CHAPTER 31 BLUNT CEREBROVASCULAR INJURIES
Over the past decade, a wealth of studies has provided the scientific rationale to promote the early screening and treatment of blunt cerebrovascular injuries (BCVI). Initially, BCVI were thought to have unavoidable, devastating neurologic outcomes, but several reports suggested anticoagulation improves neurologic outcome in patients suffering ischemic neurologic events.1,2 If untreated, carotid artery injuries (CAIs) have a stroke rate up to 50% depending on injury grade, with increasing stroke rates correlating with increasing grades of injury; vertebral artery injuries (VAIs) have a stroke rate of 20%–25%.3 Screening protocols, based on patient injury patterns and mechanism of injury, have been instituted to identify these injuries in asymptomatic patients and to initiate treatment, prior to neurologic sequelae. Current studies suggest early antithrombotic therapy in patients with BCVI reduces stroke rate and prevents neurologic morbidity.3–8
SIGNS AND SYMPTOMS
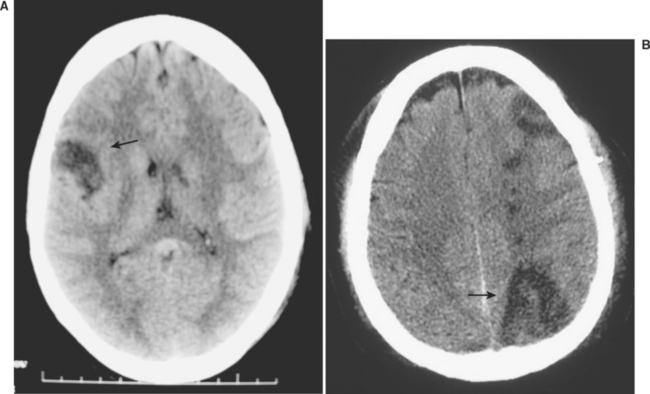
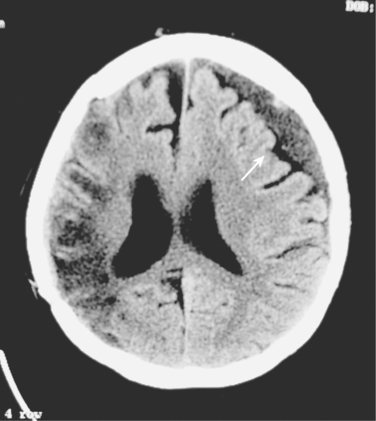
Blunt cerebrovascular injuries were first reported over 30 years ago, in patients who presented with stroke following injury.9 The patient’s symptom of cerebral ischemia or the distribution of their symptoms usually indicates the underlying cerebrovascular lesion (Figure 1). Carotid artery injuries generally result in contralateral sensorimotor deficits, which to the general practitioner is classically defined as a stroke. Aphasia occurs when the dominant hemisphere is involved, while nondominant hemisphere strokes may result in hemineglect. Vertebral artery injuries typically manifest as more vague symptomatology, namely ataxia, dizziness, vomiting, facial or body analgesia, or visual field defects. Symptoms of carotid-cavernous fistulae include orbital pain, exophthalmos, chemosis, and conjunctival hyperemia.
Although some patients may present with symptoms of BCVI-related ischemia within an hour of injury, the majority exhibit a latent period. This asymptomatic phase has been inferred based upon the time to onset of symptoms in patients with defined injuries who did not receive antithrombotic therapy. This timeframe appears to range from hours up to 14 years, but t he majority seems to develop symptoms within 10–72 hours.1,3,4,10 The goal, then, is diagnosing BCVI during this “silent period” prior to the onset stroke. After all, the theory is that if you diagnose these injuries during the asymptomatic period you can effectively treat the patient to prevent stroke. Screening for BCVI during the asymptomatic period was initially suggested in the mid-1990s after the recognition that specific patterns of injuries were associated with BCVI. Although optimal screening criteria have yet to be defined, current screening algorithms include patients considered at high risk based on their injury pattern.
MECHANISM AND PATTERNS OF INJURY
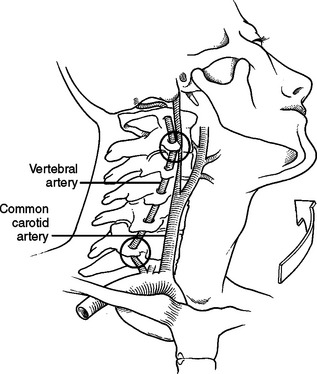
Crissey and Bernstein originally postulated three fundamental mechanisms of injury resulting in BCVI.9 The first is a direct blow to the neck. This mechanism is often seen with patients in motor vehicle collisions with inappropriately fitting seatbelts who end up with a seatbelt sign across the neck; it can also be seen in mountain bikers with a direct blow to the neck after falling during riding. The second proposed mechanism is hyperextension with contralateral rotation of the head. This is the most common mechanism causing CAI with the hyperextension resulting in a stretching of the carotid artery over the lateral articular processes of C1–C3 (Figure 2). VAI may also be due to a hyperextension-stretch injury due to the tethering of the vertebral artery within the lateral masses of the cervical spine. The third mechanism of injury is a direct laceration of the artery by adjacent fractures involving the sphenoid or petrous bones. Although originally described as the mechanism in association with CAI, this may also be the cause of VAI. With a fracture of any of the bony elements comprising the vertebral foramen, also termed the foramen transversarium, it is not surprising that the vertebral artery could be directly injured. Regardless of the type of injury mechanism, there is intimal disruption of the carotid or vertebral artery. This intimal tear becomes a nidus for platelet aggregation that may lead to emboli or vessel occlusion, and subsequent stroke.
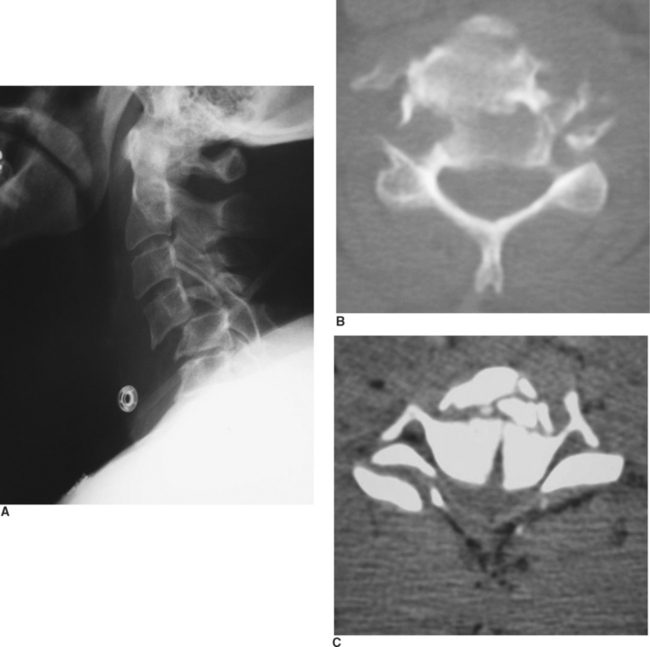
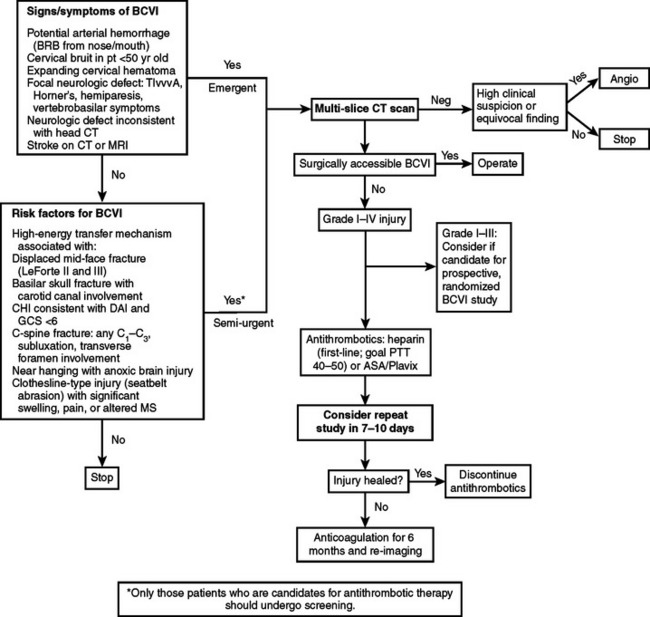
Although the mechanism of injury is important for determining patients at high risk for BCVI, the patient’s associated injuries are also critical to determine which asymptomatic patients undergo screening for BCVI. Aggressive screening for BCVI was initially suggested after recognition that specific patterns of injuries were associative. Current screening algorithms include patients with signs or symptoms, as well as those considered at high risk by injury pattern (Table 1). BCVI screening protocols have remained relatively unchanged over the past decade since their institution, aside from refinements in patients with cervical spine fractures. Initially, screening protocols included all patients with cervical spine fractures to rule out BCVI. However, there were a significant number of patients with isolated cervical spine fractures that could be managed by the local orthopedic surgeons without referral to Level I trauma facilities; therefore, we questioned the need to evaluate every patient with a cervical spine fracture.11 In our population, excluding patients who underwent screening for associated injuries or other injury patterns, only patients with three patterns of spine fracture were identified as having a BCVI, barring other symptoms of neurologic compromise: cervical spine subluxation, fractures involving the transverse foramen, and upper cervical spine fracture of C1–C3 (Figure 3). Therefore, only this subpopulation of patients with cervical spine fractures undergoes screening for BCVI.
Table 1 Denver Screening Criteria for Blunt Cerebrovascular Injuries
| Signs/Symptoms of BCVI |
BCVI, Blunt cerebrovascular injury; CT, computed tomography.
Courtesy of Denver Health Medical Center.
DIAGNOSTIC IMAGING
Using defined screening protocols, high-risk patients undergo imaging to identify BCVI. Historically, four-vessel arteriography has been the gold standard to diagnose BCVI. Undoubtedly, many clinicians question the need for subjecting patients to angiography. Angiography is labor intensive, costly, and not without risks; and, if not available at smaller hospitals, requires emergent transfer of a patient for definitive evaluation. Currently, CTA remains an unproven diagnostic modality for this injury. In particular, injuries that may be missed by such noninvasive studies are typically grade I and II injuries; however, pseudoaneurysms and occlusions have also been misdiagnosed.12,13 The risk of angiography in our screened trauma population is 0.1%, while the stroke risk of an undiagnosed grade I CAI is 8% and VAI is 6%.3,4 Recent preliminary studies report improved imaging and visualization of injuries with multislice CT scanners (16 or more slices). These promising advances in technology, which would allow noninvasive evaluation of patients, will clearly facilitate the screening process.
All patients with indications for screening, and no contraindications to antithrombotic therapy, undergo imaging as soon as possible. Patients with documented BCVI undergo repeat imaging 7–10 days after their initial diagnostic study. The importance of routine follow-up imaging is particularly salient in patients with grade I and II injuries; over half of grade I injuries completely heal, allowing cessation of antithrombotic therapy.3 While only 8% of grade II injuries heal, over 40% progress to grade III injuries despite therapy; in patients with CAI, this increase in injury grade also correlates with an increase in stroke risk. Patients with carotid or vertebral artery occlusions are not as important for reimaging, as over 80% display no change on follow-up imaging.
INJURY GRADING SCALE
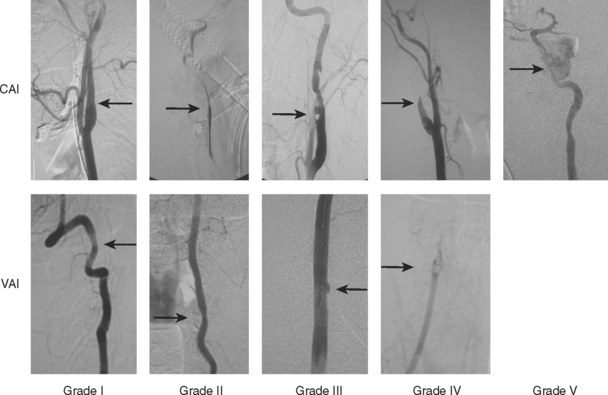
The identification of disparate outcomes associated with varied luminal irregularities comprising BCVI (dissection, occlusion, transection, and pseudoaneurysms) prompted us to propose a grading scale.10 An injury grading scale was developed to provide not only an accurate description of the injury, but also to define stroke risk by injury grade (Figure 4 and Table 2). Untreated injuries have an overall stroke rate of approximately 20%; CAIs have increasing stroke rate by increasing grade, while VAIs tend to have a more consistent stroke rate of approximately 20% for all grades of injury (Table 3).
Table 2 Denver Grading Scale for Blunt Cerebrovascular Injuries
| Grade I | Irregularity of vessel wall or dissection/intramural hematoma with less than 25% luminal stenosis |
| Grade II | Intraluminal thrombus or raised intimal flap is visualized, or dissection/intramural hematoma with 25% or more luminal narrowing |
| Grade III | Pseudoaneurysm |
| Grade IV | Vessel occlusion |
| Grade V | Vessel transection |
Courtesy of Denver Health Medical Center.
Table 3 Stroke Rate by Blunt Cerebrovascular Injury Grade
| Grade of Injury | Stroke Rate (%) | |
|---|---|---|
| Carotid arterial injury | I | 3 |
| II | 14 | |
| III | 26 | |
| IV | 50 | |
| V | 100 | |
| Vertebral arterial injury | I | 6 |
| II | 38 | |
| III | 27 | |
| IV | 28 | |
| V | 100 |
INCIDENCE OF BLUNT CEREBROVASCULAR INJURIES
Originally thought to be a rare injury, BCVIs are currently diagnosed in 1% of all blunt trauma patients. Previously, BCVI-related strokes were likely attributed to a patient’s primary head injury, rather than as a sequelae of cervical arterial injury with subsequent embolic stroke. Therefore, BCVIs were felt to be rare with an incidence less than 0.1% in all trauma patients, and with blunt CAI accounting for less than 3% of all traumatic carotid injuries. Once the patient’s neurologic changes were ascribed to a vascular source, and appropriate imaging was instituted to identify the injured artery, the incidence of BCVI identified tripled. In the first multicenter review of BCVI, performed by the Western Trauma Association, only 49 patients with carotid artery injuries were identified at 11 medical institutions over a 6-year period. With the recognition of BCVI as a specific injury causing significant morbidity and stroke-related mortality, was the associated silent period. With the advent of injury screening in asymptomatic high-risk patients, there has been a relative epidemic of BCVI diagnosed. Currently, in centers with a comprehensive screening approach, the screening yield is over 30% in high-risk populations. In our most recent Denver Health Medical Center institutional review over an 8½–year period, 0.1% of blunt injury patients presented with neurologic symptoms and 4% underwent screening angiography, with a 34% screening yield for diagnosing BCVI and an overall incidence of 1.5% in all blunt trauma victims.
ANTITHROMBOTIC TREATMENT
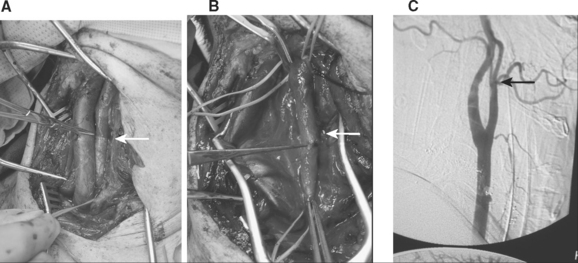
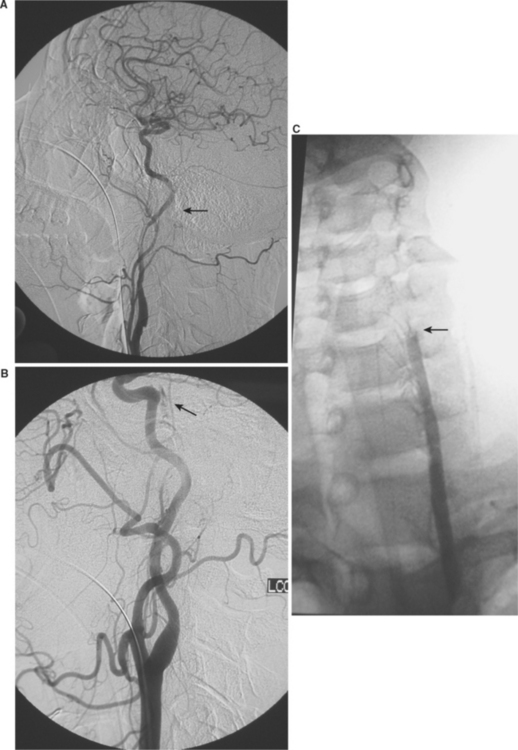
Following the recognition that BCVI were responsible for patients’ adverse neurologic events, treatment modalities were debated. If the injury occurs in a surgically accessible area of the carotid artery, particularly the common carotid artery, operative management is warranted (Figure 5). The vast majority of BCVI lesions, however, occur in surgically challenging or inaccessible areas of the blood vessels, either high within the carotid canal at the base of the skull or within the foramen transversarium (Figure 6). Such a location makes the standard vascular repair approaches including reconstruction or thrombectomy difficult if not impossible. Heparin was initially the treatment of choice for BCVI, with the assumption that this promoted clot stabilization if present and clot resolution through intrinsic fibrinolytic mechanisms, and prevented further thrombosis. Treatment with anticoagulation was shown to improve neurologic outcome in patients sustaining BCVI-related ischemic neurologic events (INEs).2,6 Initial reports, including a multicenter study by the Western Trauma Association,1 indicated that patients who were treated with anticoagulation had an improved outcome compared to those who were either not treated or had a contraindication to anticoagulation due to associated head injuries. In these studies, up to 45% of patients achieved good neurologic status, and anticoagulation therapy was independently associated with survival and improvement in neurologic outcome.
Subsequently, intravenous heparin was thought to be the treatment of choice for those asymptomatic patients with blunt injuries.3,6 Initially, standard heparinization protocols were used, but due to a moderate incidence of bleeding in multisystem trauma patients the protocol was modified (Figure 7).7,10 Currently, anticoagulation with systemic heparin is initiated using a continuous infusion of heparin at 15 U/kg/hr, without a loading dose; heparin drips are titrated to achieve a partial thromboplastin time (PTT) in 40–50 seconds. With this adjustment in the BCVI heparin protocol, less than 1% of patients have had bleeding complications necessitating transfusion in our experience.4 For patients with a contraindication to heparin, antiplatelet agents (aspirin 325 mg/day and clopidogrel 75 mg/day) have been administered. Antithrombotic therapy is not started in patients with closed head injury or intraparenchymal hemorrhage without input from the neurosurgery service. Antithrombotic therapy in patients with significant solid organ injuries or a complex pelvic fracture with associated retroperitoneal hematoma is typically not started until 24–48 hours of physiologic stability without transfusion have passed.
Currently there is controversy regarding the ideal antithrombotic therapy for any type of arterial disease—anticoagulation versus antiplatelet agents. A retrospective study by Chimowitz et al.17 indicated that warfarin is superior in patients with vertebrobasilar occlusive disease, while a more recent prospective double-blind comparison by the same authors18 demonstrated that aspirin is the therapy of choice for patients with symptomatic intracranial atherosclerotic arterial stenosis, due to equivalent stroke prevention rates as warfarin, but decreased hemorrhagic complications. A recent review of vertebrobasilar disease supported the use of antiplatelet agents in patients with arterial stenosis but warfarin in patients with severe, flow-limiting lesions or dissections.19 Which therapeutic agent is used, and whether the choice of antithrombotic should be determined by the patient’s injury grade, must continue to be evaluated in prospective studies.
Most importantly, patients who are diagnosed with BCVI early and are treated with antithrombotics almost universally avoid INE.4,8,12 The Memphis group showed a reduction in stroke rate for CAI from 64% in untreated patients to 6.8% in patients treated with antithrombotics (either anticoagulation or antiplatelet agents), and for VAI a reduction from 54% to 2.6% in treated patients.12 Our group’s most recent evaluation demonstrated a stroke rate of 0.5% in 187 patients with BCVI treated with antithrombotics, while untreated patients had an overall stroke rate of 21%.8 Although the optimal regimen remains unanswered, there appears to be equivalence between the two therapies (anticoagulation and antiplatelet agents) with regard to stroke rate.3,4,8,12
ROLE OF ENDOVASCULAR STENTS
Several isolated case reports have advocated the use of percutaneous angioplasty and stenting of carotid injuries. Not surprisingly, these case reports represent a diverse range of pathologies, symptoms, mechanisms of injury, and time to diagnosis. Although the majority appears to have patency of the stented carotid artery documented in follow-up radiographic evaluation, several cases of carotid artery occlusion following stent placement have been reported. Our most recent evaluation indicates a prohibitive stroke and carotid occlusion rate associated with carotid stents placed in acutely injured vessels.20 During the 8½–year study period, 140 patients sustained blunt carotid injury, of whom 46 (33%) had a carotid artery pseudoaneurysm. Carotid stents were placed in 23 patients, while the remaining 23 patients were treated with antithrombotic therapy alone. In the group undergoing stent placement, three patients suffered stroke and one patient sustained a subclavian artery dissection. Carotid occlusion rate in this group was 45%. In patients treated solely with antithrombotics, including heparin or antiplatelet agents, there were no ischemic neurologic events and the occlusion rate on follow-up angiography was 5%. Additionally, long-term follow-up in patients with traumatic pseudoaneurysms who were treated with anticoagulation alone is needed. Further understanding and evaluation of the role of appropriate concurrent antithrombotic therapy, as well as evolving stent technology including smaller delivery systems and covered stents, may improve the outcome for postinjury intraluminal carotid stents. In the interim, however, our experience suggests carotid stenting should be performed in selective cases and antithrombotic therapy remains the cornerstone of treatment for post-traumatic pseudoaneurysms.
LONG-TERM FOLLOW-UP AND OUTCOME
The morbidity and mortality of BCVI-related ischemic neurologic events are well documented. Historically, BCVI strokerelated permanent neurologic morbidity was greater than 80% with associated mortality rates of 40%. Modern series report lower rates of morbidity but mortality due to BCVI is significant, with CAI patients having a 13%–21% stroke-related mortality and patients with VAI-related strokes a 4%–18% mortality rate.21 A less studied variable is the impact of neurologic morbidity on the need for prolonged acute patient care. Our recent evaluation points to a greater need for discharge and overall rate of discharge to rehabilitation services in patients suffering BCVI-related INE.8 Such prolonged acute patient care increases costs to the patient, insurance companies, and ultimately to society. Performing a cost analysis of direct BCVI-related costs is difficult in these multisystem trauma patients; however, a cost analysis of patient life is even more problematic. In our series, overall mortality in patients sustaining CAI was 7% for those without neurologic event versus 32% for those with neurologic event; in patients with VAI, those without neurologic event had a mortality of 7% while those with a neurologic event had a mortality rate of 18%. The impact on mortality due to BCVI-related strokes appears independent of a patient’s associated injuries, as the ISS was not significantly different between those with and without INE.
CONCLUSIONS
Diagnosis and treatment of BCVI has evolved over the past three decades. Originally thought to be a rare occurrence, BCVIs are now diagnosed in approximately 1% of blunt trauma patients. The recognition of a clinically silent period allows for screening for injuries based on mechanism of trauma and the patient’s constellation of injuries. Currently, protocols exist for screening, hence limiting invasive procedures to those with the highest risk of injury (Figure 8). Comprehensive evaluation of patients has resulted in the early diagnosis of BCVI during the asymptomatic phase, thus allowing prompt initiation of treatment. Although the ideal regimen of antithrombotic therapy has yet to be determined, treatment with either antiplatelet agents or anticoagulation reduces the BCVI-related stroke rate. BCVI is a rare but potentially devastating injury; appropriate screening in high-risk patients should be performed and prompt treatment initiated to prevent ischemic neurologic events.
1 Cogbill TH, Moore EE, Meissner M, et al. The spectrum of blunt injury to the carotid artery: a multicenter perspective. J Trauma. 1994;37:473-479.
2 Davis JW, Holbrook TL, Hoyt DB, et al. Blunt carotid artery dissection: incidence, associated injuries, screening, and treatment. J Trauma. 1990;30:1514-1517.
3 Biffl WL, Ray CEJr, Moore EE, et al. Treatment-related outcomes from blunt cerebrovascular injuries: importance of routine follow-up arteriog-raphy. Ann Surg. 2002;235:699-706.
4 Cothren CC, Moore EE, Biffl WL, et al. Anticoagulation is the gold standard therapy for blunt carotid injuries to reduce stroke rate. Arch Surg. 2004;139:540-545.
5 Miller PR, Fabian TC, Bee TK, et al. Blunt cerebrovascular injuries: diagnosis and treatment. J Trauma. 2001;51:279-285.
6 Fabian TC, Patton JH, Croce MA. Blunt carotid injury. Importance of early diagnosis and anticoagulant therapy. Ann Surg. 1996;223:513-522.
7 Biffl WL, Moore EE, Ryu RK, et al. The unrecognized epidemic of blunt carotid arterial injuries: early diagnosis improves neurologic outcome. Ann Surg. 1998;228:462-470.
8 Cothren CC, Moore EE, Ray CE, et al. Screening for blunt cerebrovascular injuries is cost effective. Am J Surg. 2005;190:845-849.
9 Crissey MM, Bernstein EF. Delayed presentation of carotid intimal tear following blunt craniocervical trauma. Surgery. 1974;75(4):543-549.
10 Biffl WL, Moore EE, Offner PJ, et al. Blunt carotid arterial injuries: implications of a new grading scale. J Trauma. 1999;47(5):845-853.
11 Cothren CC, Moore EE, Johnson JL, Ciesla DJ, Moore JB, Burch JM. Cervical spine fracture patterns mandating angiography to rule out blunt cerebrovascular injury. Surgery. 2007;141:76-82.
12 Miller PR, Fabian TC, Croce MA, et al. Prospective screening for blunt cerebrovascular injuries: analysis of diagnostic modalities and outcomes. Ann Surg. 2002;236:386-393.
13 Malhotra AK, Camacho M, Ivatury RR, et al. Computed tomographic angiography for the diagnosis of blunt carotid/vertebral artery injury: a note of caution. Ann Surg. 2007;246:632-641.
14 Biffl WL, Egglin T, Benedetto B, Gibbs F, Cioffi WG. Sixteen-slice computed togographic angiography is a reliable noninvasive screening test for clinically significant blunt cerebrovascular injuries. J Truma. 2006;60(4):745-751.
15 Utter GH, Hollingworth W, Hallam DK, Jarvik JG, Jurkovich GJ. Sixteen-slice CT angiography in patients with suspected blunt carotid and vertebral artery injuries. J Am Coll Surg. 2006;203(6):838-848.
16 Eastman AL, Chason DP, Perez CL, McAnulty AL, Minei JP. Computed tomographic angiography for the diagnosis of blunt cervical vascular injury: is it ready for primetime? J Trauma. 2006;60(5):925-929.
17 Chimowitz MI, Kokkinos J, Strong J, et al. The Warfarin-Aspirin Symptomatic Intracranial Disease Study. Neurology. 1995;45(8):1488-1493.
18 Chimowitz MI, Lynn MJ, Howlett-Smith H, et al. Comparison of Warfarin and Aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005;352:1305-1316.
19 Savitz SI, Caplan LR. Vertebrobasilar disease. N Engl J Med. 2005;352:2618-2626.
20 Cothren CC, Moore EE, Ray CE, et al. Carotid artery stents for blunt cerebrovascular injury: risks exceed benefits. Arch Surg. 2005;140:480-486.
21 Miller RS, Patton M, Graham RM, Hollins D. Outcomes of trauma patients who survive prolonged lengths of stay in the intensive care unit. J Trauma. 2000;48:229-234.