly acquired or robust pulse sequences. Whereas some investigators have utilized T2-weighted imaging exclusively,50,55 others have sought to increase diagnostic accuracy by performing at least two complementary sequences on each patient. T2-weighted and STIR images have high sensitivity but lower specificity for the detection of osseous metastases. In contrast, T1-weighted sequences have high specificity and lower sensitivity. Lauenstein and coworkers56 found that the sensitivity and specificity of T2-weighted STIR sequences for the detection of osseous metastases to be 100% and 80%, respectively, and that the corresponding values for T1-weighted gradient recalled echo (GRE) sequences were 81% and 90%, respectively. Several investigators have taken advantage of the complementary strengths of T1- and T2-weighted MRI by performing both techniques in the same examination.52,53,57 Others have employed the same general concept by complementing T2-weighted images with an FS T1-weighted sequence obtained after administration of intravenous gadolinium contrast54 or have employed all three (T1 precontrast, T1 postcontrast, and T2-weighted sequences).58,59
In many studies, completion of multiple pulse sequences of the entire body in a reasonable time frame is facilitated by use of specialized hardware, such as a rolling table platform that utilizes a combination of stationary coils embedded in the table and a fixed anterior surface coil under which the patient moves for each anatomic station.54,56,58 In a study utilizing a rolling table platform, three sequences (T2 FSE and T1, three-dimensional GRE sequences before and after the administration of intravenous contrast) were acquired in 14.5 minutes.54 Parallel imaging techniques, based on phased-array coil technology,60 can also be used to speed image acquisition.57,61
The current trend in whole body MRI is to perform a large number of complementary pulse sequences in numerous imaging planes in approximately 1 hour or less. Dixon imaging can accomplish this objective without specialized hardware. Dixon imaging can be used to simultaneously generate three different kinds of images including T2 (no fat saturation), water-only (an FS T2 sequence), and fat-only (novel sequence in which only fat is bright) images. This has been combined with a fast T1-weighted Dixon (which again produces at least three different kinds of images in a single acquisition) and diffusion-weighted sequences to produce 56 series of images covering head, chest, abdomen/pelvis, thighs, calves, sagittal spine, and dynamic liver sequences in approximately 1 hour.49 In addition to high sensitivity for the detection of bone metastases, MRI can image soft tissues (including organs such as liver and brain), making whole body MRI a versatile imaging modality for total body imaging.54a
Whole body MRI and FDG-PET/CT are two of the newest and most comprehensive whole body imaging techniques, and research has been performed comparing their relative strengths and weaknesses for the detection of metastases. Whole body MRI has been shown to have a higher sensitivity than FDG-PET/CT for the detection of osseous metastases.43,57,58 A prospective study comparing whole body MRI and FDG-PET/CT in 98 patients found that the sensitivity of whole body MRI was greater than FDG-PET/CT for the detection of osseous and hepatic metastases, whereas whole body MRI had lower sensitivity than FDG-PET/CT for the detection of pulmonary metastases.58 In addition, FDG-PET/CT was found to be more accurate than whole body MRI for primary tumor and nodal staging. These findings suggest that the two modalities are complementary and that one of the major strengths of whole body MRI is in the evaluation of bony metastases. Future research may show that a combination of both examinations in the setting of FDG-avid tumors provides comprehensive total body imaging to the extent that few additional imaging studies are needed.
Key Points Detection
• Radiography has high specificity but low sensitivity.
• Combining radiography with the high sensitivity of bone scan, the two modalities are effective for detecting bone metastases.
• MRI effectively detects early metastases confined to the marrow, extent of disease in marrow, soft tissue extension from bone, and epidural extension.
• CT combined with SPECT and PET imaging improves lesion detection when compared with SPECT or PET alone.
• PET detects more lytic than blastic metastases, the opposite of bone scan.
Response to Therapy
Cancer response criteria are used to determine the efficacy of therapeutic agents in clinical cancer trials and can be used as general guidelines for assessing therapeutic response whether or not a patient is enrolled in a trial. The most commonly used criteria, such as the Response Evaluation Criteria In Solid Tumors (RECIST), are based on the physical measurement of metastatic lesions. Bone metastases are often located in the axial skeleton and the irregular shape of bones such as the vertebra and pelvis make physical measurement difficult. In addition, bony metastases do not always respond to therapy with a significant change in size. Therefore, these traditional methods of evaluating therapeutic response are not generally applicable to bony metastases unless a measurable soft tissue mass extends from the cortex.62 In response to the need for criteria that can be used to evaluate bony metastases, a bone-specific set of criteria were developed at the M. D. Anderson Cancer Center (MDA criteria).3
The MDA criteria include quantitative and qualitative assessments of the behavior of bone metastases (Table 33-1). Response is divided into four categories: complete response (CR), partial response (PR), progressive disease (PD), and stable disease (SD). Quantitatively, these criteria define PR as a decrease of 50% or more in the sum of the perpendicular measurements of a lesion and PD as an increase of 25% or more in this sum. The response categories are defined as follows:
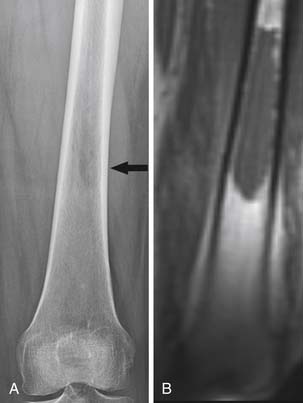
• Complete response: Complete sclerosis of initially lytic lesions on XRs or CT; return of normal bone density on XR, CT, or MRI (Figure 33-9); or normalization of tracer uptake on SS.
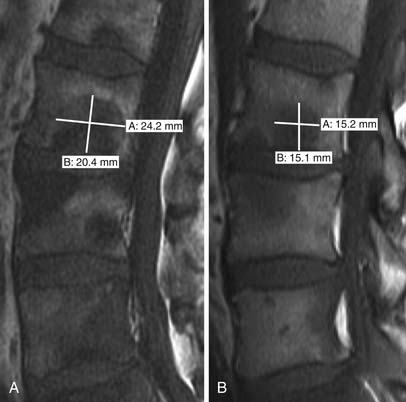
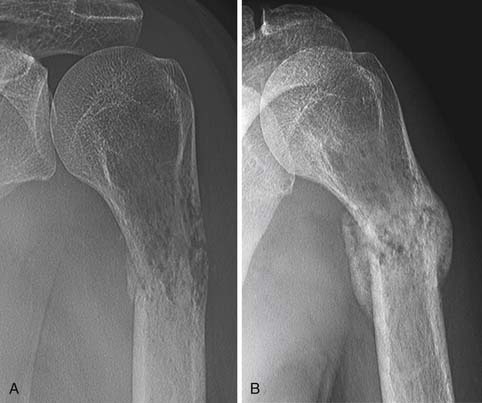
• Partial response: Development of a sclerotic rim and/or partial sclerotic fill-in of lytic metastases on XR or CT; 50% or greater decrease in the sum of measurable lesions (Figure 33-10); the subjective unequivocal decrease in the size of blastic lesions on XR or CT that cannot be accounted for by changes in obliquity or slice placement; and unequivocal decrease in tracer uptake on SS. PR also takes into account the phenomenon of “osteoblastic flare.” Interval visualization of “new” sclerotic lesions or lytic lesions with sclerotic rims, in the setting of other signs of PR, indicates the healing of previously inconspicuous metastases63 rather than disease progression (Figure 33-11). The sclerotic rims can make lesions appear to increase in size, also mimicking disease progression. Osteoblastic flare cannot be diagnosed if any preexisting bone lesions show signs of progression, such as the enlargement of lytic lesions or the development of new lytic lesions.
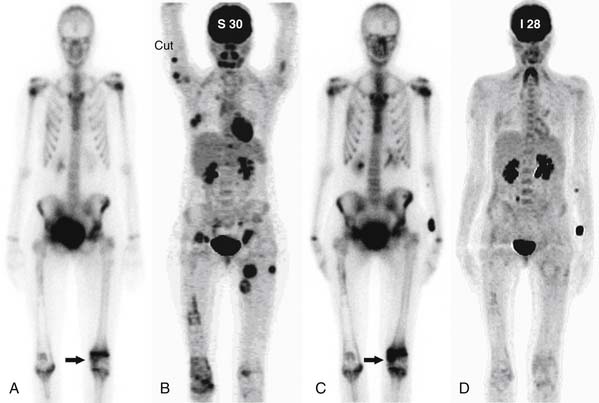
• Progressive disease: A 25% or greater increase in the size of any measurable lesion on XR, CT, or MRI; unequivocal increase in the size of unmeasurable (ill-defined) lytic or blastic lesions that cannot be accounted for by obliquity or slice placement; unequivocal increase in tracer uptake on SS; or the development of new metastases. Scintigraphic flares are usually seen within the first 3 months after therapy27,28 and increased tracer uptake on SS in this interval may need correlation with other imaging studies in order to confirm PD (Figure 33-12).
• Stable disease: No change, less than 25% increase, or less than 50% decrease in lesions, and no new lesions.
| RESPONSE CATEGORY | CRITERIA |
|---|---|
| CR | Complete sclerotic fill-in of lytic lesions on XR or CT. Normalization of bone density on XR or CT. Normalization of signal intensity on MRI. Normalization of tracer uptake on SS. |
| PR | Development of a sclerotic rim or partial sclerotic fill-in of lytic lesions on XR or CT. Sclerotic “flare”: Interval visualization of lesions with sclerotic rims or “new” sclerotic lesions in the setting of other signs of PR and absence of progressive bony disease. ≥50% decrease in measurable lesions on XR, CT, or MRI. ≥50% subjective decrease in the size of ill-defined lesions on XR, CT, or MRI. ≥50% subjective decrease in tracer uptake on SS. |
| PD | ≥25% increase in size of measurable lesions on XR, CT, or MRI. ≥25% subjective increase in the size of ill-defined lesions on XR, CT, or MRI. ≥25% subjective increase in tracer uptake on SS. New bone metastases. |
| SD | No change. <25% increase or < 50% decrease in measurable lesions. <25% subjective increase or < 50% subjective decrease in ill-defined lesions. No new bone metastases. |
Measurements are based on the sum of a perpendicular, bidimensional measurement of the greatest diameters of each individual lesion.
CR, complete response; CT, computed tomography; MRI, magnetic resonance imaging; PD, progressive disease; PR, partial response; SD, stable disease; SS, skeletal scinitgraphy; XR, radiograph.
Modified from Hamaoka T, Madewell JE, Podoloff DA, et al. Bone imaging in metastatic breast cancer. J Clin Oncol. 2004;22:2942-2953.
In comparison to the World Health Organization (WHO criteria) and International Union Against Cancer (UICC criteria), the MDA criteria were shown to be the only set of response criteria to correlate to progression-free survival in a study of 41 breast cancer patients with bone-only metastases.4 The MDA bone response criteria reflect the behavior of bone metastases as they progress or improve and can be used as guidelines for interpretation of imaging studies.
Key Points Response to therapy
• Lytic lesions typically heal with rim and/or central sclerosis.
• “Osteoblastic flare” can occur when lytic lesions heal resulting in newly visualized sclerotic lesions at the site of undetected metastases or cause lytic lesions to increase in size owing to development of rim sclerosis, mimicking disease progression.
• “Scintigraphic flare” results from increased tracer uptake in healing metastases and can mimic disease progression.
Treatment
Medical
The therapy of bone metastases is dependent on factors such as the number, location, and character of the lesions. In order to reach all of the lesions, widespread metastases are often best treated systemically with medical therapy or radiopharmaceuticals. Chemotherapy can have a positive effect on bony metastases and hormonal therapies may be administered for responsive malignancies such as breast and prostate cancer. Whereas chemotherapy and hormonal therapies treat all types of metastatic lesions, the bisphosphonates target bone. They are pyrophosphate analogues that bind to hydroxyapetite and inhibit osteoclasts, thereby preventing the bone resorption5 that occurs in both lytic and blastic metastases.12 Studies in breast cancer patients have shown that bisphosphonates, such as pamidronate (Aredia) and zoledronic acid (Zometa), reduce the development of serious skeletal-related events (SREs) such as pain, pathologic fracture, and hypercalcemia. In the 1990s, intravenous pamidronate was established as the standard of care for breast cancer patients with bone metastasis.64,65 Pamidronate delayed the first SREs for 3.5 to 6.1 months compared with placebo and the proportion of patients with skeletal complications decreased by approximately 11% to 13%. However, the effects were palliative and no survival benefit was demonstrated.64–66
Zoledronic acid is a more recently developed bisphosphonate that is more potent than pamidronate. When zoledronic acid (4 mg or 8 mg IV) was compared with pamidronate (90 mg IV), given every 3 to 4 weeks for 24 months,67 the annual incidence of skeletal complications decreased by 40% in breast cancer patients treated with zoledronic acid compared with pamidronate. Furthermore, a meta-analysis comparing numerous bisphosphonates found that zoledronic acid was as effective as pamidronate in preventing SREs.68 However, many oncologists choose zoledronic acid over pamidronate because of its shorter duration of infusion (15 min vs. 2 hr, respectively). Several studies have reported prolonged progression-free survival with bisphosphonate administration and the potential antitumor activity of bisphosphonates is an area of ongoing investigation.68,69
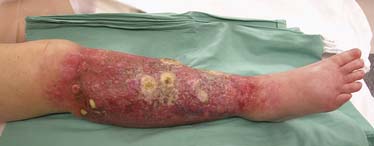
Osteonecrosis of the jaw (ONJ) is a major side effect of bisphosphonate therapy. ONJ may present in the form of nonhealing oral mucosa of the mandible or maxilla that often presents with pain and exposed bone.70,71 Bisphosphonates disrupt normal bone turnover that is dependent on the action of osteoblasts and osteoclasts. Osteoblasts produce bone matrix and become osteocytes once surrounded by mineralized bone. Osteocytes live approximately 150 days and are less able to maintain bone matrix at the end of their lifespan, allowing the development of microfractures. Osteoclasts resorb the fragile bone, allowing new osteocytes to lay down healthy bone in its place. By inhibiting osteoclast function, bisphosphonates can prevent normal bone turnover as a side effect of their main function in patients with bone metastases, which is to inhibit tumor-mediated osteolysis. This chain of events can lead to bone death and ONJ. The nitrogen-containing bisphosphonates (pamidronate and zoledronic acid) are not metabolized and are, therefore, long acting. Length of exposure to these drugs is an additional causative factor for ONJ and an incidence of 1.5% has been found in patients who received therapy for 4 to 12 months and 7.7% in patients who were treated for 37 to 48 months.71 Zoledronic acid has been more strongly implicated than pamidronate in causing ONJ.71,72 Because the iatrogenic exposure of bone (e.g., tooth extractions, periodontal procedures) is a major initiating factor for ONJ, an initial dental evaluation is required and all dental work must be completed with wounds well healed before initiating bisphosphonate therapy.73 If ONJ develops, bisphosphonates should be stopped and medical therapy such as antibiotics, use of mouth rinses, and heightened oral hygiene initiated. Surgical therapy has not been found to be more effective than medical therapy74 and aggressive débridement of necrotic bone is reserved for refractory cases75 (Figure 33-13).
Radiation
The role of radiation therapy for patients with bone metastases is palliative and is indicated for situations such as the treatment of pain that is uncontrolled with analgesics, the prevention or treatment of pathologic fractures, and the treatment of patients with epidural tumor that compresses or threatens to compress the spinal cord or nerve roots. Selection of appropriate target sites for radiation treatment can be challenging and imaging studies are useful for identifying the particular symptomatic bone(s). SS and PET/CT scanning are useful for imaging the entire skeleton19 and radiography can be used to evaluate specific, painful areas. Multiplanar MRI and three-dimensional re-formatted CT scans of the axial skeleton are helpful for identifying disease extent, and the best approach to minimize radiation toxicity to surrounding normal tissues. For vertebral metastases, MRI is preferred over radiographs and myelography because it can best identify and evaluate the extent of epidural tumor.48
External beam radiation is effective in reducing the pain of bone metastases in approximately 50% to 80% of patients.76 Owing to the potential for toxicity, the goal of palliative radiation therapy is not to eradicate all tumor cells and a moderate dose of radiation is, therefore, indicated. Many randomized trials have been conducted to determine the optimal radiation dose to palliate bone pain.77 The most widely used regimen in the United States for patients with poor prognosis is to deliver 30 Gy in 10 daily treatments over 2 weeks.76 Typically, this dose is well tolerated,76 although side effects can occur and depend on the area that is treated. For example, diarrhea is common when metastases in pelvic bones are radiated whereas nausea and esophagitis may occur during the treatment of thoracic and cervical spine.
The patient’s overall condition is considered when choosing an appropriate treatment schedule; shorter regimens can be implemented so the patient is not overly burdened by the logistics of keeping numerous appointments. For patients with widespread metastases, poor prognosis, and who are unable to keep multiple appointments, a course of 20 Gy in 5 daily treatments can be considered. Some randomized trials have found no significant difference in the efficacy of pain control upon comparison of short compared with more protracted treatment regimens.77 In the Radiation Therapy Oncology Group (RTOG) 97-14 trial, both the single-treatment (8 Gy) and the 10-daily-treatment (30 Gy) arms achieved similar rates of pain control and reduction of analgesic use.76 At 3 months, complete pain relief was achieved in approximately 17% of patients whereas partial relief was observed in an additional 49%. Differences between the two treatment regimens included a higher rate of retreatment of the same area in patients who received the single, lower dose (18% vs. 9%) but a higher rate of acute toxicity with the protracted schedule. For patients with better prognoses, higher doses of radiation therapy can be considered in order to produce a more durable response. Higher doses typically provide better local control and doses of approximately 40 Gy given over 2.5 to 3 weeks are relatively well tolerated. Reanalysis of the RTOG 97-14 trial suggested that administering a cumulative dose of 40.5 Gy resulted in significantly better pain and analgesic reduction compared with a 20-Gy cumulative dose.78
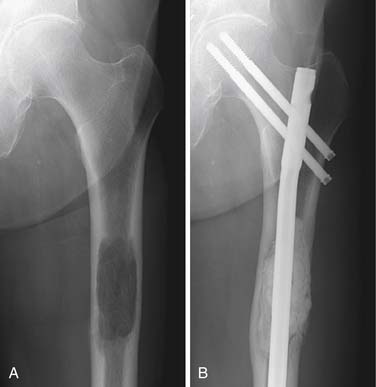
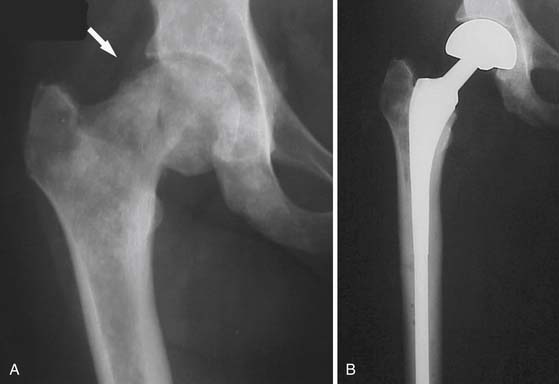
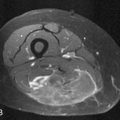
When pathologic fracture is imminent, the administration of external beam radiation can be preventive by interrupting osteoclast activity and thereby allowing the bone to reossify and regain strength (Figure 33-14). When fracture has occurred, surgical intervention is typically required to stabilize the bone (see “Surgical,” later). After the surgical repair, the metastatic lesion can be radiated to prevent continued growth of the tumor, which could again destabilize the structural integrity of the bone.
Radiation therapy is also often best combined with surgery in patients with epidural extension of tumor and subsequent compression or impending compression of the spinal cord. When a single area of the spine is involved, immediate circumferential surgical decompression of the cord before radiation therapy has been found to result in significantly better preservation of motor function than radiation alone in a prospective, randomized trial.79 Surgery is preferred in these instances because the mass effect of the radiated tumor only resolves gradually over several days. Prolonged mass effect can cause reversible edema to progress into irreversible cord ischemia. After combined treatment, 84% of the patients were ambulatory for a median duration of 122 days whereas 57% of those who received radiation alone were ambulatory for a median duration of 13 days.79 Although typically less effective than combined therapy, patients with disease at numerous spinal levels and/or a combination of poor prognosis and performance status, palliative radiation therapy alone often provides benefit.
For patients with extensive painful bone metastases refractory to analgesics and systemic therapy or patients who have failed external beam radiation, radiopharmaceuticals are another option. Strontium-89 chloride (89Sr) and samarium-153 ethylene diamine tetramethylene phosphonaic acid (153Sm) are bone-seeking beta emitters with half-lives of 50.5 days and 46.8 hrs, respectively.80 Both localize to sites of bone turnover and pretherapeutic SS can be performed in order to verify osteoblastic reaction to the metastases. Unlike 89Sr, 153Sm emits a gamma photon that permits high-quality posttherapeutic imaging (Figure 33-15). These agents are contraindicated in patients with renal insufficiency, bone marrow suppression, spinal cord compression, and pregnancy. The side effects of these agents include mild bone marrow suppression 4 to 8 weeks after the therapy, which is reflected by leukopenia and thrombocytopenia. Bone marrow toxicity is usually transient and therapy can be repeated 2 to 3 months after blood counts recover.81,82 No significant difference has been found in the palliation of bone pain with 89Sr compared with 153Sm (60-95%).81,83–85 Improved pain control is usually seen within days or weeks and can last for several months.80
Surgical/Interventional
Pathologic fracture or impending fracture is one of the most common indications for surgical treatment of bone metastases. The basic goals of surgery are stabilization, preservation of mobility, and pain relief.86 The discomfort of osseous metastases can be among the most severe that a cancer patient can experience, and the marked pain reduction after surgery typically allows patients to regain lost mobility.87
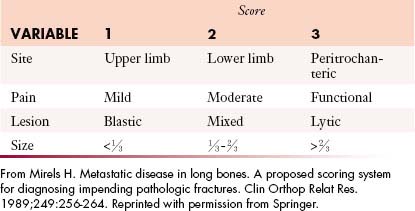
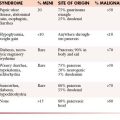
On a per-lesion basis, many factors contribute to the decision whether to intervene surgically (Figure 33-16). If a pathologic fracture is present, surgery is usually the best treatment option, but if it has not yet occurred, fracture risk must be determined in order to decide between surgery and other options. Several methods can be used to determine fracture risk, and all involve image-based analysis of the size and character of the metastasis. It has been found that painful femoral lesions that are at least 2.5 cm in length and involve the cortex are at risk for pathologic fracture88 or that lesions that occupy more than 50% of the circumference of the bone are also at considerable risk.89 Mirels’ scoring system can also be used to determine fracture risk90 (Table 33-2). This system is based on four parameters: the site of the metastasis (upper extremity, lower extremity, or peritrochanteric region), pain (mild, moderate, severe/aggravated with function), tumor type (blastic, mixed, or lytic), and lesion size (involvement of < ⅓, ⅓-⅔, or > ⅔ of the diameter of the bone). Reflecting severity, 1 to 3 points are assigned per category. A total score of 8 or greater indicates significant concern for pathologic fracture and surgery is considered the treatment of choice. A score of less than 7 suggests less risk for fracture and nonsurgical approaches such as functional bracing, protected weight-bearing, and radiation can be considered.
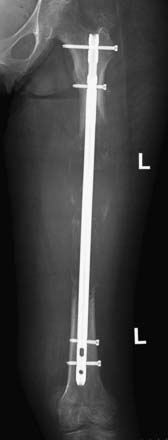
In addition to fracture risk, the expected response of bone metastasis to other therapies is a strong factor when determining the appropriateness of surgery. Response can be estimated on the basis of the histology of the primary tumor. For example, lymphoma of bone frequently resolves after chemotherapy and the bone has the capacity to recover completely. Breast, prostate, thyroid carcinoma, and multiple myeloma also tend to demonstrate positive treatment response. Conversely, renal cell carcinoma does not respond well and the lytic lesions typically progress and enlarge with time. Likelihood of response to systemic or radiation therapy is also important in determining the most appropriate type of operation. In general, surgery may be open or closed, meaning that the tumor site or fracture may be directly exposed (open) or not exposed (closed). Closed nailings are typically performed across lesions in long bones that are expected to respond well to chemotherapy or radiation. Intramedullary nails are generally preferred over side plates because nails can stabilize the entire length of the bone instead of just the area under the plate and the operation can be performed percutaneously through small incisions for the entrance sites of the nail and interlocking screws. The morbidity of such operations is minimal, although blood loss through the bone may be brisk with vascular tumors such as renal cell carcinoma, thyroid carcinoma, and myeloma. In these cases, preoperative selective arterial embolization, typically performed by interventional radiology, can significantly reduce blood loss.
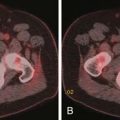
Surgical excision of the metastatic tumor, which may be done in conjunction with open nailing or more extensive reconstructive procedures, is often performed for diseases such as sarcomas, melanoma, and renal cell carcinoma that do not typically respond well to medical or radiation treatment and is performed to prevent local relapse. Common excisional procedures include curettage and en bloc resection. Curettage is a procedure in which the tumor is intralesionally removed in a piecemeal fashion, leaving the adjacent bone intact, whereas en bloc resection removes the tumor as a single intact mass, often resulting in wider surgical margins. In the setting of massive local recurrence, limb amputation may be required (Figure 33-17). Another indication for open resection of osseous metastases is for treatment of lesions that destroy the structure of the bone to such an extent that it cannot be salvaged by nailing or plating (Figure 33-18). Advantages of wide, en bloc excision include reducing the likelihood of massive local recurrence (Figure 33-19), better control of bleeding vessels, and control of refractory hypercalcemia, which can directly result from loss of large amounts of mineralized tissue.
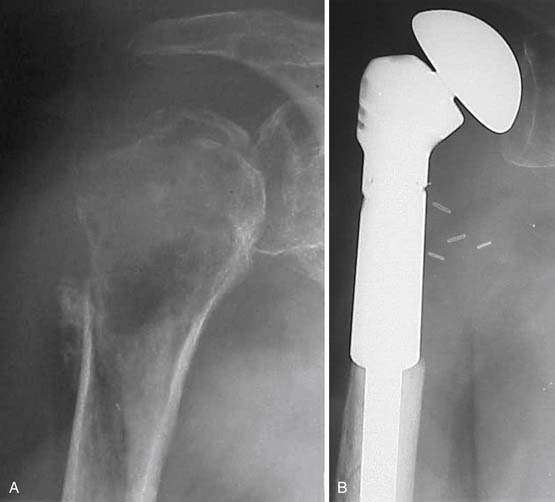
The benefits of wide excision are not achieved without a price. In addition to the increased length of surgery, more extensive wound, and greater potential for perioperative complications, there may be limb weakness and worse function after surgery. Most large skeletal defects of long bones can now be reconstructed in a straightforward manner with modular prostheses that can be easily adapted to various sizes (Figure 33-20).
Occasionally, surgical treatment of osseous metastases is performed with curative intent in addition to the goal of alleviating pain and improving mobility. Patients with renal cell carcinoma who present with a solitary metastasis in bone have a better prognosis than patients with numerous metastases91,92 and wide en bloc resection is generally recommended.92 It is commonly believed that definitive removal of the tumor can facilitate a complete cure. Whether excision of all tumor cells in the metastatic deposit can lead to cure is debatable because it is possible that patients who have a solitary osseous metastasis, particularly those who develop the metastasis years after initial diagnosis, may develop widespread disease that is simply indolent in onset. In our experience, patients with solitary renal cell metastases who undergo thorough curettage of the tumor have somewhat but not significantly worse long-term survival compared with en bloc resection.91 Curettage is a less morbid procedure than en bloc resection and is preferred if the functional deficits of en bloc resection would be substantial. Additional studies with larger patient populations are needed to further evaluate this issue.
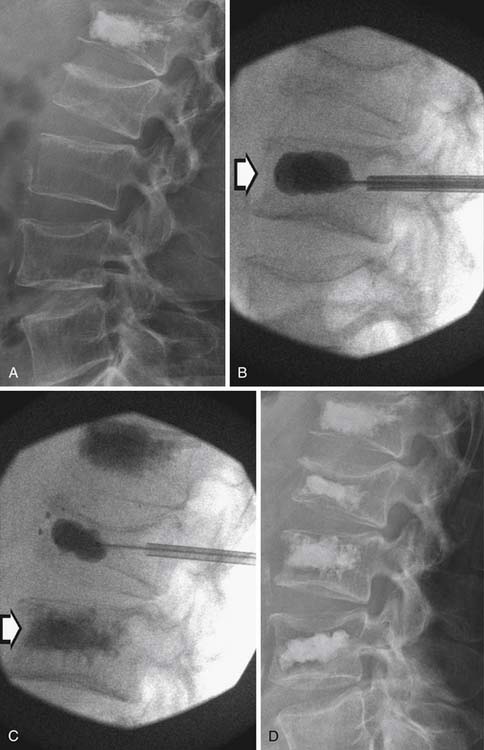
Several nonsurgical percutaneous treatment options for painful bone metastases are also available. Examples include vertebroplasty, kyphoplasty, and RFA. Vertebroplasty and kyphoplasty are performed by injecting bone cement (polymethylmethacrylate) into the collapsed vertebral body by needle, typically through the pedicles, under fluoroscopic guidance. In vertebroplasty, the cement stabilizes the segment, preventing further collapse. This has proved especially helpful for patients with multiple myeloma who may have extensive spinal disease and/or poor structural integrity, disallowing stabilization hardware. Kyphoplasty is performed in a similar manner by injecting the cement into a balloon with the goal of increasing the height of the segment in order to also help correct abnormal spinal curvature. Once the balloon is inflated, it is withdrawn and cement is injected into the cavity (Figure 33-21). Pain relief has been reported in the vast majority of patients after vertebroplasty or kyphoplasty.93–95 These procedures are contraindicated in patients with cord compression.93 Complications have been reported at approximately 0% to 8%. Most complications are related to probe insertion (e.g., hematoma/hemothorax), cement embolism (e.g., pulmonary embolism), or radiculopathies from nerve root/spinal cord compression against extravasated cement.95
RFA is a procedure by which a thermal probe is inserted into a tumor and is used to destroy the lesion by heat necrosis. RFA has been shown to provide excellent pain relief with little morbidity.96 Goetz and colleagues97 studied RFA in 43 patients with bone metastases and found that 95% experienced a clinically significant decrease in pain for up to 6 months. Lesions in this study were limited to lytic or mixed metastases owing to inability to expand the probe into hard, blastic lesions. The procedure can sometimes be complemented by percutaneous cement injection to help support the bone. RFA may be particularly advantageous in patients who have already undergone radiotherapy and are not candidates for further radiation. RFA is a valuable technique in the spectrum of surgical and interventional procedures.
Key Points Treatment
• Systemic therapy (e.g., bisphosphonates) is indicated to treat widespread bone metastases, refractory to analgesics.
• ONJ is a potential side effect of bisphosphonate therapy.
• Radiation therapy can be used to palliate limited numbers of bone metastases, and can be combined with surgery in cases of spinal cord compression or impending pathologic fracture.
• Radiopharmaceuticals can provide pain control for widespread bony metastases unresponsive to systemic therapy and analgesics
• Imaging assessed risk of pathologic fracture is a major deciding factor between surgery and other therapeutic options.
• Open surgical procedures remove the tumor whereas closed procedures simply stabilize the bone.
• Selection of the appropriate operation often depends on the expected response of the lesion to systemic or radiation therapy
• Percutaneous interventions (e.g., vertebroplasty, kyphoplasty, or RFA) should be considered for treatment of individual bone metastases
Summary
Bone metastases have a significant impact on the lives of many cancer patients. The detection of these lesions is accomplished through a variety of imaging studies. Bone scans and skeletal surveys can be used to screen the entire skeleton, whereas dedicated radiographs are helpful for evaluating specific abnormalities or painful areas. By imaging the marrow cavity, MRI can detect lesions early and is the optimal modality for determining the extent of bone metastases prior to therapy. Whole body MRI is more sensitive than bone scan for the detection of metastases and can simultaneously image organs and soft tissues. FDG-PET/CT is another whole body imaging modality that can be used to detect bone metastases and other distant metastases and for the local and nodal staging of the malignancy. Imaging is commonly used to evaluate the response of metastases to therapy, and bone-specific response criteria can be used to guide assessment. The treatment of bony metastases is a multidisciplinary endeavor that includes imaging and the expertise of the medical oncologist, radiation oncologist, and orthopedic surgeon in order to properly assess and optimize therapeutic options. Practitioners from all involved specialties benefit from an understanding of the roles of the others in order to optimize the care of cancer patients.
Key Points Summary
• CT, bone scan, MRI, and PET/CT can complement each other in detecting bone metastases.
• Response criteria provide guidelines for interpreting changes in the appearance of bone metastases after therapy.
• Widespread metastases are best treated with systemic therapy. Limited metastases are addressed by external beam radiation, surgery, or percutaneous interventions.
• Treatment of bone metastases is complex and requires a knowledge of bone imaging in conjunction with multidisciplinary approach.
1. Abrams H.L., Spiro R., Goldstein N. Metastases in carcinoma: analysis of 1000 autopsied cases. Cancer. 1950;3:74-85.
2. Coleman R.E. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res.. 2006;12:6243s-6249s.
3. Hamaoka T., Madewell J.E., Podoloff D.A., et al. Bone imaging in metastatic breast cancer. J Clin Oncol. 2004;22:2942-2953.
4. Hamaoka T., Costelloe C.M., Madewell J.E., et al. Tumour response interpretation with new tumour response criteria vs the World Health Organisation criteria in patients with bone-only metastatic breast cancer. Br J Cancer. 2010;102:651-657.
5. Langer C., Hirsh V. Skeletal morbidity in lung cancer patients with bone metastases: demonstrating the need for early diagnosis and treatment with bisphosphonates. Lung Cancer. 2010;67:4-11.
6. Batson O.V. The function of the vertebral veins and their role in the spread of metastases. Ann Surg. 1940;112:138-149.
7. Geldof A.A. Models for cancer skeletal metastasis: a reappraisal of Batson’s plexus. Anticancer Res.. 1997;17:1535-1539.
8. Kager L., Zoubek A., Kastner U., et al. Skip metastases in osteosarcoma: experience of the Cooperative Osteosarcoma Study Group. J Clin Oncol. 2006;24:1535-1541.
9. Jiya T.U., Wuisman P.I. Long-term follow-up of 15 patients with non-metastatic Ewing’s sarcoma and a skip lesion. Acta Orthop. 2005;76:899-903.
10. Enneking W.F., Kagan A. “Skip” metastases in osteosarcoma. Cancer. 1975;36:2192-2205.
11. Clain A. Secondary malignant disease of bone. Br J Cancer. 1965;19:15-29.
12. Roodman G.D. Mechanisms of bone metastasis. N Engl J Med. 2004;350:1655-1664.
13. Dorfman H., Czerniak B. Bone Tumors. St. Louis: CV Mosby; 1998. 1–33
14. Ibrahim T., Flamini E., Mercatali L., et al. Pathogenesis of osteoblastic bone metastases from prostate cancer. Cancer. 2010;116:1406-1418.
15. Madewell J.E., Ragsdale B.D., Sweet D.E. Radiologic and pathologic analysis of solitary bone lesions. Part I: internal margins. Radiol Clin North Am.. 1981;19:715-748.
16. Hortobagyi G.N., Libshitz H.I., Seabold J.E. Osseous metastases of breast cancer. Clinical, biochemical, radiographic, and scintigraphic evaluation of response to therapy. Cancer. 1984;53:577-582.
17. Ardran G.M. Bone destruction not demonstrable by radiography. Br J Radiol. 1951;24:107-109.
18. Edelstyn G.A., Gillespie P.J., Grebbell F.S. The radiological demonstration of osseous metastases. Experimental observations. Clin Radiol. 1967;18:158-162.
19. Costelloe C.M., Rohren E.M., Madewell J.E., et al. Imaging bone metastases in breast cancer: techniques and recommendations for diagnosis. Lancet Oncol. 2009;10:606-614.
20. Tehranzadeh J., Mnaymneh W., Ghavam C., et al. Comparison of CT and MR imaging in musculoskeletal neoplasms. J Comput Assist Tomogr. 1989;13:466-472.
21. Zimmer W.D., Berquist T.H., McLeod R.A., et al. Bone tumors: magnetic resonance imaging versus computed tomography. Radiology. 1985;155:709-718.
22. Arano Y. Recent advances in 99mTc radiopharmaceuticals. Ann Nucl Med. 2002;16:79-93.
23. Roodman G.D. Pathogenesis of myeloma bone disease. Blood Cells Mol Dis. 2004;32:290-292.
24. Daffner R.H., Lupetin A.R., Dash N., et al. MRI in the detection of malignant infiltration of bone marrow. AJR Am J Roentgenol. 1986;146:353-358.
25. Roodman G.D. Skeletal imaging and management of bone disease. Hematology Am Soc Hematol Educ Program. 2008:313-319.
26. Corcoran R.J., Thrall J.H., Kyle R.W., et al. Solitary abnormalities in bone scans of patients with extraosseous malignancies. Radiology. 1976;121:663-667.
27. Chao H.S., Chang C.P., Chiu C.H., et al. Bone scan flare phenomenon in non–small-cell lung cancer patients treated with gefitinib. Clin Nucl Med. 2009;34:346-349.
28. Schneider J.A., Divgi C.R., Scott A.M., et al. Flare on bone scintigraphy following Taxol chemotherapy for metastatic breast cancer. J Nucl Med. 1994;35:1748-1752.
29. Sedonja I., Budihna N.V. The benefit of SPECT when added to planar scintigraphy in patients with bone metastases in the spine. Clin Nucl Med. 1999;24:407-413.
30. Han L.J., Au-Yong T.K., Tong W.C., et al. Comparison of bone single-photon emission tomography and planar imaging in the detection of vertebral metastases in patients with back pain. Eur J Nucl Med. 1998;25:635-638.
31. Romer W., Nomayr A., Uder M., et al. SPECT-guided CT for evaluating foci of increased bone metabolism classified as indeterminate on SPECT in cancer patients. J Nucl Med. 2006;47:1102-1106.
32. Avril N. GLUT1 expression in tissue and (18)F-FDG uptake. J Nucl Med. 2004;45:930-932.
33. Mittra E., Quon A. Positron emission tomography/computed tomography: the current technology and applications. Radiol Clin North Am.. 2009;47:147-160.
34. Ohta M., Tokuda Y., Suzuki Y., et al. Whole body PET for the evaluation of bony metastases in patients with breast cancer: comparison with 99mTc-MDP bone scintigraphy. Nucl Med Commun. 2001;22:875-879.
35. Kato H., Miyazaki T., Nakajima M., et al. Comparison between whole-body positron emission tomography and bone scintigraphy in evaluating bony metastases of esophageal carcinomas. Anticancer Res.. 2005;25:4439-4444.
36. Shie P., Cardarelli R., Brandon D., et al. Meta-analysis: comparison of F-18 fluorodeoxyglucose-positron emission tomography and bone scintigraphy in the detection of bone metastases in patients with breast cancer. Clin Nucl Med. 2008;33:97-101.
37. Schirrmeister H., Guhlmann A., Elsner K., et al. Sensitivity in detecting osseous lesions depends on anatomic localization: planar bone scintigraphy versus 18F PET. J Nucl Med. 1999;40:1623-1629.
38. Yang S.N., Liang J.A., Lin F.J., et al. Comparing whole body (18)F-2-deoxyglucose positron emission tomography and technetium-99m methylene diphosphonate bone scan to detect bone metastases in patients with breast cancer. J Cancer Res Clin Oncol. 2002;128:325-328.
39. Uematsu T., Yuen S., Yukisawa S., et al. Comparison of FDG PET and SPECT for detection of bone metastases in breast cancer. AJR Am J Roentgenol. 2005;184:1266-1273.
40. Abe K., Sasaki M., Kuwabara Y., et al. Comparison of 18FDG-PET with 99mTc-HMDP scintigraphy for the detection of bone metastases in patients with breast cancer. Ann Nucl Med. 2005;19:573-579.
41. Du Y., Cullum I., Illidge T.M., Ell P.J. Fusion of metabolic function and morphology: sequential [18F]fluorodeoxyglucose positron-emission tomography/computed tomography studies yield new insights into the natural history of bone metastases in breast cancer. J Clin Oncol. 2007;25:3440-3447.
42. Shreve P.D., Grossman H.B., Gross M.D., Wahl R.L. Metastatic prostate cancer: initial findings of PET with 2-deoxy-2-[F-18]fluoro-D-glucose. Radiology. 1996;199:751-756.
43. Costelloe C.M., Chuang H.H., Madewell J.E., et al. FDG PET for the detection of bone metastases: sensitivity, specificity and comparison to other imaging modalities. PET Clin. 2010;5:281-295.
44. Costelloe C.M., Kumar R., Yasko A.W., et al. Imaging characteristics of locally recurrent tumors of bone. AJR Am J Roentgenol. 2007;188:855-863.
45. Avrahami E., Tadmor R., Dally O., Hadar H. Early MR demonstration of spinal metastases in patients with normal radiographs and CT and radionuclide bone scans. J Comput Assist Tomogr. 1989;13:598-602.
46. Aisen A.M., Martel W., Braunstein E.M., et al. MRI and CT evaluation of primary bone and soft-tissue tumors. AJR Am J Roentgenol. 1986;146:749-756.
47. Sarpel S., Sarpel G., Yu E., et al. Early diagnosis of spinal-epidural metastasis by magnetic resonance imaging. Cancer. 1987;59:1112-1116.
48. Godersky J.C., Smoker W.R., Knutzon R. Use of magnetic resonance imaging in the evaluation of metastatic spinal disease. Neurosurgery. 1987;21:676-680.
49. Ma J., Costelloe C.M., Madewell J.E., et al. Fast dixon-based multisequence and multiplanar MRI for whole-body detection of cancer metastases. J Magn Reson Imaging. 2009;29:1154-1162.
50. Eustace S., Tello R., DeCarvalho V., et al. A comparison of whole-body turboSTIR MR imaging and planar 99mTc-methylene diphosphonate scintigraphy in the examination of patients with suspected skeletal metastases. AJR Am J Roentgenol. 1997;169:1655-1661.
51. Horvath L.J., Burtness B.A., McCarthy S., Johnson K.M. Total-body echo-planar MR imaging in the staging of breast cancer: comparison with conventional methods—early experience. Radiology. 1999;211:119-128.
52. Steinborn M.M., Heuck A.F., Tiling R., et al. Whole-body bone marrow MRI in patients with metastatic disease to the skeletal system. J Comput Assist Tomogr. 1999;23:123-129.
53. Engelhard K., Hollenbach H.P., Wohlfart K., et al. Comparison of whole-body MRI with automatic moving table technique and bone scintigraphy for screening for bone metastases in patients with breast cancer. Eur Radiol. 2004;14:99-105.
54. Lauenstein T.C., Goehde S.C., Herborn C.U., et al. Whole-body MR imaging: evaluation of patients for metastases. Radiology. 2004;233:139-148.
54a. Costelloe C.M., Kundra V., Ma J., et al. Fast dixon whole-body MRI for detecting distant cancer metestasis: A preliminary clinical trial. J Magn Reson Imaging. Epub ahead of print 11 Oct 2011. DOI:10.1002/jmri.22815
54b. Balliu E., Boada M., Pelaez I., et al. Comparative study of whole-body MRI and bone scintigraphy from the detection of bone metastases. Clin Radiol. 2010;65:989-996.
55. Walker R., Kessar P., Blanchard R., et al. Turbo STIR magnetic resonance imaging as a whole-body screening tool for metastases in patients with breast carcinoma: preliminary clinical experience. J Magn Reson Imaging. 2000;11:343-350.
56. Lauenstein T.C., Freudenberg L.S., Goehde S.C., et al. Whole-body MRI using a rolling table platform for the detection of bone metastases. Eur Radiol. 2002;12:2091-2099.
57. Schmidt G.P., Schoenberg S.O., Schmid R., et al. Screening for bone metastases: whole-body MRI using a 32-channel system versus dual-modality PET-CT. Eur Radiol. 2007;17:939-949.
58. Antoch G., Vogt F.M., Freudenberg L.S., et al. Whole-body dual-modality PET/CT and whole-body MRI for tumor staging in oncology. JAMA. 2003;290:3199-3206.
59. Ohno Y., Koyama H., Nogami M., et al. Whole-body MR imaging vs. FDG-PET: comparison of accuracy of M-stage diagnosis for lung cancer patients. J Magn Reson Imaging. 2007;26:498-509.
60. Larkman D.J., Nunes R.G. Parallel magnetic resonance imaging. Phys Med Biol.. 2007;52:R15-55.
61. Schlemmer H.P., Schafer J., Pfannenberg C., et al. Fast whole-body assessment of metastatic disease using a novel magnetic resonance imaging system: initial experiences. Invest Radiol. 2005;40:64-71.
62. Eisenhauer E.A., Therasse P., Bogaerts J., et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247.
63. Amoroso V., Pittiani F., Grisanti S., et al. Osteoblastic flare in a patient with advanced gastric cancer after treatment with pemetrexed and oxaliplatin: implications for response assessment with RECIST criteria. BMC Cancer. 2007;7:94.
64. Hortobagyi G.N., Theriault R.L., Porter L., et al. Efficacy of pamidronate in reducing skeletal complications in patients with breast cancer and lytic bone metastases. Protocol 19 Aredia Breast Cancer Study Group. N Engl J Med. 1996;335:1785-1791.
65. Hortobagyi G.N., Theriault R.L., Lipton A., et al. Long-term prevention of skeletal complications of metastatic breast cancer with pamidronate. Protocol 19 Aredia Breast Cancer Study Group. J Clin Oncol. 1998;16:2038-2044.
66. Theriault R.L., Lipton A., Hortobagyi G.N., et al. Pamidronate reduces skeletal morbidity in women with advanced breast cancer and lytic bone lesions: a randomized, placebo-controlled trial. Protocol 18 Aredia Breast Cancer Study Group. J Clin Oncol. 1999;17:846-854.
67. Rosen L.S., Gordon D., Kaminski M., et al. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: a randomized, double-blind, multicenter, comparative trial. Cancer. 2003;98:1735-1744.
68. Gnant M., Mlineritsch B., Schippinger W., et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med. 2009;360:679-691.
69. Brufsky A., Bundred N., Coleman R., et al. Integrated analysis of zoledronic acid for prevention of aromatase inhibitor-associated bone loss in postmenopausal women with early breast cancer receiving adjuvant letrozole. Oncologist. 2008;13:503-514.
70. Marx R.E. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003;61:1115-1117.
71. Bamias A., Kastritis E., Bamia C., et al. Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: incidence and risk factors. J Clin Oncol. 2005;23:8580-8587.
72. Durie B.G., Katz M., Crowley J. Osteonecrosis of the jaw and bisphosphonates. N Engl J Med. 2005;353:99-102. discussion 102
73. Dimopoulos M.A., Kastritis E., Bamia C., et al. Reduction of osteonecrosis of the jaw (ONJ) after implementation of preventive measures in patients with multiple myeloma treated with zoledronic acid. Ann Oncol. 2009;20:117-120.
74. Saussez S., Javadian R., Hupin C., et al. Bisphosphonate-related osteonecrosis of the jaw and its associated risk factors: a Belgian case series. Laryngoscope. 2009;119:323-329.
75. Farrugia M.C., Summerlin D.J., Krowiak E., et al. Osteonecrosis of the mandible or maxilla associated with the use of new generation bisphosphonates. Laryngoscope. 2006;116:115-120.
76. Hartsell W.F., Scott C.B., Bruner D.W., et al. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J Natl Cancer Inst. 2005;97:798-804.
77. McQuay H.J., Collins S.L., Carroll D., Moore R.A. Radiotherapy for the palliation of painful bone metastases. Cochrane Database Syst Rev.. 2000;2:CD001793.
78. Blitzer P.H. Reanalysis of the RTOG study of the palliation of symptomatic osseous metastasis. Cancer. 1985;55:1468-1472.
79. Patchell R.A., Tibbs P.A., Regine W.F., et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366:643-648.
80. Lam M.G., de Klerk J.M., van Rijk P.P., Zonnenberg B.A. Bone seeking radiopharmaceuticals for palliation of pain in cancer patients with osseous metastases. Anticancer Agents Med Chem.. 2007;7:381-397.
81. Turner J.H., Claringbold P.G. A phase II study of treatment of painful multifocal skeletal metastases with single and repeated dose samarium-153 ethylenediaminetetramethylene phosphonate. Eur J Cancer. 1991;27:1084-1086.
82. Serafini A.N. Therapy of metastatic bone pain. J Nucl Med. 2001;42:895-906.
83. Robinson R.G., Blake G.M., Preston D.F., et al. Strontium-89: treatment results and kinetics in patients with painful metastatic prostate and breast cancer in bone. Radiographics. 1989;9:271-281.
84. Pons F., Herranz R., Garcia A., et al. Strontium-89 for palliation of pain from bone metastases in patients with prostate and breast cancer. Eur J Nucl Med. 1997;24:1210-1214.
85. Alberts A.S., Smit B.J., Louw W.K., et al. Dose response relationship and multiple dose efficacy and toxicity of samarium-153-EDTMP in metastatic cancer to bone. Radiother Oncol. 1997;43:175-179.
86. Aboulafia A.J., Levine A.M., Schmidt D., Aboulafia D. Surgical therapy of bone metastases. Semin Oncol. 2007;34:206-214.
87. Parrish F.F., Murray J.A. Surgical treatment for secondary neoplastic fractures. A retrospective study of ninety-six patients. J Bone Joint Surg Am.. 1970;52:665-686.
88. Beals R.K., Lawton G.D., Snell W.E. Prophylactic internal fixation of the femur in metastatic breast cancer. Cancer. 1971;28:1350-1354.
89. Fidler M. Prophylactic internal fixation of secondary neoplastic deposits in long bones. Br Med J. 1973;1:341-343.
90. Mirels H. Metastatic disease in long bones. A proposed scoring system for diagnosing impending pathologic fractures. Clin Orthop Relat Res.. 1989;249:256-264.
91. Lin P.P., Mirza A.N., Lewis V.O., et al. Patient survival after surgery for osseous metastases from renal cell carcinoma. J Bone Joint Surg Am.. 2007;89:1794-1801.
92. Althausen P., Althausen A., Jennings L.C., Mankin H.J. Prognostic factors and surgical treatment of osseous metastases secondary to renal cell carcinoma. Cancer. 1997;80:1103-1109.
93. Lee B., Franklin I., Lewis J.S., et al. The efficacy of percutaneous vertebroplasty for vertebral metastases associated with solid malignancies. Eur J Cancer. 2009;45:1597-1602.
94. Voggenreiter G. Balloon kyphoplasty is effective in deformity correction of osteoporotic vertebral compression fractures. Spine (Phila Pa 1976). 2005;30:2806-2812.
95. Mendel E., Bourekas E., Gerszten P., Golan J.D. Percutaneous techniques in the treatment of spine tumors: what are the diagnostic and therapeutic indications and outcomes? Spine (Phila Pa 1976). 2009;34:S93-S100.
96. Dupuy D.E., Liu D., Hartfeil D., et al. Percutaneous radiofrequency ablation of painful osseous metastases: a multicenter American College of Radiology Imaging Network trial. Cancer. 2010;116:989-997.
97. Goetz M.P., Callstrom M.R., Charboneau J.W., et al. Percutaneous image-guided radiofrequency ablation of painful metastases involving bone: a multicenter study. J Clin Oncol. 2004;22:300-306.