Chapter 7 Bone marrow biopsy
The morphological assessment of aspirated or core biopsy specimens of bone marrow is based on two principles. First, that bone marrow has an organized structure such that in normal health, bone marrow cells display distinct numerical and spatial relationships to each other. Second, that individual bone marrow cells have distinctive cytological appearances that reflect the lineage and stage of maturation. Each or all of these features may specifically be disordered in disease. The specimens obtained by bone marrow aspiration or by bone marrow trephine biopsy are very different samples (Fig. 7.1) and contribute differently to diagnosis. Trephine biopsies provide excellent appreciation of spatial relationships between cells and of overall bone marrow structure; aspirated material provides information about the numerical and cytological features of marrow cells. It is clear therefore that bone marrow aspirate and bone marrow biopsy specimens have important and complementary roles in clinical investigation and may have different relative merits in the assessment of marrow disease. Furthermore, in almost all cases marrow assessment is only one part of the overall diagnostic work-up.1–3
Aspiration of the bone marrow
Satisfactory samples of bone marrow can usually be aspirated from the sternum, iliac crest or anterior or posterior iliac spines. In the majority of patients, the procedure can be performed with local and oral analgesia without recourse to intravenous sedation.4 in most circumstances the posterior iliac spines are the preferred biopsy site and selection of this site has the advantage that, if no material is aspirated, a trephine biopsy can be performed immediately. Biopsy from the posterior iliac spine may, however, be technically difficult in subjects who are obese or immobile and puncture of the sternum is occasionally necessary.
Consent and Safety
Consent for the procedure of aspiration or trephine biopsy should take place according to local standard operating procedures, but should always include a discussion of the risks and benefits of the procedure. The risks associated with bone marrow aspiration and biopsy have been assessed using voluntary register data. Results show that risks are not dependent on operator experience and have a low incidence (around 0.1%). However, adverse events continue to be reported and may be severe. The most frequent adverse events relate to haemorrhage and are most frequently seen in the context of myeloproliferative neoplasms and thrombocytopenia or other bleeding disorders (including the use of antiplatelet agents).5,6 Particular risks are associated with aspiration from the sternum. The operator should be aware of the additional risks and contraindications associated with aspiration from that site. The sternum should not be used as a site of biopsy in children or be used in adults if there is a disorder associated with increased bone resorption, such as myeloma. Operators should also be aware that unless the needle is correctly inserted in the sternum with an appropriate guard, there is a danger of perforating the inner cortical layer and damaging the underlying large blood vessels and right atrium, with serious consequences.7
Performing a Bone Marrow Aspiration
Only needles designed for the purpose should be used for marrow aspiration (discussed later). The operator should always wear surgical gloves to obtain a biopsy of bone marrow and should take great care to avoid needlestick injuries. A marrow aspiration or trephine biopsy should be performed in accordance with local guidelines for sterile procedures. Skin around the area should be cleaned, e.g. with 70% alcohol or 0.5% chlorhexidine (5% diluted 1 in 10 in ethanol). Infiltrate the skin, subcutaneous tissue and periosteum overlying the selected site with a local anaesthetic such as 2–5 ml 2% lidocaine. Wait until anaesthesia has been achieved. With a boring movement, pass the needle perpendicularly into the cavity of the ilium at the centre of the oval posterior superior iliac spine or 2 cm posterior and 2 cm inferior to the anterior superior iliac spine. When the bone has been penetrated, remove the stilette, attach a 1 or 2 ml syringe and suck up marrow contents for making films. If a larger sample is needed (e.g. for cytogenetic or immunophenotypic analysis), attach a second 5 or 10 ml syringe and aspirate a second sample. As a rule, material can be sucked into the syringe without difficulty; occasionally it may be necessary to reinsert the stilette, push the needle in a little further and suck again. Failure to aspirate marrow – a ‘dry tap’ – suggests bone marrow fibrosis or infiltration. Computed tomography-guided marrow sampling may be helpful in patients who are obese, in whom it is difficult to locate the iliac spine.8
Because bone marrow clots faster than peripheral blood, films should be made from the aspirated material without delay at the bedside. The remainder of the material may then be delivered into a bottle containing an appropriate amount of ethylenediaminetetra-acetic acid (EDTA) anticoagulant and used later to make more films. Preservative-free heparin should be used rather than EDTA if immunophenotyping or cytogenetic studies are needed. Some material can be preserved in fixative rather than anticoagulant for preparation of histological sections (see p. 133). Fix some of the films in absolute methanol as soon as they are thoroughly dry for subsequent staining by a Romanowsky method or Perls’ stain for iron. Appropriately fixed films are also suitable for cytochemical staining (Chapter 15). If there has been a ‘dry tap’, insert the stilette into the needle and push any material in the lumen of the needle onto a slide and spread it; in lymphomas and carcinomas, especially, sufficient material may be obtained using this approach to allow a diagnosis.9 Squash preparations of marrow fragments can be a useful supplement to bone marrow films. A drop of aspirated marrow is placed in the centre of a slide and, unless the aspirate is very cellular, the fragments are concentrated by removing the more dilute part of the aspirate with a plastic pipette. A second slide is then placed on top of the first and the fragments are crushed by rotating one slide on the other. Both squashes are then fixed and stained. Bone marrow aspirates in adults can be performed from the ilium, the sternum or the spinous processes; the latter site is rarely used and the procedure is described in the 10th edition of this book.
Puncture of the Sternum
The specific risks of sternal marrow aspiration were discussed earlier (see p. 124). Puncture of the sternum must be performed with care to avoid pushing the aspiration needle through the bone. The usual site for puncture is the manubrium or the first or second parts of the body of the sternum. The manubrium is formed of rather denser bone than the body of the sternum and, in elderly subjects at least, it tends to contain more fatty marrow than is found elsewhere in the sternum. The thickness of the cortex here varies from 0.2 mm to 5.0 mm, so it may be difficult to be certain that the needle point has reached the cavity of the bone.
Comparison of Different Sites for Marrow Puncture
There is considerable variation in the composition of cellular marrow withdrawn from adjacent or different sites. Aspiration from only one site may give misleading information; this is particularly true in aplastic anaemia since the marrow may be affected patchily.10 In general, however, the overall cellularity, the haemopoietic maturation pathways and the balance between erythropoiesis and leucopoiesis are similar at all sites. In practice, it is an advantage to have a choice of several sites for puncture, particularly when puncture at one site results in a ‘dry tap’ or when only peripheral blood is withdrawn. Aspiration at a different site may yield cellular marrow or strengthen suspicion of a widespread change affecting the bone marrow, such as fibrosis or hypoplasia. In aplastic anaemia, several punctures or, much to be preferred, a trephine biopsy may be necessary to arrive at the diagnosis.
Marrow puncture needles
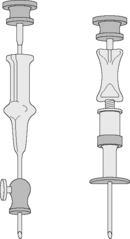
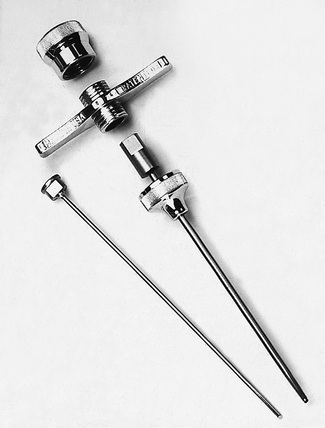
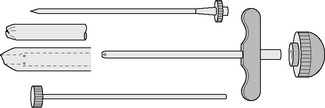
Needles should be stout and made of hard stainless steel, about 7–8 cm in length, with a well-fitting stilette and they must be provided with an adjustable guard. With reusable needles, the point of the needle and the edge of the bevel must be kept well sharpened. The most common reusable needles are the Salah and Klima needles (Fig. 7.2). A slightly larger needle with a T-bar handle at the proximal end was developed by Islam (Fig. 7.3); it provides a better grip, is more manoeuvrable and is more successful for biopsies of excessively hard (e.g. osteosclerotic) or soft (e.g. profoundly osteoporotic) bone.11 A modified version of the Islam needle has multiple holes in the distal portion of the shaft in addition to the opening at the tip to overcome sampling error when the marrow is not uniformly involved in a pathological lesion. Several types of disposable bone marrow aspiration and trephine biopsy needles are now available; their design is similar to the traditional reusable needles (Fig. 7.4). The increasing use of disposable needles by haematologists is based on considerations of safety for patient and operator.
Processing of aspirated bone marrow
Preparing Films from Bone Marrow Aspirates
Make films, 3–5 cm in length, of the aspirated marrow using a smooth-edged glass spreader of not more than 2 cm in width (Fig. 7.5). The marrow fragments are dragged behind the spreader and leave a trail of cells behind them. Spreading should be towards the area to which the label is to be applied to avoid having particles dragged to the tip of the slide, where it is difficult to examine them. If there are insufficient fragments, they can be concentrated. This is not usually necessary for marrows that are very cellular such as in acute and chronic myelogenous leukaemia and megaloblastic anaemia. Concentration of marrow can be achieved by delivering single drops of aspirate onto slides about 1 cm from one end. Most of the blood is quickly sucked off from the edge of the drop with the marrow syringe or a fine plastic pipette. The irregularly shaped marrow fragments tend to be left behind on the slide and can be lifted off with the spreader; films can then be prepared as explained earlier.
After thorough drying, fix the films of bone marrow and stain them with Romanowsky dyes, as for peripheral blood films. However, a longer fixation time (at least 20 min in methanol) is essential for high-quality staining. If a film needs to be stained urgently, fix and stain one film only and permit the others to dry thoroughly. This avoids having all films showing artefacts caused by fixation of slides before thorough drying has been achieved. Films can also be stained by Perls’ method (see p. 334) to demonstrate the presence or absence of iron.
Concentration of Bone Marrow by Centrifugation
Centrifugation can be used to concentrate the marrow cells and to assess the relative proportions of marrow cells, peripheral blood and fat in aspirated material. While concentration of poorly cellular samples is useful, especially when an abnormal cell is present in small numbers,12 it is unnecessary when the aspirated material is of average or increased cellularity. Volumetric data, too, are of little value in individual patients because of the wide range of values encountered even in health. Methods for separation of marrow cells are described on p. 65.
Preparation of Films of Post-mortem Bone Marrow
Films made of bone marrow obtained at post mortem are seldom satisfactory. If satisfactory results are to be achieved, the procedure must be carried out as soon after death as possible. When the marrow is spread in the ordinary way, the majority of the cells tend to break up and appear as smears. The rate and pattern of cellular autolysis during the first 15 h after death has been studied and the differences between the changes of post-mortem autolysis and those that occur in life as a result of blood diseases have been defined.13 Blood cells are much better preserved if a small piece of marrow is suspended in 1–2 ml of 5% bovine albumin (1 volume 30% albumin, 5 volumes 9 g/l NaCl). The suspension is then centrifuged and the deposited marrow cells are resuspended in a volume of supernatant approximately equal to, or slightly less than, that of the deposit. Films are made of this suspension in the usual way.
Examination of aspirated bone marrow
Principles of Marrow Aspirate Examination
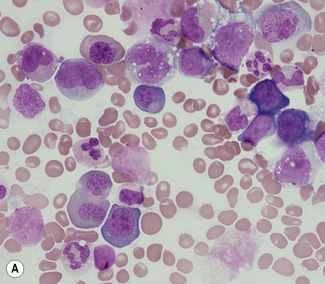
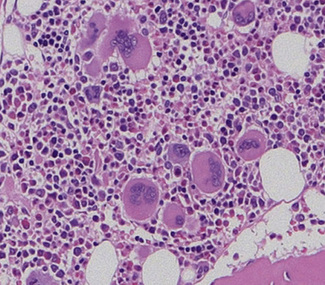
Aspirated bone marrow material spread on glass slides yields individual separate bone marrow cells that have not been subject to prior processing or been cut to form sections. The specimen may therefore be examined using an oil-immersion lens to allow excellent assessment of cytological detail. Marrow aspiration therefore has particular value where recognition of individual cells or abnormal cytological features is paramount or where individual cells need to be recognized, classified and counted. Examples where bone marrow aspiration is of major value include the cytological assessment of abnormal cell maturation of bone marrow cells or the cytological classification and numerical assessment of acute leukaemia (Fig. 7.6). Aspirated bone marrow cells are also well suited to further examination by cytogenetic, molecular or flow cytometric methods. However, the aspiration process generally disrupts structural elements within the marrow, preventing assessment of the structural relationships of normal or abnormal elements within marrow, and loose structural features such as lymphoid aggregates or granulomata are often not detected. The bone marrow aspirate may also be misleading if the cells of interest do not ‘spill’ from marrow particles and therefore do not appear on the slides. This problem is most readily apparent when marrow is subject to fibrotic processes; for example, the reticulin fibrosis of hairy cell leukaemia or the collagenous fibrosis of established myelofibrosis. An aspirate is also frequently insufficient when infiltrating cells form cohesive structures. Clumps of cells from carcinoma may on occasion be seen in aspirates, but are usually better revealed by biopsy.
Quantitative Cell Counts on Aspirated Bone Marrow
A number of values for the cell content of aspirated normal bone marrow have been given in the literature.14,15 The percentage of marrow in the sternum of healthy adults that is cellular rather than fatty is 48–79%. However, quantification of the cell content of aspirated marrow is not reliable in view of the tendency of the marrow to be aspirated in the form of particles of varying size as well as free cells and the uncontrollable factor of dilution with peripheral blood, which according to some authors may amount to 40–100% in 0.25–0.5 ml bone marrow samples.
Sources of Error and Physiological Variations
Because of the naturally variegated pattern of the bone marrow, the irregular distribution of the marrow cells when spread in films and the variable amount of dilution with blood, differential cell counts on marrow aspirated from normal subjects vary widely. Aspirating only a small volume and counting cells in the trails left behind marrow particles as they are spread on the slide minimizes the dilutional effect of blood. When there is an increase in associated reticulin, some cell types may resist aspiration or remain embedded in marrow fragments and will therefore be under-represented in the differential count. Megakaryocytes in particular are irregularly distributed and tend to be carried to the tail of the film. The chance aspiration of a lymphoid follicle would result in an abnormally high percentage of lymphocytes.16
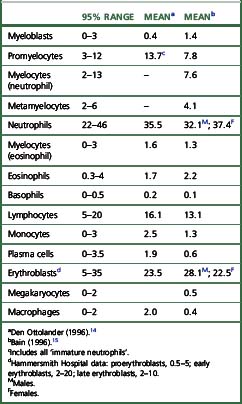
Ideally, differential counts should be performed on sectioned material, but difficulties in identification make this impractical. Methacrylate embedding offers a better opportunity for correctly identifying cells. The incidence of the various cell types is usually expressed as percentages. The normal values for cell differentials in bone marrow (Table 7.1) can only be taken as an approximate guide.14,15 The cellular composition of the bone marrow varies between normal infants, children and young adults. Variation is marked in the first year, particularly in the first month. The percentage of erythroblasts decreases from birth and at 2–3 weeks they constitute only about 10% of the nucleated cells. Myeloid cells (granulocyte precursors) increase during the first 2 weeks of life, following which a sharp decrease occurs at about the 3rd week; however, by the end of the 1st month about 60% of the cells are myeloid. Lymphocytes constitute up to 40% of the nucleated cells in the marrow of small infants; the mean value at 2 years is approximately 20%, falling to about 15% during the rest of childhood. The percentage of plasma cells is especially low from infancy up to the age of 5 years.
Reporting bone marrow aspirate films
A systematic examination of the marrow aspirate, combined with knowledge of the clinical context, provides the best chance of arriving at a diagnosis.3 Choose several of the best-spread stained films that contain easily visible marrow particles. Several particles should then be examined with a low-power (×10) objective to estimate whether the marrow is hypocellular, normocellular or hypercellular. Megakaryocytes and clumps of non-haemopoietic cells (e.g. metastatic carcinoma cells) should be looked for at this stage of the examination; they are most often found toward the tail of the film.
Systematic Scheme for Examining Bone Marrow Aspirate Films
Higher Power (×40, ×100 Oil-Immersion)
Look for areas of bone marrow necrosis.17 In necrotic areas, the cells stain irregularly, with blurred outlines, cytoplasmic shrinkage and nuclear pyknosis. Bone marrow necrosis may occur in sickle cell disease; it also occurs occasionally in lymphomas, acute lymphoblastic and chronic lymphocytic leukaemia, myeloproliferative neoplasms and metastatic carcinoma, as well as in septicaemia, tuberculosis and anorexia nervosa.18 In patients with anorexia nervosa or cachexia, there may be gelatinous transformation of the ground substance of the marrow.18
Assess the iron content of macrophages and look for iron granules in erythroid cells on a slide stained with a Perls’ stain. At least seven particles should be examined to optimally assess a bone marrow aspirate for iron stores.19 If fewer particles are available, a diagnosis of iron deficiency can only be tentative. In sideroblastic anaemia, the granules incompletely encircle the nucleus. Abnormal patterns of iron staining may also be seen in dyserythropoietic anaemias such as the thalassaemias.
Reporting Results
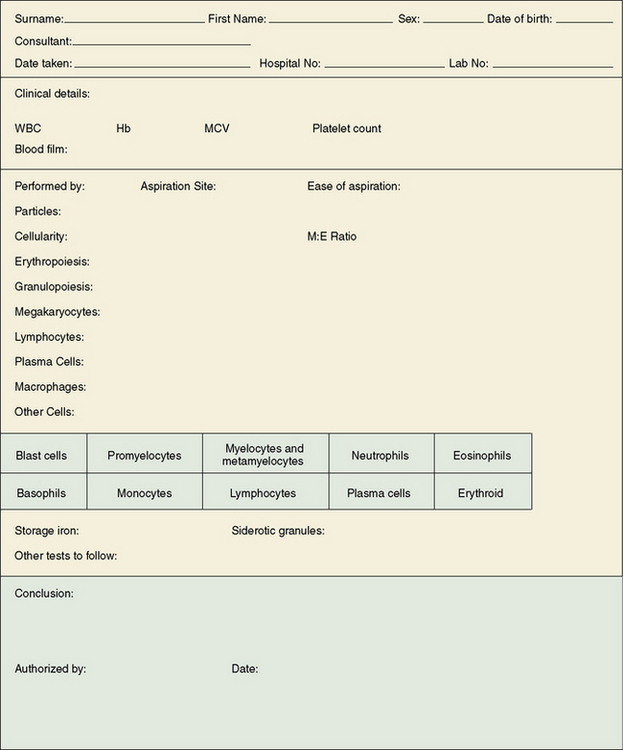
It is helpful to report bone marrow films on a printed form on which the report and conclusion can be set out in an ordered fashion (Fig. 7.7).3 Where a computerized reporting system is in use, it is useful to have a template with headings to ensure that the marrow reports are systematic and consistent. A list of the various descriptive comments that may be used can be provided in coded form to facilitate data entry. Report summaries should be intelligible to clinicians who are not haematology specialists.
Percutaneous trephine biopsy of the bone marrow
Like marrow aspirations, trephine biopsy may be carried out in the inpatient ward or in outpatient departments. The posterior iliac spine is the usual site, although the anterior iliac spine can also be used. The posterior iliac spine is said to provide samples that are longer and larger and the aspiration is less uncomfortable for the patient.20
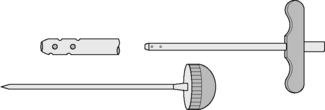
The trephine specimen is obtained by inserting the biopsy needle into the bone, using a to-and-fro rotation and then rocking the needle gently from side to side to detach a core of tissue that can be extracted within the needle. The main problems with this method are that the specimen may be crushed, thereby distorting the architecture, and it can be difficult to detach the core of bone from inside the marrow space. Trephine biopsy needles, both reusable and disposable, have been specifically designed to overcome these problems. The Jamshidi needle has a tapering end to reduce crush artefact (Fig. 7.8) and the Islam trephine has a core-securing device (Fig. 7.9). If larger specimens are needed, trephine needles that have bores of 4–5 mm may be used. Other needles occasionally used for trephine biopsy specimens are a 2 mm bore ‘microtrephine’ needle and a Vim-Silverman needle. However, compared with other needles, these yield smaller specimens of marrow that are prone to fracturing.
For the investigation of thrombocytopenia and neutropenia in neonates, sections of aspirated bone marrow can be obtained that allow assessment of marrow cellularity and architecture.21 A 19G, half-inch Osgood needle (Cadence Inc, New York) is introduced 2 cm below the tibial tuberosity. The trocar is removed and the hollow needle is advanced by twisting 2–3 mm into the marrow space. A syringe is used to apply suction to the needle until marrow appears; then the needle and syringe are withdrawn. The marrow clot is gently dislodged with the tip of a needle and placed into fixative. The specimen is processed as if it were an adult biopsy except that decalcification is not required.
Principles Behind Marrow Trephine Biopsy Examination
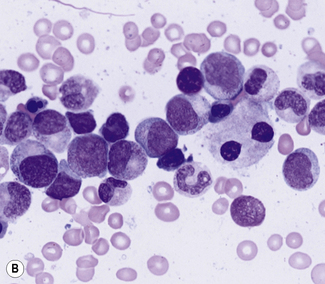
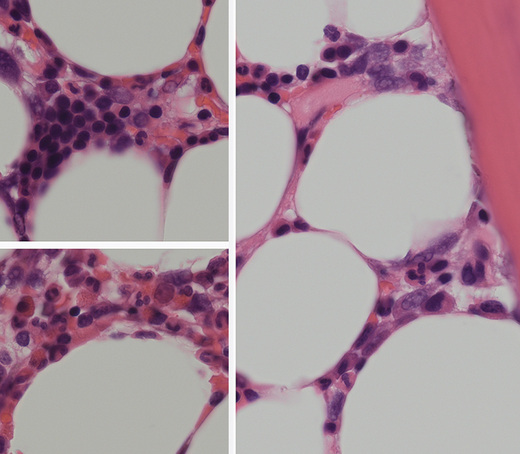
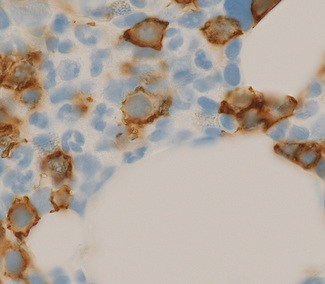
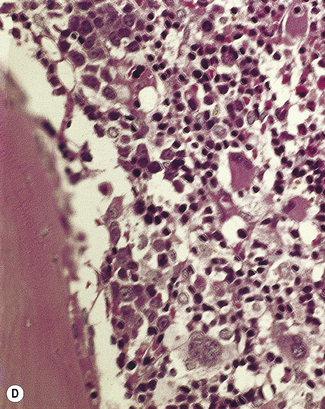
Bone marrow trephine biopsy provides a core of tissue, which is fixed and embedded to yield a histological specimen in which the structural relationships between cells, bone and bone marrow stroma are preserved. The nature of the specimen allows cellular distributions to be recognized: for example, the tendency for precursor myeloid cells to mature close to bony trabeculae or the normal development of erythroid cells in the form of small islands (Fig. 7.10). Bone marrow biopsy also delineates the abnormal distribution of cells that is characteristic of particular pathological conditions. For example, the paratrabecular collection of abnormal cells that is typical of follicular lymphoma or the clustered megakaryocytes seen in certain myeloproliferative neoplasms (Fig. 7.11). In addition, since a trephinebiopsy specimen is a core of tissue, all cells present within the specimen will be represented irrespective of fibrosis or cohesion. Cell types within a bone marrow biopsy may be recognized by cytological characteristics, but that identification is reinforced by their distribution within the biopsy. Cellular recognition on trephine biopsy sections is frequently supplemented by immunostaining in which monoclonal or polyclonal antibodies are used (with cytochemical reactions) to detect a range of lineage, maturational or cell-type restricted markers. Such markers allow confirmation of cell lineage or of maturation stage within a particular lineage (Fig. 7.12). Markers may also highlight abnormal distribution of cells: for example, markers of B-cell lineage may highlight infrequent paratrabecular aggregates of cells in cases of follicular lymphoma. Specific cell markers may highlight infrequent abnormal cells that represent residual or recurrent disease (e.g. CD72 (DBA.44) can be used to highlight neoplastic cells in hairy cell leukaemia). Finally, since trephine biopsy tissue is fixed and preserved, additional tests may be performed some time after the specimen was obtained.
Imprints from Bone Marrow Trephine Biopsy Specimens
Whenever a trephine biopsy is obtained, imprints can be taken before the specimen is transferred into fixative. This is particularly useful if the bone marrow aspirate is inadequate. The bony core is gently dabbed or rolled across the slide, which is then fixed and stained as for bone marrow smears (see p. 61). This allows immediate examination of cells that fall out of the specimen onto the slide and may provide a diagnosis several days before the trephine biopsy specimen has been processed.
Processing of Bone Marrow Trephine Biopsy Specimens
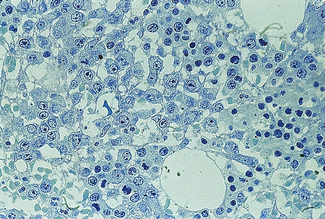
The specimen should be fixed in 10% formal saline, buffered to pH 7.0, for 12–48 h prior to decalcifying, dehydrating and embedding in paraffin wax by the usual histological procedures. Cell shrinkage and distortion from the decalcification process may obscure cellular detail. These disadvantages can be overcome by methyl methacrylate (‘plastic’) embedding (Fig. 7.13). Details of the preparation of sections of bone marrow biopsies can be found in Bain et al.22
Staining of Sections of Bone Marrow Trephine Biopsy Specimens
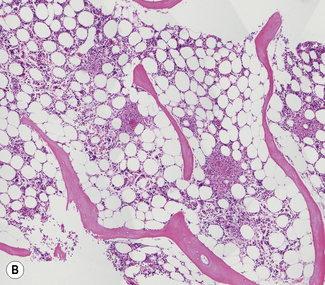
Bone marrow sections should be routinely stained with haematoxylin and eosin (H&E) and a silver impregnation method for reticulin. Sections can also be stained for iron by Perls’ reaction. H&E staining is excellent for demonstrating the cellularity (Fig. 7.14) and pattern of the marrow and for revealing pathological changes such as fibrosis or the presence of granulomata or carcinoma cells. Haemopoietic cells may be more easily identified in a Romanowsky-stained preparation. Both paraffin- and plastic-embedded specimens are suitable for immunohistochemistry.
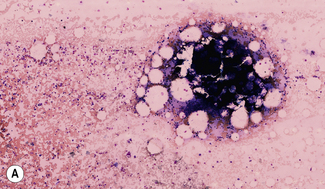
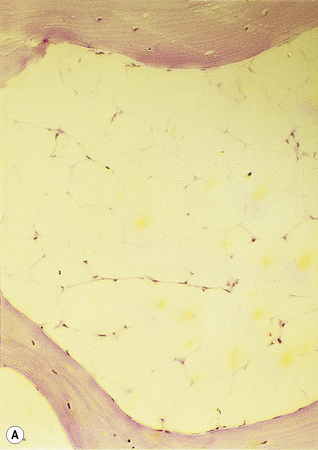
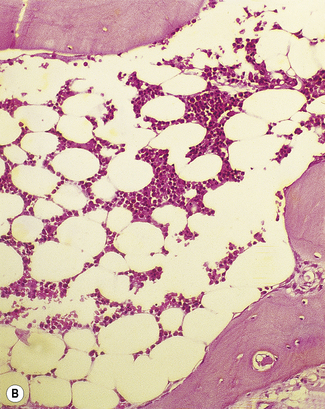
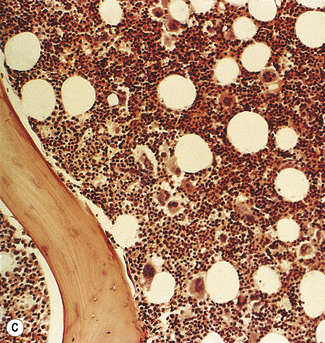
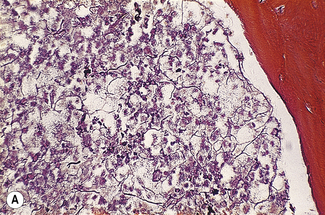
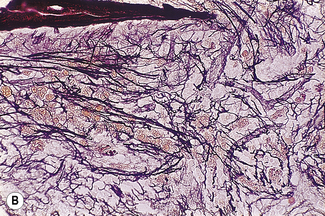
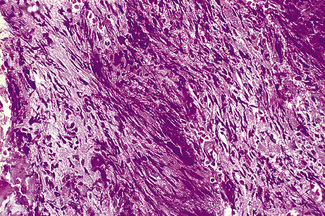
Silver impregnation stains the glycoprotein matrix, which is associated with connective tissue. The bone marrow always contains a small amount of this material, which is referred to as ‘reticulin’ and is an early form of collagen. The normal reticulin content of iliac bone marrow is shown in Figure 7.15A. An increase in marrow reticulin appears as an increase in the number, thickness and length of fibres (Fig. 7.15B). Increased reticulin deposition can occur in myeloproliferative neoplasms, particularly those associated with proliferation of megakaryocytes and in lymphoproliferative disorders, secondary carcinoma with marrow infiltration, osseous disorders such as hyperparathyroidism and Paget’s disease and inflammatory reactions.23 In primary myelofibrosis, a more ‘mature’ form of collagen is present, which, unlike reticulin, is visible on H&E staining (Fig. 7.16).
1 Bain B.J. Bone marrow aspiration. J Clin Pathol. 2001;54:657-663.
2 Bain B.J. Bone marrow trephine biopsy. J Clin Pathol. 2001;54:737-742.
3 Lee S.-H., Erber W.N., Porwit A., et al. ICSH guidelines for the standardization of bone marrow specimens and reports. International Journal of Laboratory Hematology. 2008;30:349-364.
4 Hall R. Results of bone marrow biopsy patient satisfaction survey at the Royal Bournemouth Hospital. Abstract 200. Br J Haematol. 2004;125(supp 1):62.
5 Bain B.J. Bone marrow biopsy morbidity and mortality: 2002 data. Clin Lab Haematol. 2004;26:315-318.
6 Bain B.J. Morbidity associated with bone marrow aspiration and trephine biopsy – a review of UK data for 2004. Haematologica. 2006;91:1293-1294.
7 Marti J., Anton E., Valenti C. Complications of bone marrow biopsy. Br J Haematol. 2004;124:555-563.
8 Devaliaf V., Tudor G. Bone marrow examination in obese patients. Br J Haematol. 2004;125:537-539.
9 Engeset A., Nesheim A., Sokolowski J. Incidence of ‘dry tap’ on bone marrow aspirations in lymphomas and carcinomas: diagnostic value of the small material in the needle. Scand J Haematol. 1979;22:417-422.
10 Ferrant A. Selective hypoplasia of pelvic bone marrow. Scand J Haematol. 1980;25:12-18.
11 Jacobs P. Choice of needle for bone marrow trephine biopsies. Hematology Reviews. 1995;9:163-168.
12 Fillola G.M., Laharrague P.F., Corberand J.X. Bone marrow enrichment technique for detection and characterization of scarce abnormal cells. Nouv Rev Fr Hématol. 1992;34:337-341.
13 Hoffman S.B., Morrow G.W.Jr, Pease G.L., et al. Rate of cellular autolysis in postmortem bone marrow. Am J Clin Pathol. 1964;41:281-286.
14 Den Ottolander G.J. The bone marrow aspirate of healthy subjects. Br J Haematol. 1996;95:574-575.
15 Bain B. The bone marrow aspirate of healthy subjects. Br J Haematol. 1996;94:206-209.
16 Maeda K., Hyun B.H., Rebuck J.W. Lymphoid follicles in bone marrow aspirates. Am J Clin Pathol. 1977;67:41-48.
17 Kiraly J.F., Wheby M.S. Bone marrow necrosis. Am J Med. 1976;60:361-368.
18 Smith R.R.L., Spivak J.L. Marrow cell necrosis in anorexia nervosa and involuntary starvation. Br J Haematol. 1985;60:525-530.
19 Hughes D.A., Stuart-Smith S.E., Bain B.J. How should stainable iron in bone marrow films be assessed? J Clin Pathol. 2004;57:1038-1040.
20 Hernándes-Garciá M.T., Hernández-Nieto L., Pérez-González E., et al. Bone marrow trephine biopsy: anterior superior iliac spine versus posterior superior iliac spine. Clin Lab Haematol. 1993;15:15-19.
21 Sola M., Rimsza L., Christensen R. A bone marrow biopsy technique suitable for use in neonates. Br J Haematol. 1999;107:458-460.
22 Bain B., Clark D.C., Wilkins B.S. Bone Marrow Pathology, 4th ed. Oxford: Wiley-Blackwell; 2010.
23 McCarthy D.M. Fibrosis of the bone marrow: content and causes. Br J Haematol. 1985;59:1-7.