9
Bone Healing
Robert C. Manske and Gary A. Shankman
1. Identify and describe the phases of bone healing.
2. Discuss the objectives that serve as the foundation of fracture management and bone healing.
3. Define osteoblasts, osteoclasts, and osteocytes.
4. Define and discuss Wolff’s law.
5. Discuss stress deprivation, immobilization, and normal physiologic stress as they apply to fracture healing.
6. Define three complications of bone healing.
7. Outline and describe six areas of descriptive organization of classifying fractures.
8. Describe the five types of pediatric fractures defined by Salter-Harris.
9. Define pathologic fractures and list four types.
10. Discuss how osteoporosis affects fractures.
12. List common methods of fracture fixation, fixation devices, and fracture classifications.
13. Discuss clinical applications of rehabilitation techniques used during bone healing.
STRUCTURE AND FUNCTION
Bone is an intense metabolically active tissue. Bone has unique mechanical characteristics that are determined primarily by the structural components of bone tissue. Chemically complex, bone tissue is approximately 65% mineral and 35% organic matrix. The major organic constituent of bone is type I collagen, representing about 90% of the dry weight of bone.35 The remaining 10% is composed of noncollagenous matrix proteins, lipids, phospholipids, proteoglycans, and phosphoproteins. The principal inorganic component of bone is a crystalline calcium phosphate hydroxyapatite.35 This compound is generally brittle, tolerating only small amounts of deformation before fracture. The remaining tissue volume includes fluid-filled vascular channels and cellular spaces.12
Bone Cells
There are three types of bone cells. Osteoblasts are functionally distinct cells that form bone matrix (osteoid) and synthesize type I collagen,35 and are commonly found on a bony surface. These cells are unique in that they have a large volume of endoplasmic reticulum, Golgi apparatus, and mitochondria to synthesize collagen and secrete matrix proteins.35
Osteoblasts and osteocytes are distinguished more by their location than by their structure and function.15 Osteoblasts are responsible for synthesis, deposition, and mineralization of bone. They are derived from bone marrow and secrete procollagen on all active bone surfaces. Once the osteoblast is done forming bone, it can become an osteocyte.
Osteocytes actually are osteoblasts that are embedded within newly formed mineralized bone matrix.35 Chemically, osteocytes differ from osteoblasts in that they demonstrate fewer organelles and a greater nucleus to cytoplasmic ratio.35 These cells represent approximately 90% of mature skeletal tissue and metabolically function to control extracellular concentrations of calcium and phosphorus.35 Osteoid osteocyte, and osteocytic osteoblasts are cells that are in an intermediate changeover from osteoblast to osteocyte, thus the pure distinction between cells may be more related to developmental stage than differing cell types.37
Osteoclasts are giant cell multinucleated bone resorption cells. Osteoclasts synthesize a specific acid phosphatase enzyme and also produce hydrogen ions, which in turn lower the pH environment.35 The reduced pH increases the solubility of the crystalline phosphate-hydroxyapatite that functions to remove the organic matrix crystals via acid proteolytic degradation.35 These enzymes remove a thick layer of osteoid covering, allowing osteoclasts to bind to bone and begin resorption if needed.5
Types of Bone
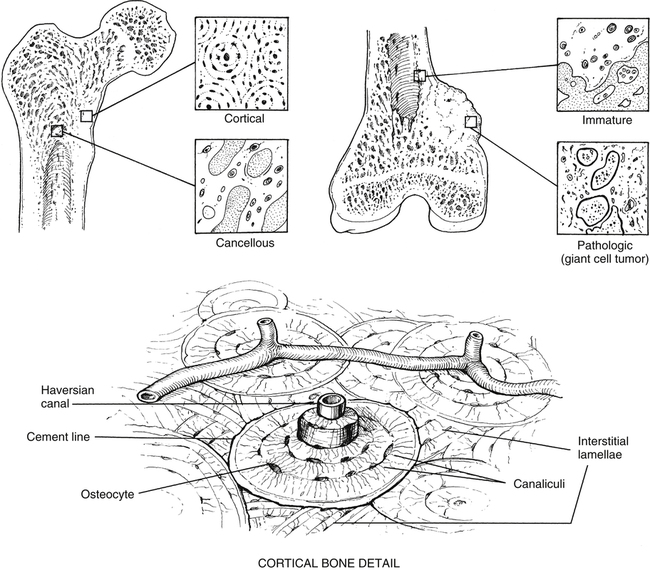
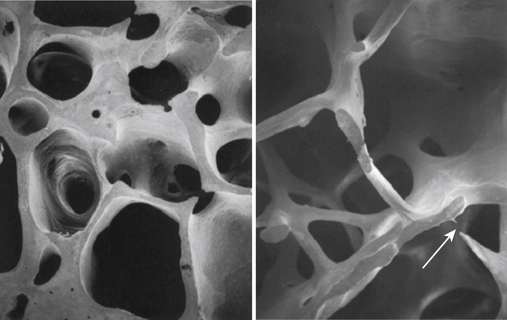
The microscopic organization and classification of bone tissue involves two distinct forms that change or adapt as we age: (1) normal, mature lamellar bone; and (2) weak, fragile, immature woven bone (Fig. 9-1).
Woven bone is structurally immature, embryologically (primary) fragile, and weak with a random disorganized collagen arrangement.3 This random arrangement of collagen fibers allows strength in all directions, while preferring strength in none specifically. Therefore this form of bone is not nearly as strong as mature bone. This specific type of bone is more commonly seen in embryos and newborns, but can also be seen in the adult during fracture repair callus, bone tumors, and various bone pathologies.35
One of the unique features of woven bone is that it can be deposited without any previous part of a cartilaginous model existing.23 Woven bone’s primary function is to provide temporary, quick acting mechanical support for injured skeletal tissue.
Very early after birth (approximately 2 months to 4 years), woven bone slowly remodels into organized, structurally mature lamellar bone. A clinically relevant feature of woven bone is that its specific mechanical behavior is termed isotropic; that is, woven bone does not conform to Wolff’s law, so its biomechanical reactions to applied forces and stress is similar in all planes.3 The disorganized arrangement of collagen fibers in woven bone strongly contributes to this unique characteristic (Fig. 9-2).3
Lamellar bone refers to mature remodeled woven bone. Mature bone begins formation at about 1 month after birth11 and comprises most of the skeleton by age 4 years.2 The collagen arrangement in lamellar bone is highly structured and organized. This gives normal mature lamellar bone anisotropic mechanical properties that are stress oriented, which characteristically respond to Wolff’s law.3
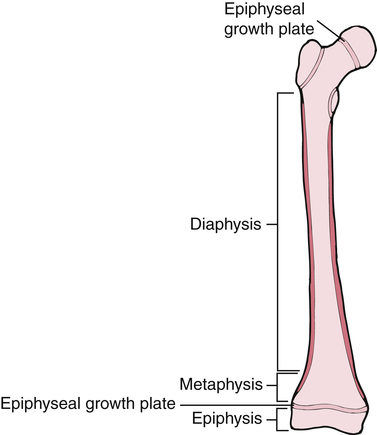
Bones are not only classified into various types, but also by their shape. Long bones are considered to have greater length than width. Examples of long bones are the femur and the humerus. These bones are tube shaped with widening at each end. Long bones have a diaphysis, which is the tubular shaped midportion that houses cancellous bone and the intermedullary canal where blood cells are formed (Fig. 9-3). The proximal and distal ends of the long bone widen to form a metaphysis, which continues to widen even more into the epiphysis or the end of the bone. In growing children, before skeletal maturity, the metaphysis and the epiphysis are separated by an epiphyseal growth plate. This can be a common area of fracture before skeletal maturity, at which time the two ends fuse and demarcation is not detectable.
Macroscopic Structure of Bone
Osseous tissue is structurally organized as either cancellous (trabecular bone) or cortical bone (compact bone). Cancellous bone is located at the metaphysis of long bones, as well as the vertebrae and other cuboid bones.3
Cortical bone, also known as compact bone, is located in the diaphyseal portion of long bones. Compact bone is very dense and hard on all surfaces, allowing it to function both structurally and for protection. Approximately 80% to 90% of the volume of cortical bone is calcium.17 The major structural subunit of cortical-compact bone is the osteon. As described by Bostrom and colleagues,3 the osteon is a central component of the haversian system.
Haversian bone is the most complex type of cortical bone. It is composed of vascular channels circumferentially surrounded by lamellar bone. This complex arrangement of bone around the vascular channel is called the osteon. The osteon is an irregular, branching, and anastomosing cylinder composed of a more or less centrally placed neurovascular canal surrounded by cell permeated layers of bone matrix.3 Primary osteons are those that develop into mature bone, while as new haversian canals are constantly formed, secondary osteons form on top of the old. Conversely, trabecular or cancellous bone is uniformly less dense with a large surface area and is significantly metabolically more “active” than cortical bone.3 In fact, even though cortical bone (compact bone) has four times the mass of trabecular bone, the metabolic activity of trabecular bone is eight times greater than that of cortical bone.3 Because bone turnover is a surface-oriented event, the profound surface area of trabecular bone explains the greater cellular exchange of cancellous bone.3
Bone Remodeling
The phenomenon of cellular turnover or remodeling of bone is a process that occurs on the surface of various portions of bone (periosteal, endosteal, trabecular, and haversian canal).35 Generally, bone remodeling is a lifelong activity that responds to mechanical stress (torsion bending, compression, tension) according to Wolff’s law.3 In simplified terms, Wolff’s law states that intermittent physiologic loads applied to bone stimulate adaptive responses. Removal of mechanical forces on the bone has the opposite effect. That is, when bone does not receive appropriate physiologic stress, osteoclastic activity overwhelms osteoblast production, which in turn reduces bone mass. The Hueter-Volkmann law is the reverse of Wolff’s law and is more specifically applied to compression and tensile forces acting on physeal growth plates. Simply stated, this law suggests that compression forces limit bone growth, whereas tensile stress stimulates growth.3
Vascular Supply to Bone
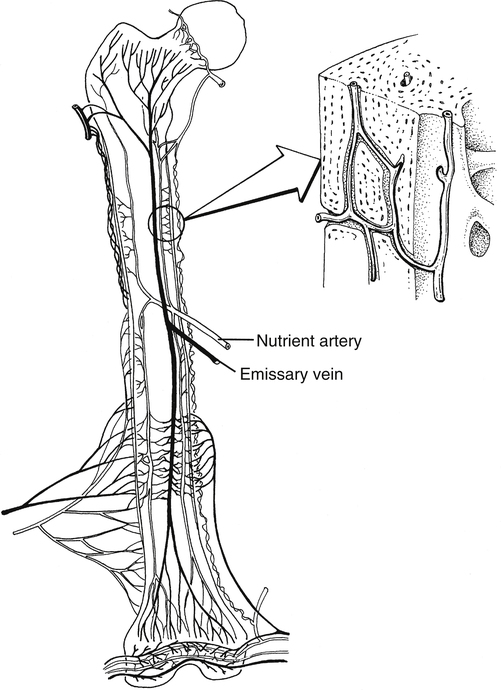
The adult skeletal system receives between 5% and 10% of the body’s total cardiac output.35 Bone receives blood supply from three distinct but interconnected systems: (1) the nutrient artery, (2) metaphyseal–epiphyseal system, and (3) periosteal systems (Fig. 9-4).
The arterial vascular system provides the origin of the nutrient system via a nutrient foramen in the diaphysis of long bones. The total number of nutrient vessels and foramen varies with each bone.3 The nutrient arteries enter the medullary space, then ascend and descend into the arterioles within the endosteal surface supplying the diaphyseal area of long bones.3 The metaphyseal–epiphyseal system is supplied by a periarticular complex system of the genicular arteries that penetrates the thin cortices of the metaphysis of long bones.3 Muscular attachment to the periosteal cortical sites of bone provides nutrition through the periosteal capillary system.3
Fractures, internal fixation devices, external fixation, and prosthetic joint implants devitalize the microcirculation of the cortical–periosteal and endosteal portion of the bone. The resultant ischemia of bone can lead to nonunion and bone infections.3 These important clinical ramifications of bone circulation disruption are evident when initiating therapeutic interventions of early weight bearing and closed kinetic chain (CKC) resistance exercise after fractures or joint replacement.
OSTEOPOROSIS
Osteoporosis is an age-related heterogeneous bone disease characterized by decreased bone tissue. Osteoporosis occurs when osteoblast (bone formation) activity is surpassed by osteoclast (bone resorption) activity (Fig. 9-5).21 This creates weaker bones that are subjected to greater rates of fracture. More than 1 million fractures a year can be attributed to osteoporosis27; vertebral body compression fractures are the most common.
Causes of osteoporosis may be multifactorial, including hormonal alterations following menopause, prolonged immobilization, diseases, and prolonged steroid administration. Women are at greater risk for developing osteoporosis for several reasons. Age-related cortical bone loss generally begins at about age 40 years,21 with the rate thereafter being approximately 0.5% annually for both men and women. However, because of lowered estrogen during menopause,21 women lose bone at a rate of 2% to 8% annually, resulting in a much greater total loss.
Poor absorption of calcium that leads to decreased bone mineralization is called osteomalacia.27 Causes include calcium-deficient diet, accelerated calcium loss, and malabsorption of calcium.21 Femoral neck fractures are common in patients with osteomalacia.27
A frail, elderly woman with osteoporosis suffers resultant multilevel thoracic vertebral body compression fractures. Bed rest with relative immobilization is ordered. She suffers further decrease in bone strength caused by immobilization. Rehabilitation is complicated by osteoporosis and fractures. Combined with the general overall negative effects of immobilization on the body’s systems, the effects of immobilization on the remaining skeletal tissue interfere with exercise, sitting, progressive ambulation, and functional activities, forming a vicious cycle that will need to be broken in order for a functional recovery.
CLASSIFICATIONS OF FRACTURES
By definition a fracture is any abnormal disruption in the normal anatomic continuity of bone. Therefore the classification of fractures takes into account the following criteria32,33:
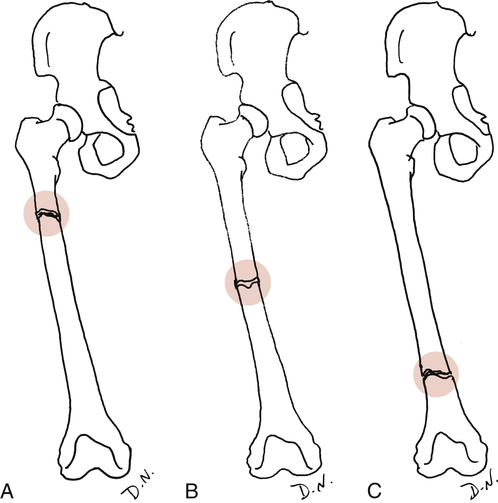
 Site of injury: The area of insult on the bone itself. An epiphyseal fracture describes the site, as does an intraarticular fracture or diaphyseal (shaft) fracture. Generally the site is described as the proximal, middle, or distal portion of a bone (Fig. 9-6).
Site of injury: The area of insult on the bone itself. An epiphyseal fracture describes the site, as does an intraarticular fracture or diaphyseal (shaft) fracture. Generally the site is described as the proximal, middle, or distal portion of a bone (Fig. 9-6).
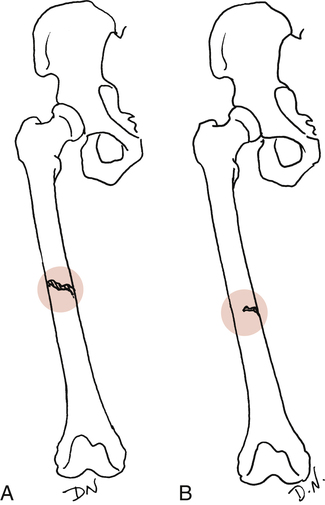
 Extent of injury: Complete or incomplete (Fig. 9-7). As the name implies, a complete fracture traverses the bone entirely. Incomplete fractures are commonly described as hairline cracks or greenstick fractures.
Extent of injury: Complete or incomplete (Fig. 9-7). As the name implies, a complete fracture traverses the bone entirely. Incomplete fractures are commonly described as hairline cracks or greenstick fractures.
 Configuration or direction of abnormality: The direction of the fracture. In a transverse fracture, the fracture line goes straight across (horizontally) through the bone; an oblique fracture crosses the bone diagonally. A spiral fracture describes a torsion or rotational injury where the fracture line literally spirals through the bone. An impacted fracture is a long-axis compression injury where the fracture fragments are forced together. The fracture is classified as comminuted when more than two fragments are present.
Configuration or direction of abnormality: The direction of the fracture. In a transverse fracture, the fracture line goes straight across (horizontally) through the bone; an oblique fracture crosses the bone diagonally. A spiral fracture describes a torsion or rotational injury where the fracture line literally spirals through the bone. An impacted fracture is a long-axis compression injury where the fracture fragments are forced together. The fracture is classified as comminuted when more than two fragments are present.
 Relationship of fracture fragments to each other: Can be displaced, nondisplaced, angulated, twisted, rotated, or overriding. An example is an avulsion fracture where a portion of bone is pulled away as part of a musculotendinous attachment or ligament–bone attachment.
Relationship of fracture fragments to each other: Can be displaced, nondisplaced, angulated, twisted, rotated, or overriding. An example is an avulsion fracture where a portion of bone is pulled away as part of a musculotendinous attachment or ligament–bone attachment.
 Relationship of fracture fragments to the environment: Whether the injury is open (compound fracture) or closed (simple).
Relationship of fracture fragments to the environment: Whether the injury is open (compound fracture) or closed (simple).
 Complications: Resulting in delayed union, nonunion, or malunion of the fracture fragments. An uncomplicated course of healing is called uneventful.
Complications: Resulting in delayed union, nonunion, or malunion of the fracture fragments. An uncomplicated course of healing is called uneventful.
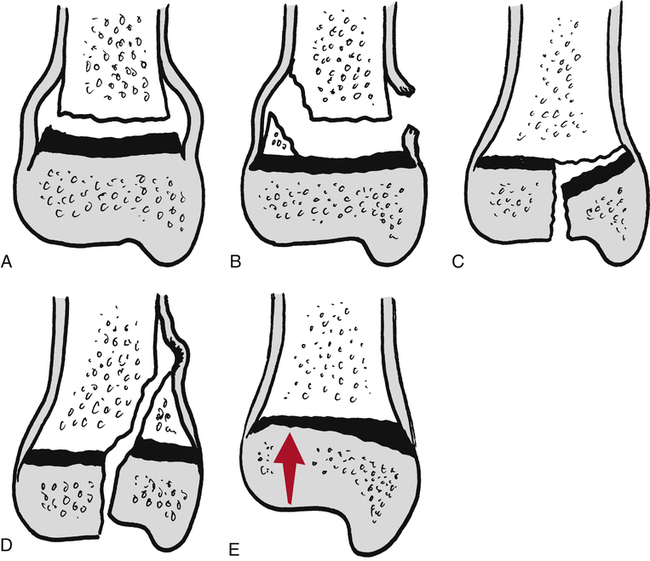
Salter’s fracture classification is outlined in Figure 9-8. Type I Salter-Harris fractures, modified by Rang,27,31–33 are transverse fractures through the physis. Type II fractures are the same as type I, but also have a metaphyseal fragment. Type III fractures involve both the physis and epiphysis. Typically these are intraarticular fractures. Type IV fractures are the same as type III but include the metaphysis. These are significant injuries that can lead to a reduction in bone growth. Type V fractures are severe injuries classified as crush injuries to the physis. This type of fracture also can lead to the arrest of growth. The PTA should also be aware of the Arbeitsgemeinschaft für Osteosynthesefragen (AO) classification system, which is universally applied to both fracture patterns and the devices used for internal fixation. This system will be discussed later in this chapter.26
Pathologic fractures are caused by tumors27 (malignant or primary bone disease), osteoporosis (most common), microtrauma from repetitive overload (stress fractures), or metastatic bone disease (second most common). These usually occur in the elderly person secondary to osteoporosis and can happen spontaneously or with very minor trauma.27
COMPONENTS OF BONE HEALING
Most of the adult skeleton (80%) is composed of compact, cortical bone. Approximately 20% of the skeleton is cancellous or spongy bone. Compact bone is extremely dense and unyielding to bending, whereas cancellous bone is more elastic and less dense than cortical bone.27
The normal dynamic process of bone synthesis and resorption is termed remodeling. Remodeling and stress occur together, which profoundly influences bone shape, density, internal architecture, and external configuration.1,18,22,27 With normal bone remodeling, weight-bearing forces and muscular contractions provide the stress needed for bone formation and adaptation. The absence of physiologic loading, or stress, is detrimental to bone, ligament, cartilage, muscle, and tendons, as well as the cardiorespiratory system. Wolff’s law1 states that intermittently applied stress, as well as changes in function of bone, causes definite changes.4,22 After a fracture, treatment may involve rigid cast immobilization and non–weight bearing over a period of weeks. This decreased stress on the bone causes rapid osteoclast activity (bone resorption) and decreased osteoblast activity.28 Therefore although the removal of stress is paramount for healing, the decrease in external force also causes significant bone loss.27 Bone loss caused by immobilization is reversible after progressive weight bearing, active motion, resistive exercise, and vertical loading (CKC exercise).27 It is recommended that strict, long-term, rigid cast immobilization be minimized whenever possible.
Although normal stresses promote bone development, excessive stress also can lead to gradual bone resorption.22 Stress that is unrelenting and does not allow for osteoblastic repair of bone can lead to pathologic accelerated bone resorption and eventual stress fracture.1,18,22
Bone remodeling is also influenced by electrical charges when forces are applied to bone. The piezoelectric effect describes a negative electric charge toward the concave, or compression, side of a force applied to a bone. An electropositive charge is seen on the tension, or convex, side of the bone. The negative-charge side responds by stimulating osteoblasts, whereas the positive-charge side (tension side) responds by increasing osteoclast activity.27
BONE INJURY AND REPAIR
The spontaneous natural course of fracture repair can be described as interfragmentary stabilization by periosteal and endosteal callus formation and by interfragmentary fibrocartilage differentiation, restoration of continuity and bone union by intramembranous and endochondral ossification, substitution of avascular and necrotic areas by haversian remodeling, modeling of the fracture site, and functional adaptation.1,3,4,8,10,20
Phases of Fracture Repair
In general terms most fractures heal with a combination of primary cortical healing and secondary–indirect healing. The phases of fracture repair are divided into six sequences of events that ultimately lead to functional remodeling of the injured bone.20 Phase I is inflammation and hematoma formation, Phase II is chondrocyte formation and angiogenesis, Phase III is cartilage and calcification, Phase IV is cartilage removal, Phase V is bone formation, and Phase VI is bone remodeling.20
Phase I is characterized by fibrin clot formation. The fracture site creates formation of the inflammatory response. Because of increased vascularity from the injury, a hematoma forms and begins the process of clot formation. The presence of the fibrin clot between fracture fragments acts as a rich source of activating inflammatory molecules essential to the complex cascade of intense cellular activity required for fracture repair. Many cellular processes are involved during this phase that are responsible for attracting, regulating, and differentiating cells that serve to propagate the development of cartilage and revascularization of bone. Animal models demonstrate intramembranous and endochondral bone formation during the first 2 weeks after fracture.20 This early mass of tissue formed during bone healing is termed a callus. The callus is an early precursor to mature calcified bone.
Angiogenesis (neovascularization) is critical for delivering oxygen, nutrients, inflammatory cells, and fibroblasts to stimulate and support the healing fracture. The vascular response to a healing fracture varies over time. Initially, the three distinct vascular systems of bone are disrupted significantly, reducing blood flow. Over the next several days after fracture, the circulation increases and peaks at approximately 2 weeks.20 Recognize that although there is a relative increase in vascular budding, the dramatic volume of cellular activity creates a state of hypoxia at the fracture that is highly conducive for cartilage formation.20
Process of Bone Healing
Bone healing can be characterized by the following sequence of events:
 Bleeding occurs, and a hematoma results.
Bleeding occurs, and a hematoma results.
 Granulation tissue is formed by the hematoma (soft callus formation).
Granulation tissue is formed by the hematoma (soft callus formation).
 Osteoblasts produce new bone, and a bony or hard callus is formed.
Osteoblasts produce new bone, and a bony or hard callus is formed.
 The callus is gradually reabsorbed, and the anatomic contour of the bone is regained.
The callus is gradually reabsorbed, and the anatomic contour of the bone is regained.
As with soft tissue, the immediate inflammatory response lasts 24 to 48 hours and is characterized by the development of granulation tissue, blood clotting, and fibroblast and osteoblast proliferation.4,27 The repair phase of bone healing signals the development of bone scarring, or callus formation, which is usually detected within the first 2 weeks after injury. The degree of callus formation depends on anatomic alignment of the fragments and the degree and quality of immobilization. If motion occurs through the fracture site during this phase, a soft bone callus (primarily of cartilage) bridges the fragments.4,27 This type of callus also forms if the union between the fragments is poor, even when immobilization prevents motion.
Primary cortical healing (hard callus) forms with anatomic alignment and fragment apposition, immobilization, and appropriately applied progressive stress. The remodeling phase of bone healing is an extraordinarily long process that can take up to several years to complete and is strongly influenced by Wolff’s law.1
Effects of Immobilization on Bone Tissue
A major goal for bone healing to occur is to immobilize the fracture site. However, when bone tissue does not receive physiologic loads (ambulation, vertical loads, and muscle contractions), the normal remodeling processes are negatively affected. The rate of normal bone remodeling changes when immobilization lasts slightly longer than 1 week.10 The “turnover” or remodeling of bone during immobilization is characterized by a loss of calcium, resulting in localized bone loss. Immobilization leads to a reduction in the hardness of bone related to the duration of immobilization.10 By 3 months, bone strength is only 55% to 60% of normal.36 The relationship between soft tissue structures and bone during periods of immobilization reflects the interdependence of muscular contractions, forces acting on joints, compressive loads, circulation (blood flow affecting nutrition), and motion in maintaining bone remodeling equilibrium.
Complications
Occasionally the process of bone healing leads to three distinct complications:
In delayed union, the dynamic biological repair processes of bone healing occur at a slower rate than anticipated. Brashear and Raney4 describe delayed union as clinically detectable when firm callus is not present at 20 weeks (for fractures of the tibia and femur) and at 10 weeks (for fractures of the humerus). An important cause of delayed union is “inadequate or interrupted immobilization.”4 Rehabilitation can significantly affect the healing process either positively or negatively. If therapeutic interventions are begun too soon or too vigorously, delayed union may occur because of excessive motion occurring at the fracture site. Unfortunately, the trade-off when caring for delayed bone union cases is the need to provide extended periods of immobilization for healing. However, cast braces and walking casts can be used to provide the weight bearing (Wolff’s law) necessary to enhance healing. In nonunion, the healing processes have stopped. Nonunion occurs when there is a significant and severe associated soft-tissue trauma, poor blood supply, and infections.
In malunion, healing results in a nonanatomic position (Fig. 9-9). Malunion is caused by ineffective immobilization and failure to maintain immobilization for an adequate period of time. For appropriate bone healing to occur, anatomic alignment (apposition) of fragments, adequate fixation (external or internal), and length of time of immobilization must occur in concert. If these factors are not balanced, the chance of complications is increased. Regardless of the type of complication, fracture healing fails or is delayed 5% to 10% of the time.6,9
Fracture Fixation: Biology and Biomechanics
The precise application of various internal fixation devices (intermedullary rods, bone plates, and compression screws) to stimulate primary cortical healing are not without inherent risks. The surgical placement of bone plates requires “stripping” the periosteum where the screws and plates are to be fixed. This process obviously devascularizes the periosteal blood supply. In addition, the areas beneath the plate increase the porosity of bone and weaken the mechanical and structural behavior, as well as the architecture of the injured bone. With intramedullary rod application, the medullary canal must be “reamed” to allow for proper anatomic placement of the rod. Again the nutrient arterial system, metaphyseal–diaphyseal system, and medullary circulation are disrupted. During the surgical placement of screws and plates, the surgeon attempts to apply these devices by hand-held screw devices and manual torque wrenches instead of using high-speed drills that might create thermal necrosis of bone and soft tissue, which could contribute to increased bone devitalization.34,36
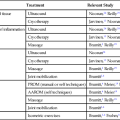
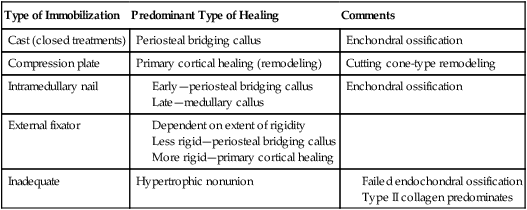
Conversely, nonoperative fracture management involves a complex series of interactions, which stimulate the thermal, chemical, mechanical, and electrical environment of periosteal and cortical bone (Table 9-1).8,20
Table 9-1
Type of Fracture Healing Based on Type of Stabilization
| Type of Immobilization | Predominant Type of Healing | Comments |
| Cast (closed treatments) | Periosteal bridging callus | Enchondral ossification |
| Compression plate | Primary cortical healing (remodeling) | Cutting cone-type remodeling |
| Intramedullary nail |
From Brinker MR, Miller MD: Fundamentals of orthopaedics, Philadelphia, 1999, Saunders.
The second phase is the development of the soft callus. Phase II is characterized by the soft-tissue compliance provided the peripheral structures surrounding the fracture fragments. The formation, organization, and gradual maturation of cellular activity in and around the injury site provides for greater stability.20 As stated by Latta, Sarmiento, and Zych,20 “peripheral structures supply early vascularization and soft tissue repair. Peripheral soft tissues provide a compliant strength which leads to reduction of acute symptoms long before there is radiographic evidence of healing.” Phase III demonstrates a radiographic confirmation of thin “hard callus” (bony ridge).
External Fixation and Fracture Repair
External fixators are used for a wide variety of clinical situations and allow for modification of stiffness and rigidity of fixation during fracture healing.4,30
The geometric composite construct of these elaborate frame configurations usually can be altered by several orders of magnitude. Several factors significantly contribute to the stability, rigidity, stiffness, and compression of the fracture site, as well as the mechanical properties of the frame.30 The number of pins used; the length, diameter, and type of pin material; the number of side bars set in the spacing between pins; the spacing between side bars and bone surface; pin–bone contact; and proximal–distal pin spacing all contribute to the material characteristics: stability, stiffness, and compression of the fracture site.8,30
Fracture healing characteristics using external fixators depend on the rigidity obtained by the device. Overall, the more rigid the fixation, the earlier the radiographic confirmation of bone union. Less rigid fixators create greater periosteal callus formation, whereas rigid fixators characteristically stimulate direct primary cortical fracture healing.8,30
As stated, a unique advantage of external fixation is the ability to alter or modify rigidity during various stages of fracture healing. Several models demonstrate the effect of dynamization (mechanisms used to decrease stiffness of the fixation or mechanisms that allow for controlled micromotion between fracture fragments) on blood flow and bone callus formation.8,20,25,30,35 A 25% increase in fracture site micromotion resulted in a 400% increase in regional blood flow and substantially more callus formation with animals receiving semirigid external fixators compared with rigid external fixation.30
Bone Fixation Devices
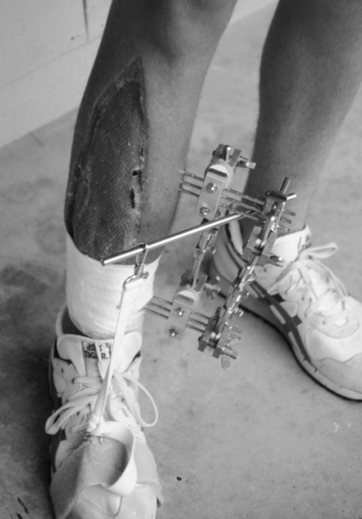
The remarkable sensitivity of bone to normal biological stress is well-known.1,22,27,33 After fractures, an attempt is made to stabilize the fracture, bring the fragments together by apposition (approximation) and alignment, and remove or minimize forces that may slow the normal healing process. Two general methods of immobilization are used in the treatment of fractures. In one method, the area is immobilized by external fixation devices. This method involves the use of casts, traction, splints, and braces and the external fixation devices employed with significant open (compound comminuted) fractures where the risk of infection is present (Fig. 9-10). These ominous-looking devices help fix the fracture site while allowing care of the open wounds with skin grafts, tissue flaps, or débridement. These are classified as external fixation devices because the pins used to immobilize the fracture do not contact the fragments directly, but are used to hold the bone segments in rigid alignment and anatomic apposition. With closed (simple) fractures, various rigid lightweight fiberglass casts, plaster casts, hinged-plaster casts, and adjustable-range hinged braces are used for immobilization.
The second method of immobilization uses internal fixation devices and is best for displaced fractures where external fixation does not provide the degree of immobilization necessary to effect healing. (See Box 9-1 for the background on the AO classification system, designed by the Association for the Study of Internal Fixation [ASIF or AO].) Internal fixation is called open reduction with internal fixation (ORIF) and involves surgically exposing the fracture site to reduce, approximate, and align the bone fragments. The materials used for ORIF procedures include metals (stainless steel and metal alloys of cobalt–chromium–molybdenum and titanium) and nonmetals (high-density polyethylene, polymethylmethacrylate, silicones, and ceramics). Metal internal fixation devices frequently are combinations of screws, staples, pins, nails, tension-band wires, and various plates. The placement of these materials can affect the delivery of certain therapeutic agents, such as ultrasound. Also, a hole or tunnel defect in a bone, with or without a screw in it, effectively reduces the overall strength of the bone up to 50%.27 Even after screw removal, the bone will not regain normal strength for up to 1 year.27
Metal internal fixation devices occasionally loosen and can “back out,” as is the case with screws. If the PTA notes signs of hardware loosening (pain, swelling, or crepitus), he or she must immediately consult with the supervising physical therapist (PT) and physician. Many metal fixation devices are designed to be left in place, as with most plates, but these devices should be removed if metal allergy reactions occur. Table 9-2 depicts the various internal fixation devices.
Table 9-2
| Type | Use |
| Compression plate | Diaphyseal fractures |
| Intramedullary rod | Lower extremity diaphyseal fractures (femur, tibia); removed at 1 or 2 years |
| Reconstruction plate | Used in pelvic and distal humerus fractures |
| Tension wires | Patella fractures and olecranon fractures |
| Sliding hip screws | Intertrochanteric hip fractures |
| Condylar screws | Distal femur fractures |
| Cannulated screws | Femoral neck fractures |
From Miller M: Review of orthopaedics, Philadelphia, 1992, Saunders.
Factors Influencing Bone Healing
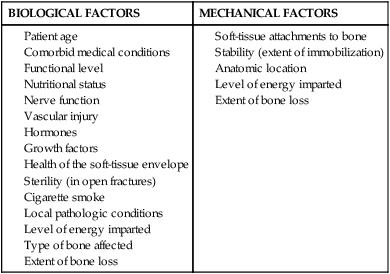
Several key factors are implicated in delayed biologic fracture repair. Tobacco smoke, malnutrition, inadequate reduction of the fragments, excessive motion from inadequate immobilization, poor vascular supply, soft-tissue interposition between fracture fragments, and infection significantly limit spontaneous fracture repair.3,8
Cigarette smoke is a vasoconstrictor that may reduce blood supply to the injury site. In addition, smoking directly interferes with osteoblast formation.36 Protein, malnutrition, and calorie deprivation reduce skeletal muscle mass and may contribute to reduced periosteal and cortical bone formation.8 Poor reduction of the fracture fragments and inadequate immobilization create excessive motion and reduced vascular supply to the bone (Box 9-2).36 Occasionally a sleeve of soft tissue becomes interposed within the fracture site, significantly reducing both primary, cortical, and secondary–periosteal bridging callus, and rendering the fragments devitalized.
From Brinker MR, Miller MD: Fundamentals of orthopaedics, Philadelphia, 1999, Saunders.
Infectious organisms have many deleterious effects on both implants and the host bone environment. Orthopedic implant loosening, bone resorption, bone destruction, and reactive periosteal elevation can occur in the presence of infection.8
Stimulation of Fracture Repair
Conversely, cadaveric bone allografts must be sterilized chemically. This process renders all cells nonviable. In both autografts and allografts, the implanted graft acts as a scaffold to support the growth of host bone tissue. This process is known as passive osteoconduction.8 Gradually the implant bone tissue becomes revascularized with stimulation and transportation of osteoblasts into the new graft. This process is referred to as osteoinduction.8 Although the sequence of events is similar (passive osteoconduction, gradual revascularization, and osteoinduction) with both graft materials, allogenic bone requires a longer time for creeping substitution to take place. Additional materials used for osteoconduction that support vascular ingrowth, growth, attachment, division, and remodeling of bone include ceramics, bioactive glass, and synthetic polymers.8
Ceramic bone graft substitutes include hydroxyapatite and tricalcium phosphate. Some of these graft substitutes are formed from marine coral and crystalline hydroxyapatite. These bone substitutes generally have been shown to be as effective as autograft bone.8
Two clinically relevant methods of fracture healing augmentation are electromagnetic field application and low-intensity ultrasound. The use of exogenously applied pulsed electromagnetic fields over nonunion fractures is based on the piezoelectric effect in response to load deformation of crystalline–collagen component’s of Wolff’s law.8 The use of specific electrical stimulation waveforms to induce bone formations is supported by scientific investigation and clinical observation that electric stimulation affects enchondral bone formation and connective tissue repair.
Basic science research suggests that the use of nonthermal, low-intensity ultrasound (30 mW/cm2) has very strong biological value to bone healing due to signal transduction and gene expression; stimulation of chondroblasts and osteoblasts and increasing blood flow; and enhancement of greater mechanical and histologic influence on enchondral and periosteal bone healing.16 Various studies have demonstrated that use of brief low-intensity ultrasound on the order of 20 minutes per day can reduce the time to fracture union.8,14,19,24,29
However, a recent systematic review7 of the use of low-intensity pulsed ultrasound for fractures that assessed randomized controlled trials determined that many of the studies had only moderate to low quality evidence for its use. Studies rarely included functional endpoints, and two of the highest quality studies showed no difference in functional outcome with the use of ultrasound.
Clinical Application of Rehabilitation Techniques During Bone Healing
Occasionally, electrical muscle stimulation (EMS) is used during cast immobilization to help retard atrophy and maintain strength. A small “window” is cut in the cast to allow the application of the electrodes. The patient is instructed to isometrically contract simultaneously with the electrically evoked muscle contraction. The benefits of electrical stimulation on muscle tissue during immobilization are controversial. The piezoelectric effect is enhanced by applying a negatively charged electrode to stimulate osteoblast activity, which is called direct current (any current in which electrons flow in one direction). An externally applied electrode with an external power source is called inductive coupling.27
Continuous passive motion (CPM) devices are used in some cases of intraarticular or extraarticular fractures of the tibia and femur.13 Although this appears to conflict with the notion of secure immobilization leading to bone healing, Salter and colleagues34 found positive effects of CPM on the development of chondrocytes and the reduction of intraarticular synovial adhesions when judiciously applied to healing intraarticular fractures.
The goals of rehabilitation programs during immobilization of healing fractures are as follows:
 Improve the overall fitness of the patient
Improve the overall fitness of the patient
 Promote motion of unaffected, nonimmobilized joints
Promote motion of unaffected, nonimmobilized joints
 Minimize muscle atrophy (isometrics and muscle stimulators)
Minimize muscle atrophy (isometrics and muscle stimulators)
 Maintain or improve muscular strength
Maintain or improve muscular strength
 Protect the healing structures; avoid unwanted, premature, or excessive motion
Protect the healing structures; avoid unwanted, premature, or excessive motion
 Teach safe and effective transfers and gait activities (with cumbersome long-leg plaster casts or external fixators)
Teach safe and effective transfers and gait activities (with cumbersome long-leg plaster casts or external fixators)
GLOSSARY
Cortical bone Eighty percent of adult skeleton.
Osteoclasts Resorb bone. Bone resorption generally is more rapid than bone formation.
Osteocytes Ninety percent of mature skeleton. Former osteoblasts that serve to maintain bone.