CHAPTER 75 Bladder, prostate and urethra
URINARY BLADDER
RELATIONS
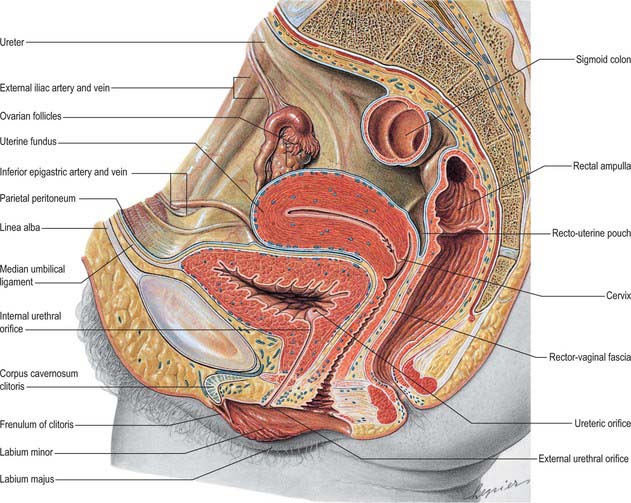
The base of the bladder is triangular and located posteroinferiorly. In females it is closely related to the anterior vaginal wall (Fig. 75.1; see Fig. 77.25B); in males it is related to the rectum although it is separated from it above by the rectovesical pouch, and below by the seminal vesicle and vas deferens on each side and Denonvillier’s fascia (Fig. 75.2A,B). The neck, which is most fixed, lies most inferiorly, 3–4 cm behind the lower part of the symphysis pubis and just above the plane of the inferior aperture of the lesser pelvis. The bladder neck is essentially the internal urethral orifice, which lies in a constant position, independent of the varying positions of the bladder and rectum. In males the neck rests on, and is in direct continuity with, the base of the prostate; in females it is related to the pelvic fascia, which surrounds the upper urethra. In both sexes the apex of the bladder faces towards the upper part of the symphysis pubis. The median umbilical ligament (urachus) ascends behind the anterior abdominal wall from the apex to the umbilicus, covered by peritoneum to form the median umbilical fold (see below).
The anterior surface of the bladder is separated from the transversalis fascia by fat in the potential retropubic space (of Retzius). This is more adherent to the bladder than to the anterior surface of the prostate, which aids reliable identification of the region of the bladder neck surgically. In males, each inferolateral surface is related anteriorly to the pubis and puboprostatic ligaments. In females the relations are similar, except that the pubovesical ligaments replace the puboprostatic ligaments. The inferolateral surfaces are not covered by peritoneum. The triangular superior surface is bounded by lateral borders from the apex to the ureteric entrances, and by a posterior border which joins them. In males the superior surface is completely covered by peritoneum, which extends slightly onto the base and continues posteriorly into the rectovesical pouch and anteriorly into the median umbilical fold: it is in contact with the sigmoid colon and the terminal coils of the ileum (Figs 75.1, 75.2 and 75.3; see page 1105). In females the superior surface is largely covered by peritoneum, which is reflected posteriorly onto the uterus at the level of the internal os (the junction of the uterine body and cervix), to form the vesicouterine pouch. The posterior part of the superior surface, devoid of peritoneum, is separated from the supravaginal cervix by fibroareolar tissue.
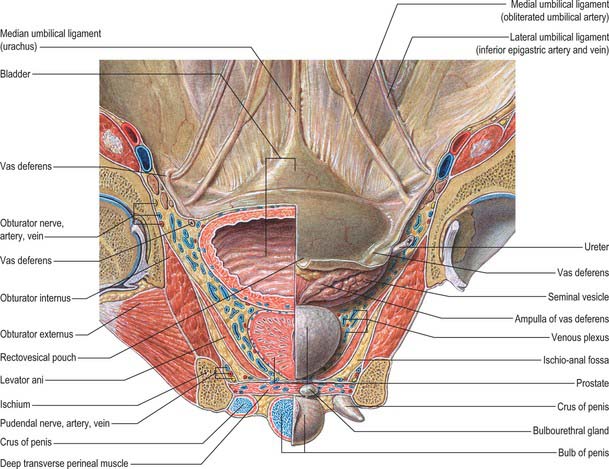
Fig. 75.3 Internal aspect of anterior abdominal wall and male anterior pelvic viscera.
(From Sobotta 2006.)
As the bladder fills it becomes ovoid. Anteriorly, it displaces the parietal peritoneum from the suprapubic region of the abdominal wall. Its inferolateral surfaces become anterior and rest against the abdominal wall without intervening peritoneum for a distance above the symphysis pubis which varies with the degree of distension, but is commonly 5–7 cm. The distended bladder may be punctured just above the symphysis pubis without traversing the peritoneum (suprapubic cystostomy): surgical access to the bladder through the anterior abdominal wall is usually by this route. The summit of the full bladder points up and forwards above the attachment of the median umbilical ligament, so that the peritoneum forms a supravesical recess of varying depth between the summit and the anterior abdominal wall: this recess often contains coils of small intestine. At birth, the bladder is relatively higher than in the adult because the true pelvis is shallow, and the internal urethral orifice is level with the upper symphysial border. The bladder is then abdominal rather than pelvic, and extends about two-thirds of the distance towards the umbilicus. Urine samples may therefore be obtained in children by performing suprapubic needle puncture. The bladder progressively descends with growth, and reaches the adult position shortly after puberty. Congenital abnormalities of the bladder are described on page 1312 (see also Fig. 78.11).
LIGAMENTS OF THE BLADDER
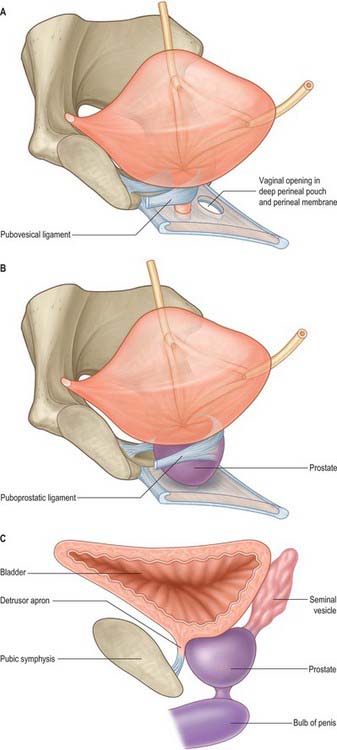
In both sexes, stout bands of fibromuscular tissue, the pubovesical ligaments, extend from the bladder neck to the inferior aspect of the pubic bones. They are derived from the detrusor muscle, part of the detrusor apron (Fig. 75.4A,B). In the female, they constitute the superior extensions of the pubourethral ligaments. In the male, the detrusor apron is described as an extension of detrusor that extends over the anterior surface of the prostate, and condenses distally and anteriorly to form the puboprostatic ligaments.
The apex of the bladder is connected to the umbilicus by the remains of the urachus, which forms the median umbilical ligament (Fig. 75.3). It is composed of longitudinal muscle fibres derived from the detrusor, and becomes more fibrous towards the umbilicus. It usually maintains a lumen lined with epithelium which persists into adult life but is only rarely complicated by a urachal cyst, sinus, fistula or adenocarcinoma. From the superior surface of the bladder the peritoneum is carried off in a series of folds, the ‘false’ ligaments of the bladder. Anteriorly there are three folds, the median umbilical fold over the median umbilical ligament (urachus) and two medial umbilical folds over the obliterated umbilical arteries. The inferior epigastric vessels are lateral to these folds on the anterior abdominal wall.
BLADDER INTERIOR
Vesical mucosa
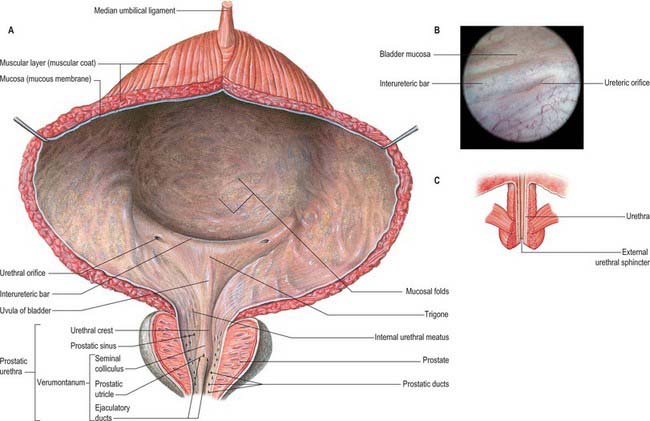
Almost all of the vesical mucosa (Fig. 75.5A–C) is attached only loosely to subjacent muscle: it folds when the bladder empties, and the folds are stretched flat as it fills. Over the trigone, immediately above and behind the internal urethral orifice, it is adherent to the subjacent muscle layer and is always smooth. The anteroinferior angle of the trigone is formed by the internal urethral orifice, its posterolateral angles by the ureteric orifices. The superior trigonal boundary is a slightly curved interureteric bar, which connects the two ureteric orifices and is produced by the continuation into the vesical wall of the ureteric internal longitudinal muscle. Laterally this ridge extends beyond the ureteric openings as ureteric folds, produced by the terminal parts of the ureters which run obliquely through the bladder wall. At cystoscopy the interureteric crest appears as a pale band and is a guide to the ureteric orifices.
Trigone
The smooth muscle of the trigone consists of two distinct layers, sometimes termed the superficial trigonal muscle and deep trigonal detrusor muscle. The latter is composed of muscle cells, indistinguishable from those of the detrusor, and is simply the posteroinferior portion of the detrusor muscle proper. The superficial trigonal muscle represents a morphologically distinct component of the trigone, which, unlike the detrusor, is composed of relatively small diameter muscle bundles that are continuous proximally with those of the intramural ureters. The superficial trigonal muscle is relatively thin but is generally described as becoming thickened along its superior border to form the interureteric bar. Similar thickenings occur along the lateral edges of the superficial trigone. In both sexes the superficial trigone muscle becomes continuous with the smooth muscle of the proximal urethra, and extends in the male along the urethral crest as far as the openings of the ejaculatory ducts.
Ureteric orifices
The slit-like ureteric orifices are placed at the posterolateral trigonal angles (Fig. 75.5A). In empty bladders they are approximately 2.5 cm apart, and 2.5 cm from the internal urethral orifice: in distension these measurements may be doubled.
BLADDER NECK
Female
The female bladder neck (Fig. 75.5C) consists of morphologically distinct smooth muscle. The large diameter fasciculi characteristic of the detrusor are replaced in the region of the bladder neck by small diameter fasciculi which extend obliquely or longitudinally into the urethral wall. In the normal female, the bladder neck sits above the pelvic floor supported predominantly by the pubovesical ligaments (Fig. 75.4A), the endopelvic fascia of the pelvic floor and levator ani. These support the urethra at rest; with elevated intra-abdominal pressure the levators contract, increasing urethral closure pressure to maintain continence. This anatomical arrangement commonly alters after parturition and with increasing age, such that the bladder neck lies beneath the pelvic floor, particularly when the intra-abdominal pressure rises, which means that the mechanism described above fails to maintain continence and women may experience stress incontinence as a result of urethral hypermobility.
Male
In the male (Fig. 75.4B), the bladder neck is completely surrounded by a circular collar of smooth muscle, with its own distinct adrenergic innervation, which extends distally to surround the preprostatic portion of the urethra. Distinct from the smooth muscle bundles which run in continuity from the bladder neck down to the prostatic urethra, and from the smooth muscle within the prostate, these smooth muscle bundles surround the bladder neck and preprostatic urethra. The bundles which form this ‘preprostatic sphincter’ are small in size compared with the muscle bundles of the detrusor and are separated by a relatively larger connective tissue component rich in elastic fibres.
VASCULAR SUPPLY AND LYMPHATIC DRAINAGE
Arteries
The bladder is supplied principally by the superior and inferior vesical arteries (see Fig. 77.3A,B), derived from the anterior trunk of the internal iliac artery, supplemented by the obturator and inferior gluteal arteries. In the female additional branches are derived from the uterine and vaginal arteries.
Veins
The veins which drain the bladder (see Fig. 77.3A,B) form a complicated plexus on its inferolateral surfaces and pass backwards in the lateral ligaments of the bladder to end in the internal iliac veins.
Lymphatics
Lymphatics which drain the bladder (see Fig. 77.3A,B) begin in mucosal, intermuscular and serosal plexuses. There are three sets of collecting vessels, most of which end in the external iliac nodes. Vessels from the trigone emerge on the exterior of the bladder to run superolaterally. Vessels from the superior surface of the bladder converge to the posterolateral angle and pass superolaterally to the external iliac nodes (some may go to the internal or common iliac group). Vessels from the inferolateral surface of the bladder ascend to join those from the superior surface or run to the lymph nodes in the obturator fossa. Minute nodules of lymphoid tissue may occur along the vesical lymph vessels.
INNERVATION
The nerves supplying the bladder arise from the pelvic plexuses, which are a mesh of autonomic nerves and ganglia on the lateral aspects of the rectum, internal genitalia and bladder base. They consist of both sympathetic and parasympathetic components, each of which contains both efferent and afferent fibres. The innervation of the bladder has been reviewed in some detail by Mundy (1999).
Efferent fibres
The urinary bladder (including the trigonal detrusor muscle) is profusely supplied with nerves which form a dense plexus among the detrusor muscle cells. The majority of these nerves contain AChE and occur in abundance throughout the muscle coat of the bladder. Axonal varicosities adjacent to detrusor muscle cells possess features which are considered to typify cholinergic nerve terminals, and contain clusters of small (50 nm diameter) agranular vesicles, occasional large (80–160 nm diameter) granulated vesicles and small mitochondria. Terminal regions approach to within 20 nm of the surface of the muscle cells and may be partially surrounded by Schwann cell cytoplasm, or more often are naked nerve endings. The human detrusor muscle possesses a sparse supply of sympathetic noradrenergic nerves which generally accompany the vascular supply and only rarely extend among the myocytes. Non-adrenergic, non-cholinergic nerves have been identified, and a number of other neurotransmitters or neuromodulators have been detected in intramural ganglia, including the peptide somatostatin. The superficial trigonal muscle is associated with more noradrenergic (sympathetic) fibres than cholinergic (parasympathetic) nerves, a difference that supports the view that the superficial trigonal muscle should be regarded as ‘ureteric’ rather than ‘vesical’ in origin. However it must be emphasized that the superficial trigonal muscle forms a very minor part of the total muscle mass of the bladder neck and proximal urethra in either sex and is probably of little significance in the physiological mechanisms which control these regions.
MICROSTRUCTURE
Lining epithelium or urothelium
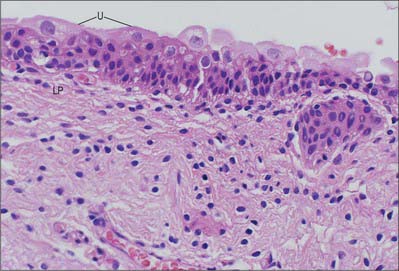
Urothelium (transitional epithelium) is 4–7 cells thick; it may appear to be attenuated to 2–3 cells thick when the bladder is fully distended. It contains three distinctive cell layers, a basal layer, an intermediate layer and a superficial (‘umbrella’ cell) layer (see Fig. 75.8). The basal layer consists of small cuboidal cells from which the upper layers arise. The intermediate layers are polygonal and possess the capacity to stretch and flatten. The superficial layer forms a protective, almost impermeable surface for the bladder mucosa and consists of large, sometimes multinucleated, cells displaying degenerative changes in their cytoplasm; these cells are ultimately exfoliated into the urine. The apical surface of the umbrella cell layer is covered by 16 nm protein particles packed hexagonally to form 2D crystals of asymmetric unit membranes, (AUMs), that contribute to the permeability barrier function of the urinary bladder, preventing reabsorption of urine across the urothelium into the bloodstream. Islands or nests of urothelium may become separated from the surface during development and are found embedded in the underlying lamina propria. These are called von Brunn’s nests and may undergo central degeneration to form cysts (cystitis cystica).
MALE URETHRA
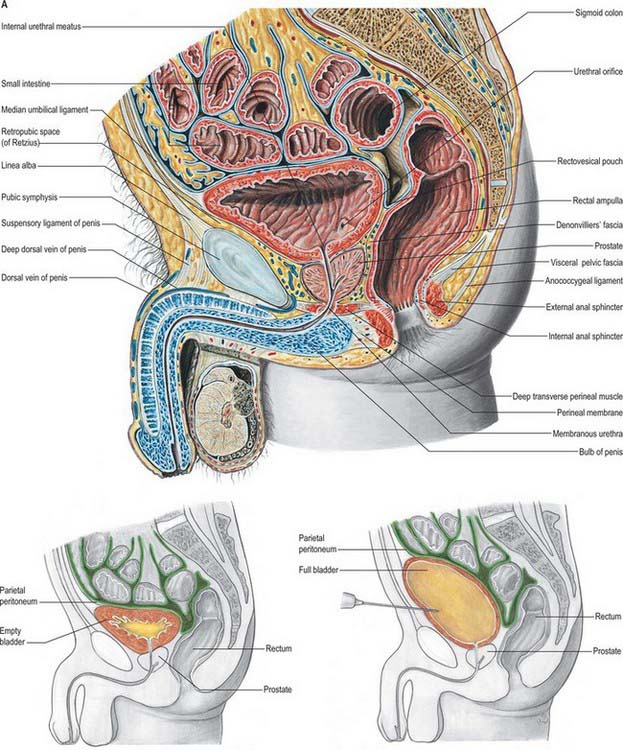
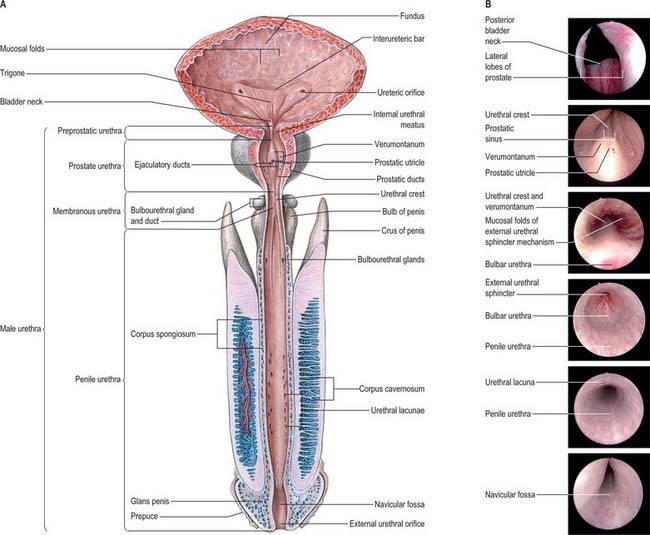
The male urethra (Fig. 75.6A,B) is 18–20 cm long, and extends from the internal orifice in the urinary bladder to the external opening, or meatus, at the end of the penis. It may be considered in two parts. The anterior urethra is approximately 16 cm long and lies within the perineum (proximally) and the penis (distally), surrounded by the corpus spongiosum. The posterior urethra is 4 cm long and lies in the pelvis proximal to the corpus spongiosum, where it is acted upon by the urogenital sphincter mechanisms. Functionally both parts act as a conduit.
Posterior part
Preprostatic urethra
The preprostatic urethra (Fig. 75.6B; endoscopic views of bladder neck and urethral crest) is approximately 1 cm in length, and extends from the base of the bladder to the prostate. Small periurethral glands at this site may contribute to benign prostatic hyperplasia (BPH) and symptoms of outflow obstruction in older men.
Prostatic urethra
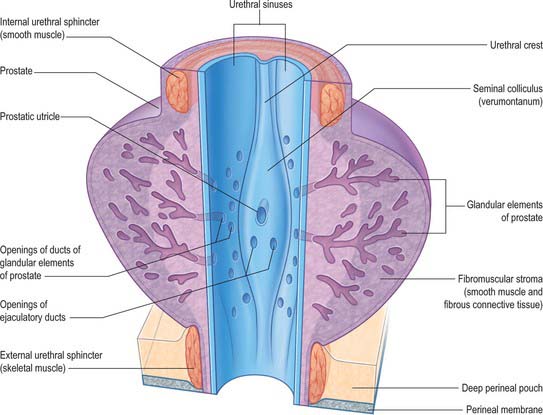
The prostatic urethra (Fig. 75.7; see Fig. 75.14) is 3–4 cm in length and tunnels through the substance of the prostate, closer to the anterior than the posterior surface of the gland. It is continuous above with the preprostatic part and emerges from the prostate slightly anterior to its apex (the most inferior point of the prostate). Throughout most of its length the posterior wall possesses a midline ridge, the urethral crest, which projects into the lumen causing it to appear crescentic in transverse section. On each side of the crest there is a shallow depression, the prostatic sinus, the floor of which is perforated by the orifices of 15–20 prostatic ducts. An elevation, the verumontanum (seminal colliculus), is seen at about the middle of the length of the urethral crest: it is used as a surgical landmark for the urethral sphincter during trans-urethral resection for benign enlargement of the prostate. At this point the urethra turns anteriorly by 35° and contains the slit-like orifice of the prostatic utricle. On both sides of, or just within, this orifice are the two small openings of the ejaculatory ducts. The prostatic utricle is a cul-de-sac 6 mm long, which runs upwards and backwards in the substance of the prostate behind its median lobe. Its walls are composed of fibrous tissue, muscular fibres and mucous membrane, the latter pitted by the openings of numerous small glands. The prostatic utricle develops from the paramesonephric ducts or urogenital sinus, and is thought to be homologous with the vagina of the female (p. 1338). It is sometimes called the ‘vagina masculina’, but the more usual view is that it is a uterine homologue and hence the term ‘utricle’. The lowermost part of the prostatic urethra is fixed by the puboprostatic ligaments and is therefore immobile.
Anterior part
The anterior or spongiose part of the urethra lies within the corpus spongiosum penis (p. 1271). In the flaccid penis it is about 15 cm long and extends from the end of the membranous urethra to the external urethral orifice on the glans penis. It starts below the perineal membrane at a point anterior to the lowest level of the symphysis pubis as the bulbar urethra, the widest part of the urethra, surrounded by bulbospongiosus. The bulbourethral glands open into the bulbar urethra approximately 2.5 cm below the perineal membrane. The urethra next curves downwards as the penile urethra. It is a narrow, transverse slit when empty, and has a diameter of approximately 6 mm when passing urine. It is dilated at its termination within the glans penis where it is known as the navicular fossa. The external urethral orifice is the narrowest part of the urethra, and is a sagittal slit, about 6 mm long, bounded on each side by a small labium.
VASCULAR SUPPLY AND LYMPHATIC DRAINAGE
Lymphatic drainage
Vessels from the posterior urethra pass mainly to the internal iliac nodes (see Fig. 77.3A,B); a few may end in the external iliac nodes. Vessels from the membranous urethra accompany the internal pudendal artery. Vessels from the anterior urethra accompany those of the glans penis, ending in the deep inguinal nodes. Some may end in superficial nodes, others may traverse the inguinal canal to end in the external iliac nodes.
INNERVATION
The prostatic plexus supplies the smooth muscle of the prostate and prostatic urethra. On each side it is derived from the pelvic plexus and lies on the posterolateral aspect of the seminal vesicle and prostate. Lesser cavernous nerves pierce the bulb of the corpus spongiosum proximally to supply the penile urethra. The greater cavernous nerves carry the sympathetic supply which causes contraction of the preprostatic sphincter during ejaculation and prevents reflux of ejaculate into the bladder. The parasympathetic preganglionic fibres are axons from neurones in the second to fourth sacral spinal segments. The nerve supply of the external urethral sphincter is controversial. It is generally believed to be supplied by neurones in Onuf’s nucleus and by perineal branches of the pudendal nerve lying on the perineal aspect of the pelvic floor; in both instances the axons arise from neurones in S2, 3 and 4. Fibres from Onuf’s nucleus (somatic) travel with the pelvic plexus on each side until they branch off and run on the pelvic aspect of the pelvic floor to enter the membranous urethra.
MICROSTRUCTURE
The epithelium lining the preprostatic urethra and the proximal part of the prostatic urethra is a typical urothelium (Fig. 75.8). It is continuous with that lining the bladder, and with the epithelium lining the ducts of the prostate and bulbourethral glands, the seminal vesicles, and the vasa deferentia and ejaculatory ducts. These relationships are important in the spread of urinary tract infections.
The epithelium changes below the openings of the ejaculatory ducts to a pseudostratified or stratified columnar type, which lines the membranous urethra and the major part of the penile urethra. Mucus-secreting cells are common throughout this epithelium and frequently occur in small clusters in the penile urethra. Branching tubular paraurethral glands secrete protective mucus onto the urethral epithelial lining and are especially numerous on its dorsal aspect. In older men, many of the deep recesses of the urethral mucosa contain concretions similar to those found within prostatic glands (see Fig. 75.15A). Towards the distal end of the penile urethra the epithelium changes once again, becoming stratified squamous in type with well-defined connective tissue papillae. This epithelium also lines the navicular fossa and becomes keratinized at the external meatus. The epithelial cells lining the navicular fossa are glycogen-rich. This may provide a substrate for commensal lactobacilli which, as in the female vagina provide a defence against pathogenic organisms.
FEMALE URETHRA
MICTURITION AND URINARY CONTINENCE
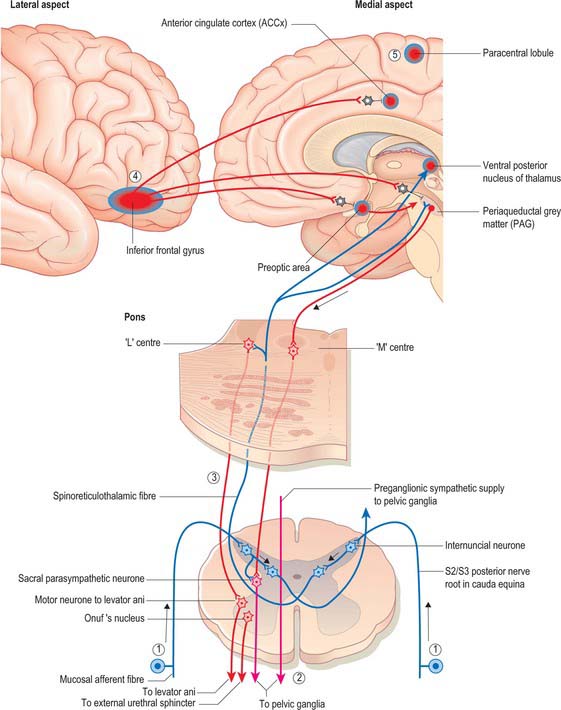
The central integration of the nervous control of the bladder and urethra is essential for normal micturition (Fig. 75.9).
Mean bladder capacity in adult males varies around 400 ml, but micturition commonly occurs at smaller volumes. Voluntary control is imposed from the inferior frontal gyrus of the cerebral cortex. Filling to 500 ml may be tolerated; beyond this level, pain caused by tension in the bladder wall leads to the urgent desire to micturate. The pain is referred to the cutaneous areas supplied by T10–L2, S2–4, including the lower anterior abdominal wall, perineum and penis. Threshold afferent stimulation activates the micturition centre in the rostral pons (the M-centre) (Fig. 75.9) which drives preganglionic parasympathetic neurones in the intermediolateral grey column of the second, third and fourth sacral spinal segments via descending spinal pathways. The axons of these neurones run to the inferior hypogastric plexus in the pelvic splanchnic nerves: they synapse on postganglionic neurones in ganglia lying within the plexus and in the wall of the bladder. Postganglionic axons ramify throughout the thickness of the detrusor smooth muscle coat. When stimulated, they release acetylcholine which activates muscarinic receptors in the detrusor layer of the bladder wall and produces the sustained bladder contraction required for micturition. The distal urethral sphincter maintains urethral closure.
Urinary continence in the male
Urinary continence at the level of the membranous urethra is mediated by the radial folds of urethral mucosa, the submucosal connective tissue, the intrinsic urethral smooth muscle, the striated external urethral sphincter and the pubourethral component of levator ani. The external urethral sphincter represents the point of highest intraurethral pressure in the normal, contracted state. The striated muscle component of the external urethral sphincter is devoid of muscle spindles. The striated muscle fibres themselves are unusually small in cross-section (15–20 μm diameter), and are physiologically of the slow twitch type, unlike the pelvic floor musculature, which is a heterogeneous mixture of slow and fast twitch fibres of larger diameter. The slow twitch fibres of the external sphincter are capable of sustained contraction over relatively long periods of time and actively contribute to the tone which closes the urethra and maintains urinary continence. They are innervated by neurones that lie in Onuf’s nucleus in the anterolateral grey matter of the second to fourth sacral spinal segments. Their firing is controlled centrally by a storage centre within the rostral pons (the L-centre) (Fig. 75.9). Just before the onset of voiding, the external urethral sphincter is relaxed by central inhibition of Onuf’s nucleus. Relaxation of the urethra is currently believed to be due to the release of nitric oxide from parasympathetic nerves in the bladder neck and urethra.
PROSTATE
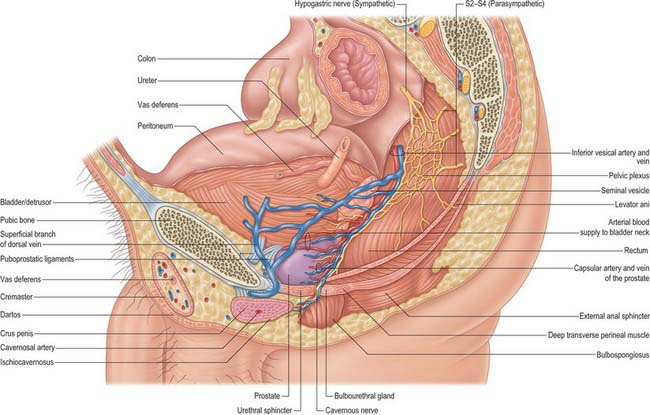
The prostate is a pyramidal fibromuscular gland which surrounds the prostatic urethra from the bladder base to the membranous urethra. It has no true fibrous capsule, but is enclosed by visceral fascia containing neurovascular tissue (Fig. 75.10A,B). The fascia is firmly adherent to the gland and is continuous with a median septum and with numerous fibromuscular septa which divide the glandular tissue into indistinct lobules.
The prostate lies at a low level in the lesser pelvis, behind the inferior border of the symphysis pubis and pubic arch (Figs 75.2A, 75.11) and anterior to rectourethralis and the rectal ampulla, through which it may be palpated. Being somewhat pyramidal, it presents a base or vesical aspect superiorly, an apex inferiorly, and posterior, anterior and two inferolateral surfaces. The prostatic base measures about 4 cm transversely. The gland is 2 cm in anteroposterior and 3 cm in its vertical diameters, and weighs about 8 g in youth, but almost invariably enlarges with the development of BPH: it usually weighs 40 g, but sometimes as much as 150 g or even more, after the first five decades of life. The small prostate without BPH is described as a croissant shape (short anterior commissure, prominent apical notch and posterior lip of prostatic tissue), and the enlarged gland is more doughnut-shaped. The shape of the prostate affects the relationship of the prostatic apex to the external urethral sphincter. This relationship is important when removing the prostate at radical prostatectomy for cancer, and anastomosing the bladder to the urethra to maintain sphincter integrity. The external urethral sphincter is flush to a large doughnut-type gland so a perpendicular incision will separate the prostate and external urethral sphincter accurately. In a small prostate the external urethral sphincter fills the defect in the anterior aspect of the prostate, so a perpendicular incision at the level of the posterior lip of the croissant shaped gland will excise much external urethral sphincter and leave the patient incontinent.
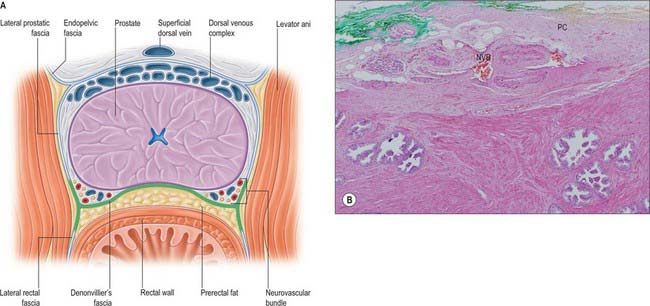
The anterior surface lies in the arch of the pubis, separated from it by the dorsal venous complex (Santorini’s plexus) and loosely attached adipose tissue. It is transversely narrow and convex, extending from the apex to the base. Near its superior limit it is connected to the pubic bones by the puboprostatic ligaments. The urethra emerges from this surface anterosuperior to the apex of the gland. The anterior part of the prostate is relatively deficient in glandular tissue and is largely composed of fibromuscular tissue. The anterior and lateral aspects of the prostate are covered by a layer of fascia derived from the endopelvic fascia on each side, called the lateral prostatic fascia. This is adherent medially to the prostate, continues posteriorly over the lateral aspect of the prostate, neurovascular bundles and rectum (lateral rectal fascia) and passes distally over the urethra (Fig. 75.10A). The prostatic venous plexus (Fig. 75.11) lies between this extension of the endopelvic fascia and the prostate. Anteroinferiorly the parietal and visceral fasciae of the prostate merge and blend with the puboprostatic ligaments. The anterior surface of the prostate and associated vascular plexus is covered by the detrusor apron.
The posterior surface of the prostate is transversely flat and vertically convex. Near its superior (juxtavesical) border is a depression where it is penetrated by the two ejaculatory ducts. Below this is a shallow, median sulcus, usually considered to mark a partial separation into right and left lateral lobes. It is separated from prerectal fat in the prerectal space (Figs 75.2A, 75.10A), and rectum by Denonvillier’s fascia, a condensation of pelvic fascia which develops by obliteration of the rectovesical peritoneal pouch, and by loose yellow fatty areolar tissue. The rectovesical pouch is obliterated from below upwards as fetal life progresses, forming Denonvillier’s fascia: at birth this fascia separates the prostate, the seminal vesicles and the ampullae of the vasa deferentia from the rectum. The superior limit of Denonvillier’s fascia is the peritoneum of the rectovesical pouch. Laterally Denonvillier’s fascia fuses with the lateral pelvic fascia: anterior to Denonvillier’s fascia, the fascia is called the lateral prostatic fascia, and posterior to Denonvillier’s fascia it is called the lateral rectal fascia.
The prostate is traversed by the urethra and ejaculatory ducts, and contains the prostatic utricle. The urethra enters the prostate near its anterior border and usually passes between its anterior and middle thirds. The ejaculatory ducts pass anteroinferiorly through its posterior region to open into the prostatic urethra (p. 1250).
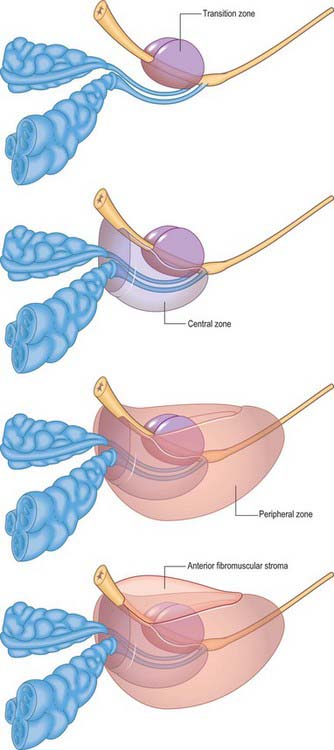
ZONAL ANATOMY OF THE PROSTATE
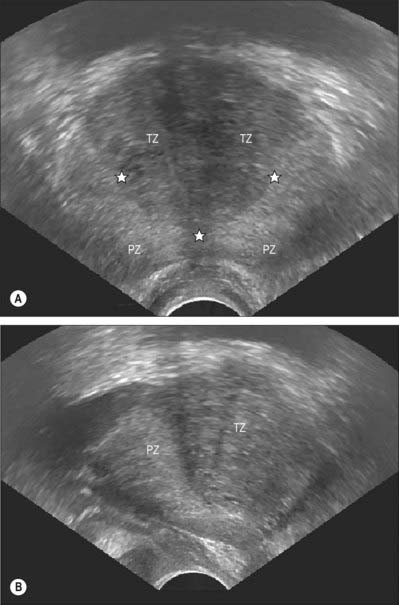
From an anatomical, and particularly from a morbid anatomical perspective, the glandular tissue may be subdivided into three distinct zones (Figs 75.12–75.14), peripheral (70% by volume), central (25% by volume), and transitional (5% by volume). Non-glandular tissue (fibromuscular stoma) fills up the space between the peripheral zones anterior to the preprostatic urethra. The central zone surrounds the ejaculatory ducts, posterior to the preprostatic urethra, and is more or less conical in shape with its apex at the verumontanum. The transitional zone lies around the distal part of the preprostatic urethra just proximal to the apex of the central zone and the ejaculatory ducts. Its ducts enter the prostatic urethra just below the preprostatic sphincter and just above the ducts of the peripheral zone. The peripheral zone is cup-shaped and encloses the central transitional zone and the preprostatic urethra except anteriorly, where the space is filled by the anterior fibromuscular stroma. Simple mucus-secreting glands lie in the tissue around the preprostatic urethra, above the transitional zone and surrounded by the preprostatic sphincter. These simple glands are similar to those in the female urethra and unlike the glands of the prostate.
The zonal anatomy of the prostate is clinically important because most carcinomas arise in the peripheral zone, whereas BPH affects the transitional zone, which may grow to form the bulk of the prostate. BPH begins as micronodules in the transitional zone which grow and coalesce to form macronodules around the inferior margin of the preprostatic urethra, just above the verumontanum. Macronodules in turn compress the surrounding normal tissue of the peripheral zone posteroinferiorly, creating a ‘false capsule’ around the hyperplastic tissue which coincidentally provides a plane of cleavage for its surgical enucleation. As the transitional zone grows, it produces the appearance of ‘lobes’ on either side of the urethra. In due course, these lobes may compress or distort the preprostatic and prostatic parts of the urethra and produce symptoms. The central zone surrounding the ejaculatory ducts is rarely involved in any disease. It shows certain histochemical characteristics which differententiate it from the rest of the prostate: it is thought to be derived from the Wolffian duct system (much like the epididymi, vasa deferentia and seminal vesicles), whereas the rest of the prostate is derived from the urogenital sinus (p. 1323).
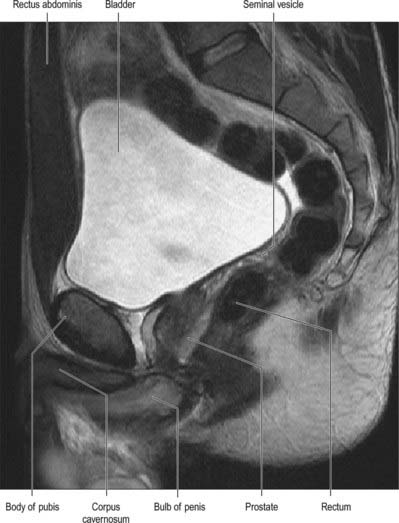
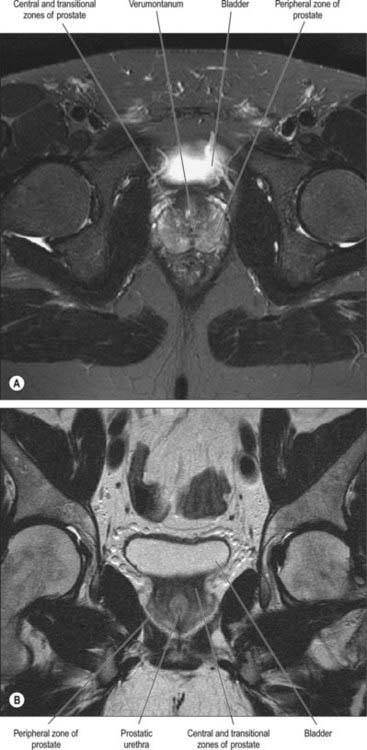
On magnetic resonance (MR) imaging the prostate gland has a zonal anatomy on T2-weighted images (Fig. 75.13). The normal peripheral zone has high signal intensity, as does fluid within the seminal vesicles. The central and transitional zones have relatively low signal and are often referred to as the ‘central gland’. The verumontanum may be seen as high signal within the central gland.
VASCULAR SUPPLY AND LYMPHATIC DRAINAGE
Arteries
The prostate is supplied by branches from the inferior vesical, internal pudendal and middle rectal arteries (Fig. 75.11). They perforate the gland along a posterolateral line from the junction of the prostate with the bladder down to the apex of the gland.
The inferior vesical artery often arises from the internal iliac artery with the middle rectal artery. It gives rise to two groups of branches, the urethral and capsular. The urethral vessels enter at the prostatovesical junction principally posteriorly at the 5 and 7 o’clock positions, but also anteriorly at 1 and 11 o’clock. This bladder neck arterial anatomy is always apparent at transurethral resection of the prostate and open removal of BPH adenomas. The capsular arteries run posterolaterally and inferiorly in the neurovascular bundles, providing perpendicular perforating vessels to the prostate. The most constant in position and prominence is the apical perforator at the prostatourethral junction, an important landmark for this point and for the neurovascular bundle at radical prostatectomy.
Veins
The veins run into a plexus around the anterolateral aspects of the prostate, posterior to the arcuate pubic ligament and the lower part of symphysis pubis, anterior to the bladder and prostate (see Fig. 76.22). The chief tributary is the deep dorsal vein of the penis. The plexus also receives anterior vesical and prostatic rami (which connect with the vesical plexus and internal pudendal vein), and drains into vesical and internal iliac veins.
INNERVATION
The prostate receives an abundant nerve supply from the inferior hypogastric (pelvic) plexus (Figs 75.10A, 75.11). The prostatic capsule is covered by numerous nerve fibres and ganglia posterolaterally, forming a crescenteric periprostatic nerve plexus. The greatest density of nerves is found in the preprostatic sphincter; fewer fibres are found in the anterior fibromuscular stroma, and the peripheral zone is the least densely innervated. Nerves containing neuropeptide Y and vasointestinal polypeptide (VIP) are localized in the subepithelial connective tissue, in the smooth muscle layers of the gland, and in the walls of its blood vessels. Neurovascular bundles containing autonomic nerves which supply the prostate, seminal vesicles, prostatic urethra, ejaculatory ducts, corpora cavernosa, corpus spongiosum, membranous and penile urethra and bulbourethral glands are closely applied to, but separable from, the posterolateral margins of the prostate. They are intimately related to the prostatic fascia. These nerves may be damaged during radical prostate surgery for organ-confined prostate cancer, producing impotence (p. 1277), or sacrificed as part of wide local excision of the prostate.
The somatic pudendal nerve (p. 1092) supplies the external urethral sphincter. The branches enter at the 5 and 7 o’clock positions, and must be avoided when placing anastomotic sutures to bring the bladder and urethra together during a radical prostatectomy. Sensory branches pass through the penile hilus in association with the dorsal venous complex and dorsal vein of the penis. They may be damaged during ligation of the dorsal vein during radical prostatectomy, which results in an anaesthetic penis.
MICROSTRUCTURE
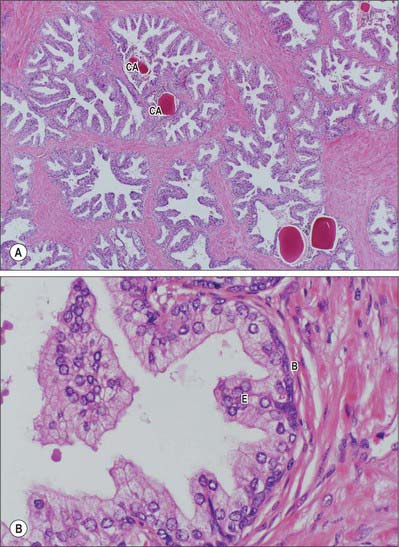
Prostatic ducts open mainly into the prostatic sinuses in the floor of the prostatic urethra. They have a bilayered epithelium, the luminal layer is columnar and the basal layer is populated by small cuboidal cells. Small colloid amyloid bodies (corpora amylacea) are frequent in the follicles (Fig. 75.15A,B). Prostatic and seminal vesicular secretions form the bulk of seminal fluid. Prostatic secretions are slightly acid, and contain acid phosphatase, amylase, prostate specific antigen, fibrinolysin and zinc. Numerous neuroendocrine cells, containing neuronespecific enolase, chromogranin and serotonin, are present in the glandular epithelium: their numbers decline after middle age and their function is unknown.
Histological sections just above the level of the verumontanum reveal two concentric, partially circumurethral, zones of glandular tissue. The larger outer zone is the peripheral zone and has long, branched glands, whose ducts open mainly into the prostatic sinuses. The inner zone is the transitional zone and consists of glands whose ducts open on the floor of the prostatic sinuses and colliculus seminalis, and a group of simple mucosal glands which surround the preprostatic urethra. Anteriorly, in the prostatic isthmus, the peripheral zone and submucosal glands are absent.
Brooks JD, Eggener SE, Chao W-E. Anatomy of the rectourethralis muscle. Eur Urol. 2002;41:94-100.
Chancellor MB, Yoshimura N. Physiology and pharmacology of the bladder and urethra. Walsh PC, et al. Campbell’s Urology Study Guide, 2nd edn, Philadelphia: Saunders, 2002. Chapter 23.
Klutke CG, Siegel CL. Functional female pelvic anatomy. Urol Clin North Am. 1995;22(3):487-498.
Meyers RP. Detrusor apron, associated vascular plexus, and avascular plane: Relevance to radical retropubic prostatectomy – Anatomic and surgical commentary. Urology. 2002;59:472-479.
Mundy AR, Fitzpatrick J, Neal D, George N. Structure and function of the lower urinary tract. In: The Scientific Basis of Urology, Chapter 11. Oxford: Isis Medical Media; 1999:217-242.
The prostate and benign prostatic hyperplasia. In: Mundy AR, Fitzpatrick J, Neal D, George N. The Scientific Basis of Urology, Chapter 13. Oxford: Isis Medical Media; 1999:257-276.