CHAPTER 271 Bone Metabolism and Osteoporosis and Its Effects on Spinal Disease and Surgical Treatments
Osteoporosis
Osteoporosis is a disease of unbalanced bone metabolism that results in low bone density with subsequently increased bone fragility and propensity for fractures. The World Health Organization (WHO) has defined osteoporosis as bone density that is 2.5 standard deviations (SD) below normal healthy bone. Loss of bone density to between 1 and 2.5 SD below normal has been defined as osteopenia. Osteoporosis is estimated to currently affect 10 million Americans, and an additional 18 million with significantly low bone density are deemed to be at high risk for the development of osteoporosis in their lifetime.1 By the year 2020, it is predicted that 14 million adults older than 50 years will have osteoporosis.2 Osteoporosis is most prevalent in North America and Europe; however, as overall life expectancy increases worldwide, the incidence of osteoporosis will similarly increase. Even though osteoporosis may be considered a normal process of aging, it is by far the most prevalent metabolic bone disease.
The primary concern for patients with osteoporosis is the increased risk for fractures. Osteoporosis-associated fractures most commonly involve the hip, spine, or wrist. More than 1.5 million osteoporotic fractures occur in the United States yearly.3 It is estimated that the annual incidence of hip fractures in the United States will exceed 6.3 million cases by the year 2050.4 Osteoporosis-related fractures can result in significant disability. Only a third of patients regain their premorbid level of function after a hip fracture, and a third require placement in a nursing home within 1 year of the fracture.5–7 Twenty percent of patients are no longer living 1 year after a hip fracture.5,8 Besides functional disability and chronic pain, osteoporotic fractures can result in significant anxiety, depression, emotional distress, decreased quality of life, and impaired social well-being.
The increased risk for vertebral compression fractures (VCFs) in patients with osteoporosis is of particular concern to spine care providers. Approximately half of all osteoporotic fractures are spine related.9 VCFs are responsible for 150,000 hospital admissions, 161,000 doctors visits, and more than 5 million days of restricted activity annually.10 It is estimated that 25% of women older than 50 years will suffer a symptomatic VCF during their lifetime.9 Although many VCFs are essentially asymptomatic or cause limited symptoms, they can also carry significant morbidity, with chronic pain related to the injury developing in up to a third of patients.11 In addition, VCFs can lead to progressive sagittal-plane deformity with concomitant reduced lung capacity. Kyphosis caused by severe or multilevel fractures can also alter the biomechanical stress at other segments and lead to increased risk for additional fractures.12–14 This disease is associated with 23% higher mortality in women older than 65 years than in age-matched controls, with additional fractures contributing to increasing mortality.15
Osteoporosis also places a significant burden on national health care expenditures. The estimated health care cost for osteoporosis-associated fractures was $13.8 billion in 1995 and increased to $17 billion in 2001.2,16 This figure includes hospital and nursing home expenses, but the majority of the cost is for inpatient medical care. The projected national health care expenditure for osteoporosis is predicted to rise to $50 billion by the year 2040.2
Pathophysiology
Pathophysiology of Osteoporosis
The National Institutes of Health Consensus Conference defined osteoporosis as a skeletal disorder characterized by compromised bone strength, as reflected in the integration of bone density and bone quality, that predisposes to an increased risk for fracture.1 Bone density is determined primarily by two factors: one’s peak bone mass and the degree of bone loss throughout one’s lifetime. Bone quality is multifactorial and is dependent on one’s bony architecture, mineralization, ratio of bone formation to resorption, and accumulation of damage. Fractures are defined as mechanical failure as a result of a force or load applied to bone. Pathophysiologically, osteoporosis is a disease of decreased bone mass in the absence of a mineralization defect. Therefore, overall bone mass decreases while the remaining bone maintains normal calcification. Bone loss occurs when the rate of bone resorption is greater than that of new bone formation. With aging, osteoclastic resorption exceeds osteoblastic activity. The net effect is progressive loss of skeletal bone mass, which generally begins by the fourth decade.
Ethnicity appears to be related to risk for the development of osteoporosis. White women have the greatest risk for osteoporosis, and this segment of the population also has a greater risk for vertebral and nonvertebral fractures than African American, Native American, and Asian women.17,18 African American women have higher overall bone mineral density (BMD) than white women throughout their lifetime. This discrepancy is reflected in a lower lifetime risk for hip fracture in African American women than in white women (6% for African American women versus 14% for white women).1
Clinical Findings and Diagnosis
Bone Mineral Density
A patient’s BMD is compared with a Z score (BMD for healthy gender- and age-matched controls) and a T score (BMD for normal healthy young controls at peak bone mass). The WHO defines a BMD of less than 1 SD below the T score as being within normal limits (osteopenia). Osteoporosis is defined as a BMD greater than 2.5 SD below the T score. Severe osteoporosis is a BMD that is greater than 2.5 SD below the T score with at least one osteoporosis-related fracture. Based on the WHO classification, it is estimated that 94% of women older than 75 years meet the criteria for osteopenia, with 38% of women in this age group having osteoporosis.19 BMD values also correlate with fracture risk. Each 1-SD decrease in age-adjusted BMD measurement equates to a 1.5-fold increase in fracture risk.
Biochemical Markers
Biochemical markers are primarily useful for determining risk for the development of osteoporosis and for assessing responsiveness to therapy. Serum markers are generally less variable than urinary markers, which need to be corrected based on creatinine clearance. Studies have demonstrated that levels of bone-specific alkaline phosphatase, osteocalcin, and NTx are higher in postmenopausal women than in premenopausal women.20 With bisphosphonate alendronate therapy, however, alkaline phosphatase, osteocalcin, and CTx levels have been shown to decrease 40% to 50% over a period of 6 to 12 months.21 NTx was the most responsive marker for measuring therapeutic response. Additional studies have suggested that NTx and CTx correlate significantly with BMD and risk for fracture.20
Conservative and Medical Management
Preventive measures remain among the most important and effective strategies for managing osteoporosis. Adequate nutrition with an appropriate balance of calcium and vitamin D is essential for optimizing bone quality. Calcium supplementation in the form of calcium carbonate or calcium citrate is primarily effective for postmenopausal women. Vitamin D supplementation is also beneficial, with one study demonstrating that 1200 mg of calcium and 600 to 800 IU of vitamin D result in a 40% decrease in hip fractures and a 16% decrease in mortality.22 Regular weight-bearing, impact exercise increases peak bone mass and thereby reduces the risk for osteoporosis. Wolff’s law states that bone forms by appositional growth in areas of increased stress. With impact loading, differences in electronegative potential occur across compressed surfaces, which subsequently stimulates bone formation. Subjects randomized to aerobics and weight-training programs demonstrate a 5.2% increase in spine density over subjects treated only with calcium supplementation.23 Strength training also increases bone density in both the spine and hips, whereas immobilization decreases overall bone mass. An active exercise program of jogging and stair climbing in postmenopausal women receiving calcium supplementation resulted in a 5.2% increase in BMD at 9 months.23 The control group of patients treated with just calcium supplementation experienced a 1.4% loss in BMD. The average BMD in smokers is 1% to 3% lower than that in nonsmokers, with the number of pack-years being inversely correlated with BMD. Although the mechanism is unclear, tobacco use may alter the local acidic environment to facilitate osteoclastic breakdown of hydroxyapatite. Chronic alcohol use also results in increased bone loss. Low vitamin D from malnutrition and decreased activation as a result of chronic liver disease may explain the effect of long-standing alcohol consumption on bone loss.
Bisphosphonates are the first-line pharmacologic agent for osteoporosis. They are analogues of pyrophosphate and function to inhibit osteoclastic activity. Early-generation bisphosphonates, such as etidronate and clodronate, are nonselective and inhibit both bone formation and resorption equally. Second-generation drugs (pamidronate, alendronate) have more selective antiresorptive activity and demonstrate a 50% reduction in spinal and hip fractures.24 Alendronate therapy is associated with a 5% to 7% increase in spinal bone mass at 2 years. Risedronate and zoledronate are third-generation bisphosphonates that preferentially function at sites of active bone resorption.
Estrogen hormonal replacement therapy (HRT) significantly increases BMD in postmenopausal women. However, estrogen HRT is associated with a significant risk for breast cancer, stroke, and deep venous thrombosis, which was found to outweigh its benefit in the treatment of osteoporosis. Estrogen-alone HRT (without progestin) is also known to increase the incidence of endometrial cancer. Selective estrogen receptor modulators (SERMs) are agents that preserve the beneficial effects of estrogen on bone metabolism while having antiestrogenic effects on breast and endometrial tissue. Raloxifene, a SERM, significantly decreases bone resorption. In particular, raloxifene in combination with the bisphosphonate alendronate has proved to be more effective in improving lumbar BMD than either agent alone.25
Surgical Treatment of Osteoporotic Vertebral Compression Fractures
Clinical studies of both vertebroplasty and kyphoplasty demonstrate significant beneficial results. Vertebroplasty is associated with a 70% to 90% success rate in relieving pain.26–29 Complications from vertebroplasty are reported in less than 10% of patients and include increased pain, radiculopathy, spinal cord compression, pulmonary embolism, infection, and rib fracture. The risk for extravasation of cement after vertebroplasty is widely variable and estimated to be 30% to 67%.30–33 Kyphoplasty demonstrates similar positive results, with rates of pain relief ranging from 88% to 100%.28,34–37 In an ongoing multicenter study from 1998 to 2000, 603 VCFs in 304 patients were treated by kyphoplasty.28 At an average 18 months’ follow-up, 90% of the patients had symptomatic and functional improvement.
Kyphoplasty is primarily differentiated from vertebroplasty in its potential for restoring vertebral body height and improving overall sagittal alignment. The average restoration of vertebral height with kyphoplasty is reported to be 30% to 35%.36,38 In the aforementioned multicenter study of 603 VCFs treated by kyphoplasty, of fractures with a 15% or greater estimated loss of vertebral height, treatment improved vertebral body height from an average of 68% of predicted height to 84% of predicted height after treatment.28 Other studies counter that although kyphoplasty may improve local kyphosis, the overall effect on global sagittal alignment is minimal. In a retrospective study of 65 patients undergoing one- to three-level kyphoplasty, Pradhan and colleagues found that kyphoplasty improved the local deformity at the fracture level by an average of 7.3 degrees, or 63% of the preoperative kyphosis.39 They found, however, that the overall angular correction decreased to 2.4 degrees, or 20% of the preoperative kyphosis, when measured one level above and below the fractured level. At three levels above and below the fracture level, the correction decreased to just 1.0 degrees, or 8% of the preoperative kyphosis. The researchers surmised that the majority of the local angular and height correction becomes negated at more distant levels by the relatively softer intervertebral disks. As a result, the radiographic improvement in overall sagittal alignment with kyphoplasty is modest at best. A meta-analysis of the literature revealed that both kyphoplasty and vertebroplasty result in a significant improvement in visual analog pain scores, with an average decrease of 5.68 for vertebroplasty and 4.60 for kyphoplasty.40 The risk for extravasation of cement with vertebroplasty was 19.7% but just 7.0% with kyphoplasty. The significant difference in risk for leakage of cement is hypothesized to be due to the injection of higher viscosity cement into the preformed cavity with kyphoplasty.
Patients with osteoporosis-related VCFs are at risk for the development of additional fractures at other spinal levels. Without surgical intervention, additional VCFs will develop in 19.2% of patients within 1 year.41,42 Because both vertebroplasty and kyphoplasty involve relatively rigid cement, the altered biomechanics of the augmented vertebra may create greater stress at weakened adjacent segments. Studies have demonstrated that the relative risk for the development of additional VCFs at a level adjacent to a previous vertebroplasty is 4.62 times greater than that at nonadjacent levels.43 In a meta-analysis of the literature on vertebroplasty and kyphoplasty, the risk for fracture at an adjacent level was 17.9% after vertebroplasty and 14.1% after kyphoplasty.40 Although both vertebroplasty and kyphoplasty result in improved clinical outcomes for patients with chronically painful VCFs, the future of vertebral augmentation will probably involve the use of osteobiologic agents and biocompatible cement.
Osteoporosis and Implications for Spinal Instrumentation
With increases in life expectancy, more patients of advanced age are undergoing spine surgery for a variety of indications, including degenerative, deforming, traumatic, rheumatologic, infectious, and oncologic disease. Spine surgery in this population frequently involves instrumented stabilization and reconstruction. However, the use of rigid spinal fixation in the setting of osteoporosis can pose significant technical challenges.44
Biomechanical studies have demonstrated that screw pullout strength is directly related to BMD.45–47 As BMD decreases, so does the force required for axial pullout of pedicle screws. Pedicle screws have been compared with other spinal implants such as laminar hooks and sublaminar wires in the setting of low BMD. Coe and coworkers found that laminar hooks demonstrated better resistance to failure with a posteriorly directed force than did either pedicle screws or wiring. Unlike pedicle screws or wires, the load to failure for laminar hooks did not correlate with the measured BMD.48 They observed that pedicle screws failed by stripping the cancellous bone within the pedicle track, consistent with the predominant effect of osteoporosis on cancellous bone. Hooks, however, required failure of the inner cortical bone of the lamina, which is relatively spared by osteoporosis.
Strategies for Spinal Fixation in Osteoporosis
Screw Placement
Several techniques can be used to reduce the risk for screw failure in patients with osteoporotic bone. Longer screws achieve better fixation in low-density bone than shorter screws do. Hackenberg and associates demonstrated that a screw length of 50 mm had significantly greater pullout strength than did 35-mm screws.45 Similarly, increasing the screw diameter also improves resistance to failure.47 However, the benefit of a larger screw diameter in settings of low BMD was observed only in patients with milder osteoporosis. In severe osteoporosis, the pullout force was low regardless of screw diameter. Pullout resistance for pedicle screws relies largely on the volume of cancellous bone engaged between the threads of the screw. With osteoporosis, however, the cancellous bone is less dense, thereby providing suboptimal fixation. Placing pedicle screws so that they achieve purchase in cortical bone increases their resistance to failure. Bicortical pedicle screws provide up to an additional 30% pullout strength in comparison to unicortical screws by engaging the ventral cortex of the vertebral body.49
Triangulation of bilateral pedicle screws is another technique for increasing the load to failure.50,51 Ruland and colleagues found that triangulated screws connected by a transverse plate provide better pullout resistance than do laminar hooks or a single pedicle screw.51 With parallel screw placement, the resistance to pullout for each screw is dependent on the volume of cancellous bone between the threads of the screw. With screw triangulation and a transverse connector, the pullout strength of the construct is contributed by the volume of bone within the trapezoid area in the vertebral body formed by the triangulated screws. With a larger volume of cancellous bone available for resistance to pullout, triangulated screws provide up to twice the pullout strength of a single pedicle screw.
Appropriate pilot hole preparation before screw placement is another technique for improving screw purchase. Tapping or drilling the pilot hole results in removal of bone within the pedicle track. Even screw insertion, removal, and subsequent reinsertion of the same screw decrease the mechanical insertion torque by more than 34%.52 Carmouche and coworkers found that tapping the pilot hole with the same-diameter drill bit as the screw significantly decreases pullout resistance in comparison to not tapping or undertapping.53 They observed no significant difference in pullout resistance between undertapping and not tapping the pilot hole. When compared with same-size tapping, undersizing the tap by 0.5 and 1.0 mm increases insertional torque by 47% and 93%, respectively.54 Interestingly, undertapping demonstrates less benefit in the thoracic region than in the lumbar spine.53 The authors attributed this observation to the less overall available cancellous bone within the smaller thoracic pedicle in the setting of osteoporosis. Therefore, the size of the tap had little effect, and they surmised that thoracic pedicle screws are probably more dependent on cortical purchase with the pedicle walls.
Screw Augmentation with Hooks
Augmentation of screw-rod constructs with hooks combines the optimal three-dimensional control of pedicle screws with the improved pullout resistance of hooks. In a study of construct stiffness obtained from load deformation curves, pedicle screws with a laminar hook demonstrated significantly more stiffness than a pedicle screw alone.55 Halvorson and colleagues found that a pedicle screw with offset hooks at two adjacent levels improves fixation and doubles the expected pullout force.46 A hook at the inferior laminar edge of the same level and a second hook one or two levels above, when connected to a rod, function as a three-point load-sharing device, even as the screw begins to displace. This particular construct in osteoporotic bone results in resistance to failure that is equal to or greater than that of a single pedicle screw in normal bone. A pedicle screw with a single laminar hook at the same level, however, does not increase the load to failure as compared with the expected value. They observed that as the screw began to displace, the hook functions as a pivot and fails to carry any load.
Screw Augmentation with Cement
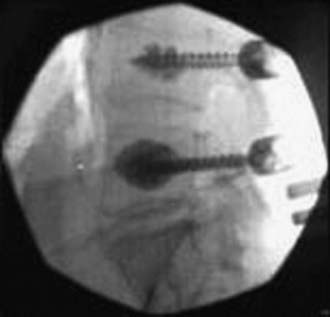
Cement augmentation of the screw track into the pedicle and vertebral body additionally increases fixation in osteoporotic bone (Fig. 271-1). Zindrick and coworkers demonstrated that even stripped pedicle screws regain within 5% of their baseline pullout strength in cadavers when PMMA is injected into the screw track.49 Sarzier and associates observed that PMMA injection resulted in increased pullout resistance in osteoporotic cadavers with a BMD at least 2 SD below normal.56 The increase in cancellous bone density with PMMA often led to an increase in pullout strength that exceeded the strength of the cortical bone. As a result, loading to failure caused a fracture of the vertebral body at the pedicle-body junction.
Screw augmentation with cement carries the risk of extravertebral or intracanal leakage of cement. The injection needle is generally inserted to a depth that is at least 5 mm less than the intended screw length so that accidental violation of the ventral vertebral cortex is avoided. Higher viscosity cement is used to reduce the likelihood of cement leakage. One to 3 mL of cement is generally recommended, with an increase in cement injection failing to demonstrate any significant benefit in pullout strength.57 Large-volume cement injection is avoided to prevent excess extravasation of cement.
Burval and coworkers found that performing a kyphoplasty technique with an inflatable bone tamp before cement augmentation further improves pullout resistance.58 Similar to conventional kyphoplasty for osteoporotic vertebral fractures, an inflatable bone tamp is used to create a cavity within the vertebral body. High-viscosity cement is injected into the cavity, followed by the insertion of pedicle screws. Pedicle screws with kyphoplasty cement augmentation demonstrate almost twice the pullout strength of screws augmented with standard cement injection and 255% better fixation than noted with unaugmented screws.
Fenestrated taps and screws facilitate cement injection while reducing the risk for retrograde migration of cement out of the pedicle track. Frankel and colleagues found that injecting PMMA through a fenestrated tap before screw placement increased pullout strength by 119% in primary procedures and by 162% in salvage procedures.57 McKoy and An contend that PMMA functions as primarily a bone void filler and not as a true adhesive.59 Therefore, injecting cement after preparation of the pilot hole only fills the void of the pedicle track. Subsequent insertion of a pedicle screw into the doughy curing cement simply coats the screw threads, thereby effectively reducing screw purchase. Alternatively, injecting cement through a cannulated fenestrated screw after insertion of the screw allows the cement to infiltrate the bone and ensures that the cement remains within the vertebral body.
Osteobiologic cement is an area of interest and development for screw augmentation. PMMA is brittle, toxic, permanent, and difficult to remove in situations requiring revision surgery. In addition, PMMA exhibits a high exothermic polymerization temperature that can induce thermal injury in surrounding structures. Alternatively, calcium phosphate is an osteobiologic agent that is bioresorbable and therefore becomes integrated in the natural process of bony remodeling. Renner and associates demonstrated that injection of 3 mL of calcium phosphate significantly improves pedicle screw pullout strength in comparison to unaugmented screws.60 In a revision model, screw augmentation with calcium phosphate resulted in pullout strength that was similar to that of unaugmented screws placed for the first time. In comparing PMMA and calcium phosphate for screw augmentation, Rohmiller and colleagues observed that calcium phosphate augmentation results in a 167% improvement in screw pullout strength in comparison to unaugmented screws.61 PMMA demonstrated a 199% increase in pullout strength over unaugmented screws; however, this difference was not statistically significant when compared with calcium phosphate augmentation.
Additional Techniques: Multiple Points of Fixation, Appropriate Release, Anterior Reconstruction
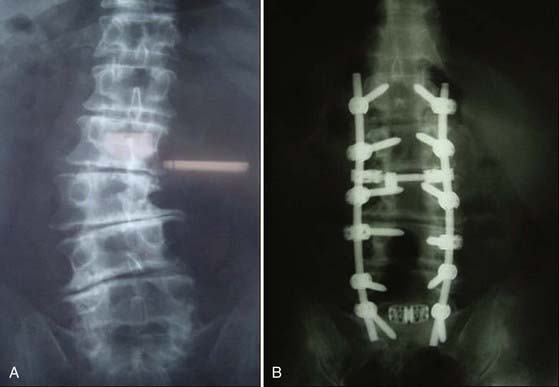
Several strategies exist for decreasing the risk for screw failure when using pedicle screws in osteoporotic bone and conditions of increased stress. Extending the number of segments included in the screw-rod construct distributes the loading forces across multiple fixation points (Fig. 271-2). Increasing the number of points of fixation decreases the stress applied to each individual screw and consequently minimizes the risk for pullout failure at each segment.62 This principle is relevant when performing procedures to correct deformity, such as rod derotation maneuvers. With rod derotation, forces are applied directly to the screws by the rotating bent rod to achieve spinal correction. Particularly in situations of anterior column failure such as a vertebral body fracture, pedicle screws are exposed to large cantilever bending loads, which may result in screw breakage or pullout at the distal ends of the construct.62
Anterior column support also decreases the biomechanical loading of pedicle screws in weakened bone. With reconstruction of the anterior column, the load is shared by the graft or cage and less stress is directed toward the pedicle screw–rod construct. However, in osteoporosis, subsidence of the graft or cage into weakened vertebral end plates can lead to collapse of the anterior column, kyphosis, and deformity. Particularly in osteoporotic bone, cage placement should ideally contact the peripheral apophyseal ring, where the stronger cortical bone is more supportive of compressive loads than the weaker central portion. Increasing the diameter of the cage and ensuring at least 30% coverage of the vertebral body maximize the cage-bone contact area and optimize anterior column support.63,64
Novel Screw Designs: Expandable Screws, Hollow Monaxial Screws
McKoy and An studied an expandable screw in cadaveric mechanical testing.65 The screw design involved a cannulated screw that is placed transpedicularly into the vertebral body. Placement of a smaller inner screw down the cannulated center causes flanges at the distal part of the screw to flare outward and expand within the vertebral body. The authors found that the expandable screw resulted in a 76% increase in holding strength in comparison to conventional pedicle screws. Other studies have demonstrated that expandable pedicle screws markedly improve pullout strength up to 50% in low-BMD bone versus standard pedicle screws.66 However, in patients with severely low BMD, expandable screws are unable to overcome the extreme biomechanical disadvantage and result in failure. Augmentation of expandable screws with PMMA injected through the cannulated portion of the screw increases mean axial pullout resistance by 200% in severely osteoporotic bone.67 Screw revision, however, remains an issue in the clinical application of both expandable and injectable screws.
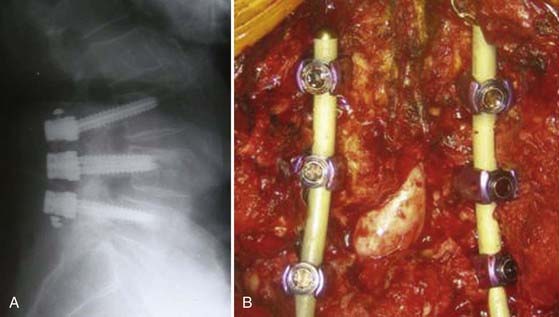
Anterior thoracolumbar screw designs have been explored to improve fixation in osteoporotic bone. Continuous cyclic loading of anterior screw constructs in porous, brittle bone can lead to screw cutout. Novel screw designs incorporate increased surface area for the screw-bone interface to improve the load-bearing cross-sectional area. Hollow monaxial screws are designed either with a cylindrical spiral blade or as a hollow-perforated cylinder (Fig. 271-3). This allows increased screw-bone contact, as well as promotes ingrowth of bone within the screw. Various hollow monaxial screws have demonstrated promising results in comparison to conventional screws in biomechanical testing on low-BMD specimens.68–70
Semirigid Fixation
Implants composed of stainless steel and titanium result in a supraphysiologic degree of stiffness.71 As the spine is loaded in various axes of motion, the rigid instrumentation restricts mobility at the instrumented levels and creates increased stress at the screw-bone interface. The lack of flexibility with nonmalleable instrumentation can also accelerate degeneration at adjacent motion segments, shield bone grafts from stress, and therefore prevent arthrodesis.
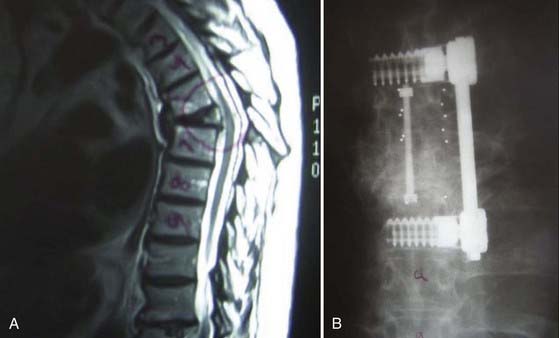
Semirigid fixation allows spinal stabilization while permitting some degree of flexibility. Polyetheretherketone (PEEK) has a modulus of elasticity between that of cortical and cancellous bone, thus theoretically mimicking the modulus of elasticity of the native environment. With PEEK rod instrumentation, some degree of motion is permitted while resisting marked flexion, extension, axial rotation, or lateral bending. As a result, semirigid fixation may provide sufficient stabilization to facilitate bony fusion while permitting enough flexibility to offload stress at the screw-bone interface or adjacent segments (Fig. 271-4).
Osteobiologic Agents
BMPs are native growth factors involved in the recruitment and induction of bone-forming precursors to facilitate arthrodesis. Recombinant BMPs are now commercially available (InFuse, Medtronic Sofamor Danek, Memphis, TN, and osteogenic protein-1 [OP-1], Stryker Biotech, Hopkinton, MA) and have been demonstrated in laboratory and clinical studies to result in successful spinal fusion. In an ovariectomized rat model of osteoporosis and posterolateral spinal fusion, BMP-7 with a collagen composite carrier led to successful arthrodesis in comparison to controls.72 In an ovariectomized sheep model of osteoporosis, injection of BMP-7 into osteopenic vertebral bodies resulted in histologic evidence of increased bone, as well as improved mechanical stiffness.73 Although the use of BMP is not currently approved by the FDA as a primary osteobiologic agent for promoting early fusion in the setting of osteoporosis, growth factors such as BMP may present a potential avenue for enhancing fusion in patients with poor bone quality.
Burval DJ, McLain RF, Milks R, et al. Primary pedicle screw augmentation in osteoporotic lumbar vertebrae: biomechanical analysis of pedicle fixation strength. Spine. 2007;32:1077-1083.
Coe JD, Warden KE, Herzig MA, et al. Influence of bone mineral density on the fixation of thoracolumbar implants. A comparative study of transpedicular screws, laminar hooks, and spinous process wires. Spine. 1990;15:902-907.
Cook SD, Salkeld SL, Stanley T, et al. Biomechanical study of pedicle screw fixation in severely osteoporotic bone. Spine J. 2004;4:402-408.
Cooper C, Atkinson EJ, O’Fallon WM, et al. Incidence of clinically diagnosed vertebral fractures: a population-based study in Rochester, Minnesota, 1985-1989. J Bone Miner Res. 1992;7:221-227.
Cortet B, Cotten A, Boutry N, et al. Percutaneous vertebroplasty in the treatment of osteoporotic vertebral compression fractures: an open prospective study. J Rheumatol. 1999;26:2222-2228.
Cummings SR, Melton LJ3rd. Epidemiology and outcomes of osteoporotic fractures. Lancet. 2002;359:1761-1767.
Eck JC, Nachtigall D, Humphreys SC, et al. Comparison of vertebroplasty and balloon kyphoplasty for treatment of vertebral compression fractures: a meta-analysis of the literature. Spine J. 2008;8:488-497.
Eyre DR. Bone biomarkers as tools in osteoporosis management. Spine. 1997;22:17S-24S.
Frankel BM, D’Agostino S, Wang C. A biomechanical cadaveric analysis of polymethylmethacrylate-augmented pedicle screw fixation. J Neurosurg Spine. 2007;7:47-53.
Fribourg et al Fribourg D, Tang C, Sra P, et al. Incidence of subsequent vertebral fracture after kyphoplasty. Spine. 2004;29:2270-2276.
Garfin SR, Yuan HA, Reiley MA. New technologies in spine: kyphoplasty and vertebroplasty for the treatment of painful osteoporotic compression fractures. Spine. 2001;26:1511-1515.
Goldhahn J, Reinhold M, Stauber M, et al. Improved anchorage in osteoporotic vertebrae with new implant designs. J Orthop Res. 2006;24:917-925.
Hasegawa K, Takahashi HE, Uchiyama S, et al. An experimental study of a combination method using a pedicle screw and laminar hook for the osteoporotic spine. Spine. 1997;22:958-962.
Kado DM, Browner WS, Palermo L, et al. Vertebral fractures and mortality in older women: a prospective study. Study of Osteoporotic Fractures Research Group. Arch Intern Med. 1999;159:1215-1220.
Lane JM. Osteoporosis: medical prevention and treatment. Spine. 1997;22:32S-37S.
Majd ME, Farley S, Holt RT. Preliminary outcomes and efficacy of the first 360 consecutive kyphoplasties for the treatment of painful osteoporotic vertebral compression fractures. Spine J. 2005;5:244-255.
McLain RF. The biomechanics of long versus short fixation for thoracolumbar spine fractures. Spine. 2006;31:S70-S79.
, 2001 NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785-795.
Sarzier JS, Evans AJ, Cahill DW. Increased pedicle screw pullout strength with vertebroplasty augmentation in osteoporotic spines. J Neurosurg. 2002;96:309-312.
Zindrick MR, Wiltse LL, Widell EH, et al. A biomechanical study of intrapeduncular screw fixation in the lumbosacral spine. Clin Orthop Relat Res. 1986;203:99-112.
1 NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785-795.
2 Lane NE. Epidemiology, etiology, and diagnosis of osteoporosis. Am J Obstet Gynecol. 2006;194:S3-11.
3 Gill SS, Einhorn TA. Osteoporosis and bone physiology. In: Herkowitz HN, Garfin SR, Eismont FJ, et al, editors. Rothman-Simeone: The Spine. Philadelphia: WB Saunders; 2006:108-121.
4 Cummings SR, Melton LJ3rd. Epidemiology and outcomes of osteoporotic fractures. Lancet. 2002;359:1761-1767.
5 Leibson CL, Tosteson AN, Gabriel SE, et al. Mortality, disability, and nursing home use for persons with and without hip fracture: a population-based study. J Am Geriatr Soc. 2002;50:1644-1650.
6 Magaziner J, Simonsick EM, Kashner TM, et al. Predictors of functional recovery one year following hospital discharge for hip fracture: a prospective study. J Gerontol. 1990;45:M101-M107.
7 Riggs BL, Melton LJ3rd. The worldwide problem of osteoporosis: insights afforded by epidemiology. Bone. 1995;17:505S-511S.
8 Vestergaard P, Rejnmark L, Mosekilde L. Increased mortality in patients with a hip fracture—effect of pre-morbid conditions and post-fracture complications. Osteoporos Int. 2007;18:1583-1593.
9 Cooper C, Atkinson EJ, O’Fallon WM, et al. Incidence of clinically diagnosed vertebral fractures: a population-based study in Rochester, Minnesota, 1985-1989. J Bone Miner Res. 1992;7:221-227.
10 Mathis JM, Barr JD, Belkoff SM, et al. Percutaneous vertebroplasty: a developing standard of care for vertebral compression fractures. AJNR Am J Neuroradiol. 2001;22:373-381.
11 Mazanec DJ, Podichetty VK, Mompoint A, et al. Vertebral compression fractures: manage aggressively to prevent sequelae. Cleve Clin J Med. 2003;70:147-156.
12 Heaney RP. The natural history of vertebral osteoporosis. Is low bone mass an epiphenomenon? Bone. 1992;13(suppl 2):S23-S26.
13 Leech JA, Dulberg C, Kellie S, et al. Relationship of lung function to severity of osteoporosis in women. Am Rev Respir Dis. 1990;141:68-71.
14 Schlaich C, Minne HW, Bruckner T, et al. Reduced pulmonary function in patients with spinal osteoporotic fractures. Osteoporos Int. 1998;8:261-267.
15 Kado DM, Browner WS, Palermo L, et al. Vertebral fractures and mortality in older women: a prospective study. Study of Osteoporotic Fractures Research Group. Arch Intern Med. 1999;159:1215-1220.
16 Ray NF, Chan JK, Thamer M, et al. Medical expenditures for the treatment of osteoporotic fractures in the United States in 1995: report from the National Osteoporosis Foundation. J Bone Miner Res. 1997;12:24-35.
17 Barrett-Connor E, Siris ES, Wehren LE, et al. Osteoporosis and fracture risk in women of different ethnic groups. J Bone Miner Res. 2005;20:185-194.
18 Cauley JA, Lui LY, Ensrud KE, et al. Bone mineral density and the risk of incident nonspinal fractures in black and white women. JAMA. 2005;293:2102-2108.
19 Glaser DL, Kaplan FS. Osteoporosis: definition and clinical presentation. Spine. 1997;22:12S-16S.
20 Eyre DR. Bone biomarkers as tools in osteoporosis management. Spine. 1997;22:17S-24S.
21 Garnero P, Shih WJ, Gineyts E, et al. Comparison of new biochemical markers of bone turnover in late postmenopausal osteoporotic women in response to alendronate treatment. J Clin Endocrinol Metab. 1994;79:1693-1700.
22 Chapuy MC, Arlot ME, Duboeuf F, et al. Vitamin D3 and calcium to prevent hip fractures in the elderly woman. N Engl J Med. 1992;327:1637-1642.
23 Dalsky GP, Stocke KS, Ehsani AA, et al. Weight-bearing exercise training and lumbar bone mineral content in postmenopausal women. Ann Intern Med. 1988;108:824-828.
24 Lane JM. Osteoporosis: medical prevention and treatment. Spine. 1997;22:32S-37S.
25 Johnell O, Scheele WH, Lu Y, et al. Additive effects of raloxifene and alendronate on bone density and biochemical markers of bone remodeling in postmenopausal women with osteoporosis. J Clin Endocrinol Metab. 2002;87:985-992.
26 Cortet B, Cotten A, Boutry N, et al. Percutaneous vertebroplasty in the treatment of osteoporotic vertebral compression fractures: an open prospective study. J Rheumatol. 1999;26:2222-2228.
27 Cyteval C, Sarrabere MP, Roux JO, et al. Acute osteoporotic vertebral collapse: open study on percutaneous injection of acrylic surgical cement in 20 patients. AJR Am J Roentgenol. 1999;173:1685-1690.
28 Garfin SR, Yuan HA, Reiley MA. New technologies in spine: kyphoplasty and vertebroplasty for the treatment of painful osteoporotic compression fractures. Spine. 2001;26:1511-1515.
29 Heini PF, Walchli B, Berlemann U. Percutaneous transpedicular vertebroplasty with PMMA: operative technique and early results. A prospective study for the treatment of osteoporotic compression fractures. Eur Spine J. 2000;9:445-450.
30 Chiras J, Depriester C, Weill A, et al. [Percutaneous vertebral surgery. Technics and indications.]. J Neuroradiol. 1997;24:45-59.
31 Cotten A, Dewatre F, Cortet B, et al. Percutaneous vertebroplasty for osteolytic metastases and myeloma: effects of the percentage of lesion filling and the leakage of methyl methacrylate at clinical follow-up. Radiology. 1996;200:525-530.
32 Deramond H, Depriester C, Galibert P, et al. Percutaneous vertebroplasty with polymethylmethacrylate. Technique, indications, and results. Radiol Clin North Am. 1998;36:533-546.
33 Weill A, Chiras J, Simon JM, et al. Spinal metastases: indications for and results of percutaneous injection of acrylic surgical cement. Radiology. 1996;199:241-247.
34 Lane JM, Johnson CE, Khan SN, et al. Minimally invasive options for the treatment of osteoporotic vertebral compression fractures. Orthop Clin North Am. 2002;33:431-438. viii
35 Ledlie JT, Renfro M. Balloon kyphoplasty: one-year outcomes in vertebral body height restoration, chronic pain, and activity levels. J Neurosurg. 2003;98:36-42.
36 Lieberman IH, Dudeney S, Reinhardt MK, et al. Initial outcome and efficacy of “kyphoplasty” in the treatment of painful osteoporotic vertebral compression fractures. Spine. 2001;26:1631-1638.
37 Theodorou DJ, Theodorou SJ, Duncan TD, et al. Percutaneous balloon kyphoplasty for the correction of spinal deformity in painful vertebral body compression fractures. Clin Imaging. 2002;26:1-5.
38 Majd ME, Farley S, Holt RT. Preliminary outcomes and efficacy of the first 360 consecutive kyphoplasties for the treatment of painful osteoporotic vertebral compression fractures. Spine J. 2005;5:244-255.
39 Pradhan BB, Bae HW, Kropf MA, et al. Kyphoplasty reduction of osteoporotic vertebral compression fractures: correction of local kyphosis versus overall sagittal alignment. Spine. 2006;31:435-441.
40 Eck JC, Nachtigall D, Humphreys SC, et al. Comparison of vertebroplasty and balloon kyphoplasty for treatment of vertebral compression fractures: a meta-analysis of the literature. Spine J. 2008;8:488-497.
41 Fribourg D, Tang C, Sra P, et al. Incidence of subsequent vertebral fracture after kyphoplasty. Spine. 2004;29:2270-2276.
42 Lindsay R, Silverman SL, Cooper C, et al. Risk of new vertebral fracture in the year following a fracture. JAMA. 2001;285:320-323.
43 Trout AT, Kallmes DF, Kaufmann TJ. New fractures after vertebroplasty: adjacent fractures occur significantly sooner. AJNR Am J Neuroradiol. 2006;27:217-223.
44 Skinner R, Maybee J, Transfeldt E, et al. Experimental pullout testing and comparison of variables in transpedicular screw fixation. A biomechanical study. Spine. 1990;15:195-201.
45 Hackenberg L, Link T, Liljenqvist U. Axial and tangential fixation strength of pedicle screws versus hooks in the thoracic spine in relation to bone mineral density. Spine. 2002;27:937-942.
46 Halvorson TL, Kelley LA, Thomas KA, et al. Effects of bone mineral density on pedicle screw fixation. Spine. 1994;19:2415-2420.
47 Soshi S, Shiba R, Kondo H, et al. An experimental study on transpedicular screw fixation in relation to osteoporosis of the lumbar spine. Spine. 1991;16:1335-1341.
48 Coe JD, Warden KE, Herzig MA, et al. Influence of bone mineral density on the fixation of thoracolumbar implants. A comparative study of transpedicular screws, laminar hooks, and spinous process wires. Spine. 1990;15:902-907.
49 Zindrick MR, Wiltse LL, Widell EH, et al. A biomechanical study of intrapeduncular screw fixation in the lumbosacral spine. Clin Orthop Relat Res. 1986;203:99-112.
50 Hadjipavlou AG, Nicodemus CL, al-Hamdan FA, et al. Correlation of bone equivalent mineral density to pull-out resistance of triangulated pedicle screw construct. J Spinal Disord. 1997;10:12-19.
51 Ruland CM, McAfee PC, Warden KE, et al. Triangulation of pedicular instrumentation. A biomechanical analysis. Spine. 1991;16:S270-S276.
52 Polly DWJr, Orchowski JR, Ellenbogen RG. Revision pedicle screws. Bigger, longer shims—what is best? Spine. 1998;23:1374-1379.
53 Carmouche JJ, Molinari RW, Gerlinger T, et al. Effects of pilot hole preparation technique on pedicle screw fixation in different regions of the osteoporotic thoracic and lumbar spine. J Neurosurg Spine. 2005;3:364-370.
54 Kuklo TR, Lehman RAJr. Effect of various tapping diameters on insertion of thoracic pedicle screws: a biomechanical analysis. Spine. 2003;28:2066-2071.
55 Hasegawa K, Takahashi HE, Uchiyama S, et al. An experimental study of a combination method using a pedicle screw and laminar hook for the osteoporotic spine. Spine. 1997;22:958-962.
56 Sarzier JS, Evans AJ, Cahill DW. Increased pedicle screw pullout strength with vertebroplasty augmentation in osteoporotic spines. J Neurosurg. 2002;96:309-312.
57 Frankel BM, D’Agostino S, Wang C. A biomechanical cadaveric analysis of polymethylmethacrylate-augmented pedicle screw fixation. J Neurosurg Spine. 2007;7:47-53.
58 Burval DJ, McLain RF, Milks R, et al. Primary pedicle screw augmentation in osteoporotic lumbar vertebrae: biomechanical analysis of pedicle fixation strength. Spine. 2007;32:1077-1083.
59 McKoy BE, An YH. An injectable cementing screw for fixation in osteoporotic bone. J Biomed Mater Res. 2000;53:216-220.
60 Renner SM, Lim TH, Kim WJ, et al. Augmentation of pedicle screw fixation strength using an injectable calcium phosphate cement as a function of injection timing and method. Spine. 2004;29:E212-216.
61 Rohmiller MT, Schwalm D, Glattes RC, et al. Evaluation of calcium sulfate paste for augmentation of lumbar pedicle screw pullout strength. Spine J. 2002;2:255-260.
62 McLain RF. The biomechanics of long versus short fixation for thoracolumbar spine fractures. Spine. 2006;31:S70-S79.
63 Closkey RF, Parsons JR, Lee CK, et al. Mechanics of interbody spinal fusion. Analysis of critical bone graft area. Spine. 1993;18:1011-1015.
64 Hasegawa K, Abe M, Washio T, et al. An experimental study on the interface strength between titanium mesh cage and vertebra in reference to vertebral bone mineral density. Spine. 2001;26:957-963.
65 McKoy BE, An YH. An expandable anchor for fixation in osteoporotic bone. J Orthop Res. 2001;19:545-547.
66 Cook SD, Salkeld SL, Whitecloud TS3rd, et al. Biomechanical evaluation and preliminary clinical experience with an expansive pedicle screw design. J Spinal Disord. 2000;13:230-236.
67 Cook SD, Salkeld SL, Stanley T, et al. Biomechanical study of pedicle screw fixation in severely osteoporotic bone. Spine J. 2004;4:402-408.
68 Ferguson SJ, Winkler F, Nolte LP. Anterior fixation in the osteoporotic spine: cut-out and pullout characteristics of implants. Eur Spine J. 2002;11:527-534.
69 Goldhahn J, Reinhold M, Stauber M, et al. Improved anchorage in osteoporotic vertebrae with new implant designs. J Orthop Res. 2006;24:917-925.
70 Schramm M, Krummbein S, Kraus H, et al. Anterior vertebral body screw pullout testing with the hollow modular anchorage system—a comparative in vitro study. Biomed Tech (Berl). 2003;48:356-361.
71 Highsmith JM, Tumialan LM, Rodts GEJr. Flexible rods and the case for dynamic stabilization. Neurosurg Focus. 2007;22(1):E11.
72 Lu J, Bhargav D, Wei AQ, et al. Posterolateral intertransverse spinal fusion possible in osteoporotic rats with BMP-7 in a higher dose delivered on a composite carrier. Spine. 2008;33:242-249.
73 Phillips FM, Turner AS, Seim HB3rd, et al. In vivo BMP-7 (OP-1) enhancement of osteoporotic vertebral bodies in an ovine model. Spine J. 2006;6:500-506.