Advances in Anesthesia, Vol. 28, No. 1, 2010
ISSN: 0737-6146
doi: 10.1016/j.aan.2010.07.005
Peripheral Blocks of the Chest and Abdomen
Thoracic paravertebral block
History
Pioneered by Sellheim in 1905 [1], the technique for thoracic paravertebral blockade (TPVB) commonly used today was originally described by Kappis in 1919 [2]. During the early part of the 20th century, the TPVB was used as a diagnostic tool to differentiate between different causes of abdominal pain [3,4], as well as to treat conditions including angina pectoris, supraventricular tachycardias, and bronchial asthma in addition to providing relief from pain associated with surgery, traumatic injuries, herpes zoster, malignancy, and sympathetic dystrophies [5]. Despite the versatility and early enthusiasm for the TPVB, it became less frequently used during the mid-twentieth century as refinements in general anesthetic agents and monitoring techniques outpaced developments in local/regional anesthesia.
More recently, there has been renewed interest in regional techniques to overcome persistent limitations of general anesthesia (GA) and systemic opioid-based analgesic techniques. Eason and Wyatt [6] renewed interest in the TPVB specifically with their 1979 description of a technique for placing catheters within the thoracic paravertebral space (TPVS). At present, developments in ultrasound (US) imaging technology and increasing operator experience with interventional US techniques are contributing to increased TPVB use. In addition, intriguing preliminary data suggesting that TPVBs may be associated with a reduction in the recurrence rate of breast cancer following surgical excision [7] may potentially increase surgeons’ requests for TPVBs in their patients.
Indications
The TPVB has been used to provide surgical anesthesia for superficial procedures of the chest or abdomen. It is most commonly used to provide surgical anesthesia for breast surgery, including radical mastectomy procedures [8,10]. The TPVB can serve as an alternative to systemic or epidural analgesia following thoracotomy, thoracoscopy, laparotomy, open cholecystectomy, and liver or kidney surgery [11–16]. It may be especially useful in situations where epidural techniques may not be possible (such as in patients with spinal trauma [17,18] or previous posterior fusion of the thoracic spine). A paravertebral catheter can be used to provide and extend the duration of analgesia after such procedures or for painful conditions including multiple traumatic rib fractures [19,21] or herpes zoster [5,22].
Contraindications
Specific contraindications for TPVB are few. TVPB should be avoided in patients with ipsilateral empyema or severe disease of the contralateral lung. Contraindications to any regional anesthetic/analgesic technique (overlying infection or neoplasm, allergy to local anesthetics, patient or surgeon refusal, inability to obtain informed consent) apply to the TVPB as well. Patients who have significantly abnormal anatomy may be at an increased risk for complications such as inadvertent pleural or dural puncture. The use of TPVBs in patients with coagulopathy or receiving anticoagulant medications is controversial, and the TPVB currently is not considered safer than neuraxial techniques [23]. It is possible that that there is less potential for neurologic injury than with central neuraxial techniques. That a hematoma confined to the central neuraxial space could lead to spinal cord injury is well known. However, a hematoma in the TPVS could possibly extend to the adjoining epidural space through the intervertebral foramina and subsequently cause compression of the spinal cord. In addition, because the TPVS is within the thoracic cage (deep to the ribs/transverse processes), applying external pressure may not control bleeding, and significant blood loss into the pleural cavity could occur.
Specific Risks
Risks specific to the TPVB include hemothorax/pneumothorax [24,26], epidural or intrathecal injection [27–30], mediastinal puncture [31,32] and systemic local anesthetic toxicity [33]. Of importance, it is possible that pneumothoraces resulting from pleural puncture during TPVB placement may be subclinical before patient discharge and develop slowly (more likely for single-injection techniques using small-gauge needles). Thus, patients should be advised to seek medical care immediately should they experience shortness of breath, and to avoid air travel or other significant changes in atmospheric pressure soon after TPVB placement.
Performing bilateral blocks could theoretically increase the risks for several reasons: bilateral sympathetic blockade can produce hemodynamic effects (bradycardia and hypotension) similar to epidural techniques [34], the increased doses of local anesthetics used to achieve bilateral blockade may increase the risk of systemic local anesthetic toxicity [33,35], and there is a risk of bilateral hemothorax/pneumothorax. Rostral or caudal spread of anesthetic within the paravertebral space can produce a block of cervical or lumbar nerve roots causing a motor, sensory, or sympathetic block of the arm (cervical plexus or stellate ganglion block) or leg [36,37]. The inferior boundary of the TPVS is controversial, however, as the origin of the psoas muscle may prevent spread below T12. In addition, anesthetic fluid injected in the lower part of the TPVS could spread via the epidural space to effect a lumbar plexus block [38], or via the transversalis fascia to the celiac ganglion, and produce splanchnic vasodilation and hypotension [39].
Anatomy
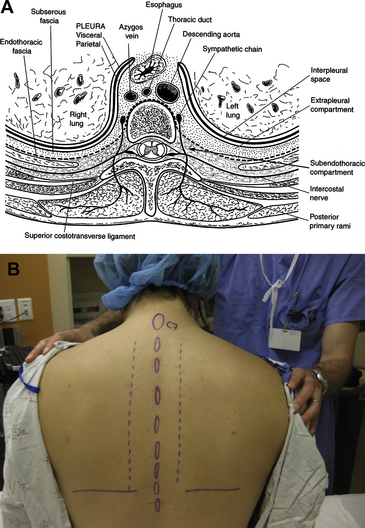
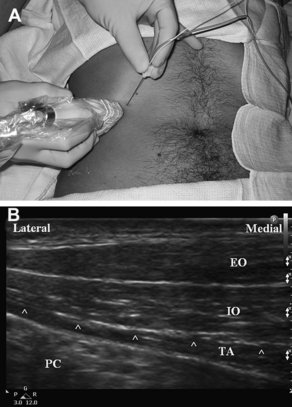
The TPVS is a wedge-shaped triangular space located on both sides of the thoracic vertebral column. The anterolateral boundary is formed by the parietal pleura and the base (medial boundary) is formed by the posterolateral aspect of the vertebral body, intervertebral disc, and the intervertebral foramina. The posterior border is formed by the superior costotransverse ligament, which extends from the lower border of the transverse process above to the upper border of the transverse process below. The TPVS communicates with the epidural space medially via the intervertebral foramina, and at the tips of the transverse processes the TPVS is continuous with the intercostal space laterally. The TPVS contains the proximal intercostal nerves (as continuation of the ventral rami), the dorsal rami, the sympathetic chain, the intercostal vessels, the endothoracic fascia, and adipose tissue (Fig. 1A) [37,40].
For anesthesia and/or analgesia of the chest and/or abdominal wall, blocks can be performed from the first thoracic to the first lumbar spinal level. Local anesthetics spread easily to adjacent levels and occasionally spread to the contralateral space via the prevertebral fascia or to the epidural space medially [41,43]. The extent of rostral/caudad spread of local anesthetic is determined by the volume of fluid injected for single-injection techniques as well as the number of levels blocked for multiple-injection techniques [44,45]. Local anesthetics placed within the TPVS act on somatic fibers as well as the sympathetic chain. As a result, TPVBs can have greater hemodynamic effects (such as vasodilation or bradycardia) than more peripheral truncal blocks, such as those of individual intercostal nerves [46].
Techniques
Landmarks
After determining the appropriate level(s) to be blocked based on the surgical procedure, the midline is marked, and a line parallel to the midline is marked 2.5 cm lateral on the side(s) to be blocked (Fig. 1B). After sterile preparation of the skin, subcutaneous infiltration of local anesthetic is performed along the paramedian line. Infiltration may be performed with chloroprocaine to minimize the risk of systemic local anesthetic toxicity while allowing infiltration of the entire area. The block needle is then introduced along this line at the level of the respective spinous processes and perpendicular to all skin planes, and it is advanced until its tip contacts the posterior surface of the transverse process. The depth to the transverse process varies with patient size and block level [47,48]. In an adult patient of normal body habitus, the needle should not be advanced past 4 cm without bony contact. If no bony contact is made, the needle should be reoriented cephalad or caudad to seek bony contact. After contact, the depth of contact is noted, the needle is partially withdrawn slightly, and oriented slightly more cephalad or caudad to “walk off” the transverse process. The needle is advanced 1 to 1.5 cm past the depth of bony contact, and local anesthetic is injected. Using a needle with depth markings on the shaft, or placing a “depth guard” on the needle may be helpful. A distinct “pop” may be felt as the needle tip enters the paravertebral space (through the costotransverse ligament), though this is not consistent.
It is important to not advance the needle more than 1.5 cm past the depth of bony contact, as this could lead to pleural puncture. The needle should not be redirected medially, as this can increase the risk of epidural or intrathecal injection [40]. Whether it is better to perform multiple small-volume injections or a single large-volume injection remains controversial. Multiple-injection techniques may be associated with an increased likelihood of block success [44,49], though each needle pass carries a risk of puncturing the pleura. Single-injection techniques may decrease this risk, although injecting a large volume of local anesthetic in a single paravertebral space may promote contralateral or epidural spread of anesthetic and produce more severe hemodynamic effects [50].
Surgical placement of catheters within the paravertebral space under direct vision [51], and video assistance during thoracoscopic procedures [52] have also been described.
Loss of resistance
The technique is identical to that described for the landmark-based approach, except that loss of resistance (LOR) is used to confirm positioning of the needle tip within the paravertebral space [17]. The LOR technique generally uses larger-gauge needles, and is especially well suited to placing catheters within the paravertebral space for continuous blocks [6]. A “pressure measurement technique” has been advocated as an objective and reproducible means to identify the TPVS [53]. This technique relies on measuring pressure changes during phases of the respiratory cycle, with a classic “pressure inversion” (pressure during expiration higher than during inspiration) indicating needle tip position within the TPVS. Fluoroscopy [42] may be used as a supplementary method of confirming correct needle or catheter placement within the TPVS.
Nerve stimulation
The technique is essentially identical to that described for landmark-based and LOR techniques, with the addition of electrical neurostimulation as an additional confirmatory end point [54]. High stimulating currents (5 mA, 2 Hz, 0.3 ms pulse width) may be used initially, and contraction of the paraspinal muscles may be observed before the needle tip makes contact with the transverse process. Stimulation can be used to confirm positioning of the needle tip within the TPVS, as contractions of appropriate intercostal/abdominal muscles may be observed at low stimulating currents (0.4–0.6 mA). Because these contractions may be subtle and difficult to see (especially in obese patients) it may be helpful to have an assistant palpate the patient’s intercostal muscles during block placement. Slight movements of the needle tip within the TPVS may help elicit contractions by moving the needle tip closer to nerve roots. It is important that these movements should not be “in-and-out” as this could increase the risk or pleural puncture. Rather, “side-to-side,” “up-and-down,” or rotational maneuvers should be used. It is also helpful to use insulated needles designed for use with a nerve stimulator.
Ultrasonography
As an aid to traditional techniques, ultrasonography can be helpful for measuring the depth to the transverse process and pleura as well as planning the trajectory of the needle, which may be especially helpful in patients with abnormal anatomy. Jamieson and Mariano [55] published a case report of 2 TPVBs placed using a nerve-stimulator guided technique after a US prescan. Pusch and colleagues [56] reported a series of 22 patients who had TPVBs placed using a landmark-based technique after measuring the depth to the transverse process and pleura at the T4 level. These studies found that the US prescan reliably measured the depth to the transverse process. Hara and colleagues [57] reported a series of 25 patients with TPVBs placed at the T4 and T1 level using an LOR technique in combination with real-time US guidance, using ultrasonography to guide the needle to make contact with the transverse process. After contact was made, the needle was advanced without US visualization and LOR was used to confirm entry of the needle tip into the TPVS.
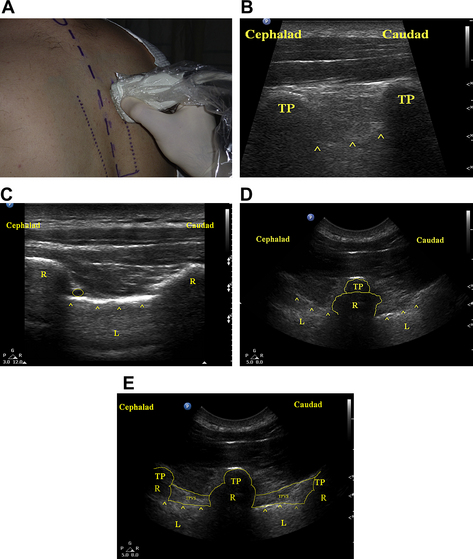
To obtain a long-axis view of the TPVS, the US transducer is placed in a sagittal orientation (parallel to the long axis of the spine, Fig. 2A) and moved medially and laterally to identify the spinous processes, the transverse processes, the ribs, and the pleura (Fig. 2B). By scanning medially and laterally, the transverse process and rib (Fig. 2C) can be identified, and the spatial relationship of the rib and transverse process can be delineated (Fig. 2D). This parasagittal view has the advantage of allowing simultaneous visualization of multiple levels. Use of a curved-array US transducer may provide a “wider” field of view (more levels). It may be difficult to use an in-plane needling technique with this view as the transducer’s “footprint” (50 mm) may make it difficult to pass the needle’s tip between the transverse processes (Fig. 2E). Use of a transducer with a smaller “footprint” (20–25 mm) may eliminate this problem, however. Alternatively, this technique can be used to guide the needle to contact the transverse process, and it can then be “walked off” using needle depth or LOR to confirm entry of the needle’s tip into the TPVS. This long-axis view can also be helpful to determine the extent of spread of local anesthetic within the TPVS. After the block is performed, the anesthetic fluid can be seen as a hypoechoic (dark) stripe superficial to the pleura. Scanning rostrally and caudally, the superior and inferior limits of local anesthetic spread can be determined.
O’Riain and colleagues [58] described a similar parasagittal long-axis in-plane technique and reported block success in 8 of 9 patients undergoing breast surgery. All patients received a continuous TPVB placed at the T3 level on the operative side. The TPVS was expanded with saline and a catheter was then passed 3 cm past the needle’s tip. If there was no hemodynamic response to a standard test dose, 10 mL of 0.25% bupivacaine was injected through the catheter. The number of dermatomes blocked using this technique was not formally assessed. All patients had GA for the surgical procedure. No complications were reported. The investigators stated that needle visualization may be challenging using this approach due to the “acute angle the needle must take to enter between adjacent transverse processes.” Although the reported block success rate was high in this series, the small number of patients, the fact that blocks were not used for surgical anesthesia, and the lack of a control group (TPVBs performed without US guidance) make it difficult to make definitive statements regarding the efficacy of this approach. Confirmation with larger series and prospective comparisons with standard approaches are still needed.
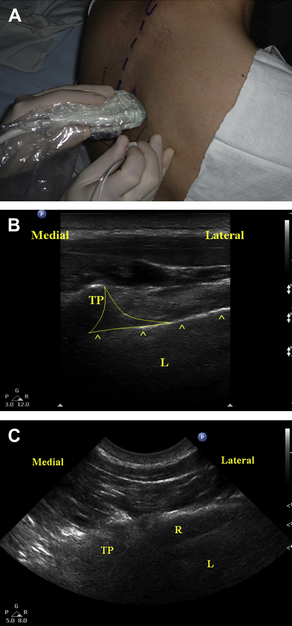
To obtain a short-axis view of the TPVS, the transducer is placed in an axial orientation (perpendicular to the long axis of the spine, Fig. 3A). With the transducer in this orientation, the spinous processes, laminae, transverse processes, ribs, and pleura can be seen simultaneously (Fig. 3B). Because these structures cannot be seen simultaneously using the long-axis view, this type of imaging may allow better appreciation of the TPVS. The TPVS can be imaged using this view by scanning in a rostral or caudal orientation until the rib is not in view (Fig. 3C). The rib and pleura may appear similar on the US image, and must be distinguished from one another for the block to be performed safely. The rib can usually be identified by the characteristic acoustic dropout “shadowing” seen during US imaging of bone (hyperechoic surface, US waves unable to penetrate past the surface, see Fig. 3C). In contrast, the parenchyma of the lung can usually be visualized deep to the hyperechoic pleura. In addition, the “sliding lung sign” can help confirm the identity of the pleura. By having the patient take a deep breath, the sliding of the parietal and visceral pleura can be seen. After confirmation of the relevant anatomic relationships, the needle tip can then be positioned in the TVPS using an in-plane needling technique by inserting the needle from the lateral edge of the transducer (see Fig. 3A). This short-axis in-plane technique has the advantage of consistently allowing visualization of the needle as it is advanced toward the paravertebral space. However, this approach should be used with great caution, as failure to maintain visualization of the needle’s tip could allow the needle tip to pass through the intervertebral foramen into the central neuraxial space or the spinal cord itself.
Luyet and colleagues [59], using the short-axis in-plane technique, placed styleted catheters in the TPVS of 20 cadavers. In all cases, the relevant anatomy and needle were easily visualized, and the catheters were easy to pass. However, during the subsequent anatomic dissection, a mixture of local anesthetic and contrast dye injected through 9 of the 20 catheters was seen in the epidural space, the pleural cavity, or the mediastinum. Although it is not clear how this could impact blocks performed in clinical situations, the high incidence of concerning patterns of local anesthetic spread in this study highlight that caution should be exercised using this technique.
Renes and colleagues [60] recently described a similar technique in a series of 36 patients undergoing various surgical procedures of the chest or abdomen. Each block was placed at a level deemed appropriate for the surgical procedure. Under real-time US guidance, the needle’s tip was positioned within the TVPS. Fifteen milliliters of 0.75% ropivacaine was then injected through the needle and a catheter passed 2 cm past the needle’s tip. Placement of the catheter was confirmed by injecting an additional 5 mL of 0.75% ropivacaine with US visualization of spread within the TPVS. A blinded observer assessed block success, and catheter placement was confirmed radiographically by injecting contrast dye through the catheter tip. Renes and colleagues reported a block success rate of 100% with reduced cold sensation in a median of 6 dermatomal segments, and all catheters were radiographically well -positioned. One patient had evidence of epidural spread of contrast dye, but had no sensory changes on the contralateral side. All patients in this series reported adequate pain relief, and no complications were reported. No control group was included for comparison.
Outcomes Data
Several studies have shown improved early recovery for patients undergoing breast surgery with TPVBs with sedation as compared with patients undergoing similar procedures under GA [8–10,61–63]. Patients in the TPVB/sedation groups had significantly less rest and/or dynamic pain, nausea, and sedation. In addition, patients in the TPVB/sedation groups were able to be discharged home earlier than patients who had received GA. Although the risk of complications from the TPVB was generally low in these studies, one pneumothorax was reported (in a series of 100 patients) [61], so caution is warranted in settings where surgical backup may not be available if urgent thoracostomy is necessary. A 1% risk of pneumothorax may be unacceptably high for elective minor procedures, especially in an ambulatory setting.
TPVB/sedation has also been compared with GA for other types of surgical procedures including inguinal hernia repair [64,65], ventral hernia repair [11], and endovascular abdominal aortic aneurysm (AAA) repair [66]. For these procedures, patients had similar improvements in pain control and early recovery. In addition, TPVB/sedation was associated with a reduced risk of hypotension in patients undergoing endovascular AAA repair compared with GA, suggesting that this type of anesthetic may be a good alternative for patients who may not tolerate GA.
Two studies have compared TPVBs to spinal anesthesia (SA) for inguinal hernia repair [67,68]. Both studies found that patients in the TPVB group had longer anesthesia-related times and block onset times. The TPVB groups required higher doses of propofol for sedation during surgery, but these differences were not clinically significant. Both studies also found that patients in the TPVB group had lower postoperative pain scores, longer times to first-rescue analgesic dose, shorter time to recovery of ambulation, and faster discharge from the postanesthesia care unit (PACU). In the study that reported rates of urinary retention [68], no patients in the TPVB group (30 patients) required bladder catheterization, compared with 5 patients (of 30) in the SA group.
Several studies have shown that TPVBs provide better pain control than systemic opioid-based analgesics following many types of surgical procedures of the chest and abdomen, including breast surgery [69,70], thoracotomy or thoracoscopy [13,71,72], laparoscopic or open cholecystectomy [12,73], open hepatic resection [16] or radiofrequency ablation of liver masses [74], transhepatic biliary drainage procedures [75], and major kidney surgery [15]. These studies all showed that compared with patients receiving systemic opioid-based analgesics alone, patients who received TPVBs had lower pain scores, decreased opioid consumption, and a lower incidence of postoperative nausea and vomiting (PONV). The duration of analgesia from single-injection techniques may be short-lived, however, as one study found no differences in pain scores between groups following patient discharge from the PACU [70]. This result suggests that continuous techniques may be more effective [76], but there have been no comparisons of single-injection with continuous techniques to date.
Few trials have compared TPVBs and local infiltration of local anesthetic or more selective peripheral nerve blocks. One trial found that compared with continuous wound infusions of ropivacaine, single-injection ropivacaine TPVBs provided superior early pain control and a lower incidence of PONV following modified radical mastectomy, but patients with wound infusions had lower pain scores 16 and 24 hours after surgery [77]. A continuous TPVB group was not included for comparison. Two studies have compared TPVBs with ilioinguinal/iliohypogastric (II/IH) blocks for pain control following inguinal hernia repair under GA [78,79]. In both studies TPVBs were performed before surgery and II/IH blocks were performed during surgery. Both of the studies found that patients in the TPVB group had lower pain scores and required fewer opioid analgesics, and neither study reported any complications in either group.
Numerous trials have compared TPVBs with thoracic epidural analgesia (TEA) following major thoracic surgery. In a recent meta-analysis, Davies and colleagues [80] pooled data from 10 trials involving a total of 520 patients, and found no significant differences between study groups’ pain scores from 4 to 8, 24, or 48 hours postoperatively with similar doses of supplemental morphine usage between groups at all time points. The TPVB groups had lower risks of pulmonary complications (odds ratio [OR] 0.36), urinary retention (OR 0.23), nausea and vomiting (OR 0.47), and hypotension (OR 0.23). In addition, the TPVB groups had lower block failure rates (OR 0.28). All differences were highly statistically significant (P values .03 to <.0001). It was concluded that TPVBs provided similar pain relief compared with TEA but have a more favorable side effect profile. These findings are in agreement with those of an earlier systematic review of regional techniques for post-thoracotomy analgesia by Joshi and colleagues [81], which found that TPVBs provided similar pain relief to TEA but was associated with a lower risk of pulmonary complications and hypotension. Of note, this study found that TPVBs were associated with lower rates of pulmonary complications than systemic analgesic regimens, whereas TEA was not. In another systematic review, Scarci and colleagues [82] reported that compared with TEA, TPVBs were associated with improved pulmonary function, lower plasma cortisol concentrations, shorter operative times, lower rates of technical failure or catheter displacement, and a reduction in the incidence and severity of hypotension. In a recent meta-regression analysis of different TPVB techniques for post-thoracotomy analgesia, Kotze and colleagues [45] analyzed 25 trials involving a total of 763 patients, and found that higher doses of local anesthetic were associated with improved pain control and faster recovery of pulmonary function. These investigators also found that compared with intermittent bolus dosing, regimens involving continuous infusions of local anesthetic provided better pain relief, whereas the addition of adjuvants such as clonidine or fentanyl did not improve the analgesic effect of the TPVB.
In a prospective trial of 20 patients undergoing open cholecystectomy, patients were randomly assigned to receive either a continuous TPVB or TEA for postoperative pain control [83]. There were no differences between groups with regard to postoperative hemodynamic stability or pulmonary function; however, patients in the TPVB group had higher pain scores and required more opioid analgesics than patients in the TEA group. One trial compared left-sided TPVB with TEA for pain control following elective robotic-assisted coronary artery bypass grafting via a left-sided minithoracotomy approach [84]. There were no differences in outcomes between groups with regard to pain control, hemodynamic stability, or complications. There was a trend toward improved pulmonary function in the TPVB group, but this did not reach statistical significance.
Additional studies have also suggested that TPVBs may reduce the risk of chronic postsurgical pain (CPSP) following breast surgery. In a 1-year follow-up study of patients enrolled in a previous study comparing TPVB/sedation to GA for surgical anesthesia for breast surgery, Kairaluoma and colleagues [85] found that patients who had received TPVBs had significantly decreased prevalence of pain symptoms as well as a decreased intensity of pain with movement and at rest. Iohom and colleagues [86] studied the effect of TPVB on acute and chronic pain following breast surgery with axillary clearance; they followed patients for 10 weeks postoperatively and found that patients who received GA and TPVBs were less likely to develop CPSP than patients who received GA and systemic analgesics alone (0/14 in the TPVB group, 12/14 in the GA group, P = .009). This study also investigated the role that nitric oxide production could play in the development of CPSP, but no correlation between perioperative plasma nitric oxide levels and risk of subsequent CPSP was demonstrated. However, the results of this study could be confounded by the fact that patients in the TPVB group also received parecoxib after induction of GA and celecoxib for up to 5 days postoperatively, whereas patients in the GA group did not.
One of the most intriguing outcomes examined recently is the potential effect TPVBs may have on the rates of breast cancer recurrence. A recent retrospective analysis [7] of patients undergoing surgical excision of malignant breast lesions showed a significant reduction in the rates of local recurrence during a 36-month follow-up period (6% vs 24%, P = .007). This effect could be caused by a blunted stress response to surgery, as patients undergoing breast cancer surgery with TPVBs have been shown to have lower serum concentrations of glucose, cortisol, and C-reactive protein than patients treated with systemic opioids [87]. However, in this study TPVBs did not reduce the serum levels of the vascular endothelial growth factor (VEGF) or prostaglandin-E2 (PGE2). Others have proposed that the TPVB leads to a reduction in substance P production with decreased tumor cell proliferation and neoangiogenesis via activation of tumor neurokinin-1 receptors [88], though they have not yet tested these hypotheses in vitro or in vivo. It does appear that the TPVB may indeed contribute to the lower rates of recurrence, as serum from patients with TPVBs undergoing excision of malignant breast lesions inhibited proliferation of estrogen-receptor negative breast cancer cells in vitro [89]. The authors wish to emphasize that although these findings are very intriguing, confirmation with large-scale prospective studies is necessary. The design of these studies has been proposed [90], and patient recruitment is currently under way.
Transversus abdominis plane block
History
In 2001, Rafi [91] described a percutaneous technique to deposit local anesthetic solution via the iliolumbar triangle of Petit to produce an “abdominal field block.” Based on their findings from cadaveric anatomic studies as well as clinical and imaging studies in volunteers, in 2007 McDonnell and Laffey [92] coined the term “transversus abdominis plane” block (commonly abbreviated as TAP block) to describe this novel block. Since that that time, the TAP block has been increasingly used by anesthesiologists as a means of providing postoperative somatic analgesia for adult and pediatric patients undergoing a wide array of abdominal surgical procedures (see later discussion).
Indications
The TAP block has been shown to be effective for reducing pain following many types of abdominal surgery including cesarean delivery [93], open retropubic prostatectomy [94], appendectomy [95], abdominal hysterectomy [96], vertical midline laparotomy with colon resection [97], and laparoscopic cholecystectomy [98]. To date, large series of prospective studies examining use of the TAP block for surgical anesthesia have not been published. The efficacy of the TAP block for providing analgesia after surgical procedures involving incisions above the umbilicus remains unclear (see Anatomy section).
Specific Risks
Specific risks of the TAP block are predominantly related to intraperitoneal needle placement with injury to intra-abdominal organs. Jankovic and colleagues [99] reported a case of inadvertent intraperitoneal catheter placement without injury to viscera, and Farooq and Carey [100] reported a case of liver trauma during TAP block. Both cases involved blocks performed using a posterior, landmark-based “2-pop” technique.
Anatomy
The ventral rami of spinal nerve roots T6 to L1 give rise to the thoracolumbar nerves, which subsequently course through the lateral abdominal wall within a potential space defined superiorly by the costal margin, inferiorly by the iliac crest, medially by the lateral border of the rectus abdominis muscle (linea semilunaris), superficially by the internal oblique muscle, and deep by the transversus abdominis muscle (Fig. 4A). The lumbar triangle of Petit is defined by the posterior border of the external oblique muscle anteriorly, and the lateral border of the latissimus dorsi muscle posteriorly. The base of the triangle is defined by the iliac crest (Fig. 4B). Jankovic and colleagues [101] performed an anatomic study in cadavers to more precisely describe the position and size of the triangle. These investigators found that the triangle is more posterior than previous descriptions of McDonnell and Rafi (mean distance posterior to mid-axillary line 9.3 cm, range 4–15.1 cm). It was also noted that the thoracolumbar nerves were not in the TAP in this posterior location, but had entered the TAP at the level of the mid-axillary line. Small branches of the subcostal arteries within the triangle in 16 of 24 specimens were also noted. Rozen and colleagues [102] performed detailed dissections of the TAP in 20 cadaveric hemi-abdominal walls. These investigators demonstrated an extensive fascial layer between the internal oblique and transversus abdominis in all specimens. This layer was not adherent to the internal oblique, and bound down the thoracolumbar nerves on its deep surface to the transversus abdominis below. Segmental nerves T6 to T9 were observed to enter the TAP between the midline and anterior axillary line. At the level of the anterior axillary line, the segmental nerves of T9 to L1 had branched extensively, with every segmental origin contributing to at least 2 nerves within the TAP. Multiple locations were found where there was extensive communication between segmental nerves. Interconnections between subcostal and intercostal nerves (T6–T9) within the TAP form an “intercostal plexus,” while T9 to L1 contribute to plexi lateral to the deep circumflex iliac artery (“TAP plexus”) and alongside the deep inferior epigastric artery located within the rectus abdominis muscle (“rectus sheath plexus”).
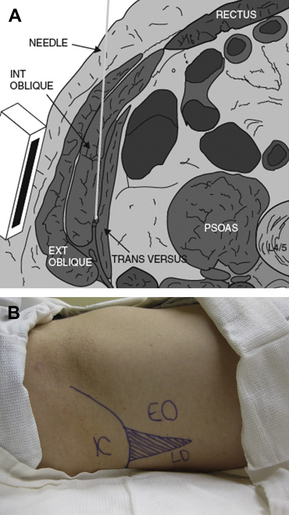
Fig. 4 Anatomy of the transversus abdominis plane (TAP). (A) Diagram shows anatomy of the TAP and needle trajectory in the TAP between the internal oblique and the transversus abdominis muscles. Ext Oblique, external oblique muscle; Int Oblique, internal oblique muscle; Transversus, transversus abdominis muscle. (Data from Ecole Polytechnique Fédérale de Lausanne, Switzerland, Visible Human Web Server. Available at: http://visiblehuman.epfl.ch) (Reprinted from Tran TM, Ivanusic JJ, Hebbard P, et al. Determination of spread of injectate after ultrasound-guided transversus abdominis plane block: a cadaveric study. Br J Anaesth 2009;102(1):123–7; with permission.) Note the location of the US transducer on the abdominal wall and the tangential (relative to the peritoneal cavity) trajectory of the needle inserted from the medial aspect of the transducer. (B) Surface anatomy for TAP block via the posterior approach. The lumbar triangle of Petit (shaded) is defined by the external oblique muscle (EO) anteriorly, the latissumus dorsi muscle (LD) posteriorly, and the iliac crest (IC) inferiorly. The needle insertion site is the center of the triangle.
McDonnell and colleagues [103] evaluated the spread of injectate within the TAP both in a cadaver model and using radiographic imaging in healthy volunteers. These investigators reported a sensory block extending from T7 to L1 dermatomes in the volunteers, and have reported satisfactory analgesia with this technique even for surgical procedures using a vertical midline incision extending above the umbilicus [97]. However, Hebbard [104] and Shibata and colleagues [105] performed separate retrospective audits and, based on their experiences, suggested that the TAP block performed just above the iliac crest does not reliably provide sensory analgesia above the umbilicus. Hebbard described a new “oblique subcostal” approach to the TAP, and reported that this modified approach resulted in a more cephalad distribution of the block [106]. Tran and colleagues [107] and Barrington and colleagues [108] confirmed these findings in separate studies examining the distribution of dye injected into the TAP of cadavers. These studies found that the spread of dye was limited to the T10-L1 nerve roots with the originally described US-guided posterior TAP approach, but from T9 to T11 if a US-guided subcostal approach was used. Multiple-injection techniques within the TAP (as the needle was advanced from medial to lateral) were found to be more likely to involve the T7 and T8 segmental nerves.
Lee and colleagues [109] recently published an observational study of patients receiving bilateral US-guided TAP blocks for analgesia following major abdominal surgery. Patients received the TAP blocks via either a posterior or oblique subcostal approach. This study found that patients in the oblique subcostal group had a larger number of affected dermatomal segments, and that the oblique subcostal approach was more likely to block more cephalad segments (median block height T8 vs T10 in the posterior-approach group, P = .001).
Techniques
Landmark-based loss-of-resistance technique
The lumbar triangle of Petit is marked following identification of muscular and bony landmarks by palpation. A short-bevel needle is inserted in the center of the triangle perpendicular to all skin planes. A classic “2-pop” end point is used to determine correct placement of the needle’s tip within the TAP. The “pops” indicate the fascia under the external oblique (first) and internal oblique (second). Typically, large volumes of local anesthetic (up to 20 mL in adults, 0.2 mL/kg in children) are then injected incrementally in the TAP. Caution should be used with injection of large volumes of anesthetic, and doses of local anesthetic should be kept below recommended maximum dosing guidelines (eg, 2.5 mg/kg for bupivacaine, and so forth) especially in the case of bilateral blocks. Kato and colleagues [110] have shown that the US-guided TAP blocks in patients under GA resulted in significant increases in the plasma levels of local anesthetic (peak plasma lidocaine levels above therapeutic range for antiarrhythmic effect), though it is not known if similar levels are encountered in patients who are awake at the time of block placement.
Ultrasound-guided technique
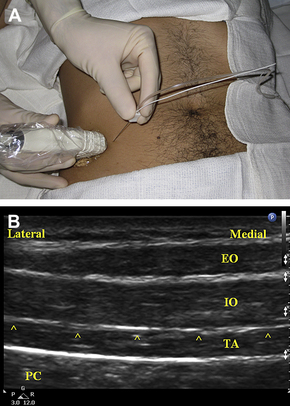
With a linear-array, high-frequency transducer placed in an axial orientation on the lateral abdominal wall (Fig. 5A), the US image can show the subcutaneous tissues, the internal and external oblique muscles, the transversus abdominis muscle, and the peritoneum and viscera (Fig. 5B). The muscular layers of the lateral abdominal normally have a characteristic “3-stripe” appearance. The internal and external oblique muscles usually have a characteristic striated appearance, whereas the transversus abdominis usually appears relatively hypoechoic (dark) and homogeneous. Walter and colleagues [111] reported variability of the layers of the abdominal wall in Petit’s area, and the best US image may be obtained with the transducer somewhat more anterior than directly over the triangle. The implications of injecting anesthetic anterior to the triangle remain unclear.
For the posterior approach to the TAP block, the US transducer is positioned axially over the iliac crest along the mid-axillary line (see Fig. 5A). The transducer’s position is adjusted as necessary until the classic 3-stripe image of the muscular layers of the abdominal wall is obtained (see Fig. 5B). This 3-stripe image may be more difficult to find in obese patients, as the muscles are deeper due to abundant overlying adipose tissue, and the muscles may be atrophic and as a result relatively thin. If the 3-stripe image is difficult to obtain, the transversus abdominis muscle can often be identified by first locating the peritoneal cavity, then looking superficially. Peristaltic movements of the intestine can be seen within the peritoneal cavity, and the transversus abdominis is the muscular layer directly overlying the parietal peritoneum, though in very obese patients a thick layer of preperitoneal fat can be mistaken for the transversus abdominis muscle. Alternatively, the transducer can initially be placed more medially over the rectus abdominis muscle. By scanning laterally, the posterior capsule of the rectus sheath can be demonstrated, dividing to envelop the transversus abdominis and internal oblique muscles. The anterior capsule can be seen splitting to envelop the external and internal oblique muscles (the internal oblique muscle’s fascial aponeurosis contributes to both the anterior and posterior capsules of the rectus sheath above the level of the arcuate line).
An in-plane needle approach is typically used, and the needle is usually inserted from the medial aspect of the transducer (see Figs. 4 and 5); this results in a tangential needle trajectory that may decrease the likelihood of puncturing the peritoneum (see Fig. 4A). Once the needle’s tip penetrates the fascia (seen as a hyperechoic line) between the internal oblique and transversus abdominis muscles, local anesthetic is injected incrementally into the TAP. The local anesthetic may initially be seen spreading into the substance of the transversus abdominis muscle itself. In this case, the needle should be withdrawn slightly so that the local anesthetic spreads in the TAP. The local anesthetic must be deposited deep to the fascia that binds the thoracolumbar nerves onto to the transversus abdominis muscle, which will lift off from the transversus abdominis as more fluid is injected. The key to a successful block is not to accept anesthetic spread above this fascial layer, as the thoracolumbar nerves are below it. The needle should under no circumstances be advanced through the transversus abdominis, as injury to intraperitoneal structures could result.
The sonoanatomy of the abdominal wall in the subcostal region is usually similar. However, local anesthetic can be deposited between the posterior capsule and the belly of the rectus abdominis muscle (similar to the rectus sheath block, see later discussion) if the transversus abdominis muscle (normally posterior to the rectus abdominis muscle at this level) cannot be identified in this location. For the subcostal approach, the US transducer is placed obliquely below the costal margin (Fig. 6A) along the lateral border of the rectus muscle (linea semilunaris). The muscular layers are again demonstrated (Fig. 6B). The transversus muscle may be located more medially at this level. Usually it is demonstrated posterior to the belly of the rectus abdominis muscle. If the transversus cannot be identified, local anesthetic can be injected between the belly of the rectus and the posterior capsule of the rectus sheath. A multiple-injection technique with injections both in this location and in the TAP along the costal margin described above may result in more extensive spread of local anesthetic [108]. This spread may be due to the injections being both medial and lateral to the anterior axillary line (see Anatomy section). Catheter placement may be performed in a manner similar to that described earlier for the posterior TAP block. The location of the catheter should be selected based on the dermatomal levels involved in the surgical incision.
Outcomes Data
In several small prospective randomized controlled trials, the TAP block has been shown to improve pain control and decrease opioid consumption following several abdominal surgical procedures, including large bowel resection via a vertical midline incision [97], cesarean delivery through a Pfannenstiel incision [93], abdominal hysterectomy through a low transverse incision [96], open appendectomy [95], and laparoscopic cholecystectomy with all port sites at or below the umbilicus [98,112]. Several other case reports and case series have reported reduced pain scores and decreased morphine consumption compared with historical controls for other abdominal procedures such as retropubic prostatectomy [94].
Petersen and colleagues [113] recently conducted a systematic review of studies that have examined the effect of the TAP block on postoperative pain. These investigators identified 7 prospective studies involving a total of 364 patients. The studies overall had good methodological quality (median score 5/5, range 2–5) and validity (median score 14/16, range 2–15) as evaluated by the Oxford Quality and Oxford Pain Validity Scales. Three studies involved blocks performed using the traditional landmark-based technique. Four studies involved US-guided blocks. In 6 studies, bilateral blocks were performed. Meta-analysis of patient outcomes was performed, and significant reductions in pain scores at rest and with movement, as well as morphine consumption during the first 24 hours postoperatively, were demonstrated for patients in the TAP block groups. Of note, subgroup analysis revealed a significant difference in reduction of morphine consumption between landmark-based and US-guided blocks. Landmark-based blocks resulted in a greater reduction in mean morphine consumption (−38 mg vs −11 mg). It is not clear if this difference was due to a difference in the location of injection with the 2 techniques, or if it reflects specific differences related to the surgical procedures included in these trials.
Future Directions
The TAP block has not been used extensively for surgical anesthesia, and comparisons with GA or SA for suitable surgical procedures such as inguinal or umbilical hernia repair could be helpful. To date, no study has formally compared the various approaches for the TAP block (eg, posterior vs subcostal). Studies comparing different techniques (eg, landmark-based with LOR vs ultrasonography) could help define their relative efficacy, success rates, and other advantages and/or limitations. Direct comparisons between TAP blocks and TPVBs or neuraxial techniques such as TEA are also needed [114] to evaluate their relative success rates, analgesic effects, side effect profiles, the incidence of complications or side effects, and to reveal any additional advantages of efficiency into daily clinical practice. Although one study has shown increased plasma levels of local anesthetic following US-guided TAP blocks [110], the pharmacokinetics of various local anesthetics within the TAP remains unclear. The effect of adding epinephrine to local anesthetics on plasma levels has not been investigated. No study to date has evaluated the effects of adding adjuvants such as opioids, bicarbonate, or clonidine to the local anesthetic solution.
References
[2] F. Mandl. Paravertebral block. New York: Grune and Stratton; 1946.
[32] S.S. Wyatt, R.A. Price. Complications of paravertebral block. Br J Anaesth. 2000;84(3):424.
[37] J. Richardson, P.A. Lonnqvist. Thoracic paravertebral block. Br J Anaesth. 1998;81(2):230-238.
[40] M.K. Karmakar. Thoracic paravertebral block. Anesthesiology. 2001;95(3):771-780.
[92] J.G. McDonnell, J.G. Laffey. Transversus abdominis plane block. Anesth Analg. 2007;105(3):883.
This research was funded internally by the Department of Anesthesiology and Perioperative Medicine, Oregon Health and Sciences University.