Bone and cartilage are derived from the mesoderm layer of the embryo and are specialised forms of connective tissue. Together with other connective tissue and skeletal muscle they constitute the musculoskeletal system. The musculoskeletal system is involved in producing movement and stability of the body by transmitting and resisting forces produced by, for instance, muscle contraction and gravity. Common features of bone and cartilage are that they contain connective tissue fibres and ground substance, forming an extracellular matrix which is relatively rigid compared with the rest of the body and is able to resist mechanical stress. In the extracellular matrix, collagen fibres are important in resisting tensile forces and elastin fibres are capable of deformation under stretching forces (and they recoil when the force is reduced).
Cartilage
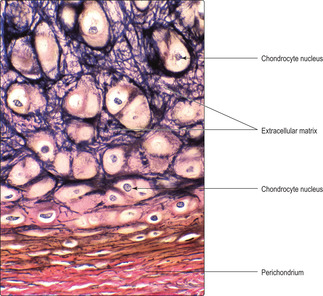
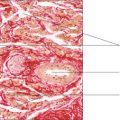
Cartilage has cellular and non-cellular components. Cartilage cells (chondrocytes) lie in spaces, known as lacunae, surrounded by cartilage matrix (the non-cellular component) (
Fig. 9.1). The matrix is produced by cartilage cells and it is a semi-solid material composed of ground substance comprising large molecules (e.g. glycosaminoglycans and proteoglycans), water and connective tissue fibres. Cartilage in many regions is surrounded by a thin layer of connective tissue known as the perichondrium, which is composed mainly of fibroblasts (
Fig. 9.1). However, there are sites where perichondrium is not adjacent to cartilage, e.g. on the articulating surfaces of bone (
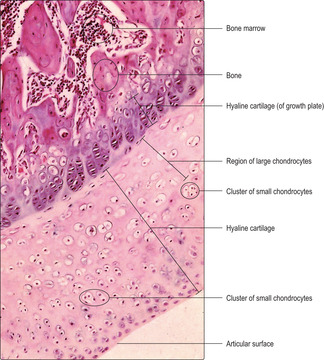
Fig. 9.2). Blood capillaries are not present in cartilage and the oxygen and nutrients required by chondrocytes diffuse through the matrix from nearby capillaries in the perichondrium.
There are three types of cartilage defined by the major type of fibre present:
■ hyaline cartilage contains mainly type II collagen fibres and is the most abundant type of cartilage in the body
■ elastic cartilage contains type II collagen and elastin fibres
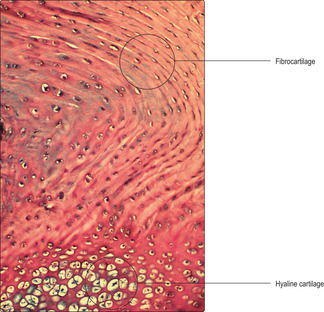
■ fibrocartilage contains type I collagen fibres.
Hyaline cartilage
Hyaline cartilage is present in a wide range of locations and is important in resisting forces and in supporting soft tissues. It forms the articulating surfaces of bones in synovial joints (
Fig. 9.2), where it provides a smooth, friction-free surface at which movements occur, and it acts as a shock absorber. Hyaline cartilage also aids respiration by providing rigidity in the larynx, trachea and bronchi, thus ensuring their patency during inspiration (
Chapter 11). It is also present at the anterior ends of ribs where movements of the rib cage occur during respiration. In the embryo, hyaline cartilage forms a temporary skeleton which eventually is largely replaced by bone (see below).
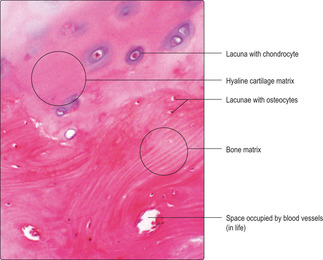
There are two cell types in hyaline cartilage: chondroblasts and chondrocytes. Chondroblasts are able to undergo mitosis and secrete extracellular cartilage matrix. They are very active metabolically and may appear in small clusters. Such clusters (
Fig. 9.2) contain new cells produced by mitosis from one initial cell in the region. Chondrocytes are the mature form of chondroblasts and are no longer able to undergo mitosis but can still secrete and modify cartilage matrix. The main chemical components of hyaline cartilage matrix are proteoglycans, glycosaminoglycans and hyaluronic acid, which all attract water molecules. This composition allows hyaline cartilage to resist compressive forces and act as a shock absorber. The type of collagen fibres (type II) and their orientation in hyaline cartilage are important in resisting mechanical forces. The appearance of hyaline cartilage is described as ‘glassy’ and the collagen fibres are not apparent in routine histological preparations. With age, chondrocytes and the lacunae they occupy enlarge (
Fig. 9.2) and calcium salts may be deposited in the matrix. These changes may alter the mechanical properties of the cartilage.
Growth in hyaline cartilage occurs by two modes: interstitial and appositional growth. Interstitial growth (growth from within) occurs mainly in the early stage of cartilage development. In interstitial growth, cartilage cells in lacunae undergo mitosis, produce matrix and separate from one another; the matrix occupies increasingly larger regions between the cells. Appositional growth occurs at the periphery of clusters of cartilage cells, adjacent to the perichondrium. Fibroblasts in the innermost part of the perichondrium are specialised and known as chondrogenic cells as they can become chondroblasts and secrete cartilage matrix. Most cartilage in the body grows by appositional growth. However, there are exceptions. For example, articular cartilage is not invested by perichondrium (
Fig. 9.2) and grows only by interstitial growth. Furthermore, growth of long bones, which occurs up to the age of about 20
years, also occurs via interstitial growth of cartilage (see below).
Elastic cartilage
There are similarities between the structure and mode of growth of hyaline and elastic cartilage. However, chondrocytes in elastic cartilage are larger than those in hyaline cartilage and the volume of matrix is less. The matrix of elastic cartilage contains type II collagen fibres, but in addition it has an abundance of elastin fibres which confers on the cartilage a degree of deformability and recoil. The elastin fibres may be readily displayed using special stains (
Fig. 9.1). Elastic cartilage is present in, for example, the epiglottis and the external ear.
Bone
Bone plays a vital physical role in protecting delicate underlying body structures, e.g. the heart and brain. Collectively, bones form the jointed skeleton of the body and, in conjunction with the attachment sites of skeletal muscles and tendons, bones act as levers and enable movements to occur. Although bone is hard and apparently inert, it is able to remodel in response to changes, e.g. in the stresses acting upon it, particularly during growth, exercise and after fracture. In addition, calcium in bone acts as a store for use by the rest of the body if calcium uptake in the diet is inadequate. Bone also provides the framework for body shape (along with fat and muscle) and spaces in bones, filled with bone marrow, are sites where blood cell formation (haematopoiesis) occurs (
Chapter 8).
Bone consists of several types of bone cell and associated bone matrix. Most bone cells in adults are osteocytes and they lie in lacunae embedded in extracellular bone matrix. Although bone matrix resembles cartilage matrix (
Fig. 9.4) it has organic and inorganic components. The organic components of bone matrix include type I collagen fibres, proteoglycans and glycosaminoglycans. The inorganic component of bone matrix consists largely of calcium hydroxyapatite (Ca
10(PO
4)
6(OH)
2). The hydroxyapatite is deposited alongside the collagen fibres and this produces the rigid hardness of bone. In comparison with cartilage matrix, the composition of bone matrix restricts the diffusion of gases, nutrients and waste molecules. However, bone matrix is permeated by small blood vessels (capillaries), an arrangement that ensures osteocytes receive sufficient oxygen and nutrients.
There are four major cell types in bone: osteoprogenitor cells, osteoblasts, osteocytes and osteoclasts.
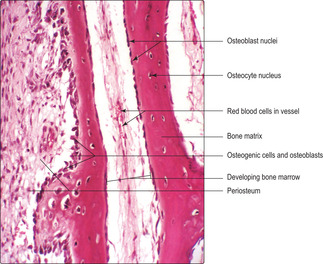
■ Osteoprogenitor cells are fibroblast-like cells in the inner layer of the periosteum adjacent to the surface of bone: they function as stem cells. In actively growing bone or after fracture they undergo mitosis. Some offspring cells differentiate into osteoblasts whilst others remain as osteoprogenitor cells.
■
Osteoblasts (
Fig. 9.5) are present at the surface of bone matrix (and the surface of hyaline cartilage matrix in growing bones; see below). Their appearance depends on their level of activity in synthesising new matrix: inactive osteoblasts have little cytoplasm but active ones may appear polygonal or cuboidal in shape. Active osteoblasts have an abundance of rough endoplasmic reticulum in their cytoplasm, reflecting their activity in secreting the organic components of bone matrix. Newly formed bone matrix, deposited onto the surface of existing matrix, does not contain calcium salts and is known as osteoid. Rapidly, osteoid becomes mineralised by the addition of calcium salts and this forms bone matrix. Osteoblasts are essential in this calcification process, which confers rigidity on bone.
In contrast to the growth of cartilage, bone growth occurs only by the appositional method of adding matrix onto the surface of existing matrix. Once osteoblasts are surrounded by bone matrix they become osteocytes and do not undergo mitosis; thus, interstitial growth does not occur.
■
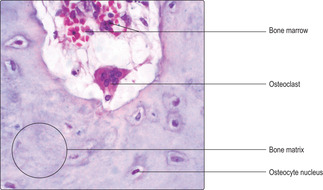
Osteoclasts are involved in the remodelling of bone such as that occurring during growth, development and fracture repair. Unlike other bone cells which are derived from fibroblast-like cells, osteoclasts are derived from blood monocytes. Osteoclasts are large, multinucleated cells (
Fig. 9.7) and they lie on the surface of bone matrix, sometimes occupying small depressions known as Howship’s lacunae. Osteoclasts secrete enzymes onto the bone matrix that digest the matrix releasing ions and small molecules, e.g. calcium and phosphate ions and amino acids. The released small molecules enter blood vessels, circulate and are available for use elsewhere in the body.
The activity of osteoclasts is under the control of two hormones (
Chapter 14) that ensure circulating blood levels of calcium are regulated. Parathyroid hormone stimulates osteoclasts to resorb bone and this raises blood calcium. Osteoclast activity is inhibited by calcitonin from the parafollicular cells of the thyroid gland and this lowers blood calcium.
Types of bone
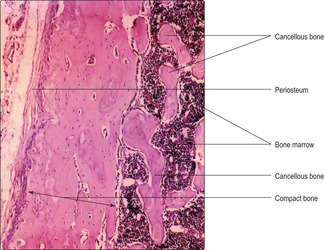
Most bones in the body contain compact (dense) and cancellous (spongy) bone. Compact bone is very dense compared with cancellous bone (
Fig. 9.8). In a typical adult long bone, e.g. the humerus, compact bone forms an outer collar along the shaft (diaphysis), whereas cancellous bone constitutes the less dense interior. Cancellous bone forms most of the ends of long bones (the epiphyses) in adults although compact bone may form a thin rim. (Hyaline cartilage is also a component of epiphyses of bones involved in synovial joints and it covers the joint surface.) Spaces within cancellous bone (
Fig. 9.8) are filled by bone marrow, which is involved in blood cell formation, and fat cells; the number of fat cells increases with age.
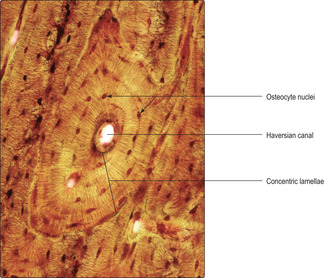
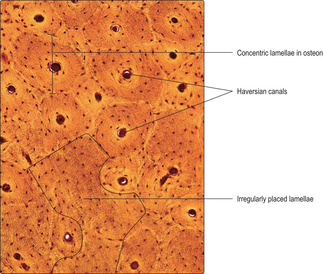
Compact bone is based on an arrangement of bone cells and matrix known as the Haversian system. This arrangement consists of many thin layers (lamellae) of bone (Fig. 9.6) arranged concentrically as cylinders which lie along the length of the shaft of long bones. This arrangement gives maximal resistance to forces acting along the bone and is particularly important in resisting compression due to weight bearing. Osteocytes, in lacunae, are embedded within the lamellae and lie in concentric circles which are apparent if the cylinders are sectioned across their length, i.e. transversely (
Figs 9.6 and
9.9). At the centre of each cylinder of lamellae are small blood and lymphatic vessels and nerves in a space known as a Haversian canal (
Figs 9.6 and
9.9). Cytoplasmic processes of osteocytes extend through very small channels (canaliculi) in the bone matrix (
Fig. 9.6) and via this route exchange gases and molecules with blood in vessels in a nearby Haversian canal. The whole unit of a Haversian canal plus lamellae is known as an osteon. Although Haversian systems have such a highly regular arrangement of osteons, in many regions the cylinders are packed together with irregular lamellae of bone (
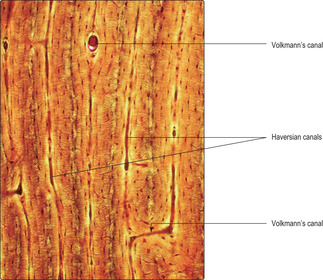
Fig. 9.9). The regularity of the cylinders of osteons is also apparent when they are sectioned along their length (
Fig. 9.10). In addition, canals (Volkmann’s) carry vessels and nerves perpendicular to Haversian canals. The presence of blood vessels in bone matrix ensures all osteocytes have adequate oxygen and nutrients, without which they die and the bone matrix degenerates.
Cancellous bone consists of thin spicules (trabeculae) of bone that project as a meshwork from compact bone (
Fig. 9.8). This type of bone has a random lamellar structure with osteocytes located in lacunae but it does not have Haversian or Volkmann’s canals. The osteocytes are nourished via diffusion from blood vessels in the marrow.
Bone formation and growth
Bone formation (ossification) and growth of bones begins in utero and continues up to the age of 20 or so. After this time, ossification and bone growth stops but the components of matrix are gradually removed and/or replaced throughout life. In addition, at any stage, the shape of bones may be remodelled in response to various factors including dietary changes, strenuous exercise or physical damage. Bone formation and growth occurs by two methods: intramembranous and endochondral ossification.
Intramembranous ossification
Endochondral ossification
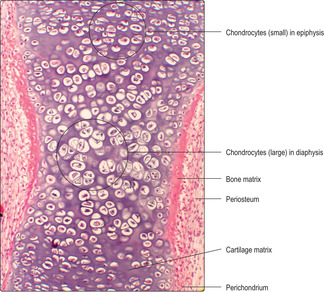
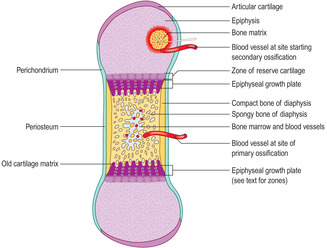
The majority of bones in the skeletal system are formed, and grow, by endochondral ossification. In this process a ‘model’ of hyaline cartilage, comprising chondrocytes and cartilage matrix in the shape of the adult bone, develops first (
Fig. 9.11). The cartilage model grows and gradually bone matrix and bone cells replace the cartilage in the diaphysis. In growing long bones the diaphysis is separated from each epiphysis by an epiphyseal growth plate (EGP) of cartilage. This is the region where many bones grow in length until adulthood.
There are several stages in endochondral ossification.
1.
Hyaline cartilage model forms (Fig. 9.11).
■ Some bones (e.g. the femur) begin to form in utero at about 6weeks (in humans).
■ Appositional and interstitial growth occurs in the cartilage model and shape develops.
■ Chondrocytes in the centre of the model increase in size.
■ Calcium salts are deposited in the matrix around the older chondrocytes, which eventually die (see below).
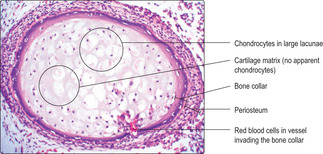
2. Collar of bone forms (
Fig. 9.12).
■ Osteogenic cells and blood vessels develop in the perichondrium.
■ The osteogenic cells become osteoblasts which form a collar of bone (bone cells and matrix) around the diaphysis. This is described as a primary ossification centre.
■ The perichondrium in the region of the bone becomes periosteum.
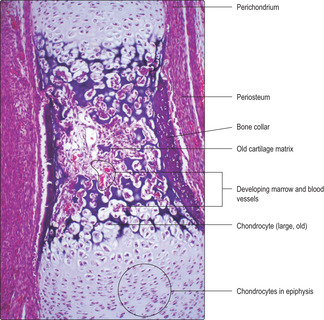
3. Invasion of the bone collar occurs (
Fig. 9.13).
■ Cells in the periosteum invade the collar of bone forming a ‘periosteal bud’.
■ The invasion brings osteogenic cells (and cells which form red and white blood cells and blood vessels) into the diaphysis.
4. The large old chondrocytes die and disappear; bone and bone marrow form (
Figs 9.13 and
9.14).
■ Chondrocytes die and leave a loose meshwork of cartilage matrix.
■ The invading cells from the periosteal bud enter the spaces left in old, calcified cartilage matrix.
■ Osteoblasts (developed from the invading osteogenic cells) secrete bone matrix onto the calcified cartilage matrix.
■ Osteoblasts surrounded by bone matrix become osteocytes (
Fig. 9.15).
■ Bone marrow forms from the invading cells as red and white blood cells and blood vessels develop.
5. Ossification extends.
■ Osteoblasts continue to deposit bone matrix onto cartilage matrix at each end of the diaphysis.
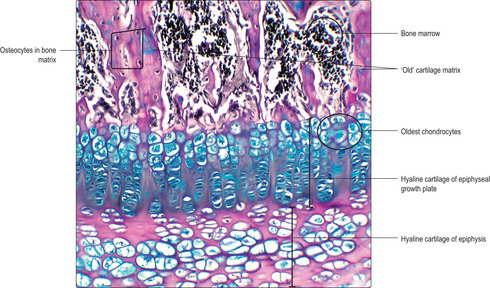
■ Chondrocytes remain as an EGP (
Fig. 9.16) at each end of a diaphysis for several years. Chondrocytes produced by mitosis in these plates are responsible for the growth in length of bones until adulthood.
Growth in length of bone at epiphyseal plates
■ Zone of reserve cartilage. This is the hyaline cartilage connecting each EGP to the adjacent epiphysis.
■ Zone of proliferation. Chondrocytes proliferate rapidly and new chondrocytes extend as columns of cells parallel to the long axis of the bone and away from the epiphysis. This is interstitial growth. Older chondrocytes in the columns, those farthest from the reserve zone, begin to secrete cartilage matrix. By forming these new cells and matrix in columns, the length of the diaphysis is extended and a framework is formed on which bone is later deposited.
■ Zone of hypertrophy. With increasing age chondrocytes enlarge.
■ Zone of calcification and chondrocyte death. Calcium salts are deposited in the cartilage matrix and the oldest chondrocytes die, probably due to programmed cell death (apoptosis).
■
Zone of ossification. Osteoprogenitor cells migrate from the diaphysis and become osteoblasts as they secrete bone matrix onto the surface of the calcified cartilage matrix. This process extends the length of the bone of the diaphysis. Calcium salts are then deposited into the bone matrix and some osteoblasts become surrounded by bone matrix and become osteocytes (
Fig. 9.15).
Growth in width of bone
Circumferential growth of each diaphysis occurs as osteoprogenitor cells in the periosteum become osteoblasts and lay bone matrix on the outer surface of the shaft (appositional growth). At the same time osteoclasts remove bone from the inner surface of the shaft. As a result the width of the outer rim of compact bone is adjusted and does not become thicker as the overall width of the bone increases. Gradually, the adult distribution of cancellous and dense bone develops.
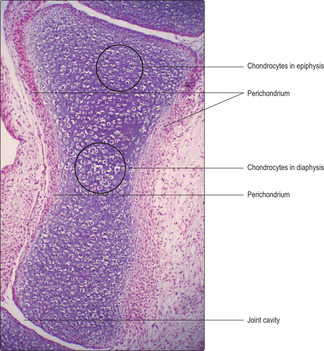
Growth of epiphyses and cessation of growth
Interstitial growth of cartilage, and appositional growth (in regions adjacent to perichondrium), increases the volume of each epiphysis. Just after birth, small regions develop where blood vessels and osteogenic cells invade some epiphyses, a process described as secondary ossification (
Fig. 9.16). Gradually, over the next 20
years or so, bone and bone marrow replace the hyaline cartilage of epiphyses, except at surfaces involved in synovial joints where hyaline cartilage remains.Epiphyses fuse with their diaphysis as bone replaces the hyaline cartilage of the growth plates. The time at which this ossification occurs differs between different bones in the body. These times are relatively constant between individuals.
Clinical notes
Genetic disorders These may affect epiphyseal growth plates and result in individuals shorter or taller than normal. (Epiphyseal growth plates and adult height may also be abnormal as a result of abnormal levels of growth hormone secreted by the pituitary gland.)
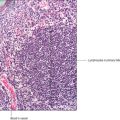
Osteoarthritis and rheumatoid arthritis These inflammatory processes that occur in cartilage are common. Osteoarthritis affects the hyaline cartilage covering the ends of articulating bones, particularly affecting weight-bearing joints such as knees, hips and ankles. In this disease the cartilage slowly degenerates with age, with underlying damage affecting the bone. In contrast, rheumatoid arthritis is a systemic autoimmune disease that affects other organs and tissues as well as joints. Because of its widespread effects, rheumatoid arthritis is much more difficult to treat. Rheumatoid arthritis, in contrast to osteoarthritis, may affect young people and may pursue a much more aggressive course.
Bone and cartilage
■ These are connective tissues with relatively rigid extracellular matrix containing collagen fibres and large molecules containing carbohydrates. Bone is more rigid than cartilage and has extensive deposits of calcium salts in the extracellular matrix.
■ Mature cartilage and bone cells (respectively, chondrocytes and osteocytes) occupy spaces (lacunae) in the matrix.
■ Osteocytes in many regions are nourished by a network of capillaries in Haversian canals.
Cartilage
■ This occurs in three types:
■ hyaline cartilage forms the smooth articulating surfaces of many bones and has a major role in skeletal development
■ elastic cartilage has a matrix containing elastin and collagen and is able to recoil after being deformed
■ fibrous cartilage provides resistance to mechanical forces.
■ Cartilage grows by appositional and interstitial growth.
Bone
■ Bone protects many organs and provides firm attachments for muscles and tendons which allows movements to occur.
■ It may be described as compact (dense) or cancellous (spongy):
■ in compact bone the matrix comprises cylinders of lamellae of bone around blood vessels in a Haversian canal
■ in cancellous bone thin trabeculae of bone matrix form a meshwork.
■ It begins to develop in utero as a hyaline cartilage model and becomes bone by endochondral ossification. (A few bones form directly by intramembranous ossification.)
■ In endochondral ossification the hyaline cartilage model grows by interstitial and appositional growth.
■ The cartilage is replaced as chondrocytes die and osteogenic cells become osteoblasts which deposit bone matrix on old cartilage matrix.
■ Up to about 20years of age cartilage persists in some bones as an epiphyseal growth plate (EGP) between the diaphysis and an epiphysis. Growth in the length of bones is a result of cartilage cells proliferating in EGPs and bone matrix being deposited on old cartilage matrix.
■ Osteoclasts digest bone and are involved in remodelling during growth, development and repair.