Blepharitis
Classification
Historical Classification of Blepharitis
Blepharitis represents one of the most common anterior segment disorders encountered in ophthalmology. Data from the National Disease and Therapeutics Index reported 590 000 patient visits in 1982 due to blepharitis.1 More recent studies have shown that ophthalmologists and optometrists observe blepharitis in 37–47% of their patients.2,3 Indeed, epidemiologic data from one study in Britain indicated that blepharitis and conjunctivitis account for 71% of ocular cases of inflammation that presented to the emergency room.4
Despite the prevalence of blepharitis in both presentation and contribution to ocular conditions, the etiology of blepharitis remains largely unknown. Available evidence suggests that the etiology is most likely multifactorial and this has led to a fair amount of variation in classification of the disease (Table 8.1). Historically, Elsching first described the condition in 19085 and Thygeson classified blepharitis into different types in 1946.6 Thygeson initially described the entity as a ‘chronic inflammation of the lid border’ and further divided it into two general categories: squamous and ulcerative. He went on to describe findings associated with seborrheic blepharitis, staphylococcal blepharitis and a combination of the two clinical entities. Thygeson established the association of blepharitis with abnormal Staphylococcus colonization and attributed infection of the meibomian glands to be the primary cause of blepharitis.6
Table 8.1
Summary of Major Proposals for Classification of Chronic Blepharitis
| Primary Study Author(s) | Year Published | Proposed Classification System |
| Thygeson | 1946 | Ulcerative and squamous. |
| McCulley | 1982 | Classification of blepharitis into 6 categories: (1) staphylococcal blepharitis, (2) seborrheic blepharitis, (3) mixed seborrheic with staphylococcal, (4) seborrheic with meibomian seborrhea, (5) seborrheic blepharitis with secondary meibomianitis, and (6) primary meibomianitis. |
| Huber-Spitzy | 1991 | Classification into 3 groups based on clinical features: (1) blepharitis sicca, (2) blepharitis seborrheica and (3) blepharitis ulcerosa. |
| AAO Preferred Practice Patterns | 2003 | Blepharitis divided into two main categories based on anatomic delineation of lid margin: (1) anterior and (2) posterior blepharitis, then further subcategorized by presentation (anterior blepharitis referring to staphylococcal and seborrheic, posterior blepharitis referring to meibomian gland dysfunction). |
| Mathers | 2004 | Using cluster analysis, categorized blepharitis into one of 9 groups based on meibomian gland dropout, lipid volume, Schirmer test value, evaporation, and lipid viscosity. |
| Shapiro and Abelson | 2006 | Standardized photograph grading scale for blepharitis and meibomitis based on anatomical classifications. |
Interestingly, over three decades passed before McCulley et al. first reported blepharitis caused by non-infectious means. Studies conducted by McCulley and others revealed that the disease of blepharitis encompassed much more than infection of the meibomian glands.7 In fact, one study, comparing 26 control patients to 26 patients with chronic blepharitis, demonstrated that all of the blepharitis patients possessed a generalized sebaceous gland dysfunction, which included the meibomian glands.8 Further, investigators of these initial studies noted that stagnation of the meibomian glands seemed to cause a defect in the tear lipid layer, resulting in a superficial punctate keratopathy consistent with tear film deficiency states.7 Further investigations led to the notion that the disease of blepharitis encompassed several factors in addition to Staphylococcus aureus that were not of an infectious nature.
Subsequently, McCulley and colleagues designed a more elaborate classification system based on the intense study of changes induced in the lid, lashes, hair follicles, meibomian glands, conjunctiva and cornea in blepharitis patients.7 They divided blepharitis into six distinct categories: (1) staphylococcal blepharitis, (2) seborrheic blepharitis, (3) mixed seborrheic and staphylococcal, (4) seborrheic with meibomian seborrhea, (5) seborrheic blepharitis with secondary meibomianitis, and (6) primary meibomianitis.4 The authors noted the distinct clinical features of each entity that separated them into different categories. For instance, those patients with staphylococcal blepharitis tended to have relatively more inflammation of the anterior portion of the lid, but for a shorter duration, compared to the other categories. Further more, unlike patients with primary or secondary meibomitis, these patients were culture positive for either S. aureus or S. epidermidis (compared to controls). Strikingly, those patients with seborrheic blepharitis, of any type demonstrated a 95% incidence of associated seborrheic dermatitis, while staphylococcal patients had relatively no dermatologic findings.7 This detailed classification and study greatly expanded on the early observations of Thygeson and emphasized the complex nature of the disease.
In 1991, Huber-Spitzy proposed a simplified classification compared to McCulley, consisting of only three groups based on clinical features: (1) blepharitis sicca, (2) blepharitis seborrheica and (3) blepharitis ulcerosa.9 The authors described blepharitis sicca as a local eczematous disease, consisting of only superficial inflammation with dry scaling of the lid margin.9 On the other hand, blepharitis seborrheica was characterized as having marked inflammation with large ‘greasy scales’ and excessive sebaceous gland secretions. Finally, the most severe form, blepharitis ulcerosa, was diagnosed only when the follicles of the lashes were encrusted with thickly matted, hardened crusts which frequently resulted in bleeding on forceps removal.9
Further research detailing the coexistence of blepharitis with dry eye, led Mathers and colleagues to create a multifaceted classification system for blepharitis, ocular surface disease and dry eye, based on cluster analysis of several different variables.10 In 2004, the investigators presented data suggesting that by assessing meibomian gland dropout, lipid volume, Schirmer test value, evaporation, and lipid viscosity, patients could be placed in one of nine distinct diagnostic groups.9 These groups were identified as: (1) obstructive MGD with rosacea and dry eye, (2) obstructive MGD and dry eye, (3) seborrheic MGD, (4) seborrheic MGD with dry eye, (5) seborrheic, obstructive MGD with dry eye, (6) low evaporation and dry eye, (7) high evaporation and high schirmer’s test, (8) low Schirmer’s, high evaporation and dry eye, and (9) normal Schirmer’s high evaporation and dry eye.
In 2003, the American Academy of Ophthalmology advocated the anatomic model of classification that many ophthalmologists were using to divide blepharitis into two main categories, based on anatomic delineation of lid margin: anterior and posterior blepharitis.11 The AAO preferred practice pattern then further subcategorized blepharitis by its presentation, i.e., anterior blepharitis, encompassing both staphylococcal and seborrheic blepharitis and posterior blepharitis, referring mainly to meibomian gland dysfunction.11
More recently, Shapiro and Abelson devised a photographic standardized scale for blepharitis and meibomitis based on anatomical classifications.12 These anatomical classifications include assessing the lash follicles, dermis, eyelid, vascularity, mucocutaneous junction, meibomian gland orifices and tarsal conjunctiva. Digital images were reviewed by a panel of clinicians and were arranged from least severe to most severe; representative images were then selected to generate a scale of 0 to 3 or 0 to 4 (normal to severe) and subsequently used for several FDA studies.13,14
Anterior Blepharitis
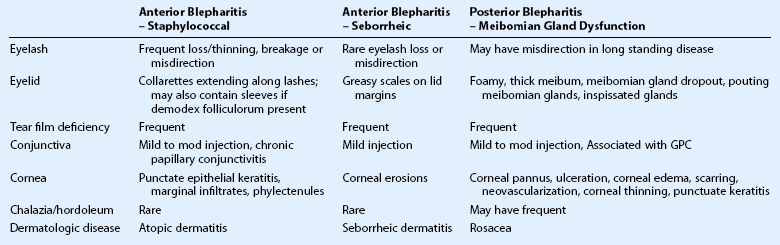
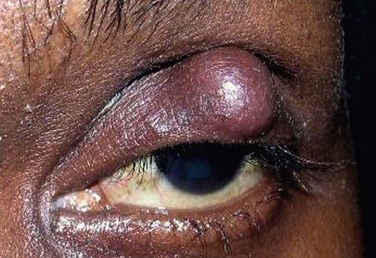
Common symptoms of blepharitis include burning, itching, a gritty or foreign body sensation, crusting and redness or irritation of the lid margins. However, there is significant overlap of these symptoms in all forms of blepharitis, and therefore, clinical features must be utilized to distinguish between different etiologies of blepharitis (Table 8.2). Of note, the symptoms of blepharitis are traditionally bilateral and any unilateral presentation, marked asymmetry or resistance to therapy, should alert the clinician to the presence of other diseases masquerading as blepharitis, such as sebaceous cell carcinoma (Fig. 8.1).15
Staphylococcal Blepharitis
Staphylococcal blepharitis is typically characterized by scaling, crusting, and erythema of the lid margin.16 This form of anterior blepharitis tends to occur more often in females and at a slightly younger age than seborrheic blepharitis (mean age 42, compared with 51 years old).7
Clinical Features
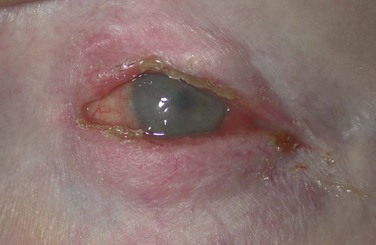
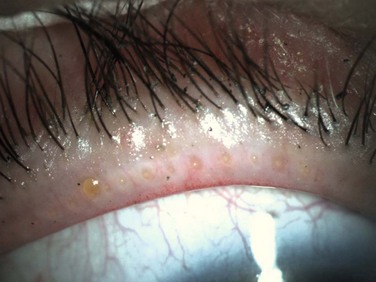
Collarettes are typically found in staphylococcal infection. Staphylococcal debris and white blood cells congeal to form hard, brittle fibrinous scales at the base of the lash. As the lash grows, these scales encircle the lash and form what is known as collarettes (Fig. 8.2).11 In addition, sleeves may be found along the lash shaft, which are representative of Demodex follicularum. The parasite mite is found in both normal patients and those with blepharitis; as such, its role in blepharitis is unclear.11 In addition, dilated blood vessels at the base of the lashes produce erythema and chronic inflammation of the anterior lid, which may lead to changes, such as notching and thickening of the lid, loss or thinning of lashes, and misdirected lashes.17
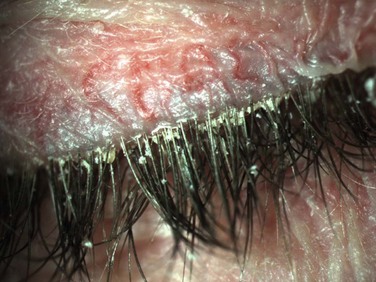
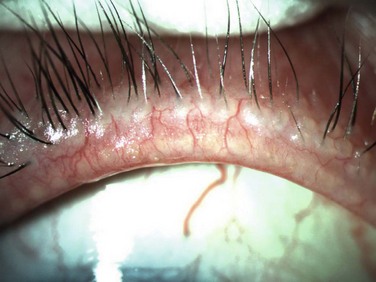
The lids in staphylococcal blepharitis tend to be more inflamed than other forms of blepharitis and in some cases, external hordoleum or chalazia may occur from acute inflammation of the surrounding meibomian glands.5 In addition, the conjunctiva in staphylococcal blepharitis may appear mild to moderately hyperemic and may demonstrate a chronic papillary conjunctivitis, thought to occur from release of the bacterial toxins.4 Further more, this form of anterior blepharitis may also be associated with punctate epithelial keratitis, marginal infiltrates or ulcerations and phlyctenular keratitis (Fig. 8.3).7
Infectious Etiology
Classically, staphylococcal blepharitis is considered to be the form of blepharitis associated with bacterial colonization of the anterior lid margin. The most common organisms isolated from patients with chronic blepharitis include S. epidermidis, Propionibacterium acnes, Corynebacterium and S. aureus.7,18 Some studies have shown that though S. epidermidis is isolated in high concentrations from both normal patients and those with blepharitis, S. aureus seems to be isolated in greater frequency in patients with a clinical diagnosis of staphylococcal blepharitis.7 However, other studies suggest that rather than having higher concentration of one bacterial isolate, blepharitis patients have a more heavily colonized lid surface than normal controls.16 Additionally, there has been speculation that staphylococcal toxins may be responsible for the symptoms of blepharoconjunctivitis. Yet, a study by Seal et al. demonstrated that S. aureus colonized 6% of normal lids without producing blepharitis, even though all isolates produced alpha toxin.18
Seborrheic Blepharitis
On the other hand, seborrheic blepharitis has been associated with an overproduction of sebum, leading to greasy scaling of the anterior eyelid. Seborrheic blepharitis tends to occur in an older age group, compared to staphylococcal blepharitis and does not appear to have a gender predilection.19 Overall, there is less inflammation than staphylococcal blepharitis, unless there is a staphylococcal superinfection.7 McCulley et al. reported that in seborrheic blepharitis, the meibomian glands had dilated ductules with normal secretions.7 Approximately one-third of patients with seborrheic blepharitis had keratoconjunctivitis sicca4 and corneal erosions have been reported in up to 15% of cases.20
Associated Conditions
For the most part, staphylococcal blepharitis does not have any definitive associated systemic conditions. Both anterior and posterior blepharitis are associated with rosacea, but rosacea is more often found in posterior disease. Some patients with chronic atopy have increased susceptibility to the development of chronic staphylococcal infections and appear to be predisposed to developing staphylococcal blepharitis as well.17
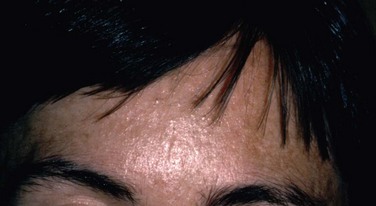
On the contrary, as McCulley et al.7 noted, a great preponderance of seborrheic blepharitis patients also have seborrheic dermatitis. Seborrheic dermatitis is a common, chronic condition characterized by symmetric erythematous inflammation, with scaling that is often greasy in areas of the skin with a high concentration of sebaceous glands.21 Most patients with seborrheic blepharitis and dermatitis present with similar yellow, greasy scaling on the eyelids, eyebrows, and scalp (Fig. 8.4). Other commonly affected areas include the ears, sides of the nose, chest, axilla, and inguinal area.20
All categories of blepharitis appear to be associated with some degree of aqueous tear deficiency or keratoconjunctivitis sicca.22,23
Posterior Blepharitis
Contrary to patients with anterior blepharitis, patients with meibomian gland disease have inflammation associated with the posterior lid margin. In meibomian gland dysfunction (MGD), the glands tend to be minimally inflamed, but the meibomian orifices are dilated with stagnant secretions. Clinically, posterior blepharitis patients seem to have more pronounced symptoms with fewer distinct clinical signs, in comparison to anterior blepharitis patients. The subcommittee for diagnosis and classification of MGD, at the International workshop on meibomian gland dysfunction, noted that MGD encompasses myriad signs including meibomian gland dropout, altered composition of meibum, and changes in lid morphology.24
Meibomian Gland Dropout
Though meibomian gland dropout increases with age in normal patients, it has been postulated that this occurs at an increased rate in patients with severe MGD, leading to an evaporative dry eye state with its associated symptoms.20 Several techniques have been described to quantify meibomian gland dropout, including meiboscopy, meibography, and confocal microscopy.20 Meiboscopy involves a clinical examination with transilluminated biomicroscopy of the glands,25 while meibography captures images of the glands using near-infrared light and camera.26Confocal microscopy has also been described to assess meibomian gland structure and changes in MGD.27,28 Ibrahim et al. proposed parameters for confocal microbioscopic analysis of meibomian gland function and dropout, including meibomian gland acinar longest diameter, shortest diameter, and inflammatory cell density.29
Altered Biochemical Composition of Meibum
In obstructed meibomian gland disease, studies have shown the lipid secretions to be altered, leading to thickening of the secretions, plugging of the glands and pouting of the meibomian orifices.30 The secretions of meibomian glands in posterior blepharitis range from a cloudy, turbid fluid, to a granular substance to inspissated glands with a ‘toothpaste-like consistency’ that may be extruded as a plug or curled thread.19 In addition, there may be ‘meibomian foam,’ a frothy accumulation, which is postulated to be attributed to the presence of soaps in the tear film.31 Dougherty and McCulley et al. reported in several studies the alterations of specific polar and nonpolar lipid secretions of meibomian glands, compared to normal controls and even within different classifications of blepharitis, as the rationale behind the varying symptomatology and classification of blepharitis.31,32 For instance, the investigators have proposed that the increase in oleic acid found in the meibum of patients with posterior blepharitis may be responsible for complaints of increased burning sensation in this subset of patients.33 Further more, they showed a significant difference in the fatty wax and sterol ester fraction of meibum, which represents a large portion of the total lipid secretion from the meibomian glands in patients with chronic blepharitis.33
Changes in Lid Morphology
Pouting of the meibomian glands serves as an early, pathognomonic sign of morphological changes in the lid that occur in MGD (Fig. 8.5).25 The meibomian orifice becomes elevated above the surface of the lid, secondary to obstruction of the terminal ducts and extrusion of the abnormal meibum described earlier. These changes are compounded by retroplacement of the meibomian orifices behind the mucocutaneous junction over time.31 The orifices may become ovally elongated and result in duct exposure in severe cases.31 Additional lid changes in MGD include rounding, notching, dimpling, telangiectasia, increased vascularity of the posterior lid margin, and epithelial ridging between gland orifices.25 With chronic inflammation, hyperemia, lid thickening, and irregularity of lid contour may occur. As a result, secondary changes in the anterior lid margin may occur, such as loss of eyelashes, crusting of the lid margin, and hyperkeratinization of the mucocutaneous junction from squamous metaplasia.34
Associated Conditions
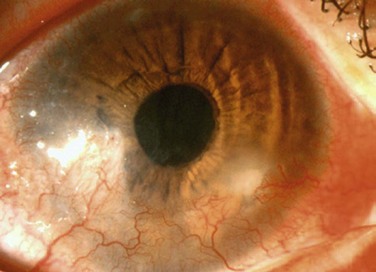
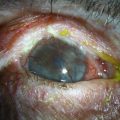
A large number of patients with posterior blepharitis also present with rosacea.35 Rosacea is a chronic skin condition characterized by persistent pustules, papules, erythema, telangiectasia, and sebaceous gland hypertrophy.17 Typically, dilated, telangiectatic blood vessels are found on the nose, cheeks and forehead. Ocular rosacea may be present with or without chronic skin changes and is typically associated with obstructed meibomian glands and telangiectatic vessels (Fig. 8.6). Sequelae of ocular rosacea include corneal pannus, dendritic keratopathy, corneal edema, scarring, neovascularization, thinning, lipid deposition, phlyctenules, ulceration, and perforation (Fig. 8.7).34 These potentially grave complications of ocular rosacea, highlight the importance of recognition and treatment of this disease in association with posterior blepharitis.
Chalazia are also more common in patients with posterior blepharitis. On pathology, the lesion appears as a localized, chronic granulomatous reaction to extravasated meibomian gland secretions from a plugged gland (Fig. 8.8).16 Therefore, it would follow that chalazia tend to occur more often in uncontrolled posterior blepharitis with plugging of the meibomian glands. Clinicians must be aware of the rare but ominous masquerade conditions that may mimic chalazia, such as sebaceous cell carcinoma and Merkle cell carcinoma.
Patients with giant papillary conjunctivitis secondary to contact lens use, may also have a greater incidence of posterior blepharitis.35
References
1. National Disease and Therapeutics Index (NDTI), IMS America, Dec 1982.
2. Hom, MM, Martinson, JR, Knapp, LL, et al. Prevalence of meibomian gland dysfunction. Optom Vis Sci. 1990;67:710–712.
3. Lemp, MA, Nichols, KK. Blepharitis in the United States 2009: a survey based perspective on prevalence and treatment. Ocul Surf. 2009;7(Suppl. 2):S1–S14.
4. Edwards, RS. Ophthalmic emergencies in a district general hospital casualty department. Br J Ophthalmol. 1987;71:938–942.
5. Mathers, WD, Shields, WJ, Sachdev, MS, et al. Meibomian gland dysfunction in chronic blepharitis. Cornea. 1991;10:277–285.
6. Thygeson, P. Etiology and treatment of blepharitis. Arch Ophthalmol. 1946;36:445–477.
7. McCulley, JP, Dougherty, JM, Deneau, DG. Classification of chronic blepharitis. Ophthalmology. 1982;89:1173–1180.
8. McCulley, JP, Sciallis, GF. Meibomian keratoconjunctivitis. Am J Ophthalmol. 1977;84:788–793.
9. Huber-Spitzy, V, Baumgartner, I, Böhler-Sommeregger, K, et al. Blepharitis – a diagnostic and therapeutic challenge. A report on 407 consecutive cases. Graefes Arch Clin Exp Ophthalmol. 1991;229:224–227.
10. Mathers, WD, Choi, D. Cluster analysis of patients with ocular surface disease, blepharitis, and dry eye. Arch Ophthalmol. 2004;122:1700–1704.
11. American Academy of Ophthalmology Cornea/External Disease Panel. Preferred Practice Pattern Guidelines. San Francisco, CA: Blepharitis—Limited Revision, AAO; 2011.
12. Torkildsen, GL, Cockrum, P, Meier, E, et al. Evaluation of clinical efficacy and safety of tobramycin/dexamethasone ophthalmic suspension 0.3%/0.05% compared to azithromycin ophthalmic solution 1% in the treatment of moderate to severe acute blepharitis/blepharoconjunctivitis. Curr Med Res Opin. 2011;27:171–178.
13. Comparative study of AzaSite plus compared to AzaSite alone and dexamethasone alone to treat subjects with blepharoconjunctivitis. http://clinicaltrials.gov/ct2/show/NCT00754949, 2012.
14. A single-center, double-masked, randomized, vehicle controlled study to evaluate the safety and efficacy of testosterone 0.03% ophthalmic solution compared to vehicle for the treatment of meibomian gland dysfunction. http://clinicaltrials.gov/ct2/show/NCT00755183?term=single-center+testosterone&rank=1, 2012.
15. Akpek, EK, Polcharoen, W, Chan, R, et al. Ocular surface neoplasia masquerading as chronic blepharoconjunctivitis. Cornea. 1999;18:282–288.
16. Raskin, EM, Speaker, MG, Laibson, PR. Blepharitis. Infect Dis Clin North Am. 1992;6:777–787.
17. Ghanem, VC, Mehra, N, Wong, S, et al. The prevalence of ocular signs in acne rosacea: comparing patients from ophthalmology and dermatology clinics. Cornea. 2003;22:230–233.
18. Seal, D, Ficker, L, Ramakrishnan, M, et al. Role of staphylococcal toxin production in blepharitis. Ophthalmology. 1990;97:1684–1688.
19. Bron, AJ, Benjamin, L, Snibson, GR. Meibomian gland disease. Classification and grading of lid changes. Eye. 1991;5(Pt 4):395–411.
20. Peralejo, B, Beltrani, V, Bielory, L. Dermatologic and allergic conditions of the eyelid. Immunol Allergy Clin North Am. 2008;28:137–168. [vii].
21. Bernardes, TF, Bonfioli, AA. Blepharitis. Semin Ophthalmol. 2010;25:79–83.
22. Shine, WE, McCulley, JP. Keratoconjunctivitis sicca associated with meibomian secretion polar lipid abnormality. Arch Ophthalmol. 1998;116:849–852.
23. Bron, AJ, Tiffany, JM. The contribution of meibomian disease to dry eye. Ocul Surf. 2004;2:149–165.
24. Tomlinson, A, Bron, AJ, Korb, DR, et al. The international workshop on meibomian gland dysfunction: report of the diagnosis subcommittee. Invest Ophthalmol Vis Sci. 2011;52:2006–2049.
25. Robin, JB, Jester, JV, Nobe, J, et al. In vivo transillumination biomicroscopy and photography of meibomian gland dysfunction; a clinical study. Ophthalmology. 1985;92:1423–1426.
26. Nichols, JJ, Berntsen, DA, Mitchell, GL, et al. An assessment of grading scales for meibography images. Cornea. 2005;24:382–388.
27. Matsumoto, Y, Sato, EA, Ibrahim, OM, et al. The application of in vivo laser confocal microscopy to the diagnosis and evaluation of meibomian gland dysfunction. Mol Vis. 2008;14:1263–1271.
28. Wang, Y, Le, Q, Zhao, F, et al. Application of in vivo laser scanning confocal microscopy for evaluation of ocular surface diseases: lessons learned from pterygium, meibomian gland disease, and chemical burns. Cornea. 2011;30:525–528.
29. Ibrahim, OM, Matsumoto, Y, Dogru, M, et al. The efficacy, sensitivity, and specificity of in vivo laser confocal microscopy in the diagnosis of meibomian gland dysfunction. Ophthalmology. 2010;117:665–672.
30. Jackson, WB. Blepharitis: current strategies for diagnosis and management. Can J Ophthalmol. 2008;43:170–179.
31. Dougherty, JM, Osgood, JK, McCulley, JP. The role of wax and sterol ester fatty acids in chronic blepharitis. Invest Ophthalmol Vis Sci. 1991;32:1932–1937.
32. McCulley, JP, Shine, WE. Eyelid disorders: the meibomian gland, blepharitis, and contact lenses. Eye Contact Lens. 2003;29(Suppl. 1):S93–S95.
33. Smith, RE, Flowers, CW, Jr. Chronic blepharitis: a review. CLAO J. 1995;21:200–207.
34. Lee, WB, Darlington, JK, Mannis, MJ, et al. Dendritic keratopathy in ocular rosacea. Cornea. 2005;24:632–633.
35. Martin, NF, Rubinfeld, RS, Malley, JD, et al. Giant papillary conjunctivitis and meibomian gland dysfunction blepharitis. CLAO J. 1992;18:165–169.