Chapter 23 Bipolar radiofrequency cold ablation turbinate reduction for obstructive inferior turbinate hypertrophy
1 INTRODUCTION
Nasal obstruction secondary to inferior turbinate hypertrophy is a common compounding problem for many patients with obstructive sleep apnea and/or snoring (OSA/S).
2 PATIENT SELECTION
With respect to inferior turbinate hypertrophy, the relative size of the inferior turbinate along its full length must be assessed. It is not uncommon to find patients with relatively normal-appearing anterior inferior turbinates, but with very pronounced posterior (tail) cobblestoned turbinate hypertrophy. The relationship of the inferior turbinate hypertrophy to nasal septal deviation in particular should be assessed. Some patients with septal deviation may yet still be candidates for inferior turbinate reduction alone if the turbinate component is felt to contribute substantially more to the nasal obstruction. Unfortunately, no clear-cut testing modality will define the individual contributions to nasal obstruction for these anatomic factors. However, we and others have found that a topical nasal decongestant test with neosynephrine or oxymetazoline may help identify patients more likely to benefit from inferior turbinate reduction. Patients must be cautioned that this pharmacologic turbinate reduction is supraphysiological and may exaggerate what is achievable with mechanical inferior turbinate reduction. Those patients who demonstrate an improvement in their subjective sense of nasal breathing and/or objectively demonstrate improvement in their nasal patency (as measured by acoustic rhinometry or nasal endoscopy) are more likely to achieve benefit with inferior turbinate reduction alone. We avoid mixing topical lidocaine in conjunction with topical decongestants because it may confound the patient’s subjective assessment of their nasal breathing. A small fraction of patients will have limited improvement with topical decongestion, still demonstrating large inferior turbinates. These patients often have a large bony (concha) inferior turbinate and may be better candidates for submucous resection techniques. Patients with significant nasal septal deviation (especially in the anterior or mid-nasal cavity), sinonasal polyposis or adenoid hypertrophy are often not good candidates for inferior turbinate reduction alone.
3 OUTLINE OF PROCEDURE
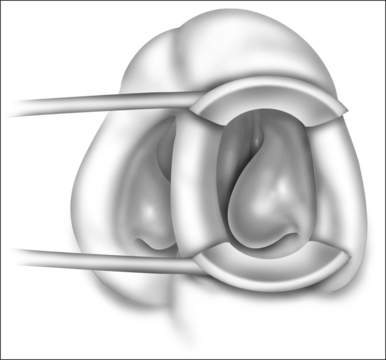
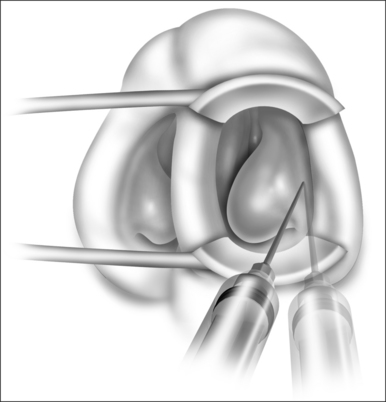
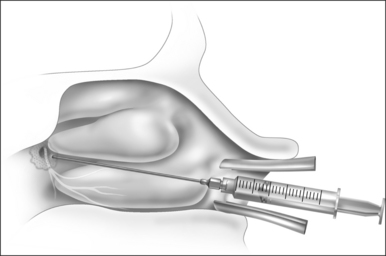
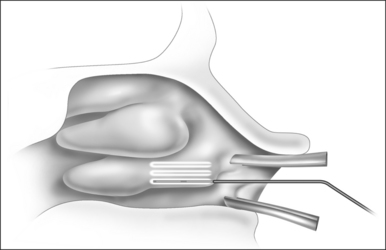
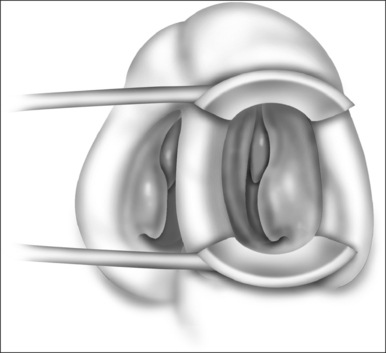
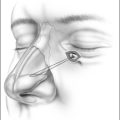
Patients are positioned upright in the procedure chair in the clinical examination room. Visualization of the anterior aspect of the nasal cavity is readily provided by a nasal speculum and headlight illumination as seen in Figure 23.1. The inferior turbinate dimensions both in terms of cross-sectional area occupied and anterior–posterior length of hypertrophy are determined. Infrequently, preoperative sedation or preoperative pre-emptive pain medication may be provided for anxious individuals. The procedure for anesthesia of the nasal cavity is a very important component of outpatient coblation inferior turbinate reduction and actually takes up much of the operative time. The nasal cavity is sequentially anesthetized, first with spray application of a lidocaine/phenylephrine mixture. After a few minutes, this is then followed by a direct contact anesthesia applied in the form of a cottonoid pledget soaked in cetaccainephenylephrine which is placed in the anterior one half of the nasal cavity, maximizing contact with the inferior turbinate. After an additional few minutes have elapsed, these pledgets are removed, an additional 2–3 ml up 2% lidocaine has infiltrated in each anterior inferior turbinate head, extending from the mucosal surface to the periosteum of the inferior concha. Additionally, we have found that further anesthetic injections just lateral to the piriform aperture, slightly anterior to the anterior head of the inferior turbinate, provide additional anesthesia to the infraorbital nerve branches to the inferior turbinate, increasing patient comfort and tolerance for the procedure. Occasionally, even after these steps with anesthesia, patients still may have some pain with the coblation procedure, especially as the middle to posterior aspects of the inferior turbinate are treated. In these patients, it is helpful to provide a sphenopalatine nerve block with a slightly bent spinal needle as indicated in Figure 23.2. For a sphenopalatine nerve block, 1–2 ml of 1% lidocaine with epinephrine are instilled just posterior and slightly inferior to the posterior attachment of the middle turbinate. This is best done with a 3 ml injection syringe coupled to a slightly bent 22-gauge spinal needle (Fig. 23.3). On occasion, endoscopic guidance may facilitate this posterior nerve block. Alternatively, a transoral, greater palatine foramen approach to the sphenopalatine ganglion for this additional nerve block may be employed.
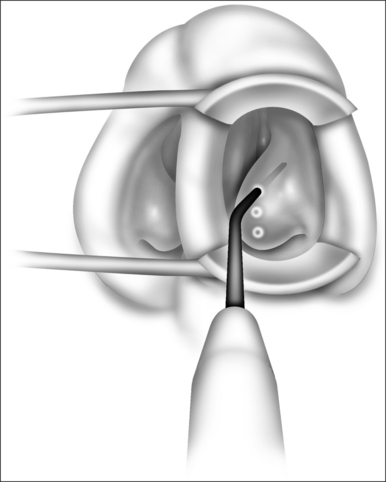
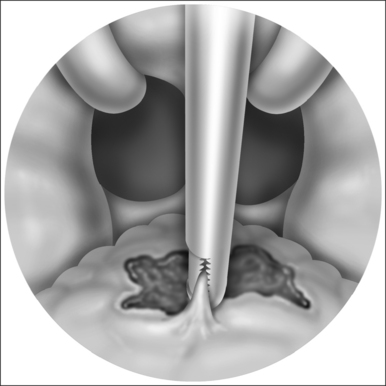
For typical inferior turbinate reduction procedures, the coblation radiofrequency controller (ENTec/Arthrocare Corp., Sunnyvale, CA) is set to a power level of 6. We prefer to use this turbinate setting and conduct multiple passes rather than increasing power level for larger turbinates. Next, a small amount of nasal saline gel is placed on the tip of the coblation wand. This nasal saline gel as a conductive medium is essential to provide smooth entry into the surface of the inferior turbinate without thermally related damage. Using a small nasal speculum to dilate and protect the limen nasi, the coblation wand is placed against the anterior aspect of the inferior turbinate. The wand is activated by the foot pedal, and the probe is carefully passed into the inferior turbinate in a submucosal plane (Figs 23.4 and 23.5). Depending on the degree of turbinate hypertrophy and the desired volume of tissue reduction, anything from one to six passes may be made. The coblation wand is maintained in the active state as the wand is removed from the turbinate, thereby providing additional exit point hemostasis. For each pass, approximately 10–15 seconds are required. The same procedure is repeated for the contralateral turbinate. Again, the desired volume of tissue reduction may be achieved by adjusting the number of passes and depth of entry into the inferior turbinate. Commonly, though not always, there is a visual reduction in turbinate size immediately during the procedure (Fig. 23.6). Any minor oozing after the procedure is handled by replacement of the cottonoid pledget into the nasal cavity. We commonly leave a cottonoid pledget in the nasal cavity to be removed by the patient approximately 4–6 hours later, depending on the degree of minor mucosal oozing.
5 SUCCESS RATE OF PROCEDURE AND WHAT TO DO IF THE PROCEDURE FAILS
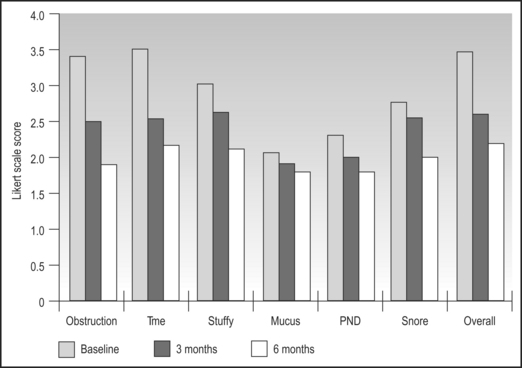
Success of inferior turbinate reduction may be measured on many scales including degree of relief in terms of structuring, time spent with nasal obstruction, nasal stuffiness, mucus production, postnasal discharge, snoring and overall symptoms. With follow-up ranging from 6 months and beyond, the vast majority of patients perceive significant benefit from coblation inferior turbinate reduction with statistically and clinically significant decreases in most nasal symptoms. For example, nasal obstruction, the amount of time spent with nasal obstruction and nasal stuffiness decreased by 43%, 35% and 27% after a single treatment with coblation inferior turbinate reduction. Overall nasal symptoms decreased by 34% at 6 months follow-up. Overall, seven out of 10 patients can expect overall perceived clinical success with one treatment, and an additional two patients out of 10 will obtain substantial clinical improvement after a second treatment (Fig. 23.7).
1. Atef A, Mosleh M, El Bosraty H, et al. Bipolar radiofrequency volumetric tissue reduction of inferior turbinate: does the number of treatment sessions influence the final outcome? Am J Rhinol. 2006;20:25-31.
2. Berger G, Gass S, Ophir D. The histopathology of the hypertrophic inferior turbinate. Arch Otolaryngol Head Neck Surg. 2006;132:588-594.
3. Bhattacharyya N, Kepnes LJ. Bipolar radiofrequency cold coblation turbinate reduction for obstructive inferior turbinate hypertrophy. Operat Tech Otolaryngol Head Neck Surg. 2002;13:170-174.
4. Bhattacharyya N, Kepnes LJ. Clinical effectiveness of coblation inferior turbinate reduction. Otolaryngol Head Neck Surg. 2003;129:365-371.
5. Cavaliere M, Mottola G, Iemma M. Comparison of the effectiveness and safety of radiofrequency turbinoplasty and traditional surgical technique in treatment of inferior turbinate hypertrophy. Otolaryngol Head Neck Surg. 2005;133:972-978.
6. Farmer SE, Eccles R. Understanding submucosal electrosurgery for the treatment of nasal turbinate enlargement. J Laryngol Otol. 2006:28:1-28:8.
7. Grutzenmacher S, Lang C, Mlynski G. The combination of acoustic rhinometry, rhinoresistometry and flow simulation in noses before and after turbinate surgery: a model study. ORL J Otorhinolaryngol Relat Spec. 2003;65:341-347.
8. Zonato AI, Bittencourt LR, Martinho FL, et al. Upper airway surgery: the effect on nasal continuous positive airway pressure titration on obstructive sleep apnea patients. Eur Arch Otorhinolaryngol. 2006;263:481-486.