CHAPTER 4 Blood, lymphoid tissues and haemopoiesis
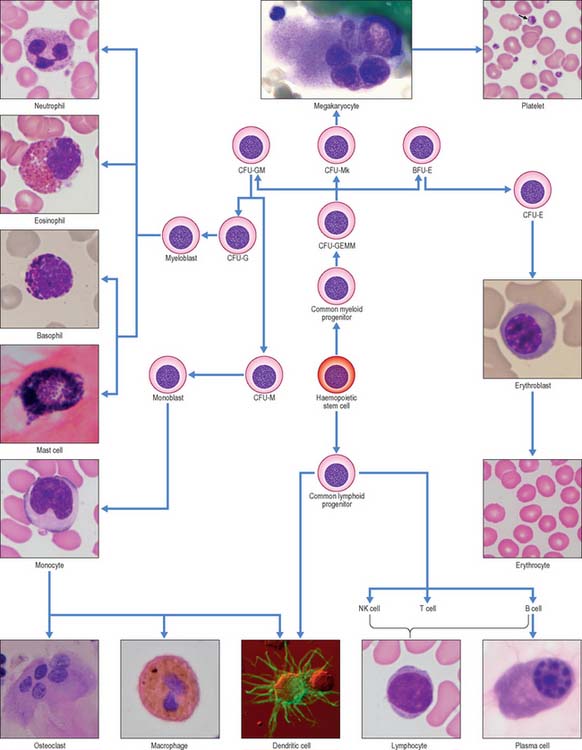
In postnatal life blood cells are formed in the bone marrow. Haemopoiesis produces red cells (erythrocytes), and a wide variety of defensive cells (white blood cells, or leukocytes). The latter include neutrophil, eosinophil and basophil granulocytes, B lymphocytes and monocytes. T lymphocytes develop in the thymus from bone marrow-derived progenitors. Platelets are produced in the bone marrow as cellular fragments of megakaryocytes. Only erythrocytes and platelets are generally confined to the blood vascular system, whereas all leukocytes can leave the circulation and enter extravascular tissues. The numbers of cells doing so increases greatly during inflammation caused by local infections and diseases.
CELLS OF PERIPHERAL BLOOD
BLOOD
Plasma
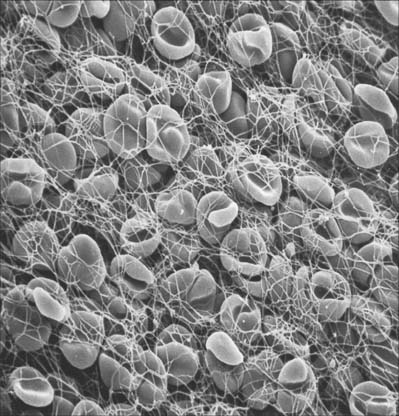
The precipitation of the protein fibrin from plasma to form a clot (Fig. 4.1) is initiated by the release of specific materials from damaged cells and blood platelets in the presence of calcium ions. If blood or plasma samples are allowed to stand, they will separate into a clot and a clear yellowish fluid, the serum. Clot formation is prevented by removal of calcium ions, e.g. by addition of citrate, oxalate or various organic calcium chelators (EDTA, EGTA) to the sample. Heparin is also widely used as an anticlotting agent, because it interferes with another part of the complex series of chemical interactions which lead to fibrin clot formation.
ERYTHROCYTES
Each erythrocyte is a biconcave disc (Fig. 4.1, Fig. 4.2) with a mean diameter in dried smear preparations of 7.1 μm; in fresh preparations the mean diameter is 7.8 μm, decreasing slightly with age. Mature erythrocytes lack nuclei. They are pale red by transmitted light, with paler centres because of their biconcave shape. The properties of their cell coat cause them to adhere to one another by their rims to form loose piles of cells (rouleaux). In normal blood, a few cells assume a shrunken star-like, crenated form: this shape can be reproduced by placing normal biconcave erythrocytes in a hypertonic solution, which causes osmotic shrinkage. In hypotonic solutions erythrocytes take up water and become spherical; they may eventually lyse to release their haemoglobin (haemolysis), leaving red cell ghosts.
Haemoglobin
Haemoglobin (Hb) is a globular protein with a molecular mass of 67 kDa. It consists of globulin molecules bound to haem, an iron-containing porphyrin group. The oxygen-binding power of haemoglobin is provided by the iron atoms of the haem groups, and these are maintained in the ferrous (Fe++) state by the presence of glutathione within the erythrocyte. The haemoglobin molecule is a tetramer, made up of four subunits, each a coiled polypeptide chain holding a single haem group. In normal blood, five types of polypeptide chain can occur, namely; α, β and two β-like polypeptides, γ and δ. A third, β-like η chain is restricted to early fetal development. Each haemoglobin molecule contains two α-chains and two others, so that several combinations, and hence a number of different types of haemoglobin molecule, are possible. For example, haemoglobin A (HbA), which is the major adult class, contains 2α- and 2β-chains; a variant, HbA2 with 2α and 2δ chains, accounts for only 2% of adult haemoglobin. Haemoglobin F (HbF), found in fetal and early postnatal life, consists of 2α- and 2γ-chains. Adult red cells normally contain less than 1% of HbF.
Lifespan
The recognition of effete erythrocytes by macrophages appears to depend in part on the exposure of normally inaccessible parts of membrane proteins, enabling autoantibodies to these erythrocyte senescence antigens to bind to them and flag them for macrophage removal. Red cells are destroyed at the rate of 5 × 1011 cells a day and are normally replaced from the bone marrow (see Fig. 4.12) at the same rate.
LEUKOCYTES
Leukocytes (white blood cells) belong to at least five different categories (see Fig. 4.12), and are distinguishable by their size, nuclear shape and cytoplasmic inclusions. In practice, leukocytes are often divided into two main groups, namely those with prominent stainable cytoplasmic granules, the granulocytes, and those without.
Granulocytes
This group consists of eosinophil granulocytes, with granules which bind acidic dyes such as eosin; basophil granulocytes, with granules which bind basic dyes strongly; and neutrophil granulocytes, with granules which stain only weakly with either type of dye. Granulocytes (Fig. 4.3) all possess irregular or multilobed nuclei and belong to the myeloid series of blood cells (p. 77 see Fig. 4.12).
Basophil granulocytes
Slightly smaller than other granulocytes, basophil granulocytes are 10–14 μm in diameter, and form only 0.5–1% of the total leukocyte population of normal blood, with a count of 25–200/μl. Their distinguishing feature is the presence of large, conspicuous basophilic granules. The nucleus is somewhat irregular or bilobed, and is usually obscured in stained blood smears by the similar colour of the basophilic granules. The granules are membrane-bound vesicles which display a variety of crystalline, lamellar and granular inclusions: they contain heparin, histamine and several other inflammatory agents, and closely resemble those of tissue mast cells (see p. 36). Both basophils and mast cells have high affinity membrane receptors for IgE and are therefore coated with IgE antibody. If this binds to its antigen it triggers degranulation of the cells, producing vasodilation, increased vascular permeability, chemotactic stimuli for other granulocytes, and the symptoms of immediate hypersensitivity, e.g. in allergic rhinitis (hay fever). Despite these similarities, basophils and mast cells develop as separate lineages in the myeloid series, from haemopoietic stem cells in the bone marrow. Evidence from experimental animal models suggests that they are closely related (see Fig. 4.12) but studies on mast cell disorders in humans indicate that their lineages diverge from a more distant ancestral progenitor (Kocabas et al 2005).
Mononuclear leukocytes
Lymphocytes
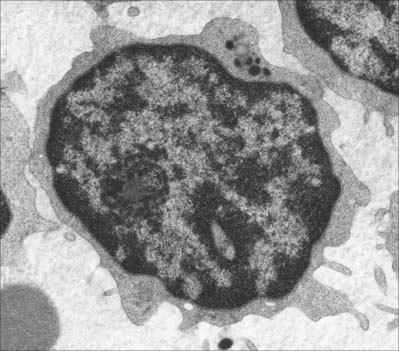
Lymphocytes (Fig. 4.4, see Fig. 4.6, see Fig. 4.12) are the second most numerous type of leukocyte in adulthood, forming 20–30% of the total population (1500–2700/μl of blood). In young children they are the most numerous blood leukocyte. Most circulating lymphocytes are small, 6–8 μm in diameter; a few are medium-sized and have an increased cytoplasmic volume, often in response to antigenic stimulation. Occasionally, cells up to 16 μm are seen in peripheral blood. Lymphocytes, like other leukocytes, are found in extravascular tissues (including lymphoid tissue); however, they are the only white blood cells which return to the circulation. The lifespan of lymphocytes ranges from a few days (short-lived) to many years (long-lived). Long-lived lymphocytes play a significant role in the maintenance of immunological memory.
Blood lymphocytes are a heterogeneous collection mainly of B and T cells and consist of different subsets and different stages of activity and maturity. About 85% of all circulating lymphocytes in normal blood are T cells. Primary immunodeficiency diseases can result from molecular defects in T and B lymphocytes (reviewed in Cunningham-Rundles & Ponda 2005). Included with the lymphocytes, but probably a separate lineage subset, are the natural killer (NK) cells. NK cells most closely resemble large T cells morphologically.
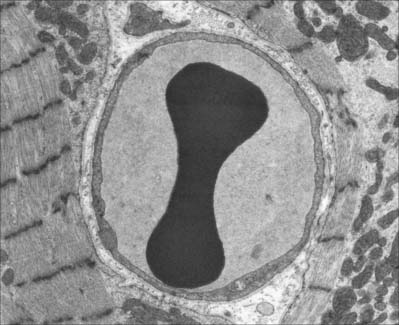
Small lymphocytes (both B and T cells) contain a rounded, densely staining nucleus which is surrounded by a very narrow rim of cytoplasm, barely visible in the light microscope. In the electron microscope (Fig. 4.4), few cytoplasmic organelles can be seen apart from a small number of mitochondria, single ribosomes, sparse profiles of endoplasmic reticulum and occasional lysosomes: these features indicate a low metabolic rate and a quiescent phenotype. However, these cells become motile when they contact solid surfaces, and can pass between endothelial cells to exit from, or re-enter, the vascular system. They migrate extensively within various tissues, including epithelia (Fig. 4.5).
B cells
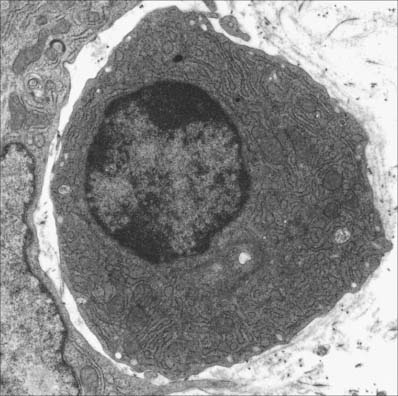
B cells and the plasma cells that develop from them synthesize and secrete antibodies which can specifically recognize and neutralize foreign (non-self) macromolecules (antigens), and can prime various non-lymphocytic cells (e.g. neutrophils, macrophages and dendritic cells) to phagocytose pathogens. B cells differentiate from haemopoietic stem cells in the bone marrow. After deletion of autoreactive cells, the selected B lymphocytes then leave the bone marrow and migrate to peripheral lymphoid sites (e.g. lymph nodes). Here, following stimulation by antigen, they undergo further proliferation and selection, forming germinal centres in the lymphoid tissues. Following this, some B cells differentiate into large basophilic (RNA-rich) plasma cells, either within or outside the lymphoid tissues. Plasma cells produce antibodies in their extensive rough endoplasmic reticulum (Fig. 4.6) and secrete them into the adjacent tissues. They have a prominent pale-staining Golgi complex adjacent to an eccentrically-placed nucleus, typically with peripheral blocks of condensed heterochromatin resembling the numerals of a clock (clock-faced nucleus) (see Fig. 4.12). Other germinal centre B cells develop into long-lived memory cells capable of responding to their specific antigens not only with a more rapid and higher antibody output, but also with an increased antibody affinity compared with the primary response.
Antibodies are immunoglobulins, grouped into five classes according to their heavy polypeptide chain. Immunoglobulin G (IgG) forms the bulk of circulating antibodies. Immunoglobulin M (IgM) is normally synthesized early in immune responses. Immunoglobulin A (IgA) is present in breast milk, tears, saliva and other secretions of the alimentary tract, coupled to a secretory piece (a 70 kDa protein) which is synthesized by the epithelial cells and protects the immunoglobulin from proteolytic degradation: IgA thus contributes to mucosal immunity. IgA deficiency is relatively common, particularly in some ethnic groups (reviewed in Woof & Kerr 2006). Immunoglobulin E (IgE) is an antibody which binds to receptors on the surfaces of mast cells, eosinophils and blood basophils; it is found only at low concentrations in the circulation. Immunoglobulin D (IgD) is found together with IgM as a major membrane-bound immunoglobulin on mature, immunocompetent but naïve (prior to antigen exposure) B cells, acting as the cellular receptor for antigen.
T cells
Functional groups of T cells are classified according to the molecules they express on their surfaces. The majority of cytokine-secreting helper T cells express CD4, while cytotoxic T cells are characterized by CD8. Regulatory T cells coexpress CD4 and CD25. The CD (cluster of differentiation) prefix provides a standard nomenclature for all cell surface molecules. At present, more than 330 different CD antigens have been designated: each one represents a cell surface molecule that can be identified by specific antibodies. Further details of the classification are beyond the scope of this publication and are given in Male et al (2006).
Cytotoxic T cells
Cytotoxic T lymphocytes (which express CD8 on their surface) are responsible for the direct cytotoxic killing of target cells (e.g. virus-infected cells): the requirement for direct cell–cell contact ensures the specificity of the response. Recognition of antigen, presented as a peptide fragment on MHC class I molecules, triggers the calcium-dependent release of lytic granules by the T cell. These lysosome-like granules contain perforin which forms a pore in the target cell membrane. They also contain several different serine protease enzymes (granzymes) which enter the target cell via the perforin pore and induce the programmed cell death (apoptosis; p. 24) of the target.
Regulatory T cells
A third population of T cells, ‘regulatory’ T or ‘Treg’ cells has been identified within the last decade (reviewed in O’Garra & Vieira 2004). These CD4+, CD25+ cells have an immunomodulatory function and can dampen the effector functions of both cytotoxic and helper T cells. Regulatory T cells are produced in the thymus and are an important additional mechanism for maintaining self-tolerance. Treg function is antigen-specific and depends upon direct cell–cell contact. Molecules secreted or induced by Treg cells, such as interleukin (IL)-10 or transforming growth factor (TGF) β, may also play an important role in mediating Treg suppressive effects on the immune system. Similar regulatory T cells can be induced in the periphery and may be important in the induction of oral tolerance to ingested antigens as well as tolerance to tissue specific molecules that are not expressed in the thymus.
Natural killer (NK) cells
Natural killer (NK) cells have functional similarities to cytotoxic T cells. However they lack other typical lymphocyte features, and do not express antigen-specific receptors. They normally form only a small percentage of all lymphocyte-like cells and are usually included in the large granular lymphocyte category. When mature, NK cells have a mildly basophilic cytoplasm. Ultrastructurally, their cytoplasm contains ribosomes, rough endoplasmic reticulum and dense, membrane-bound vesicles 200–500 nm in diameter with crystalline cores. These contain the protein perforin (cytolysin), which is capable of inserting holes in the plasma membranes of target cells, and granzymes (serine proteases), which trigger subsequent target cell death by apoptosis. NK cells are activated to kill target cells by a number of factors. They can recognize and kill antibody coated target cells via a mechanism termed antibody-dependent cell-mediated cytotoxicity (ADCC). They also have receptors that inhibit NK destructive activity when they recognize MHC class I on normal cells. When NK cells detect the loss or downregulation of MHC class I antigens on certain virus-infected cells and some tumour cells, they activate apoptosis-inducing mechanisms which enable them to attack these abnormal cells, albeit relatively non-specifically. For further reading, see Vivier et al 2008.
Platelets
Platelets play an important role in haemostasis. When a blood vessel is damaged, platelets become activated, evert their membrane invaginations to form lamellipodia and filopodia, and aggregate at the site of injury, plugging the wound. They adhere to each other (agglutination), and to other tissues. Adhesion is a function of the thick platelet coat and is promoted by the release of ADP and calcium ions from the platelets in response to vessel injury. The contents of released alpha granules, together with factors released from the damaged tissues, initiate a complex sequence of chemical reactions in the blood plasma, which leads to the precipitation of insoluble fibrin filaments in a three-dimensional meshwork, the fibrin clot (Fig. 4.1). More platelets attach to the clot, inserting extensions of their surfaces, filopodia, deep into the spaces between the fibrin filaments, to which they adhere strongly. The platelets then contract (clot retraction) by actin–myosin interactions within their cytoplasm, and this concentrates the fibrin clot and pulls the walls of the blood vessel together, which limits any further leakage of blood. After repair of the vessel wall, which may be promoted by the mitogenic activity of PDGF, the clot is dissolved by enzymes such as plasmin. Plasmin is formed by plasminogen activators in the plasma, probably assisted by lysosomal enzymes derived from the lambda granules of platelets. Platelets typically circulate for 10 days before they are removed, mainly by splenic macrophages.
LYMPHOID TISSUES
LYMPH NODES
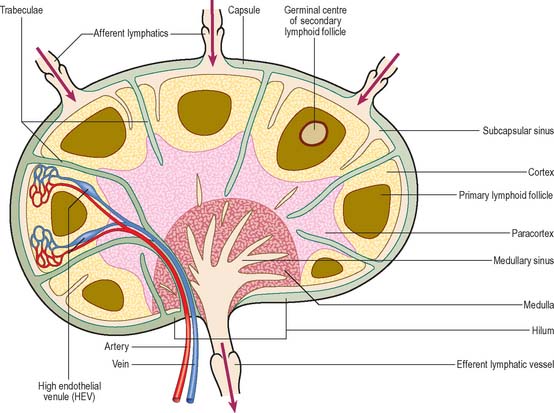
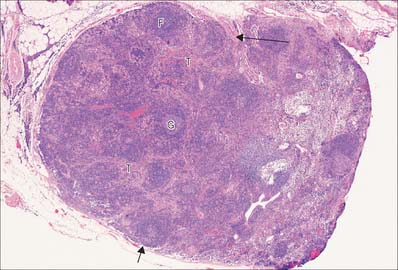
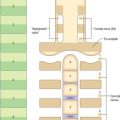
Lymph nodes (Fig. 4.7) are encapsulated centres of antigen presentation and lymphocyte activation, differentiation and proliferation. They generate mature, antigen-primed, B and T cells, and filter particles, including microbes, from the lymph by the action of numerous phagocytic macrophages. A normal young adult body contains up to 450 lymph nodes, of which 60–70 are found in the head and neck, 100 in the thorax and as many as 250 in the abdomen and pelvis. Lymph nodes are particularly numerous in the neck, mediastinum, posterior abdominal wall, abdominal mesenteries, pelvis and proximal regions of the limbs (axillary and inguinal lymph nodes). By far the greatest number lie close to the viscera, especially in the mesenteries.
Microstructure
Lymph nodes (Fig. 4.8) are small, oval or kidney-shaped bodies, 0.1–2.5 cm long, lying along the course of the lymphatic vessels. Each usually has a slight indentation on one side, the hilum, through which blood vessels enter and leave and the efferent lymphatic vessel leaves. Several afferent lymphatic vessels enter the capsule around the periphery. Lymph nodes have a highly cellular cortex and a medulla which contains a network of minute lymphatic channels (sinuses) through which lymph from the afferent lymphatics is filtered, to be collected at the hilum by the efferent lymphatic. The cortex is absent at the hilum, where the medulla reaches the surface.
The fine reticulin bundles branch and interconnect to form a very dense network in the cortex: there are fewer fibres in the germinal centres of follicles (see below). They provide attachment for various cells, mostly dendritic cells, macrophages and lymphocytes. Reticulin and the associated proteoglycan matrix are produced by fibroblasts, a few of which are associated with the fibre network.
Lymphatic and vascular supply
Lymph nodes are permeated by channels through which lymph percolates after its entry from the afferent vessels. Macrophages line the channels or migrate along the reticulin which crosses them, and so lymph is exposed to their phagocytic activities, as well as to B and T lymphocytes which lie within the various regions of a node. Afferent lymphatic vessels enter at many points on the periphery, branch to form a dense intracapsular plexus, and then open into the subcapsular sinus, a cavity which is peripheral to the whole cortex except at the hilum (Fig. 4.7). Numerous radial cortical sinuses lead from the subcapsular sinus to the medulla, where they coalesce as larger medullary sinuses. The latter become confluent at the hilum with the efferent vessel which drains the node. All of these spaces are lined by a continuous endothelium and traversed by fine reticular fibres, which support sinus macrophages.
Cells and cellular zones of lymph nodes
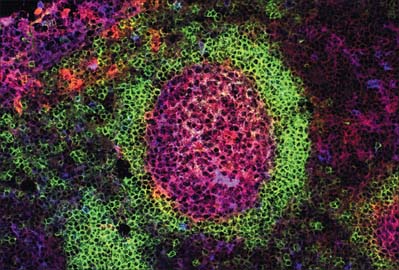
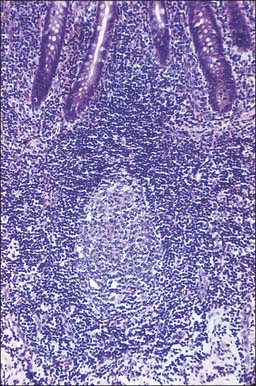
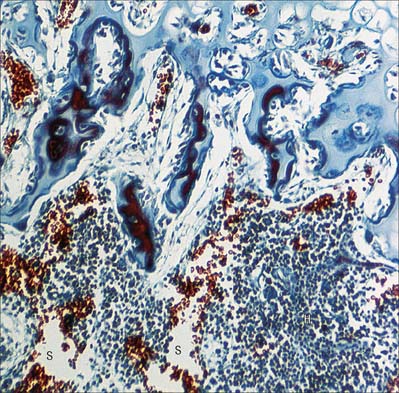
Although most of the cells in a lymph node are B and T lymphocytes, their distribution is not homogeneous. In the cortex, cells are densely packed and in the outer cortical area they form lymphoid follicles or nodules (Fig. 4.8), which are populated mainly by B cells and specialized follicular dendritic cells (FDC) (see Fig. 4.15). The number, degree of isolation and staining characteristics of follicles vary according to their state of antigenic stimulation. A primary follicle is uniformly populated by small, quiescent lymphocytes, whereas a secondary follicle has a germinal centre (Fig. 4.9) which is composed mainly of antigen-stimulated B cells which are larger, less deeply staining and more rapidly dividing than those at its periphery.
The role of the germinal centre is to provide a microenvironment which allows the affinity maturation of the B cell response, so that as the immune response progresses the affinity or strength with which antibodies bind their antigen also increases. There are several zones in the germinal centre where this is allowed to happen. In the ‘dark zone’ the B cells (centroblasts) undergo rapid proliferation which is associated with hypermutation of their antibody molecules. They then move into the light zone (as centrocytes), where they can interact with the FDCs which carry intact unprocessed antigen on their surface. The centrocytes compete for binding to the antigen; those whose antibody has the highest affinity survive and the rest die. T cells are also present, helping the survival of the B cells and inducing class switching. Macrophages in the germinal centre phagocytose apoptotic lymphocytes (e.g. those B cells which die as part of the process of affinity maturation), and consequently macrophage cytoplasm becomes filled with engulfed lipid and nuclear debris.
The mantle zone (Fig. 4.9) is produced as surrounding cells are marginalized by the rapidly growing germinal centre. It is populated by cells similar to those found in primary follicles, mainly quiescent B cells with condensed heterochromatic nuclei and little cytoplasm (hence the deeply basophilic staining of this region in routine preparations; Fig. 4.10), a few helper T cells, FDCs and macrophages. After numerous mitotic divisions the selected B cells give rise to small lymphocytes, some of which become memory B cells and leave the lymph node to join the recirculating pool, while others leave to mature as antibody-secreting plasma cells either in the lymph node medulla or in peripheral tissues.
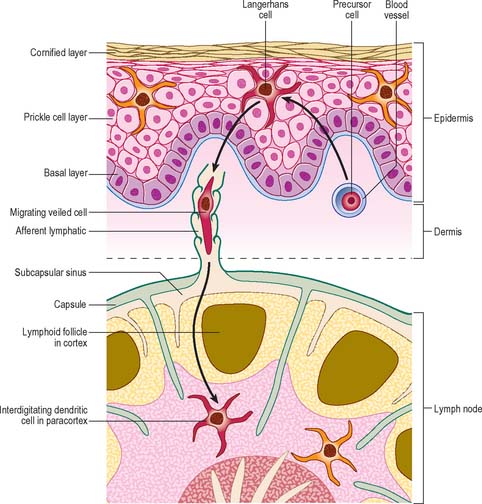
The deep cortex or paracortex lies between the cortical follicles and the medulla, and is populated mainly by T cells, which are not organized into follicles. Both CD4 and CD8 T cell subsets are present. The paracortex also contains interdigitating dendritic cells. These dendritic cells include Langerhans cells from the skin and other squamous epithelia which have migrated as veiled cells via the afferent lymphatics into the draining lymph nodes (see Fig. 4.14). Their role is to present processed antigen to T cells. The region expands greatly in T cell-dominated immune responses, when its cells are stimulated to proliferate and disperse to peripheral sites.
MUCOSA-ASSOCIATED LYMPHOID TISSUE (MALT)
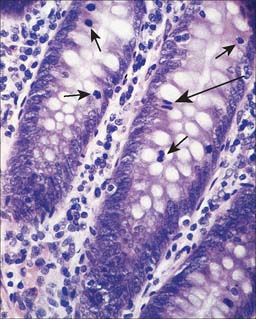
Throughout the body, MALT includes an extremely large population of lymphocytes, principally because of the size of the alimentary tract. The lymphoid cells are located in the lamina propria and in the submucosa as discrete follicles or nodules. More scattered cells, derived from these follicles, are found throughout the lamina propria and in the base of the epithelium (Fig. 4.5, Fig. 4.10). MALT includes macroscopically visible lymphoid masses, notably the peripharyngeal lymphoid (Waldeyer’s) ring of tonsillar tissue (palatine, nasopharyngeal, tubal and lingual), and the Peyer’s patches of the small intestine (see Ch. 66) which are described elsewhere. Most MALT consists of microscopic aggregates of lymphoid tissue, and lacks a fibrous capsule. Lymphocyte populations are supported mechanically by a fine network of fine type III collagen (reticulin) fibres and associated fibroblasts, as they are in lymph nodes.
Follicle-associated epithelium
Thus, many of the lymphocytes migrating between cells in the basal regions of epithelia (Fig. 4.5) are effector cytotoxic and helper T cells that have already been selected in lymphoid nodules and are engaged in immune responses. Similar cells, and activated IgA-producing B cells and plasma cells, are also scattered throughout the entire mucosal lamina propria.
HAEMOPOIESIS
BONE MARROW
Bone marrow is a soft pulpy tissue which is found in the marrow cavities of all bones (Fig. 4.11) and even in the larger Haversian canals of lamellar bone. It differs in composition in different bones and at different ages and occurs in two forms, yellow and red marrow. In old age the marrow of the cranial bones undergoes degeneration and is then termed gelatinous marrow.
Yellow marrow
Yellow marrow consists of a framework of connective tissue which supports numerous blood vessels and cells, most of which are adipocytes. A small population of typical red marrow cells persists and may be reactivated when the demand for blood cells becomes sufficiently great.
Red marrow
Red bone marrow consists of a network of loose connective tissue, the stroma, which supports clusters of haemopoietic cells (haemopoietic cords or islands) and a rich vascular supply in which large, thin-walled sinusoids are the main feature (Fig. 4.11). The vascular supply is derived from the nutrient artery to the bone, which ramifies in the bone marrow, and terminates in thin-walled arterioles from which the sinusoids arise. These, in turn, drain into disproportionately large veins. Lymphatic vessels are absent from bone marrow. The stroma contains a variable amount of fat, depending on age, site and the haematological status of the body, and small patches of lymphoid tissue are also present. Marrow thus consists of vascular and extravascular compartments, both enclosed within a bony framework from which they are separated by a thin layer of endosteal cells (p. 91).
CELL LINEAGES
Haemopoietic stem cells
Within the adult marrow there is a very small number (0.05% of haemopoietic cells) of self-renewing, pluripotent stem cells which are capable of giving rise to all blood cell types, including lymphocytes (Fig. 4.12). Although they cannot be identified morphologically in the marrow, they can be recognized in aspirates by the expression of specific cell surface marker proteins (e.g. CD34). It is thought that haemopoietic stem cells occupy specific environmental niches in the marrow associated with the endosteum of trabecular bone or with sinusoidal endothelium and that their microenvironment is important in homeostasis, the balance between self-renewal and differentiation. Stem cells can also be found (at lower concentrations) in the peripheral blood, particularly after treatment with appropriate cytokines.
Progressively more lineage-restricted committed progenitor cells develop from these ancestors (see Laiosa et al 2006 for a recent review) to produce the various cell types found in peripheral blood. The committed progenitor cells are often termed colony-forming units (CFU) of the lineage(s), e.g. CFU-GM cells give rise, after proliferation, to neutrophil granulocytes, monocytes and certain dendritic cells, whereas CFU-E produce only erythrocytes. Each cell type undergoes a period of maturation in the marrow, often accompanied by several structural changes, before release into the general circulation. In some lineages, e.g. the erythroid series, the final stages of maturation take place in the circulation, whereas in the monocytic lineage, they occur after the cells have left the circulation and entered peripheral tissues where they differentiate into macrophages and some dendritic cells.
Lymphocytes
B cell development
B cells start their development in the subosteal region of the bone marrow, and move centripetally as differentiation progresses. Their development entails the rearrangement of immunoglobulin genes to create a unique receptor for antigen on each B cell, and the progressive expression of cell surface and intracellular molecules required for mature B lymphocyte function. Autoreactive cells which meet their self-antigen within the bone marrow are eliminated. Overall some 25% of B cells successfully complete these developmental and selection processes: those that fail die by apoptosis and are removed by macrophages. Bone marrow stromal cells (fibroblasts, fat cells and macrophages) express cell surface molecules and secreted cytokines which control B lymphocyte development. The mature naïve B lymphocytes leave via the central sinuses. They express antigen receptors (immunoglobulin) of IgM and IgD classes. Class switching to IgG, A and E occurs in the periphery following antigen activation in response to signals from T helper cells.
Platelets
Platelets arise in a unique manner by the shedding of thousands of cytoplasmic fragments from the tips of processes of megakaryocytes in the bone marrow. The first detectable cell of this line is the highly basophilic megakaryoblast (15–50 μm), followed by a promegakaryocyte stage (20–80 μm), in which synthesis of granules begins. Finally, the fully differentiated megakaryocyte, a giant cell (35–160 μm) with a large, dense, polyploid, multilobed nucleus, appears. Once differentiation is initiated from the CFU-Meg, DNA replicates without cytoplasmic division (endoreduplication), and the chromosomes are retained within a single polyploid nucleus which may contain up to 256n chromosomes (where n is the haploid complement present in gametes). Megakaryocyte lineage characteristics and disorders are reviewed in Sun et al (2006).
PHAGOCYTES AND ANTIGEN-PRESENTING CELLS
Macrophages and neutrophils (see above) are specialized phagocytes. Certain dendritic cells (see Fig. 4.12), e.g. Langerhans cells of the skin and other stratified squamous epithelia, are ‘professional’ antigen-presenting cells (APCs): they take up foreign material by endocytosis and macropinocytosis, and are uniquely capable of efficiently activating naïve as well as mature T lymphocytes. Macrophages are also able to process and present antigen to lymphocytes, but are less effective than dendritic cells. In addition they play an important role in the effector arm of the immune response, clearing the infectious agent by phagocytosis. The third major cell type involved in antigen presentation and T cell activation is the B lymphocyte, which is particularly efficient at taking up antigen that binds to its surface immunoglobulin (see above). Follicular dendritic cells of lymph nodes, MALT and the spleen are capable of presenting non-processed antigen to B lymphocytes, but are not classic APCs because they cannot present antigen to helper T cells.
MACROPHAGES
Macrophages are very variable in size (generally 15–25 μm) and are found in many tissues of the body, where they constitute a heterogeneous family of cells (reviewed in Gordon & Taylor 2005). They are migrant cells in all general connective tissues, the alveolar macrophages in the lung, Kupffer cells in liver sinusoids, in bone marrow and in all lymphoid tissues. Macrophages often aggregate in subserous connective tissue of the pleura and peritoneum, where they are visible as milky spots near small lymphatic trunks. They cluster around the terminations of small (penicillar) arterioles in the spleen and are distributed, more diffusely, throughout the splenic cords.
Phagocytosis
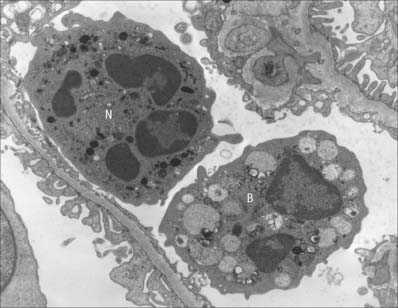
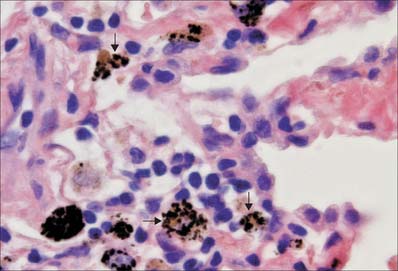
The uptake of particulate material and microorganisms is carried out by macrophages in many tissues and organs. When present in general connective tissue, they ingest and kill invading microorganisms and remove debris that has been produced as a consequence of tissue damage. They recognize, engulf and rapidly ingest apoptotic cells in all situations: the mechanism of apoptotic cell uptake does not activate the phagocyte for antigen presentation, and so the process is immunologically silent. In the lung, alveolar macrophages constantly patrol the respiratory surfaces, to which they migrate from pulmonary connective tissue (Fig. 4.13). They engulf inhaled particles including bacteria, surfactant and debris and many enter the sputum (hence their alternative names, dust cells or, in cardiac disease, heart failure cells, which are full of extravasated erythrocytes). They perform similar scavenger functions in the pleural and peritoneal cavities. In lymph nodes, macrophages line the walls of sinuses and remove particulate matter from lymph as it percolates through them. In the spleen and liver, macrophages are involved in particle removal and in the detection and destruction of aged or damaged erythrocytes. They begin the degradation of haemoglobin for recycling iron and amino acids.
DENDRITIC CELLS
There are two distinct groups of dendritic cell, myeloid dendritic cells (also known as conventional dendritic cells) and plasmacytoid dendritic cells. Both groups of cells are derived from haematopoietic stem cells. Until recently it was thought that plasmacytoid dendritic cells were derived from the lymphoid precursor cells, while the myeloid dendritic cells were derived from the myeloid progenitor cell. However, it is now apparent that these cells can be derived from either lineage, possibly from a common stem cell indicating considerable plasticity in their developmental pathways. Both cells are involved in antigen presentation, though have somewhat different functional roles in controlling both the adaptive and innate immune system. The myeloid dendritic cells are professional antigen-presenting cells (APC), which are able to process and present antigen to T lymphocytes, including naïve T cells. They are present as immature dendritic cells in the epidermis of the skin (Fig. 4.14) and other stratified squamous epithelia, e.g. the oral mucosa (Langerhans cells), and in the dermis and most other tissues (interstitial dendritic cells), where they are concerned with immune surveillance. Immature dendritic cells have an antigen-capturing function. They respond to chemotactic signals, for example defensins released by epithelial cells in the small intestine and they express pattern recognition receptors (e.g. Toll-like receptors) on their surface. Binding of bacterial molecules (e.g. carbohydrate or DNA) to these receptors stimulates the dendritic cells to migrate via the lymphatics to nearby secondary lymphoid tissues where they mature and acquire an antigen-presenting function. Mature dendritic cells are known as veiled cells when in the afferent lymphatics and the subcapsular sinuses of lymph nodes, and as interdigitating dendritic cells once they are within the lymphoid tissue proper. Their function within the secondary lymphoid tissue is to present their processed antigen to T lymphocytes, and thus to initiate and stimulate the immune response. For a review of recent research on dendritic cell function, see Colonna et al (2006).
Langerhans cells
Langerhans cells (Fig. 4.14) are one of the most well-studied types of immature dendritic cell (reviewed in Berger et al 2006). They are present throughout the epidermis of skin, where they were first described, but are most clearly identifiable in the stratum spinosum (see Ch. 7). They have an irregular nucleus and a clear cytoplasm, and contain characteristic elongated membranous vesicles (Birbeck granules). Langerhans cells endocytose and process antigens, undergoing a process of maturation from antigen-capturing to antigen-presenting cells which express high levels of MHC class I and II molecules, co-stimulatory molecules and adhesion molecules. They migrate to lymph nodes to activate T lymphocytes.
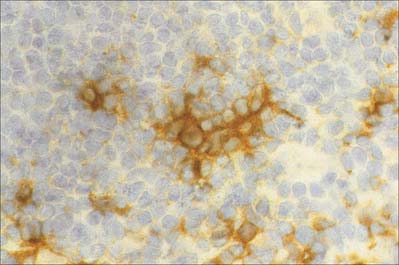
Follicular dendritic cells
Follicular dendritic cells, FDCs (Fig. 4.15), are a non-migratory population of cells found in the follicles of secondary lymphoid tissues, where they attract and interact with B cells. Unlike other dendritic cells, FDCs are not haemopoietic in origin, but are probably derived from the stromal cells of lymphoid tissues. They are unable to endocytose and process antigen, and they lack MHC class II molecules. However, Fc receptors and complement receptors CD21 and CD35 on FDCs allow the cells to bind immune complexes to their surface for subsequent presentation, as unprocessed antigen, to germinal centre B cells. Interactions between B cells, CD4 helper T cells and FDCs in the germinal centres are important in the selection of high affinity B cells and their maturation to either plasma cells or memory B lymphocytes.
Berger CL, Vasquez JG, Shofner J, Mariwalla K, Edelson RL. Langerhans cells: mediators of immunity and tolerance. Int J Biochem Cell Biol. 2006;38:1632-1636.
Colonna M, Pulendran B, Iwasaki A. Dendritic cells at the host–pathogen interface. Nature Immunol. 2006;7:117-120.
Cunningham-Rundles C, Ponda PP. Molecular defects in T- and B-cell primary immunodeficiency diseases. Nature Rev Immunol. 2005;5:880-892.
Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953-964.
Janeway C, Walport M, Travers P. Immunobiology: The Immune System in Health and Disease, 5th edn. New York: Garland Publishing, 2004.
Kocabas CN, Yavuz AS, Lipsky PE, Metcalfe DD, Akin C. Analysis of the lineage relationship between mast cells and basophile using the c-kit D816V mutation as a biologic signature. J Allergy Clin Immunol. 2005;115:1155-1161.
Laiosa CV, Stadtfelt M, Graf T. Determinants of lymphoid-myeloid lineage diversification. Ann Rev Immunol. 2006;24:705-738.
Liu Y-J. Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell. 2001;106:259-262.
Male D, Brostoff J, Roth DB, Roitt I. Immunology, 7th edn. London: Mosby, 2006.
O’Garra A, Vieira P. Regulatory T cells and mechanisms of immune system control. Nat Med. 2004;10:801-805.
Sun L, Hwang WYK, Aw SE. Biological characteristics of megakaryocytes: specific lineage commitment and associated disorders. Int J Biochem Cell Biol. 2006;38:1821-1826.
VIvier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nature Immunol. 2008;9:503-510.
Woof JM, Kerr MA. The function of immunoglobulin A in immunity. J Path. 2006;208:270-282.