151 Blood Component Therapies
Blood component therapy has had a central role in the development and practice of numerous medical advances, especially in modern surgery. It is only in more recent years that blood transfusion is no longer regarded as essential for a wide range of medical and surgical conditions. It is now possible for most uncomplicated major surgery to be conducted without allogeneic blood component therapy.1 Blood component transfusion is generally supportive therapy for the correction of one or more hematologic deficiencies until the basic disease process can be controlled or corrected. Appropriate attention to accurate diagnosis of the hematopoietic deficiency and consideration of the range of therapeutic options available and their potential hazards are essential before accepting blood component therapy as indicated.2
 Guidelines for Blood Component Therapy
Guidelines for Blood Component Therapy
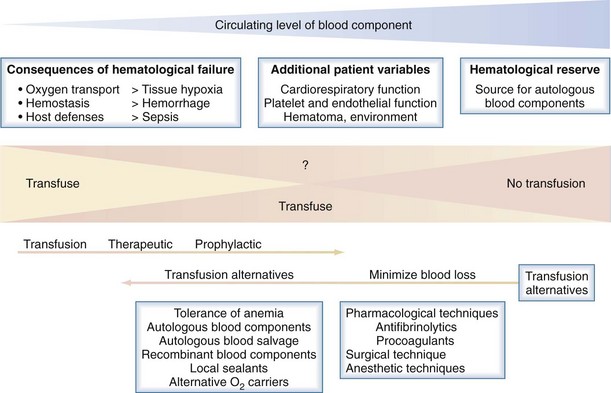
The following is a brief summary of the guidelines for use of commonly available blood components. An evidence-based approach to blood component transfusion has resulted in many long-standing transfusion dogmas being challenged and better guidelines for their use being developed for safe, effective clinical practice. Figure 151-1 illustrates the general approach to the decision to transfuse blood components, with the emphasis on patient blood management and how blood component therapy fits into the bigger picture.1
Red Blood Cell Concentrates
Appropriate and inappropriate use of red blood cell (RBC) transfusions in acute medicine has received considerable attention in recent years; however, identifying the benefits of RBC transfusion in many circumstances has been difficult.2–3 The question of the lowest safe hematocrit continues to receive considerable attention. Pushing any aspect of a system to its limits risks “sailing close to the wind” and may be appropriate in some situations but potentially hazardous in others. In an otherwise stable patient, the transfusion of RBC concentrates is likely to be inappropriate when the hemoglobin level is above 100 g/L. Their use may be appropriate when hemoglobin is in the range 70 to 100 g/L if there are other defects in the oxygen transport system. The decision to transfuse should be supported by the need to relieve clinical signs and symptoms of impaired oxygen transport and to prevent morbidity and mortality, ultimately to improve clinical outcomes. The transfusion of RBC concentrates is likely to be appropriate when hemoglobin is less than 70 g/L and the anemia is not reversible with specific therapy in the short term, but lower levels may be acceptable in patients who are asymptomatic, especially in the younger age group.
Platelet Concentrates
Platelet transfusions may benefit patients with platelet deficiency or dysfunction, and there are some general recommendations for their use.4 Prophylactic transfusion of platelet concentrates is indicated in patients with bone marrow failure when the platelet count is (1) less than 10 × 109/L and there are no associated risk factors for bleeding or (2) less than 20 × 109/L in the presence of additional risk factors. However, recent evidence suggests lower levels may be tolerated if there is no clinical evidence of hemostatic failure.
Fresh Frozen Plasma and Cryoprecipitate
Fresh frozen plasma is widely used, but there are limited specific indications for its use, and there is a dearth of evidence for efficacy in many clinical settings.5–6 The use of fresh frozen plasma may be appropriate in patients with a coagulopathy who are bleeding or at risk for bleeding when a specific therapy or factor concentrates are not appropriate or unavailable. Fresh frozen plasma generally is indicated in hemorrhaging patients for replacement of labile plasma coagulation factors (e.g., massive transfusion, cardiac bypass, liver disease, or acute disseminated intravascular coagulation [DIC]). Fresh frozen plasma is rarely indicated in vitamin K deficiency or reversal of warfarin therapy, because concentrates are now generally available.7 The use of fresh frozen plasma generally is not considered appropriate in cases of hypovolemia, in plasma exchange procedures (unless postexchange invasive procedures are planned), or in treatment of immunodeficiency states.
Plasma-Derived Products
A wide range of highly purified plasma-derived blood products is available for use in numerous clinical conditions. It is beyond the scope of this chapter to discuss their use in detail; Table 151-1 summarizes commonly used fresh and plasma-derived blood products. Fibrinogen concentrate instead of cryoprecipitate is having an increasing role in the management of hypofibrinogenemic states, depending on local availability.
| Blood Product | Main Indications |
|---|---|
| Whole blood* | Rarely indicated in acute hemorrhage if other blood products are unavailable |
| Red blood cell concentrates* | Hemorrhage and anemia |
| Leukocyte-depleted blood* | In patients having febrile reactions, to avoid leukocyte immunization in selected patients (especially patients with hematologic malignancy). Universal prestorage leukodepletion is more widely used and has the added benefit of minimizing storage lesions. |
| Platelet concentrates* | Thrombocytopenia due to marrow hypoplasia or platelet functional defect |
| Granulocyte concentrates* | Occasionally in patients with sepsis associated with profound and prolonged neutropenia secondary to marrow suppression |
| Fresh frozen plasma* | Specific or multiple plasma protein deficiencies (especially coagulation) |
| Cryoprecipitate* | Hypofibrinogenemia and rarely in factor VIII and von Willebrand disease, when concentrates are unavailable |
| 4% or 5% albumin solutions† | Plasma volume expansion. Use is controversial, and the role of albumin solutions in critically ill patients remains under deliberation.30 |
| Concentrated albumin† | Severe hypoalbuminemic states with complicating hypovolemia |
| Concentrate of coagulation factors II, VII, IX, and X† | Vitamin K–dependent factor II, IX, and X deficiency and reversal of oral vitamin K antagonists31 |
| Specific factor concentrates† | Factor VIII and IX concentrates have an established role in management of hemophilia, but others are in the process of establishing their clinical efficacy and indications. |
| Fibrinogen concentrates for hypofibrinogenemia and dysfibrinogenemia32 Antithrombin concentrates are available for thrombophilia due to antithrombin deficiency and are increasingly recommended in other disorders in which antithrombin may be depleted (e.g., DIC, MODS).31 |
|
| Gamma globulin† | Generally used intravenously for replacement in hypogammaglobulinemia or in high dosage in autoimmune disorders33 |
| Specific immune gamma globulins† | Rhesus prophylaxis, specific infection prophylaxis (e.g., tetanus, zoster, hepatitis B) |
DIC, disseminated intravascular coagulation; MODS, multiorgan dysfunction syndrome.
Recombinant Blood Products
Development and introduction of recombinant blood components continues to be one of the most exciting advances in transfusion medicine. Recombinant growth factors (cytokines) such as erythropoietin and granulocyte stimulating factors have had a major impact on managing anemia and neutropenia. There are further promising recombinant cytokines in development that could have a role in countless clinical conditions, especially as antiinflammatory and tissue-protecting agents. Recombinant hemostatic factors have improved the management of hemophilia, and recent expansion of clinical indications for the use of recombinant activated factor VII (factor VIIa)—beyond treating hemophiliac patients with coagulation factor inhibitors—is having an impact on management of a range of hemostatic disorders.8 Because factor VIIa is dependent on tissue factor, which is usually available in limited quantities within the circulation, its clinical use is generally regarded safe from a thrombosis-inducing point of view, and its use is now being recommended as a “panhemostatic agent.” Factor VIIa initiates the extrinsic coagulation pathway only when complexed to tissue factor at sites of injury. It may have a role in a wide range of hemostatic disorders (e.g., massive blood transfusion, liver disease, uremia, severe thrombocytopenia, and platelet disorders). It has been difficult to establish a sound evidence base outside the hemophilia setting for the use of rVIIa, with most experience being observational and anecdotal. Randomized controlled trial results have shown a significant reduction in transfusion requirements but could not demonstrate a reduction in mortality. There is also an increased risk of thromboembolism.
 Transfusion Management of Massive Acute Hemorrhage
Transfusion Management of Massive Acute Hemorrhage
Transfusion can be minimized with tolerance of hypotension until hemorrhage is controlled and acceptance of lower hemoglobin levels. The immediate posttransfusion function of stored red cells and hemoglobin in delivering oxygen to microcirculation and in oxygen unloading is also being questioned, with the storage age of RBCs possibly being associated with poorer clinical outcomes.9 Recent animal data point to the immediate clinical benefit of transfused red cells in treating hypovolemic shock relating more to reconstitution of the macrocirculation, with potentially adverse effects on the functional capillary density in the microcirculation.
A protocol approach to blood component therapy has generally not been recommended. However, this remains a controversial issue, with advocates for up-front protocol component therapy with red cell and hemostatic components, especially fresh frozen plasma with or without cryoprecipitate. With better understanding of coagulopathy in the critical hemorrhage setting and the importance of hypofibrinogenemia and hyperfibrinolysis, there is a reanalysis of the approach to blood component therapy. Failure of hemostasis is common in acutely bleeding patients and may be complex and multifactorial. Accumulating evidence supports the view that the pathophysiology of coagulopathy, when occurring in the context of critical hemorrhage, should be viewed as related to the primary insult or initiating event. A secondary coagulopathy may compound the problem in the resuscitated patient, such as massive stored blood transfusion, hemodilution, hypothermia, and continuing tissue hypoxia.10–11 The primary mechanisms of coagulopathy relating to the initiating event may relate to trauma, hypoxia, pregnancy, sepsis, envenomation, or antithrombotic agents.12–13 In all circumstances there is activation or inhibition of some aspect of the hemostatic system, and therapy is better informed if these varied mechanisms are better understood. Frequently, complex tests are required for definitive diagnosis, but the urgency of the situation cannot always wait for the results, and therapy may be initiated on clinical evidence with minimal laboratory information.
Many trauma patients have coagulopathy at presentation related to hypovolemic shock and not consumption or dilution. Recent evidence indicates that activation of the protein C system and hypofibrinogenemia due to secondary hyperfibrinolysis are important.14 Except when severe clotting test abnormalities are present, hemostatic laboratory parameters correlate poorly with clinical evidence of hemostatic failure. In the massively transfused patient, thrombocytopenia and impaired platelet function are the most consistent significant hematologic abnormalities, correction of which may be associated with control of microvascular bleeding. A problem with standard screening tests of coagulation function is they do not provide information about the formation of the hemostatic plug, its size, structure, or stability. Global tests of hemostatic plug formation and stability such as thromboelastography, thrombin generation tests, and clot waveform analysis in which changes in light transmission in routine activated partial thromboplastin time (APTT) are measured are of increasing use. With ongoing bleeding with associated microvascular oozing, various approaches may be taken. Having ensured that all identifiable hemostatic defects have been corrected, questions then arise as to the role of fresh blood and, more recently, recombinant activated factor VII.
 Hazards of Allogeneic Transfusion
Hazards of Allogeneic Transfusion
It cannot be overemphasized that allogeneic blood transfusion is a tissue transplant that is probably associated with the greatest range of potential hazards of any medical intervention and should only be used in circumstances in which there is good evidence that clinical outcomes will be improved.15–16
The pathophysiology of transfusion reactions can be divided broadly into three categories:
TABLE 151-2 Red Blood Cell Storage Lesions and Possible Clinical Consequences
| Storage Lesion | Potential Clinical Consequences |
|---|---|
| Alterations in red blood cell structure and function: | |
| ATP depletion | Echinospherocyte formation, increased osmotic fragility, impaired RBC deformability with adverse effects on oxygen transport and delivery |
| Microvesiculation and loss of membrane lipid, lipid peroxidation and hemolysis, and irreversible damaged RBCs | Reduced RBC viability and cell death |
| Hyperbilirubinemia, LDH, increased serum iron, free radical generation (?), hyperkalemia | |
| Reduced 2,3-DPG | Increased hemoglobin affinity for oxygen and impaired unloading |
| Decreased CD47 antigen (integrin-associated protein) expression | Reduced posttransfusion survival due to premature clearance post transfusion |
| RBC adhesion to endothelial cells | Adverse effects on microcirculatory hemodynamics |
| Storage temperature | Hypothermia unless pretransfusion warming |
| Additives: | |
| Citrate | Hypocalcemia, acid-base imbalance, initial acidosis alkalosis |
| Glucose | Hyperglycemia |
| Sodium | Hypernatremia |
| Cytokines: IL-1, IL-6, IL-8, TNF | Fever, hypotension, flushing |
| Enzymes: myeloperoxidase, elastase, arginase, secretory phospholipase A2 | Transfusion-related immunomodulation, neutrophilia |
| Reactive proteins: defensins, annexin, soluble HLA, Fas ligand, soluble endothelial cell growth factor, and others | Proinflammatory, potential “priming” for ARDS, TRALI, and MODS |
| Histamine and kinin accumulation | Hypotension, anxiety, flushing, pain syndromes, proinflammatory |
| Microaggregates and procoagulants | Blockade of reticuloendothelial system |
| Risk factor for development of ARDS, MODS, TRALI | |
| Activation of hemostasis > DIC (?), VTE (?), arterial thrombotic events (?) | |
ARDS, acute respiratory distress syndrome; ATP, adenosine triphosphate; DIC, disseminated intravascular coagulation; 2,3-DPG, 2,3-diphosphoglycerate; HLA, human leukocyte antigen; IL, interleukin; LDH, lactate dehydrogenase; MODS, multiorgan dysfunction syndrome; RBC, red blood cell; TNF, tumor necrosis factor; TRALI, transfusion-related acute lung injury; VTE, venous thromboembolism.
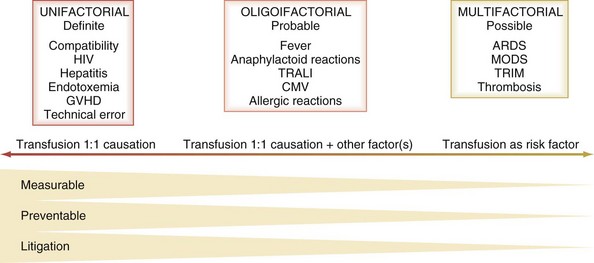
In terms of causation of an adverse clinical event, the possible role of transfusion can be classified broadly into three categories on the basis of probability (Figure 151-2):
Hemolytic Transfusion Reactions
Clinical features of hemolytic transfusion reactions are as follows:
Allergic and Anaphylactoid Reactions
Noncellular blood (plasma and plasma derivatives) components rarely are considered to be a major cause for adverse reactions to transfusion therapy, but considering the complexity of plasma and component preparation processes, a broad range of potential adverse effects is possible.18 Plasma reactions may be related to immunologic differences between the donor and the recipient; either the component is antigenic to the recipient or the plasma contains an antibody reacting with a recipient antigen. There may be physicochemical characteristics of the plasma component such as temperature, additives, alterations due to preparative processes, and accumulation of metabolites or cellular release products on storage. Clinical severity may range from minor urticarial reactions or flushing to fulminant cardiorespiratory collapse and death. Many such reactions are probably true anaphylaxis, but in others, mechanisms have been less clear, and the term anaphylactoid has been used.
Transfusion-Related Acute Lung Injury
Transfusion-related acute lung injury (TRALI) is a potentially severe complication of blood transfusion, characterized by acute respiratory distress arising within hours of a transfusion. Most patients who are well resuscitated improve within 48 hours and usually make a full recovery. The pathophysiology of TRALI is classically due to the presence of leukoagglutinating or human leukocyte antigen (HLA)-specific antibodies in the plasma of the donor of the implicated components. When complement is activated, C5a promotes neutrophil aggregation and sequestration in the lung microvasculature, causing endothelial damage. The concept of TRALI has been expanded to embrace a broader spectrum of acute lung injury after transfusion to include cases of posttransfusion lung injury in which other mechanisms may be responsible (e.g., anaphylactic reactions, cytokine reactions, platelet reactions, granulocyte transfusions, blood storage lesion).18 The patient’s lungs may be “primed” by other pathologic factors such as shock and sepsis, and transfusion becomes an additional risk factor.19
Transfusion-Associated Graft-Versus-Host Disease
Transfusion-associated graft-versus-host disease is due to infusion of immunocompetent lymphocytes, precipitating an immunologic reaction against the host tissues. It is most commonly observed in immunocompromised patients but also may be seen in recipients of directed blood donation from first-degree relatives. This response is also occasionally seen when donor and recipient are not related; homozygosity for HLA haplotypes for which the recipient is heterozygous is responsible. Transfusion-associated graft-versus-host disease is generally a devastating and fatal condition, with onset of the syndrome 2 to 4 weeks after allogeneic transfusion. Presenting signs and symptoms are fever, liver function test abnormalities, profuse watery diarrhea, erythematous skin rash, and progressive marrow failure.20
Transfusion-Related Immunomodulation
Transfusion-related immunomodulation (TRIM) is an evolving and complex area of research and new knowledge.21 Leukocytes seem to be the main blood component responsible for the immunomodulatory effects of transfusion. Space does not permit detailed analysis; however, it is likely that prestorage leukodepletion minimizes the effects. Allogeneic transfusion has been shown to be an independent risk factor for postoperative infection, with many infections being distant from the wound site, suggesting a systemic reduction in host resistance. Immunomodulation also may be responsible for increased cancer recurrence rates after surgery, but this remains controversial. The possible role of TRIM in the association between allogeneic blood transfusion and poorer clinical outcomes is discussed later.
Transfusion-Transmitted Infections
Transfusion-related infections have received much attention, and their recognition has been a driving force behind many changed blood donation and processing policies. The reader is referred to recent reviews of transfusion-transmitted infections.16,21–23
Bacterial Contamination
Bacterial contamination of stored blood can cause fulminant endotoxic shock. In recent years, the storage of platelets at room temperature has made this blood component particularly susceptible to bacterial contamination.24 The clinical features of transfusion-related endotoxic shock in a nonanesthetized patient include violent chills, fever, tachycardia, and vascular collapse with prominent nausea, vomiting, and diarrhea. Anesthetized patients may have delayed onset of symptoms, and in patients who are already febrile and on antibiotics, diagnosis can be elusive or missed.
Blood Storage Lesions and Potential Clinical Consequences
The storage lesions progressively increase until the time of expiry, and the extent of these changes is determined by the specific blood component, preservative medium, container, storage time, and storage conditions.25 Storage results in quantitative or qualitative deficiencies (or both) in blood components, which may reduce the efficacy of a transfusion. Quantitative deficiencies may result in reduced RBC survival, failure to achieve anticipated endpoints, and excessive donor exposure, increasing immunization and infection risks. Qualitative deficiency includes decreased membrane flexibility and increased adhesion to endothelium, which may impair microcirculatory hemodynamics. Reduced 2,3-diphospho-glycerate decreases hemoglobin oxygen affinity, impairing oxygen unloading.
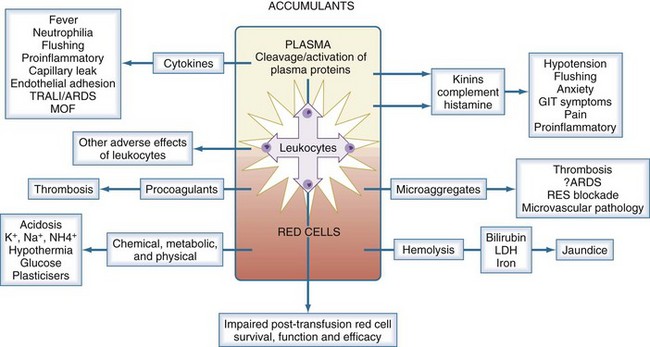
In parallel with these storage changes is an accumulation of degenerate material (e.g., microaggregates and procoagulant material), release of vasoactive agents, cytokine generation, and hemolysis (Figure 151-3). Many of the changes occurring during storage are related to the presence of leukocytes (especially granulocytes) and can be minimized by prestorage leukoreduction. The clinical significance of storage lesions continues to be debated. In some cases, the effects are widely accepted; in others, further studies are needed. There is evidence that the storage lesion is clinically significant in several respects.26 Transfusion may result in significant increases in unconjugated bilirubin and lactic dehydrogenase, neutrophilia, and saturation of serum iron. The transfusion of biologically active lipids in stored blood may be associated with development of acute lung injury in patients with predisposing conditions. Blood transfusion has been shown to be an independent risk factor for development of postinjury multiorgan failure and acute respiratory distress syndrome, and this relationship may be stronger with the age of the transfused blood. There is an increased rate of infection associated with transfusion of old blood after severe injury, suggesting that transfusion-related immunomodulation may not be related only to allogeneic transfusion but contributed to by the storage lesion. In some studies, transfusion of stored blood older than 15 days in trauma patients was a predictor of a greater likelihood of admission to the intensive care unit (ICU) and predicted a prolonged length of ICU stay. Further information about the storage lesion and the possible clinical implications is summarized in Table 151-2.
The commonly recognized potential hazards of rapid blood transfusion are as follows:
Allogeneic Transfusion as An Independent Risk Factor for Poorer Clinical Outcomes
In recent years, experimental and clinical studies have identified blood transfusion as an independent risk factor for morbidity and mortality as well as increased admission rates to ICUs, increased length of hospital stay, and additional costs. The implication of RBC transfusion as part of the problem rather than optimal therapy has challenged long-held views about the safety of allogeneic blood transfusion. It has always been assumed that blood transfusion can only be of benefit to the bleeding or anemic patient, with immunologic and infection transfusion hazards well understood and minimized. There is thus increasing evidence that TRIM and the transfusion effects of storage lesions may be responsible for poorer clinical outcomes in a range of clinical settings.27–28 There is also an association of transfusion with a higher incidence of venous thromboembolism.29 The case for this association between blood transfusion and poorer outcomes is strengthening, and evidence for the efficacy of many transfusions is being reassessed, as are studies supporting restrictive red cell transfusion policies as not jeopardizing clinical outcomes. Until these concerns are resolved, a precautionary approach should be adopted, with avoidance or minimization of allogeneic transfusion and the use of appropriate patient blood conservation techniques whenever possible.
 Basic Immunohematology
Basic Immunohematology
Key Points
Isbister JP. Decision making in perioperative transfusion. Transfus Apher Sci. 2002;27:19-28.
Thomson A, Farmer S, Hofmann A, Isbister J, Shander A. Patient blood management—a new paradigm for transfusion medicine? Vox Sang ISBT Science Series. 2009;4:423-435.
Ganter MT, Pittet JF. New insights into acute coagulopathy in trauma patients. Best Pract Res Clin Anaesthesiol. 2010;24:15-25.
Zubair AC. Clinical impact of blood storage lesions. Am J Hematol. 2010;85:117-122.
Buddeberg F, Schimmer BB, Spahn DR. Transfusion-transmissible infections and transfusion-related immunomodulation. Best Pract Res Clin Anaesthesiol. 2008;22:503-517.
Marik PE, Corwin HL. Efficacy of red blood cell transfusion in the critically ill: a systematic review of the literature. Crit Care Med. 2008;36:1-8.
1 Thomson A, Farmer S, Hofmann A, Isbister J, Shander A. Patient blood management—a new paradigm for transfusion medicine? Vox Sang ISBT Science Series. 2009;4:423-435.
2 Marik PE, Corwin HL. Efficacy of red blood cell transfusion in the critically ill: a systematic review of the literature. Crit Care Med. 2008;36(9):1-8.
3 Spiess BD. Red cell transfusions and guidelines: a Work in Progress. Hematol Oncol Clin North Am. Feb 2007;21(1):185-200.
4 Slichter SJ. Platelet transfusion therapy. Hematol Oncol Clin North Am. Aug 2007;21(4):697-729. vii
5 Kor DJ, Stubbs JR, Gajic O. Perioperative coagulation management–fresh frozen plasma. Best Pract Res Clin Anaesthesiol. Mar 2010;24(1):51-64.
6 Pape A, Stein P, Horn O, Habler O. Clinical evidence of blood transfusion effectiveness. Blood Transfus. 2009;7(4):250-258.
7 Leissinger CA, Blatt PM, Hoots WK, Ewenstein B. Role of prothrombin complex concentrates in reversing warfarin anticoagulation: a review of the literature. Am J Hematol. Feb 2008;83(2):137-143.
8 Grottke O, Henzler D, Rossaint R. Activated recombinant factor VII (rFVIIa). Best Pract Res Clin Anaesthesiol. Mar 2010;24(1):95-106.
9 Lee JS, Gladwin MT. Bad blood: the risks of red cell storage. Nat Med. 2010;16(4):381-382.
10 Maani CV, DeSocio PA, Holcomb JB. Coagulopathy in trauma patients: what are the main influence factors? Curr Opin Anaesthesiol. 2009;22(2):255-260.
11 Ganter MT, Pittet JF. New insights into acute coagulopathy in trauma patients. Best Pract Res Clin Anaesthesiol. Mar 2010;24(1):15-25.
12 Silver RM, Major H. Maternal coagulation disorders and postpartum hemorrhage. Clin Obstet Gynecol. Mar 2010;53(1):252-264.
13 Isbister GK. Procoagulant snake toxins: laboratory studies, diagnosis, and understanding snakebite coagulopathy. Semin Thromb Hemost. 2009;35(1):93-103.
14 Brohi K, Cohen MJ, Ganter MT, et al. Acute coagulopathy of trauma: hypoperfusion induces systemic anticoagulation and hyperfibrinolysis. J Trauma. 2008;64(5):1211-1217. discussion 1217
15 Hendrickson JE, Hillyer CD. Noninfectious serious hazards of transfusion. Anesth Analg. 2009;108(3):759-769.
16 Stramer SL. Current risks of transfusion-transmitted agents: a review. Arch Pathol Lab Med. 2007;131(5):702-707.
17 Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340(6):409-417.
18 Triulzi DJ. Transfusion-related acute lung injury: current concepts for the clinician. Anesth Analg. 2009;108(3):770-776.
19 Frenette PS, Mohandas N. Bad blood: a trigger for TRALI. Nat Med. 2010;16(4):382-383.
20 Ruhl H, Bein G, Sachs UJ. Transfusion-associated graft-versus-host disease. Transfus Med Rev. 2009;23(1):62-71.
21 Buddeberg F, Schimmer BB, Spahn DR. Transfusion-transmissible infections and transfusion-related immunomodulation. Best Pract Res Clin Anaesthesiol. Sep 2008;22(3):503-517.
22 Stramer SL, Hollinger FB, Katz LM, et al. Emerging infectious disease agents and their potential threat to transfusion safety. Transfusion. Aug 2009;49(Suppl. 2):1S-29S.
23 Allain JP, Stramer SL, Carneiro-Proietti AB, et al. Transfusion-transmitted infectious diseases. Biologicals. Apr 2009;37(2):71-77.
24 Palavecino EL, Yomtovian RA, Jacobs MR. Bacterial contamination of platelets. Transfus Apher Sci. Feb 2010;42(1):71-82.
25 D’Alessandro A, Liumbruno G, Grazzini G, Zolla L. Red blood cell storage: the story so far. Blood Transfus. 2010;8(2):82-88.
26 Zubair AC. Clinical impact of blood storage lesions. Am J Hematol. Feb 2010;85(2):117-122.
27 Reeves BC, Murphy GJ. Increased mortality, morbidity, and cost associated with red blood cell transfusion after cardiac surgery. Curr Opin Anaesthesiol. 2008;21(5):669-673.
28 Koch CG, Li L, Sessler DI, et al. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. 2008;358(12):1229-1239.
29 Khorana AA, Francis CW, Blumberg N, Culakova E, Refaai MA, Lyman GH. Blood transfusions, thrombosis, and mortality in hospitalized patients with cancer. Arch Intern Med. Nov 24 2008;168(21):2377-2381.
30 Caironi P, Gattinoni L. The clinical use of albumin: the point of view of a specialist in intensive care. Blood Transfus. 2009;7(4):259-267.
31 Liumbruno G, Bennardello F, Lattanzio A, Piccoli P, Rossetti G. Recommendations for the use of antithrombin concentrates and prothrombin complex concentrates. Blood Transfus. 2009;7(4):325-334.
32 Weinkove R, Rangarajan S. Fibrinogen concentrate for acquired hypofibrinogenaemic states. Transfus Med. 2008;18(3):151-157.
33 Provan D, Chapel HM, Sewell WA, O’Shaughnessy D. Prescribing intravenous immunoglobulin: summary of Department of Health guidelines. Br Med J (Clin Res Ed). 2008;337:a1831.