Blood transfusion
Principles of blood transfusion
Nevertheless, blood transfusion carries a range of potential hazards, including transfusion reactions, transmission of infection, clerical errors leading to incompatible transfusion, and potential immunosuppression in cancer patients. For replacing blood loss, stored blood has the advantage over gelatin and electrolyte solutions that it remains within the vascular compartment, although most ‘blood’ for transfusion consists of concentrated red cells with platelets and clotting factors removed. Potentially lethal side-effects mean that a decision to transfuse blood or blood products must be based on clear indications and after considering alternatives (see Reducing the need for bank blood transfusion, p. 120). The types of transfusion components available and the general indications for their use are summarised in Box 9.1.
Laboratory aspects of blood transfusion
Blood transfusion in clinical practice
Reducing the need for bank blood transfusion
Autologous transfusion
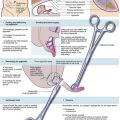
Intraoperative cell salvage (IOCS)
• In manual (unwashed) salvage, blood is collected into a reservoir containing anticoagulant before being reinfused via a fine filter
• In washed systems, an automated cell saver collects, washes, concentrates and resuspends salvaged red cells in physiological solution. This blood, with a packed cell volume of 50%, is then available for reinfusion. Some Jehovah’s Witnesses allow this, as blood is regarded as never having left the body. The technique remains controversial in malignant disease even though clinical studies have not demonstrated dissemination of metastases.
Hazards and complications of blood transfusion
Haemolytic reactions
If there is major ABO incompatibility, massive haemolysis may be fatal. Incompatibility of minor determinants causes a lesser degree of haemolysis. Almost all haemolytic reactions are caused by human error and incompatible transfusion. Clinical features are summarised in Box 9.2. The diagnosis is confirmed by blood tests—finding hyperbilirubinaemia and a positive Coombs’ test, and demonstrating a new antibody. With massive haemolysis, haemoglobinaemia and haemoglobinuria may occur. The transfusion must be halted immediately and the patient resuscitated. Oliguria is treated by osmotic diuresis (e.g. mannitol), sometimes aided by a loop diuretic.
Infection
Infection may arise from three sources: the donor, contamination during blood preparation and storage, or from the giving set or cannula site (Fig. 9.1).
Infections transmitted by donor blood or blood product transfusion
• Viral infections—hepatitis B, C and D; HIV I and II; HTLV I and II; cytomegalovirus; Epstein–Barr virus
• Bacterial infections—syphilis; brucellosis
• Protozoal infections—malaria; Chagas disease and babesiosis
The more frequent of these infections are discussed below.
Hepatitis viruses: In carriers or infected individuals, hepatitis B virus is transmitted by any blood product. Donors have been screened for hepatitis B virus (HBV) by all transfusion services in the developed world since the late 1960s and this has dramatically reduced transfusion-related hepatitis B. Despite screening, infections occasionally occur because the surface antigen HBsAg, the first marker to appear, may be absent early after infection. For this reason, patients needing multiple transfusions or blood products should be vaccinated against hepatitis B.
Human immunodeficiency virus (HIV): In 1981, the HIV epidemic and its link with blood transfusion was recognised, with reports of opportunistic pneumonias in haemophiliacs. By the mid-1980s, the risk of HIV transmission via transfused blood in the USA was as high as 1 in 2500. Luckily, transmission via surgical blood transfusion has been rare in the UK. Haemophiliacs were much less fortunate, being infected via clotting factor VIII preparations, often collected abroad.
Cytomegalovirus: Infection with cytomegalovirus is widespread, with the virus remaining latent in leucocytes. The risk of infection is significant only in immature neonates and CMV seronegative immunosuppressed patients. To minimise risk, donated blood for these groups should be screened for CMV-specific antibody. Leucodepletion removes 99.9% of white cells and is as effective in reducing the risk of CMV infection.
Protozoal infection—malaria: Transfusion-transmitted malaria remains rare in developed countries. In endemic areas it is common and most healthy blood donors are potentially infectious. The disease should be considered if a patient becomes ill after transfusion.
Variant Creutzfeldt–Jakob disease (vCJD): vCJD was first described in the UK in 1996. It differs from the familial forms of CJD and is closely linked to bovine spongiform encephalopathy in cattle (BSE), and is thought to be acquired by ingestion of infected meat. There are currently 12–15 new cases of vCJD per annum in the UK, but the number incubating the disease is unknown.
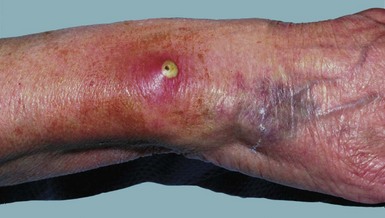
Contamination of blood or giving sets with microorganisms
Giving sets may become contaminated unless strict aseptic technique is maintained during setting-up or changing of any component of transfusion equipment. Finally, peripheral intravenous cannulae, and particularly central venous lines, may become infected by opportunistic skin commensals (e.g. coagulase-negative staphylococci) or contaminating pathogens (e.g. Staph. aureus, E. coli) causing local infection (see Fig. 9.1), bacteraemia or even systemic sepsis. Cannula sites should be inspected daily and changed at least every 72 hours. In a patient with a central venous line and pyrexia of unknown origin, the line should be removed and tested bacteriologically.