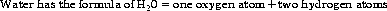Chapter 3 Bonds found in biological chemistry
Each electron orbital has a specific allocation of spaces available for electrons to fill (see Figure 2.3, p. 9). In the case of the outer orbitals, it is unusual to find all the spaces empty or full and one atom will seek out another compatible atom so that they can share their respective electrons and fill their outer orbitals. This arrangement creates great stability for the associated atoms, which is why, whenever possible, bonds are formed, between individual atoms.
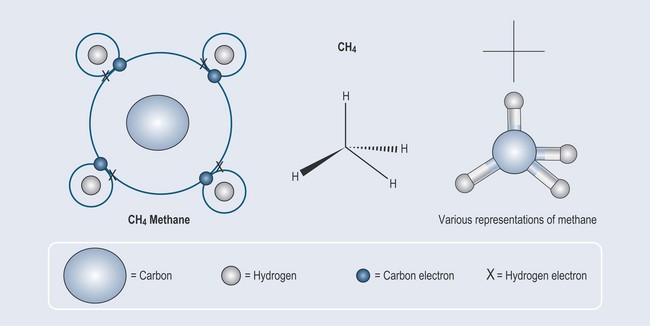
Taking the examples of hydrogen and carbon used in Chapter 2 (Figure 2.3), it is possible to see that hydrogen:
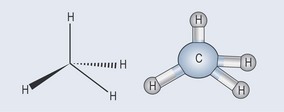
The final result of this union is that four hydrogen atoms attach themselves to one carbon atom, thus filling the outer orbitals of both the carbon and the hydrogens satisfying the needs of both elements (Figure 3.1).
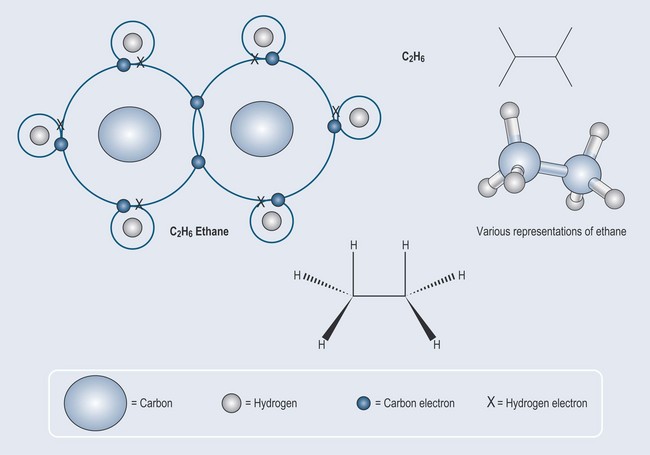
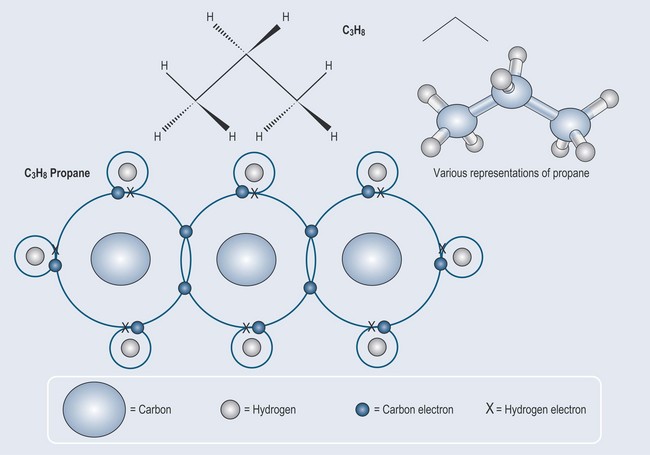
It is a matter of simple arithmetic to work out where this leads: a more complex molecule – ethane – can be formed from two carbon atoms and six hydrogen atoms Figure 3.2. The process can be continued to make an even longer compound called propane (Figure 3.3).
Note: as drawing chemicals out in this way can become complicated, there is a need for a method of abbreviation. Each figure, therefore, contains examples of various chemical shorthands used to represent these structures. This will enable you to become used to seeing them. Naming and representation will be covered in more detail in Chapter 5 ‘Nomenclature: representation of chemical structures and basic terminology’.
Covalent Bonds
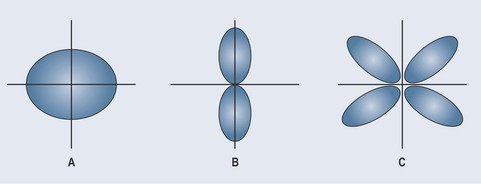
For a greater appreciation, blow up balloons of different shapes and arrange them as you see them in Figure 3.4. The different balloons are the accepted shapes of electron orbitals and the shape of the electrostatic force created by this orbital. Notice how it is possible to bend the orbital shown in B around the axis, but that in C the movement around the axis is restricted because the sides of the balloons push against one another limiting movement.
Each substrate and site has a specific shape and in many cases only certain molecules will be allowed to fit into the site, which may be on an enzyme, the surface of a cell or at a nerve ending (see Chapter 11 ‘Amino acids and proteins’, p. 85 and Chapter 19 ‘Pharmacodynamics: how drugs elicit a physiological effect’, p. 137).
Importance of Covalent Bonds
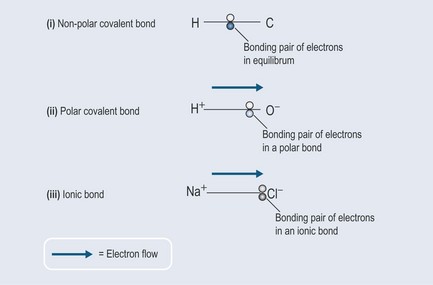
Non-polar covalent bonds (Figure 3.5(i), Figure 3.6)
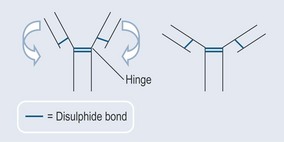
Example of relevance to pharmacology: Covalent bonds are found in protein chains where two sulphurs are joined together (disulphide bonds) (Figure 3.7). In antibody molecules, the disulphide bond firmly holds the two parts of the antibody together, allowing the rest of the structure to hinge around it. This flexibility is an important part of the function of an antibody as it allows the antibody binding sites to adapt in binding to antigens and reach more antigen sites.
Polar covalent bonds (Figure 3.5(ii))
The following is an example of the pharmacological relevance of a polar covalent bond:
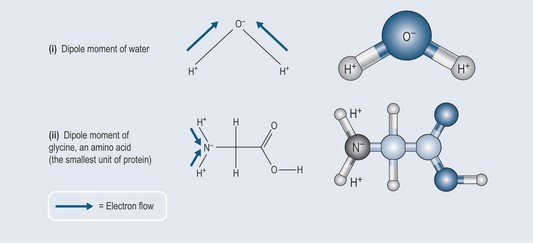
The average electron density around the oxygen atom is 10×times the amount around the hydrogen atom. This charge separation creates something called a dipole moment, as the oxygen has a partial negative charge and the hydrogen a partial positive charge (Figure 3.8(i)). Water is actually a fascinating molecule, the pharmacological importance of which will become evident as the book progresses.
The dipole moment described above also occurs in amino acids (Figure 3.8(ii); see also Chapter 11 ‘Amino acids and proteins’, p. 86) and becomes important in the temporary binding of chemicals to proteins, which are made up of amino acids, for example at enzyme binding sites or binding of chemicals to plasma proteins.
‘Oil and Water Don’t Mix’
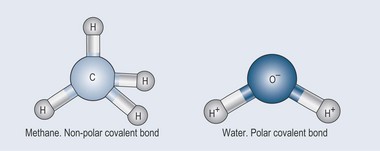
Whether a molecule is charged (polar) or not charged (non-polar) has great relevance when it comes to pharmacology. As explained above, the electron bonding between two elements such as carbon and hydrogen is equally shared so the union is well balanced. Because of this equilibrium, methane (see Figure 3.1 and Figure 3.9) has no charge (i.e. it is non-polar).
However, as you have seen, oxygen exerts a much greater electrostatic charge than hydrogen, and so draws electrons towards itself, creating a dipole moment and therefore a charged molecule (see Figure 3.5(ii) and Figure 3.9). You now know that where there is a dipole moment the molecule becomes charged.
You might have heard the phrase ‘oil and water do not mix’; more than likely this is something you will have experienced when making a vinaigrette dressing. Shaking the components of the dressing together seems to mix them, but the effect is only temporary and the mixture soon separates out into two layers, with the oily part sitting on top. Mixing two different types of oil, however, is easy and there is no separation.
Ionic Bonds (Figure 3.5(iii))
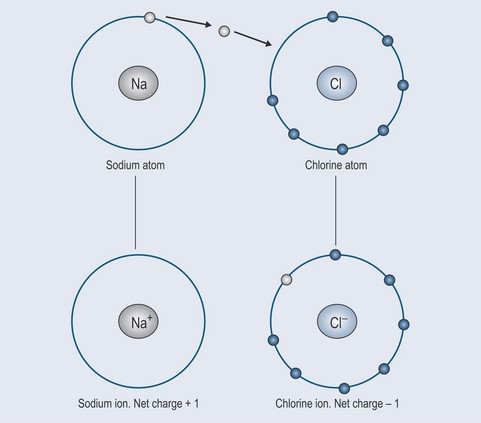
As previously discussed, all atoms like to have full outer orbitals (see Chapter 2 ‘The atom: the smallest unit of pharmacology’, p. 7). Elements such as chlorine and sodium are very unstable on their own because:
The chlorine snatches the electron from the sodium atom, making its outer orbital stable, but leaving sodium with a positive charge (Figure 3.10). The sodium atom has thus become a positively charged sodium ion (Na+). This is because the protons in the nucleus normally exactly equal the negative electrons in the outer shell (see Figure 2.1, p. 8). Sodium is now short of an electron and so, overall, gains a positive charge:
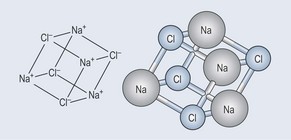
As ions of opposite charge are attracted to one another, like opposite poles of a magnet, they form a type of bond called an ionic bond. Thus a salt crystal (of the variety you use on your food) is a lattice of ionic bonds (Figure 3.11).
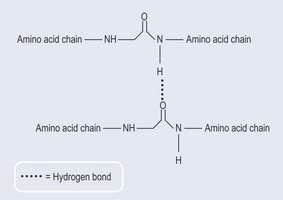
Hydrogen Bonds
Hydrogen bonds are very special as they are, strictly speaking, not true bonds but forces of attraction between the hydrogen atoms in one molecule and atoms of high electronegativity in other molecules. This situation is due to the uneven sharing of the bonding electron pair in a polar covalent bond (see Figure 3.5(ii)).
The section on polar bonds above shows that the hydrogen attached to nitrogen or oxygen becomes relatively positively charged, as the nitrogen and oxygen atoms, pulling the electrons towards them, become relatively negatively charged (see Figure 3.8).
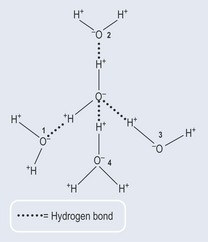
Hydrogen Bonding in Water
The relatively negatively charged oxygen atom in a water molecule is able to attract positively charged hydrogen atoms attached to other water molecules. Simple mathematics then dictates that each water molecule has the potential to form four hydrogen bonds with surrounding water molecules (Figure 3.12). This enables water to form a type of lattice even as a liquid, a phenomenon that accounts for its relatively high boiling point compared with molecules of a similar size, which are gaseous at the same temperature. It also explains why ice floats and water freezes from the top downwards.