Chapter 4 Bonds continued
Chapter 3 introduced bond formation. This chapter looks at more complex bonds, where several electrons are shared to form double or triple bonds. These bonds are relevant to the type of chemistry you will encounter in clinical practice.
Double Bonds
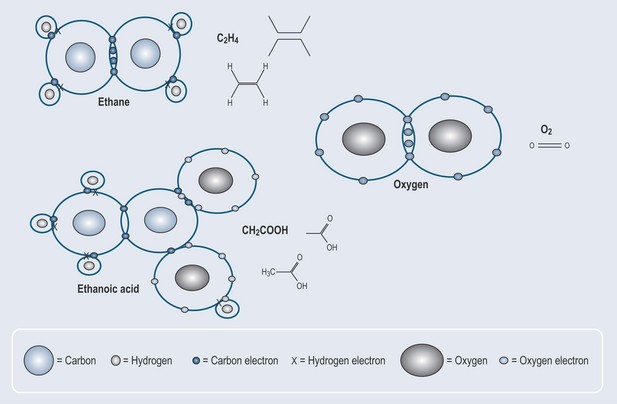
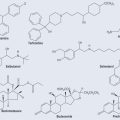
Carbon and oxygen atoms can form a double bond. Figure 4.1 gives the examples of double bonds found in pharmacology. The chemical shorthand for these structures is shown alongside the detailed diagrams of electron distribution.
Why is a double bond significant?
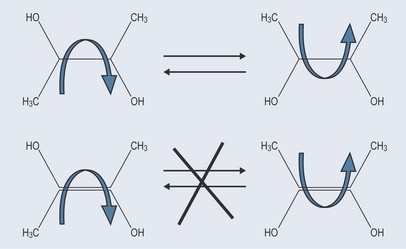
When carbon atoms are joined by a single bond, rotation can easily occur around the bond. When there is a double bond this mobility is not possible (Figure 4.2). The structure of the double bond will not allow this rotation and rigidly fixes the shape of that molecule around the double bond. This inability to change orientation is very important in pharmacology. For example, chemically active compounds might have the same molecular formula but different chemical reactions as the components of the compounds orientated differently in space. The different actions of cis and trans fatty acids on the body are important examples of geometrical isomerism (see Chapter 6 ‘Isomers’, p. 38 and Chapter 10 ‘Lipids’, p. 74).
Triple Bonds
Triple bonds (Figure 4.3) occur in nature in cyanogenic glycosides (see Chapter 24 ‘Glycosides’, p. 183) they are not as common as single or double bonds.
Aromatic Rings
What happens when alternating double and single bonds join to form a ring? If this occurs a very interesting structure called an aromatic ring forms. The bonds in an aromatic ring are particularly interesting.
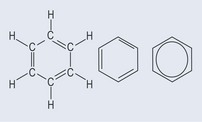
The basis of aromatic ring is benzene (Figure 4.4). In theory, benzene has three double bonds, but a molecule that is not a ring structure and has double bonds reacts very differently chemically to benzene. There are also other quibbles with bond lengths and shape, which are outside the scope of the book.
The chemical assumption that has to be made is that the electrons associated with the benzene ring are, more or less, evenly shared around the ring, so it is impossible to tell where the double and single bonds are. Thus, although the benzene ring can be thought of as a hexagon, and although the bonds are shown for the purposes of presenting a standard structure, there are, in effect, no alternating double and single bonds. In many cases, the ring is shown as the far right notation in Figure 4.4, which indicates the sharing of the electrons. This sharing gives the structure unique qualities when it comes to chemical reactions, because the hydrogen atoms around the ring can be substituted and replaced by a new functional group (see Figure 5.1, p. 30) while the benzene ring stays intact rather than breaking open and changing shape.
Long Structures
Isoprene units, which are molecules of five carbon atoms, can join together to form carbon structures that are 10, 15 or 20 carbon atoms long (see Figure 22.1, p. 167). These comprise a very important class of molecules called terpenes (see Chapter 22), which form the aromatic components of aromatherapy oils (monoterpenes; see Figure 22.3, p. 169), steroidal-type compounds (see Figure 22.7, p. 172) and carotenes (see Figure 22.8, p. 174).
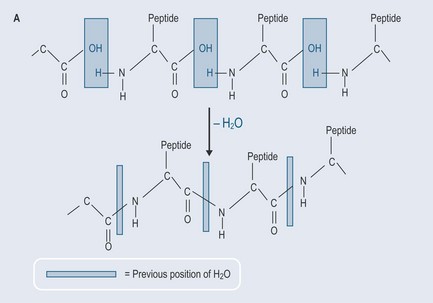
These long chains are called polymers, and are effectively repeating segments of smaller molecular units called monomers, e.g. isoprene units. Amino acids can also do this, forming polypeptide chains (polymers of amino acids), which – when they become long enough – cross-link form proteins (see Chapter 11 ‘Amino acids and proteins’).
The backbone of most of organic compounds is largely made up of carbon and hydrogen atoms (hydrocarbons) with functional groups attached to the backbone (see Figure 5.1, p. 30). As with amino acids, these functional groups are responsible for the chemical characteristics of those molecules.
The Significance of Polar and Non Polar Bonds
The body is full of water. Water is a very polar substance (see Figure 3.8, p. 16) and, therefore, if a chemical is polar it is likely to easily mix with water (see Chapter 3 ‘Bonds found in biological chemistry’, p. 16). Polar chemicals are hydrophilic (water loving).
Polarity, however, is relative and some substances have a tendency to be more polar or non-polar, depending on the environment. A good example of this can be seen in the digestive tract, where the pH in the stomach is approximately 1 and that in the small intestine is nearer 8. pH affects the absorption of the chemicals from a drug or herb by ensuring the majority of solution of the chemical is pushed towards being polar (water soluble) or non-polar (fat soluble) (see Chapter 8 ‘Acids and bases’, p. 55).
So, the more non-polar or hydrophobic a substance is, the easier it will be absorbed into the body (remember, cell membranes are largely made up of lipids). Pharmaceutical companies take this into account when they decide in which part of the digestive tract the drug needs to be absorbed. It is also worth noting as a herbalist, because changes in pH occur with age or disease, then the absorption of their herbal prescription might be affected (see Chapter 15 ‘Methods of administration’, p. 119). This will be discussed in more detail in Chapter 8 ‘Acids and bases’.
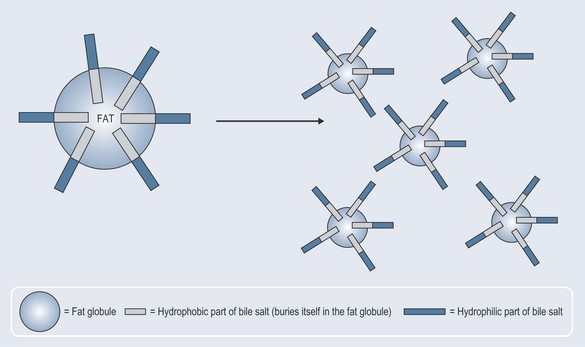
Emulsification
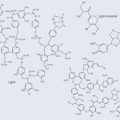
Emulsification is a very important process. A compound known as an emulsant is both hydrophobic and hydrophilic (amphipathic). For example, when fat enters the small intestine, the hydrophobic parts of the bile salts released from the bile duct latch onto the fat, leaving the hydrophilic parts free (Figure 4.5). This encourages part of the fat globule to break off and the smaller globules become surrounded once again by the bile salts. In this way, a large amount of fat (lipid) has been made available to the lipases (the enzymes that breakdown lipids) in the small intestine (i.e. the surface area has been increased). The smaller size makes it easier for the lipases to access the parts of the lipids they need to react with.
Reactions You might Encounter in Literature Related to Bonding
Hydrolysis
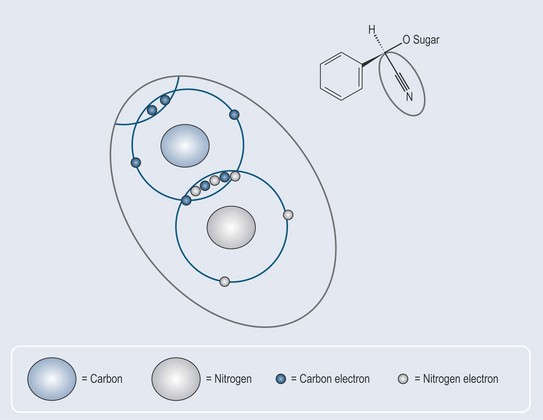
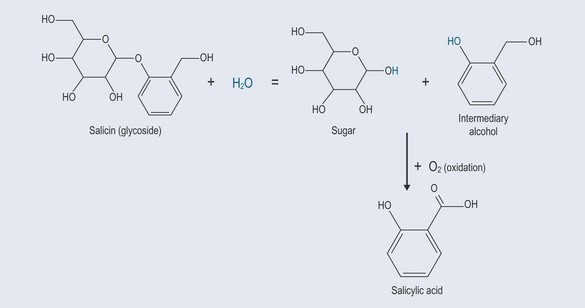
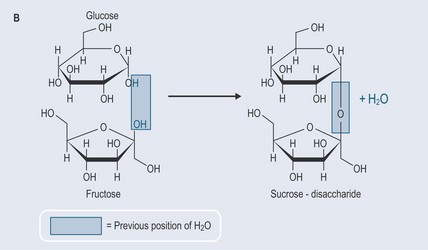
Hydrolysis occurs when the bond in an organic molecule is broken apart and the O–H component of water is used to form two new molecules. The OH group joins itself to one part from the organic molecule and the H atom from the water molecule joins to the other (Figure 4.6).
The cleaving of a sugar molecule from the organic component of herbs, many of which are attached to a sugar (see Chapter 17 ‘Metabolism’, p. 132 and Chapter 24 ‘Glycosides’, p. 181), is due to hydrolysis.