Chapter 11 Blood supply of the lumbar spine
The lumbar arteries
A pair of lumbar arteries arises from the back of the aorta in front of each of the upper four lumbar vertebrae.1,2 Occasionally, the arteries at a particular level may arise as a single common trunk which rapidly divides into right and left branches. At the L5 level, the fifth lumbar arteries arise from the median sacral artery but otherwise they resemble the other lumbar arteries.
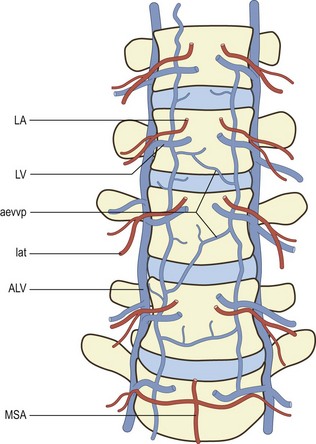
Each lumbar artery passes backwards around its related vertebral body (Fig. 11.1), lying in the concavity formed by the lateral surface of the vertebral body where it is covered by the tendinous arch of the psoas muscle. Upon reaching the level of the intervertebral foramen, the artery divides into several branches (Fig. 11.2).
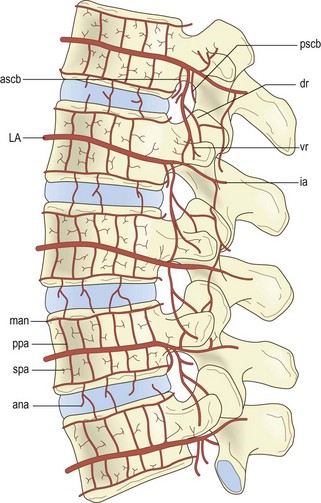
Lateral branches pass through the psoas and quadratus lumborum muscles eventually to supply the abdominal wall. Others pass with the ventral ramus and dorsal ramus of the spinal nerve supplying the paravertebral muscles innervated by these nerves. A substantial posteriorly directed branch passes below the transverse process, running perpendicular to the lateral border of the pars interarticularis of the lamina, to enter the back muscles (see Fig. 11.2).1,3 In addition to supplying the back muscles, the posterior branches of the lumbar arteries form anastomoses around the zygapophysial joints, which they supply, and plexuses that surround and supply the laminae and spinous processes.1
Opposite the intervertebral foramen, three medially directed branches arise from the lumbar artery (see Fig. 11.2). These are the anterior spinal canal branch, the posterior spinal canal branch and the radicular branch.1,3 The radicular branches are described in detail later.
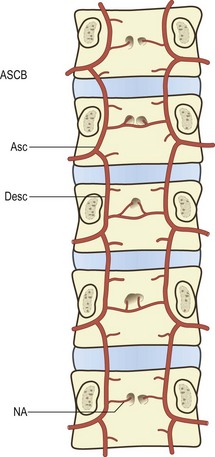
The anterior spinal canal branch at each level enters the intervertebral foramen and bifurcates into ascending and descending branches. The ascending branch crosses the intervertebral disc and circumvents the base of the pedicle above to anastomose with the descending branch from the next higher segmental level. In this way a series of arterial arcades is formed across the back of the lumbar vertebral bodies, i.e. along the floor of the vertebral canal (Fig. 11.3).
The lumbar veins
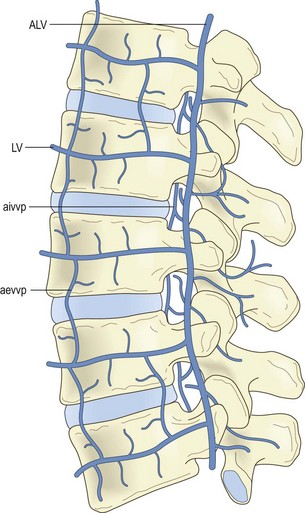
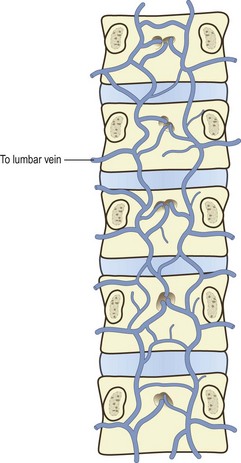
Several veins surround and drain the lumbar spine. These are the lumbar veins, the ascending lumbar veins and several vertebral venous plexuses. The lumbar veins accompany the lumbar arteries in their course around the vertebral bodies, and drain into the inferior vena cava (see Fig. 11.1). Opposite the intervertebral foramina the lumbar veins on each side communicate with the ascending lumbar vein, a long channel that runs in front of the bases of the transverse processes (Fig. 11.4). Inferiorly on each side, the ascending lumbar vein communicates with the common iliac vein while, superiorly, the right ascending lumbar vein joins the azygous vein, and the left ascending lumbar vein joins the hemiazygous vein.
Over the anterolateral aspects of the lumbar spine, a variable series of vessels interconnect the lumbar veins to form the anterior external vertebral venous plexus (see Fig. 11.4). Within the vertebral canal, two other plexuses are formed. One covers the floor of the vertebral canal and is known as the anterior internal vertebral venous plexus (Fig. 11.5). The other lines the roof of the vertebral canal and is called the posterior internal vertebral venous plexus. Within the vertebral canal these plexuses extend superiorly to thoracic levels and inferiorly to sacral levels, and at each intervertebral foramen the two internal vertebral venous plexuses communicate with the ascending lumbar veins.
Blood supply of the vertebral bodies
As each lumbar artery crosses its vertebral body, it gives off some 10–20 ascending and descending branches called the primary periosteal arteries.2 Branches of these vessels supply the periosteum and outermost walls of the vertebral body (Figs. 11.2, 11.6). Similar periosteal branches arise from the arcade of the anterior spinal canal arteries to supply the posterior wall of the vertebral body (see Figs. 11.2, 11.6).
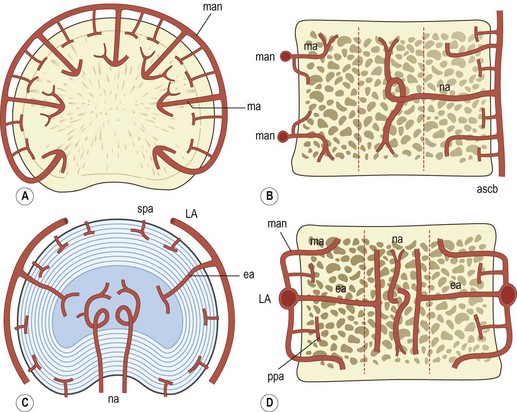
Figure 11.6 The intraosseous arteries of the lumbar vertebral bodies. (Based on Ratcliffe 1980.2) (A) Transverse section of upper or lower end of vertebral body showing the metaphysial anastomosis (man) and the sectors supplied by the metaphysial arteries (ma). (B) Midline, sagittal section showing the central distribution of the nutrient artery (na), and the peripheral distribution of the metaphysial arteries (ma) and the penetrating branches of the anterior spinal canal branches (ascb). (C) Transverse section through the middle of the vertebral body showing the central distribution of the nutrient arteries (na) augmented by equatorial branches (ea) of the lumbar artery (LA), and the superficial distribution of the secondary periosteal arteries (spa). (D) Frontal section through the middle of the vertebral body showing the central distribution of the nutrient arteries (na) and the equatorial arteries (ea), and the peripheral distribution of the metaphysial anastomosis (man), metaphysial arteries (ma) and the primary periosteal arteries (ppa) that arise from the lumbar artery (LA).
At the upper and lower ends of each vertebral body, terminal branches of the primary periosteal arteries form an anastomotic ring called the metaphysial anastomosis.2 This ring runs parallel to the superior or inferior border of the vertebral body and surrounds its anterior and lateral aspects (see Figs. 11.2, 11.6).
Branches from the metaphysial anastomosis and others from the lumbar arteries and the anterior spinal canal arteries penetrate and supply the internal parts of the vertebral body. The penetrating branches of the anterior spinal canal arteries pierce the middle of the posterior surface of the vertebral body and are known as the nutrient arteries of the vertebral body. They divide into ascending and descending branches that supply the central core of the vertebral body (see Fig. 11.6). Penetrating branches of the lumbar arteries, called the equatorial arteries, pierce the anterolateral surface of the vertebral body at its midpoint and divide into ascending and descending branches that join those of the nutrient arteries to supply the central core of the vertebra.
The peripheral parts of the upper and lower ends of the vertebral body are supplied by penetrating branches of the metaphysial anastomosis called metaphysial arteries. Several metaphysial arteries pierce the anterior and lateral surfaces of the vertebral body at its upper and lower ends, and each artery supplies a wedge-shaped region that points towards the central core of the vertebral body (see Fig. 11.6).
In the region of the vertebral endplate, terminal branches of the metaphysial arteries and the nutrient arteries form dense capillary plexuses in the subchondral bone deep to the endplate and in the base of the endplate cartilage.1,4 Details of the morphology of this plexus are not known in humans, but in dogs, certain differences occur in different regions. Over the nucleus pulposus, the capillary terminations are sessile and discoid ‘like the suckers on the tentacles of an octopus’,5 while over the anulus fibrosus the capillary terminals are less dense, smaller and simpler in appearance.5 The functional significance of these differences, however, still remains obscure.
The principal veins of the vertebral body are the basivertebral veins. These are a series of long veins running horizontally through the middle of the vertebral body (Fig. 11.7). They drain primarily posteriorly, forming one or two large veins that pierce the posterior surface of the vertebral body to enter the anterior internal vertebral venous plexus. Anteriorly, the basivertebral veins drain to the anterior external vertebral venous plexus.
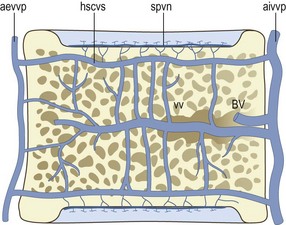
Figure 11.7 The intraosseous veins of the lumbar vertebral bodies. (Based on Crock et al. 1973.4) aevvp, anterior external vertebral venous plexus; aivvp, anterior internal vertebral venous plexus; BV, the basivertebral veins; hscvs, horizontal subchondral collecting vein system; spvn, subchondral postcapillary venous network; vv, vertical veins within the vertebral body.
In the region immediately adjacent to each vertebral endplate, the capillaries of the subchondral bone drain into a system of small veins that lies parallel to the disc–bone interface (see Fig. 11.7); this is the subchondral postcapillary venous network.1,4 Short vertical veins drain this network into a larger venous system that again lies parallel to the vertebral endplate (see Fig. 11.7); this is the horizontal subarticular collecting vein system.1,4 The veins in this system are arranged in a radial pattern that converges centrally opposite the nucleus pulposus. Here the veins turn towards the centre of the vertebral body and form the vertical veins that drain through the central core of the body to the basivertebral veins. Peripheral elements of the horizontal subarticular collecting vein system drain to the anterior external and anterior internal vertebral venous plexuses.
Blood supply of the spinal nerve roots
The lumbar spinal nerve roots receive their blood supply from two sources. Proximally, they are fed by vessels from the conus medullaris of the spinal cord. Distally, in the intervertebral foramina, they receive the radicular branches of the lumbar arteries.6–8
At their attachment to the conus medullaris, virtually each of the ventral and dorsal rootlets is supplied by a fine branch derived from the extra-medullary longitudinal vessels of the conus (Fig. 11.8) but the distribution of these small branches is limited to a few centimetres along the rootlets.7 The rest of the proximal ends of the dorsal and ventral roots are supplied by the proximal, ventral and dorsal radicular arteries (see Fig. 11.8).
The dorsal proximal radicular arteries arise from the dorsolateral longitudinal vessels of the conus (derived from the posterior spinal arteries), and the ventral proximal radicular arteries arise from the ‘accessory anterolateral longitudinal channels’ (derived from the anterior spinal artery).7 Each proximal radicular artery travels with its root but is embedded in its own pial sheath, until several millimetres from the surface of the spinal cord, it penetrates the root.7 Upon entering the root, the radicular artery follows one of the main nerve bundles along its entire length and gives off collateral branches that enter and follow other nerve fascicles. Within a root there may be one to three substantial vessels that could be named as the proximal radicular artery.
At each intervertebral foramen, the radicular branch of the lumbar artery enters the spinal nerve and then divides into branches that enter the ventral and dorsal roots (see Fig. 11.8). These vessels may be referred to as the distal radicular arteries, to distinguish them from the proximal radicular arteries arising from the conus medullaris. Each distal radicular artery passes proximally along its root, giving off collateral branches, until it meets and anastomoses with its respective proximal radicular artery. En route, the dorsal distal radicular artery forms a plexus around the dorsal root ganglion.8
Within each root, collateral branches of the proximal and distal radicular arteries communicate with one another through transverse branches (Fig. 11.9), and a particular feature of these branches in the adult is that they are coiled.7 Similarly, their parent vessels are coiled proximal and distal to the origin of each of these transverse communicating branches (see Fig. 11.9). These coils appear to be designed to accommodate the stretching of the nerve root that occurs during movements of the lumbar spine.8 They are less developed in neonates because of the relatively shorter length of the lumbar spinal nerve roots, and hence there is a lesser propensity for them to stretch.
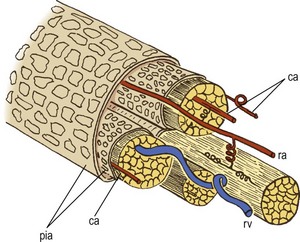
Figure 11.9 The distribution of radicular vessels in a nerve root. (Based on Parke and Watanabe 1985.7) The radicular artery (ra) runs with the nerve bundles in the nerve root, accompanied by several collateral arteries (ca) in adjacent nerve bundles. The arteries anastomose with one another through coiled junctions. The radicular vein (rv) has a sinuous course separate to that of the arteries. pia, pia mater.
The point of anastomosis between the proximal and the distal radicular arteries lies in the proximal half of each root.8 Consequently, the proximal radicular artery supplies the proximal one-third or so of the root, while the distal two-thirds are supplied by the distal radicular artery. Arterial supply, however, is neither the only nor the principal source of nutrition for the roots. Only some 35% of the glucose absorbed by a root comes from the radicular arteries. The rest is absorbed directly from the surrounding CSF.7
The veins of the nerve roots may be divided into proximal and distal radicular systems but are fewer in number than the corresponding arteries and run courses separate to those of the arteries.7 The veins tend to lie deep in the nerve bundle and assume a spiralling course (see Fig. 11.9). The proximal veins drain towards the spinal cord, while the distal veins drain towards the intervertebral foramina where they join the tributaries of the lumbar veins and the ascending lumbar veins.
Nutrition of the intervertebral disc
The intervertebral disc is not an inert structure. The cartilage cells in the nucleus pulposus and the fibroblasts in the anulus fibrosus are biologically active, albeit at a low-grade level, but this activity is essential for the constant synthesis and replacement of proteoglycans and collagen.9–12 To sustain this activity these cells require nutrition.13 However, the intervertebral discs receive no major arterial branches.
The only vessels that actually enter the discs are small branches from the metaphysial arteries which anastomose over the outer surface of the anulus fibrosus (see Fig. 11.2) but these branches are restricted to the very outermost fibres of the anulus.9 Consequently, for their nutrition, intervertebral discs are dependent on diffusion, and this diffusion takes place from the two closest available systems of vessels: those in the outer anulus, and the capillary plexuses beneath the vertebral endplates.
To reach the nucleus pulposus, nutrients like oxygen, sugar and other molecules must diffuse across the matrix of the vertebral endplate or through the anulus fibrosus. Subsequently, nutrients to the nucleus must permeate the proteoglycan matrix of the nucleus. The rate of diffusion of nutrients through these media is dependent on three principal factors: the concentration gradient of any particular substance; the resistance to diffusion offered by the endplate or the anulus fibrous; and the resistance to diffusion offered by the proteoglycans of the nucleus.13
In this respect, the permeabilities of the anulus fibrosus and the vertebral endplates differ. Virtually the entire anulus fibrosus is quite permeable to most substances but only the central portions of the vertebral endplates are permeable.11–13 However, because the surface area of the endplates is greater than that of the anulus, the relative contributions to disc nutrition from the anulus and the endplates is approximately the same. This conclusion, however, holds only for uncharged molecules which are unaffected by other processes.11–13 The diffusion of charged molecules is affected by the chemical properties of the nucleus pulposus.
The resistance to diffusion of charged molecules offered by the nucleus pulposus is a property of the high concentration of the negatively charged carboxyl and sulphate radicals in its mucopolysaccharides.11,13 Uncharged molecules like glucose or oxygen permeate readily through the proteoglycan matrix of the nucleus, but negatively charged substances, like sulphate ions and chloride ions, meet great resistance once they cross the endplates and reach the matrix. On the other hand, positively charged ions like sodium and calcium pass readily from the endplates into the matrix.
Because the concentration of mucopolysaccharides in the anulus fibrosus is less than that in the nucleus pulposus, the anulus offers less resistance to the diffusion of negatively charged molecules, and most negatively charged solutes that reach the nucleus do so via the anulus.13
Although it is generally regarded that diffusion is the principal mechanism by which nutrients reach the inner parts of the intervertebral disc,12,14 there has been some work to suggest that compression of the intervertebral disc tends to squeeze water out of it, and when the compression is released, the water returns. It is maintained by some authorities that this flux of water is capable of carrying nutrients with it.15 In particular, it has been shown in animal experiments that spinal movements, over a long time, exert a positive nutritional effect on the disc.16 It is presumable that a similar phenomenon occurs in humans but the extent to which exercise might benefit human discs, or whether it forestalls disc degeneration, still remains to be shown.
1 Crock HV, Yoshizawa H. The blood supply of the lumbar vertebral column. Clin Orthop. 1976;115:6-21.
2 Ratcliffe JF. The arterial anatomy of the adult human vertebral body: a microarteriographic study. J Anat. 1980;131:57-79.
3 Dommisse GF. The Arteries and Veins of the Human Spinal Cord from Birth. Edinburgh: Churchill Livingstone; 1975.
4 Crock HV, Yoshizawa H, Kame S. Observations on the venous drainage of the human vertebral body. J Bone Joint Surg. 1973;55B:528-533.
5 Crock HV, Goldwasser M. Anatomic studies of the circulation in the region of the vertebral end-plate in adult greyhound dogs. Spine. 1984;9:702-706.
6 Dommisse GF, Grobler L. Arteries and veins of the lumbar nerve roots and cauda equina. Clin Orthop. 1976;115:22-29.
7 Parke WW, Watanabe R. The intrinsic vasculature of the lumbosacral spinal nerve roots. Spine. 1985;10:508-515.
8 Parke WW, Gammell K, Rothman RH. Arterial vascularisation of the cauda equina. J Bone Joint Surg. 1981;63A:53-62.
9 Maroudas A, Nachemson A, Stockwell R, et al. Some factors involved in the nutrition of the intervertebral disc. J Anat. 1975;120:113-130.
10 Souter WA, Taylor TKF. Sulphated acid mucopolysaccharide metabolism in the rabbit intervertebral disc. J Bone Joint Surg. 1970;52B:371-384.
11 Urban J, Maroudas A. The chemistry of the intervertebral disc in relation to its physiological function. Clin Rheum Dis. 1980;6:51-76.
12 Urban JPG, Holm S, Maroudas A. Diffusion of small solutes into the intervertebral disc. Biorheology. 1978;15:203-223.
13 Maroudas A. Nutrition and metabolism of the intervertebral disc. In: Ghosh P, editor. The Biology of the Intervertebral Disc, vol. II. Boca Raton: CRC Press; 1988:1-37. Ch. 9
14 Holm S, Maroudas A, Urban JPG, et al. Nutrition of the intervertebral disc: solute transport and metabolism. Connect Tissue Res. 1981;8:101-119.
15 Kraemer J, Kolditz D, Gowin R. Water and electrolyte content of human intervertebral discs under variable load. Spine. 1985;10:69-71.
16 Holm S, Nachemson A. Variations in the nutrition of the canine intervertebral disc induced by motion. Spine. 1983;8:866-874.