Biophysical Properties of Gap Junctions
Structure of Gap Junction Channels
Cardiac Gap Junctions: Homomeric, Heterotypic, and Heteromeric Forms
Biophysical Properties of Cardiac Gap Junction Channels
Action Potential Propagation in the Myocardium: The Role of Connexins
Non–Voltage-Dependent Regulators of Channel Patency
Extrinsic Uncoupling Agents of Cardiac Gap Junction Channels
Background
The propagation of the cardiac action potential throughout the working myocardium is made possible by voltage-dependent Na+, Ca2+, and K+ currents and by gap junction channels. One of the roles of gap junction channels is to permit the passage of currents from cell to cell that are essential for action potential propagation throughout the working myocardium. When considering action potential propagation gap junctions can be best understood as components of the longitudinal resistance within the functional syncytium of the myocardium. Gap junction channels are in series with the cytoplasmic resistance. The intercellular resistance of gap junctions is in series with the intracellular resistance of the cytoplasm, and together they represent longitudinal resistance. Both resistances can affect conduction velocity within the heart, but it is the gap junction channels composed of connexins that dominates longitudinal resistance. Dual whole-cell patch clamp studies of cardiac myocytes have been used to quantify gap junction membrane resistance or junctional conductance in vitro. The estimates of junctional resistance for ventricular myocyte cell pairs reveal that it is often an order of magnitude less than the input resistance of an isolated myocyte, somewhere in the range of 2 to 10 MΩ or 1000 to 100 nS.1 These values must be considered under estimates because of the series resistance of the pipettes that have similar resistance values to the junctions themselves.1 In addition to creating an electrical syncytium for the heart gap junctions also allow the passage of small solutes such as cAMP that are able to affect the function of multiple systems within cardiac myocytes. To better understand how gap junction channels contribute to the normal functions of the heart and how they participate in cardiac arrhythmias and ischemia, it is necessary to first describe their structure and biophysical properties.
Structure of Gap Junction Channels
Structural analysis has revealed that gap junction channels are composed of two hemichannels or connexons linked together that provide an intercellular pathway between two adjacent cell interiors (Figure 15-1, A). Each hemichannel is composed of six connexins. Each connexin contains four membrane-spanning domains (M1-M4; see Figure 15-1, B). The N-terminus protrudes from M1 into the cytosol. M1 and M2 are connected by an extracellular loop E1, whereas M2 and M3 are connected by a cytoplasmic loop. M3 and M4 are connected by another extracellular loop (E2), and the cytoplasmic extension of M4 is the C-terminus (see Figure 15-1, B). This general model is true for all the cardiac connexins and the other human connexins. Individual gap junction channels within the atrial and ventricular myocardium are found in abundance at the intercalated discs in the form of membrane complexes or plaques containing a large number of channels, ranging from hundreds to thousands. A small portion of such a plaque is depicted in Figure 15-1, A. These plaques can also form along the lateral surfaces of myocytes in normal myocardium and can become even larger structures in stressed or diseased myocardium.2
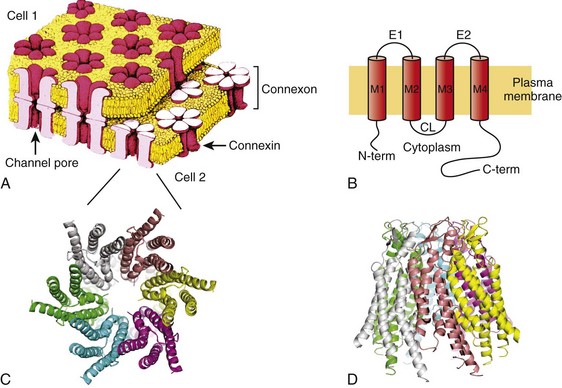
Figure 15-1 A, A portion of a junctional plaque. Yellow bilayers are the plasma membranes of two adjacent cells. Each gap junction channel is composed of two hemichannels or connexons that are themselves composed of connexins. Modified after reference.55 B, Schematic representation of a connexin with 4 membrane domains, M1-M4, 2 extracellular loops, E1 and E2, and 3 cytoplasmic domains, N-terminus, cytoplasmic loop, and the C-terminus. C, En face view the hemichannel from the cytoplasm of a cell. D, Molecular rendition of connexin26 (Cx26) viewed from within the plasma membrane of a cell. Each connexin is colored differently. (A, Modified from Makowski L, Caspar DL, Phillips WC, et al: Gap junction structures. VI. Variation and conservation in connexon conformation and packing. Biophys J 45:208–218, 1984.)
Only a few studies have been successful in elucidating the molecular structure of gap junction channels using electron crystallography. The first analysis was performed on a noncardiac connexin, Cx26, and more recently has been revisited, revealing structural detail to a resolution of approximately 0.35 nm.3 Cx43 has also been analyzed using crystallographic methods with a resolution of approximately 1.0 nm.4 Figure 15-1, C, depicts a representation of the helical membrane domains and the extracellular loops of a hemichannel as viewed from within the plasma membrane of a cell. Figure 15-1, D, illustrates the structure of Cx26 from the perspective of looking down its long axis directly into the channel from the cytoplasm where the four transmembrane domains of each connexin can be visualized. The structural analysis has not been sufficiently detailed to demonstrate clearly which membrane-spanning domains are forming the channel wall. However, the structure shown in Figure 15-1, D, again depicting the four transmembrane helices, suggests that M1, M2, M3, and even M4 are potential contributors to the lining of the pore or the cytoplasmic vestibule.
One approach that has been used to define the pore lining regions of connexins has been the substituted cysteine accessibility method (SCAM). Substituting cysteine for other amino acids thought to be contributing to the pore wall is the first step to successful use of SCAM. This step is followed by a demonstration that the substitution does not affect normal channel activity. To establish whether the substituted group is part of the pore wall, a thiol reactive agent such as maleimide or a derivative is then perfused into the preparation while monitoring channel activity. One possible outcome is altered unitary conductance consistent, with the substituted group being a component of the pore wall. In fact, the application of SCAM to connexins has provided varied results when assessing pore lining regions of the four membrane-spanning domains and extracellular loops and intracellular regions. The use of SCAM to elucidate the pore wall structure on Cx46 has generated data most consistent with pore lining domains or pore access regions in M1 and the E1 loop,5 but Cx32 SCAM data are more consistent with M3 as a major pore lining helix.5 No SCAM analysis has been performed on Cx43, Cx40, Cx45, or Cx37. Site-directed mutagenesis has also been used for Cx43 and Cx37 with various substitution strategies. In Cx43, mutations were introduced in M3 that resulted in silent channels.5 This is not conclusive evidence that M3 participates as a functional member of the pore wall, but it does not exclude the possibility. For Cx37, site-directed mutations in M3 resulted in altered conductive states, which is consistent with M3 participation in forming the pore wall, but does not exclusively demonstrate that either.6 The crystallographic and mutagenic data lead to one possible explanation: different connexins do not use the identical structural motifs in the formation of an intercellular pore despite functional similarities. The crystallographic structure shown in Figure 15-1, C, indicates that there are potential surfaces for all four domains contributing to the channel or pore and the cytoplasmic vestibule.
Cardiac Gap Junctions: Homomeric, Heterotypic, and Heteromeric Forms
Hemichannels composed of six connexins from two closed aligned cells form a linkage via the extracellular domains E1 and E2 to create a complete gap junction channel. The heart does not express all 21 identified connexins7 that are able to assemble into functional gap junction channels (see Figure 15-1, A, B). Instead, a select number of connexins are expressed within the human heart; they are Cx43, Cx40, Cx45, Cx46, and Cx37.8 Their distribution within the heart is not uniform. For example, Cx43 is abundantly expressed within the ventricles but is only sparingly expressed within the AV and SA nodes.8,9 Table 15-1 illustrates the relative connexin expression levels within the ventricles, atrium, SA node, AV node, and bundle branch/conducting system (BB/CS). Connexin 31.9, the ortholog of mouse Cx30.2 found in the mouse AV node, has thus far not been detected in the human heart. Cx30.2 has been shown to be in part responsible for conduction delay at the AV node in mice.9 The absence of Cx31.9 in the human AV node suggests that the delay in humans might be the result of reduced channel numbers or the presence of heteromeric or heterotypic forms of Cx45, Cx40, and Cx43 or possibly the existence of another unidentified connexin within the AV node whose properties mimic those of mouse Cx30.2.
Table 15-1
| Ventricles | Cx43 > Cx45 > > Cx40 > > Cx46~Cx37 |
| Atrium | Cx40 > Cx43 ~ Cx45 |
| BB/CS | Cx40 ~ Cx43 ~ Cx45 |
| AV node | Cx45 > Cx40 ~ Cx43 |
| SA node | Cx45 > Cx40 > #x003E; #x003E; > Cx43 |
BB/CS, Bundle branch/conducting system; AV, ventricular; SA, sinoatrial.
From Severs NJ, Bruce AF, Dupont E, et al: Remodelling of gap junctions and connexin expression in diseased myocardium. Cardiovasc Res 80:9–19, 2008; and Jansen JA, van Veen TA, de Bakker JM, et al: Cardiac connexins and impulse propagation. J Mol Cell Cardiol 48:76–82, 2010.
As implied in Table 15-1, myocytes within the different regions of the heart are able to coexpress connexins. For example, individual atrial myocytes express Cx40, Cx43, and Cx45 simultaneously but in differing amounts with Cx40 being the most abundantly expressed connexin. The expression of a single connexin within myocytes has the potential to generate functional gap junction channels composed of two identical hemichannels, both composed of the same connexin referred to as homomeric (i.e., homotypic) channel. Another type of gap junction channel is also possible, where each hemichannel of two opposing cells is homomeric but each cell expresses a different connexin. This type of channel is heterotypic.7 Finally, because most myocytes express at least two and often three connexins, a hemichannel can potentially contain two or possible three different connexins.7,10 This type of hemichannel is heteromeric. Two heteromeric hemichannels will form a heteromeric gap junction channel.
Biophysical Properties of Cardiac Gap Junction Channels
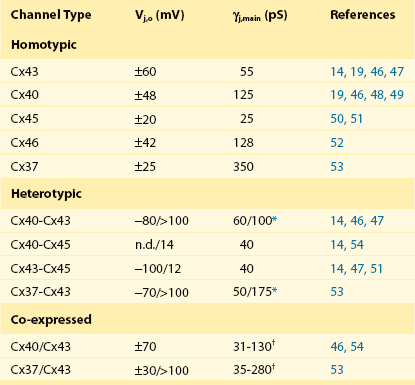
The biophysical properties of gap junction channels are best illustrated using a dual whole-cell patch clamp on isolated cell pairs. Figure 15-2, A, depicts a cell pair coupled by gap junctions with the equivalent circuit for the cell pair. All the cardiac gap junction channels have been studied in connexin-deficient cells that are then transfected with specific cardiac connexins to better understand how homotypic gap channel forms of Cx43, Cx40, Cx45, Cx46, and Cx37 behave. In all cases, each can be shown to gate closed with the application of increased transjunctional voltage (Vj). A macroscopic record of junctional currents is shown in Figure 15-2, B, for Cx45 and illustrates the decline of junctional current with sustained Vj. Ij,inst is the junctional current recorded at the onset of a voltage step, and Ij,ss is the steady state current. In some cases, individual channel activity can be observed as shown in Figure 15-2, C. Single-channel recordings for Cx43, Cx40, and Cx45 are shown. Multichannel and single-channel data have allowed the determination of unitary conductance (γj,main) for the cardiac connexins, which are listed in Table 15-2. The ability to monitor unitary events has also allowed a better understanding of voltage-dependent gating in connexins, which has been shown to have at least two distinct mechanisms: fast gating and slow gating. Fast gating is characterized by a rapid transition from an open state to a residual state (γj,residual; see dashed lines in Figure 15-2, C) or closed state, whereas slow gating is manifest as a series of subconductive states transitioning from an open or closed state.10 A systematic study on Cx30 gap junctions reported five substrate conductances unevenly spaced between γj,main and γj,residual, suggesting that each of the six connexins of a hemichannel act as a subgate.11 Macroscopic recordings of junctional currents (see Figure 15-2, B) have also been useful in dissecting the molecular mechanisms of voltage-dependent gating. Plotting the steady state currents generated in response to different Vj steps (see Figure 15-2, B) reveals the relationship between junctional conductance (gj) and transjunctional potential (Vj). gj is derived from the ratio of gj,ss/gj,inst. A Boltzmann fit of the normalized steady state junctional conductance for various amplitude voltage steps is shown in Figure 15-2, D, for Cx43, Cx40, and Cx45.
Table 15-2
Multichannel and Single-Channel Data of Different Types of Gap junction Channels
*Conductance polarity dependent.
†Conductances from homotypic, heterotypic, and heteromeric channels are possible.
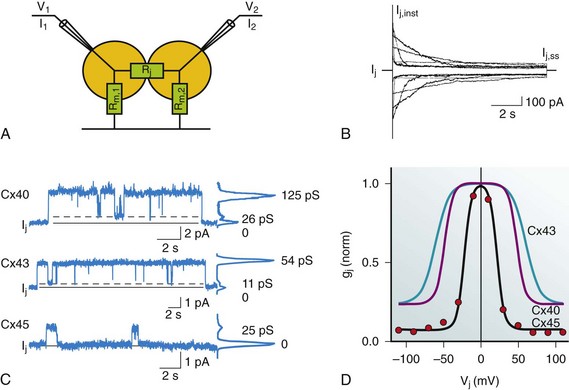
Figure 15-2 A, Dual whole-cell patch clamp equivalent circuit and superimposed cell pair. The patch electrodes ideally are significantly lower resistance than the junctional (Rj) and nonjunctional resistances (Rm). The circuit allows for the computation of junctional conductance (gj) with the following relationships: Vj = V2 − V1; I1 = Im,1 + Ij; I2 = –Ij; gj = Ij / Vj. B, Macroscopic record of homotypic Cx45 using Vj steps of ±10, ±30, ±50, ±70, ±90, and ±110 mV of 10 seconds in duration, which illustrates the voltage dependence of Cx45 and the varied time constants of inactivation with Vj. Ij,inst is the instantaneous junctional current and Ij,ss is the steady state current. C, Single-channel properties of Cx43, Cx40, and Cx45 channels. Records of single-channel currents observed in pairs of HeLa cells transfected with Cx40, Cx43, and Cx45. Solid line, zero current level; dashed line, residual current level. The current histograms (right) revealed γj,main = 125 pS and γj,residual = 26 pS for Cx40, γj,main = 54 pS and γj,residual = 11 pS for Cx43, and γj,main =24 pS for Cx45 cell pairs; pipette was filled with 120 mM K+aspartate–. D, Normalized gj plotted against Vj for Cx43, Cx40, and Cx45. The red dots represent the record shown in (B). Normalized junctional conductance (gj) is steady state conductance gj,ss normalized against the instantaneous conductance gj,inst. (Data modified from Valiunas V, Beyer EC, Brink PR: Cardiac gap junction channels show quantitative differences in selectivity. Circ Res 91:104–111, 2002; and Valiunas V, Gemel J, Brink PR, et al: Gap junction channels formed by coexpressed connexin40 and connexin43. Am J Physiol Heart Circ Physiol 281:H1675–H1689, 2001.)
An important parameter derived from the Boltzmann fit is Vj,o. This parameter represents the half inactivation voltage or that transjunctional potential where half the channels can be considered closed. The instantaneous junctional conductance remains relatively constant regardless of the voltage, implying that many if not all the gap junctions are patent when Vj is zero. Analysis of single and multichannel records of Cx43 (see Figure 15-2, C) have been used to determine open probability under chronic application of Vj between 20 and 40 mV yielding values ranging from 0.5 to 0.95, consistent with the idea that gap junction channels are in the open state when there is no applied transjunctional potential. The application of Vj greater than 50 mV reduces the mean open time, whereas mean closed time remains relatively constant, which translates into reduced open probability (Po) with increased Vj amplitude.12 This is consistent with the behavior manifest in macroscopic recordings for Cx43. Nonstationary analyses of Cx43 and Cx37 have also revealed similar results with open probabilities less than 0.95 for Cx43 and 0.7 for Cx37.13
Table 15-2 lists Vj,o and unitary conductance for the cardiac connexins, which includes homotypic, heterotypic, and heteromeric forms. The values of Vj,o for homotypic channels vary from 20 mV for Cx45 to 60 mV for Cx43. Also listed are all the Vj,o values for heterotypic forms that have thus far been determined. In addition, values for cells coexpressing Cx43/Cx40 and Cx43/Cx37 are given. Unitary conductance for the cardiac connexins varies greatly, as seen in Table 15-2. For heterotypic forms, the observed unitary conductance can be polarity dependent.14 Observation of cell pairs coexpressing connexins reveals a range of conductances consistent with channels of different connexin content.
The macroscopic junctional currents shown in Figure 15-2, B, illustrate that the currents decline (deactivation) with a prescribed time course on the order of 0.1 to 1 seconds for Cx43.15 The time constant for voltage-dependent inactivation is itself voltage dependent and becomes shorter with larger transjunctional voltage steps. The time course of voltage-dependent inactivation in junctional currents as shown in Figure 15-2, B, for Cx45 are similar to the behavior of the other cardiac connexins, although it should be noted that Cx45 has the largest inactivation time constants of the cardiac connexins, approximately 0.1 seconds for large transjunctional potentials and 3 seconds for smaller transjunctional steps.15
Heterotypic forms that have been studied often generate asymmetric voltage dependent I-V curves, an example of heterotypic Cx43-Cx45 is shown in Figure 15-3, B, along with heterotypic Cx40-Cx45 (see Figure 15-3, C) and Cx40-Cx43 (see Figure 15-3, D). Note that for one voltage polarity, a voltage-dependent deactivation or decline in junctional current is present, much like the homotypic forms. For the other polarity there is, in effect, little or no voltage-dependent closure. In the case of Cx40-Cx45 and to a lesser degree Cx43-Cx45, there is an increase in junctional current, which is best illustrated by the plots of gj versus Vj. This observation suggests that heterotypic gap junction channels have altered voltage sensing and gating relative to their homotypic parents and that Po for these forms might be significantly less than unity, or the asymmetric unitary conductance observed in heterotypic channels is itself voltage dependent.
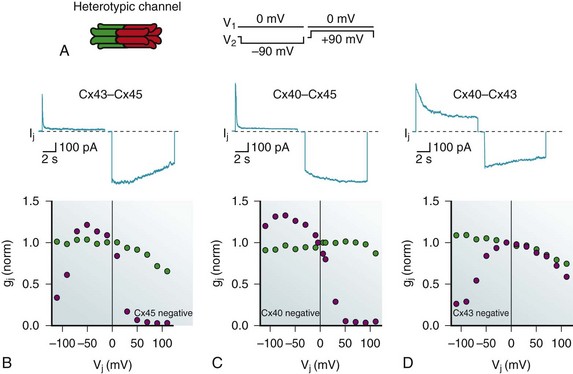
Figure 15-3 A, Rendition of the heterotypic channel where each hemichannel is homomeric, but the connexins for each are different. A voltage step profile for the records shown in B–D are illustrated to the right. B, Macroscopic current record of Cx43-Cx45 heterotypic gap junction channels. The lower panel shows the normalized gj versus Vj showing both steady state gj,ss (blue circles) and instantaneous gj,inst (green circles). Cx45 gates close when the potential in the opposing cell of a pair is more positive; therefore, it gates closed with a negative potential relative to its neighbor (Cx45 negative). C, The same paradigm for a Cx40-Cx45 heterotypic junction. D, The same paradigm for a Cx43-Cx45 heterotypic junction.
The Number of Functional Channels within the Intercalated Disc
The formation of large plaques or aggregates of channels that are closely packed together is an interesting synapselike feature of gap junctions (see Figure 15-1), and it raises a number of questions, such as are all the channels simultaneously active or are there active and inactive or silent populations? Furthermore, is each channel functioning as an identical but independent channel, or is there evidence for nonindependent, interdependent, or cooperative behavior? Or can channels shift between states that in the case of gap junctions would be moving from mostly patent to mostly closed? Recall that active gap junction channels possess high open probabilities approaching unity. Thus, accurate measurement of total junctional conductance and knowing the unitary conductance for a particular connexin allows an estimate of the total number of functioning channels operating between a cell pair. To determine whether all of channels within a plaque are functional first requires a determination of the number of channels within any one plaque; second, it requires the determination of the number of functional channels within that particular plaque. By tagging Cx43 with a fluorescent reporter such as GFP, it is possible to image plaques within cell pairs and further directly assess the junctional conductance using a dual whole-cell patch clamp. An analysis of experiments using this dual approach of imaging and electrophysiologic assessment of junctional conductance has revealed that approximately 10% of the channels are functioning within junctional membrane plaques16; furthermore, it appears that Cx43 channels displayed non-independent behaviors associated with phenomena such as transitioning between an active patent state and a silent state on the order of many seconds to minutes, which represents an example of mode shifting.12 These surprising results beg the question: why are there so many apparently silent channels (Po = 0) in presumed dynamic equilibrium with a lesser population of almost continually open (Po ≈ 1) channels? Are there conditions or circumstances in which the silent channels can be activated rapidly via phosphorylation, for example? Or are the silent channels already designated or identified for internalization as connexosomes (internalized gap junction membranes) to be trafficked to lysosomes?17 The significance of these observations might have little bearing on normal action potential propagation, but it remains unclear in regard to proarrhythmic and antiarrhythmic processes.
Cardiac Gap Junction Permeability to Ions and Other Solutes
The major intracellular ion involved in intercellular current flow via gap junctions is K+. Estimates of the number of K+ flowing through a single gap junction channel per second in response to a voltage step of approximately 23.4 mV or the equivalent of a 10× concentration gradient range from approximately 18.3 × 106 ions per channel per second for Cx40 to 8.1 × 106 ions per channel per second for Cx43 and 3.7 × 106 ions per channel per second for Cx45.18,19 A number of studies have also determined the permeability of the major cardiac connexins to a variety of exogenous fluorescent probes and endogenous second messengers such as cAMP.20
Permeability of gap junction channels has been defined qualitative and quantitative. A common qualitative approach for such studies is the use of an exogenous fluorescent probe that is introduced in one cell and detected in another cell of a pair (Figure 15-4, A). For quantification of any specific probe’s permeability, it is necessary to simultaneously measure junctional conductance and fluorescence distribution and intensity of the probe via imaging.19
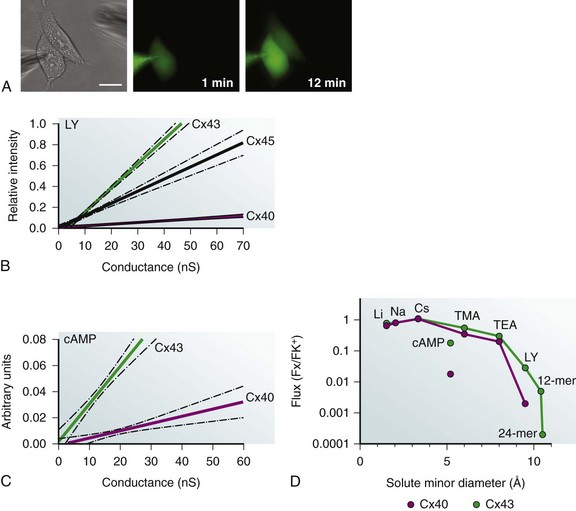
Figure 15-4 A, Three images of a cell pair where the electrode on the left contains a known concentration of fluorescent dye Lucifer yellow (LY). Once in whole-cell patch mode, fluorescence emission is monitored over time (1- and 12- minute records shown). Scale bar, 20 µm. B, Relative fluorescence intensity of LY in target cell, compared with source cell plotted versus junctional conductance for Cx43, Cx40, and Cx45. All fluorescence measurements are made at the same time interval. C, Cyclic AMP permeability to Cx43 and Cx40 versus conductance. cAMP is approximately 10-fold more permeable to Cx43 than Cx40. Arbitrary units are the ratio of current density for a cAMP activated channel before and during exposure in elevated intracellular cAMP levels. (D) Semilog plot of Li+, Na+ Cs+, cAMP, TMA, TEA and LY flux relative to K+ ion for Cx43 and Cx40. Permeability of 2 oligonucleotides (12 and 24 nucleotides long) to Cx43 is also plotted. (Data from Valiunas V, Beyer EC, Brink PR: Cardiac gap junction channels show quantitative differences in selectivity. Circ Res 91:104–111, 2002; Kanaporis G, Mese G, Valiuniene L et al: Gap junction channels exhibit connexin-specific permeability to cyclic nucleotides. J Gen Physiol 131:293–305, 2008; Valiunas V, Polosina YY, Miller H, et al: Connexin-specific cell-to-cell transfer of short interfering RNA by gap junctions, J Physiol 568(Pt 2):459–468, 2005; and Brink PR: Gap junctions in vascular smooth muscle. Acta Physiol Scand 164:349–356, 1998.)
Figure 15-4, A, shows cell to cell the transfer of fluorescent dye Lucifer yellow (LY). Comparison of LY permeability for Cx43, Cx40, and Cx45 is a notable example of selectivity, as illustrated in Figure 15-4, B. Lucifer yellow with a minor diameter of 0.95 nm and a net negative charge is greater than tenfold more permeable to Cx43 than Cx40 and threefold more permeable to Cx45.18,19 The relative fluorescence intensity in a target cell of a pair is plotted at the 12-minute mark versus the measured junctional conductance measured simultaneously in Figure 15-4, B.19
Figure 15-4, C, illustrates the differences in cyclic adenosine monophosphate (cAMP) permeability for Cx43 and Cx40, where the y-axis is the ratio of a reporter channel current density relative to a control versus junctional conductance measured simultaneously.20 CAMP permeability to Cx43 is much more permissive than Cx40.
For a number of the exogenous probes and select messengers, it has been possible to determine their flux relative to K+ for specific homotypic connexins, namely Cx43 and Cx40. Figure 15-4, D, is a semilog plot of flux of either an ion, probe, or solute relative to K+. The monovalent cations are Li+, Na+, TMA, and TEA. Lucifer yellow, cAMP, and two oligonucleotides (short interforing RNA [siRNA] mimics) represent solutes of varying size and charge. The graph illustrates what is considered a general rule for connexin channels; monovalent cations have permeabilities relative to K+ that are similar to an Eisenmann series I or II, which is much like their mobility in free solution. As solute diameter increases, differences in permeability for the same solute begin to appear between Cx43 and Cx40. The permeability of two synthetic oligonucleotides are also plotted; they are long, rod-shaped molecules (morpholinos) whose minor diameters are 1.0 to 1.1 nm.21
The differences in permeability of LY and cAMP for Cx43 and Cx40 are shown in Figure 15-4, D, and it should be noted that TMA, a larger probe but of lesser charge density than cAMP, is more permeable than cAMP with a larger smaller minor diameter for both Cx43 and Cx40. This strongly suggests selectivity based not only on size but on charge as well.
An important aspect of gap junction mediated transfer of second messengers is best illustrated when considering cAMP permeability. It has the potential to affect a multitude of cellular functions. One function is the ability to positively shift the voltage dependence of human HCN4, resulting in an increase in Po of HCN4 channels at any particular membrane potential that in turn can affect pacemaker rate within the heart.22 The cell-to-cell diffusion of cAMP might also participate in the generation of a positive inotropic effect. This scenario implies a sparse innervation density in the ventricular myocardium where only one myocyte of a number of gap junction–coupled myocytes receive autonomic input. The autonomic input to the innervated myocyte results in the rapid generation of elevated cAMP within its cytoplasm. The cAMP then diffuses to surrounding cells that are not innervated. The time to reach half concentration in adjacent cells coupled along their lateral borders would be on the order of 1 to 5 seconds, assuming a junctional permeability illustrated in Figure 15-4 for cAMP and a cytoplasmic diffusion coefficient of approximately 1 × 10−6 cm2/s.23 Such a time course is consistent with the time course for sympathetic induced inotropy of the heart. The notion of sympathetic and parasympathetic innervation density being less than one-to-one for nerve to myocyte, while never having been quantitatively assessed is consistent with observations describing low innervation density in the ventricular myocardium.24
Another possible role for connexins in the heart and other syncytial tissues is the passage of microRNAs and siRNAs able to affect gene expression and ultimately cellular phenotype.25 As a general rule, cardiac connexins can be considered to be poorly selective toward monovalent cations as an Eisenmann series 1 implies and are only moderately more selective toward the monovalent halide anions. With increased size and charge, Cx40 and Cx45 appear to be less permissive or more selective than Cx43.
Action Potential Propagation in the Myocardium: The Role of Connexins
Two questions arise when considering the role of gap junction channels in the propagation of the cardiac action potential. First, what is the relationship between gap junction number and conduction velocity? Second, does gap junction channel voltage dependence matter? The first question has been best addressed using a combination of experimental data and computational analysis. In vitro studies of cell pairs and isolated tissues have shown that a pharmacologically induced reduction in gap junctional conductance slows conduction and ultimately can block conduction, whereas increased expression enhances conduction.26 Similarly, many arrhythmias have been associated with reduced gap junction channel numbers, redistribution of junctional plaques on the lateral surfaces, or mutations within connexins.2 Using both experimental and computationally derived data, it has been shown that there is a nonlinear relationship between conduction and longitudinal resistance dominated by gap junctions. Simulation of the experimental data predicts that conduction velocity is roughly proportional to the log10 of gap junction channel number over a range of 10 to 1000 nS (Figure 15-5).
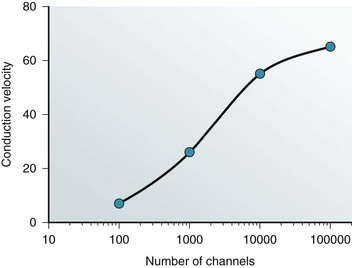
Figure 15-5 Conduction velocity is plotted against the log of the number of gap junction channels. Both simulation data and experimental data have been used to generate the depicted relationship. . (From Brink PR, Cronin K, Ramanan SV: Gap junctions in excitable cells J. Bioenerg Biomembr 28:351–358, 1996; and Cole WC, Picone JB, Sperelakis N: Gap junction uncoupling and discontinous propagation in the heart. A comparison of experimental data with computer simulations. Biophys J 53:809–818, 1988.)
The second question as to whether gap junction voltage dependence can have a role in conduction velocity requires defining current flow longitudinally within myocytes in response to a propagating action potential. This definition then allows the determination of the transjunctional voltage experienced at the intercalated disc. It is assumed that a myocyte is approximately 100 µm long (L) and has a diameter of approximately 15 µm and that myoplasmic resistance is approximately 400 Ω-cm. Thus, the longitudinal resistance of a myocyte is approximately 2 MΩ. Assuming that conduction velocity (θ) is 50 cm/s and that the maximum rate of rise for the action potential is 100 V/s, the longitudinal voltage drop along the long axis of the cell can be determined by Vcell = ([V / s] / θ) × L, or 20 mV. The longitudinal current flow is then 20 mV / 2 MΩ =10 nA. For an intercalated disc with 1.8 × 105 functioning channels or a junctional conductance of approximately 1000 nS, the amount of current flowing through each patent channel is 0.05 pA. The former assumes a channel population of homotypic Cx43 channels each with a unitary conductance of approximately 55 pS (19). Therefore, the transjunctional voltage is 1 mV per channel. For 20,000 channels the value is approximately 10 mV per channel, and for 2000 channels it is approximately 100 mV per channel. Homotypic Cx43 unitary conductances of 55 pS are observed when using K+aspartate– pipette solutions (see Table 15-2) that best mimic the myoplasmic electrolytes. The other factor to consider is the time course of the voltage dependence. It is possible for a transjunctional voltage of 10 to 20 mV or larger, as might occur with only a 1000 channels or fewer to result in voltage-dependent channel closure. To assess this possibility, it is necessary to determine how long a transjunctional voltage will persist during the passage of an action potential. What is the duration of the transjunctional voltage for an action potential conducting at 50 cm/s? For θ = 50 cm/s, the cardiac action potential will traverse a 100-µm length in 0.2 ms or 2 ms for θ = 5 cm/s. The time course of voltage-dependent closure varies from connexin to connexin, but for the cardiac connexins a 2-ms duration would result in a small reduction in junctional conductance.
It is easy to see that only with reduced numbers of functional channels would their number or voltage dependence begin to have an effect on the propagation or conduction of the cardiac action potential. In fact, the normal AV19 node delay of 0.12 seconds is thought to be in part a result of sparse gap junction density.27 Conditions such as AV block might be manifest by reduced gap junction channel number and voltage-dependent closure, effectively elevating longitudinal resistance to a point where insufficient current can spread from cell to cell to trigger continued conduction. Results from animal model systems where connexin knockouts of Cx40 have been constructed are consistent with this notion.9
Pacemaker Activity and Connexins
Besides the essential role of connexins in allowing the propagation of the cardiac action potential throughout the myocardium, they are also of paramount importance in the generation of pacing activity of the sinoatrial node (SA node). The myocytes of the SA node vary in morphology and form gap junction composed of Cx45 and to a lesser degree Cx40 (see Table 15-1). There are no easily demonstrable intercalated discs, rather the myocytes form smaller junctional plaques.9 SA node cells are characterized by phase 4 depolarization resulting from the activity of HCN4 and to a lesser degree HCN1,28 which is sufficient to elicit an action potential in the nodal cells and subsequent propagation to the surrounding atrial myocardium.
Artificial pacemaker units consisting of two coupled cells have been shown to pace at 1 Hz where one cell of a pair is a nonmyocyte cell expressing HCN, and the other is a ventricular myocyte by itself is not capable of pacemaker activity.29 The data clearly demonstrate the role of gap junctions in the initiation of pacemaker activity and further illustrate that pacing rate is unaffected over an approximately fourfold range of junctional conductance (Figure 15-6).
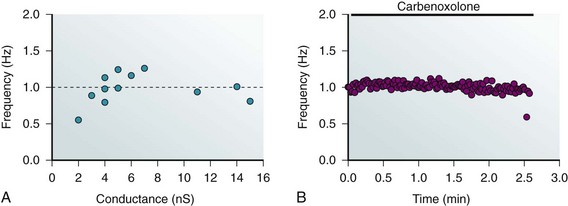
Figure 15-6 A, Pacing frequency generated by a cell transfected with HCN2 coupled to a ventricular myocyte. Frequency is plotted against the measured junctional conductance. The dashed line corresponds to an average frequency of 1 Hz. B, Carbenoxolone exposure results in a cessation of pacing. Pacing remains constant for more than 2 minutes and then abruptly stops. This is consistent with the notion that junctional conductance only affects propagation once a critically low level of coupling is attained. (Modified from Valiunas V, Kanaporis G, Valiuniene L, et al: Coupling an HCN2-expressing cell to a myocyte creates a two-cell pacing unit. J Physiol 587(Pt 21):5211–5226, 2009.)
Non–Voltage-Dependent Regulators of Channel Patency
There are two intrinsic intracellular elements that are able to affect junctional conductance: intracellular pH and intracellular calcium. Lowered intracellular pH, as occurs in ischemia,30 is known to affect many cardiac membrane channels and transporters and can effectively reduce gap junction conductance.31 Figure 15-7 shows the effect of perfusion with 100% CO2 on junctional conductance measured between a pair of cells expressing Cx43. A pH-sensitive fluorescent probe was used to determine intracellular pH (see Figure 15-7, B). Many investigators have observed that short exposures to elevated H+ results in reduced coupling with subsequent recovery, as illustrated in Figure 15-7. What is not shown in Figure 15-7 is the result of prolonged exposure of many minutes, which results in an irreversible reaction culminating in permanent channel closure, implying both rapid and slow processes triggered by H+. The majority of the connexins are similarly affected by acidification, responding to elevated intracellular H+ with reduced junctional conductance that is presumed to be the result of increased closed times and reduced open times. However, the mechanisms of H+-induced closure are apparently not universal. The mechanism of pH-induced alteration of Cx43 gap junction channel open probability has been shown to be manifest by a ball-and-chain configuration between the C-terminus and the cytoplasmic loop between membrane-spanning domains M2 and M3. In contrast, Cx40 is also pH sensitive, but the mechanism of channel closure is not mediated by a ball-and-chain–like mechanism. The mechanism by which H+ affects Cx40 channel patency has not been elucidated, but the pKa for Cx40 is essentially the same as that for Cx43 (6.7).32 The formation of heteromeric or heterotypic forms of Cx43 and Cx40 shifts the pKa to a more alkaline value of 7.0.32 The other cardiac connexins have not been studied as extensively as Cx43 and Cx40, with the exception of Cx46, for which the data are most consistent with a direct effect on the cytoplasmic surface. Rapid delivery of H+ to the cytoplasmic side of Cx46 hemichannels was accomplished using an inside-out patch clamp in combination with two Cx46 constructs, a wild type with an intact C-terminus, and a deletion mutant of Cx46 missing a significant portion of the C-terminus. Both forms had the same pH sensitivity with a pKa of 6.5, clearly suggesting a site or sites for Cx46 other than the ball-and-chain mechanism of Cx43.10
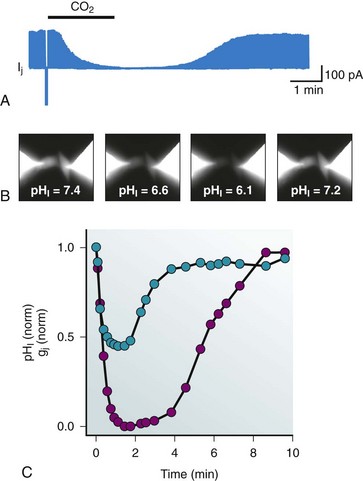
Figure 15-7 A, Junctional current is reduced when 100% CO2 is bubbled into the perfusate. The bar represents the exposure time, which is approximately 2 minutes. The time course to reduction in junctional current and subsequent recovery are not the same, but both represent examples of diffusion time within the bath and buffering capacity of the cells. B, Intracellular pH can be monitored via pH-sensitive fluorescent dyes. A HeLa cell pair expressing Cx43 is shown. C, Intracellular pH (pHi) normalized to the initial pHi (7.4) and junctional conductance (gj) normalized to the initial gj are plotted against time during 100% CO2.
Another intracellular ion able to affect Cx43 junctional conductance is calcium. Elevated intracellular calcium (500 nM to 1 µM) reduces the number of functioning Cx43 gap junction channels and eventually results in complete uncoupling. The mechanism of calcium-mediated channel closure has been the center of some controversy, but recently it has been demonstrated that calcium acts to reduce Cx43 gap junctional conductance via calmodulin.33 It is possible that calcium–pH synergy is mediated through calmodulin, but there are currently no data to support such a hypothesis. Interestingly, gap junction channels composed of Cx40 are not affected by elevated intracellular calcium and do not possess the putative calmodulin binding sites found on Cx43. In addition to affecting the gating of connexins, calcium is also permeable to connexins and has been associated with cell death within the myocardium and other syncytial tissues.34 These findings suggest that it is Cx40 that allows the spread of elevated intracellular calcium in infarcts, whereas Cx43 is the connexin working to preserve cellular integrity by isolating healthy myocytes from those that are damaged in an ischemic episode. Calcium and calmodulin sensitivity of Cx46, Cx45, and Cx37 has not been studied as completely as it has in Cx43 and Cx40.
Extrinsic Uncoupling Agents of Cardiac Gap Junction Channels
Anesthetics such as halothane have shown to reduce mean open time and increase mean closed time for Cx43 and Cx40 homotypic and heteromeric channels in a dose-dependent manner. The mechanism by which halothane reduces channel open time remains unknown, but it has been suggested that interactions at the protein-lipid interface are the most likely site of action. Another class of agents, long chained alcohols such as octanol and heptanol, are also effective in reducing junctional conductance and are thought to act via protein-lipid interactions. Other agents that are able to reduce functional gap junction numbers are carbenoxolone, glycyrrhetinic acid, quinine derivatives, retinoic acid, arachidonic acid, and spermine.35 All the aforementioned agents lack specificity and inhibit other systems within cells.35 Another approach has been the use of mimetic peptides. A recent study has shown that GAP26, a polypeptide, is able to block hemichannels of Cx43 on a time scale of seconds to minutes, and longer exposure is able to effectively reduce Cx43 gap junction–mediated coupling.36 The mimetic peptides appear to function by binding to the extracellular loops, and when the hemichannel is subsequently incorporated into a plaque or attempts to link via E1 or E2 with its counterpart in the adjacent cell, the peptide prevents formation of a functioning channel. The apparent turnover rate of approximately 30 minutes is faster than the turnover rate determined via Western blot analysis,37 but might be related connexon docking and gap junction channel assembly or disassembly associated with the scaffolding complex protein zonula occludens, ZO-1.38 Despite a lack of mechanistic understanding, these data demonstrate a potentially useful clinical tool if GAP26 is clearly shown to be gap junction specific. Direct proof of binding to the extracellular loops is a first necessary step, and tagged peptides must be used to assess whether the peptide might affect function intracellularly possibly via endosomal entry.
A potentially clinically relevant feature of mimetic peptides is manifest in Gap26, a mimetic peptide for Cx43 that has been shown to protect against induced myocardial ischemia in vivo.39 Its potential to slow conduction and create proarrhythmic activity has not been tested. Overall, extrinsic uncoupling agents have proved useful in attempting to understand how gap junction channels affect cardiac action potential propagation but, as might be expected, a reduction in the number of functioning gap junction channels results in slowed conduction and consequently the possibility of generating arrhythmogenic activity.26
Ischemia, Mutations, Arrhythmia, and Gap Junctions
Ischemia reduces or completely occludes blood flow to the myocardium, resulting in hypoxia that subsequently triggers the release of intracellular calcium and acidosis, which can result in cellular remodeling or cell death. A number of studies have demonstrated that ischemia triggers an anatomic remodeling of gap junctions within myocytes, such that fewer junctions are found in the intercalated disc and more appear on the lateral surfaces of the myocytes.40 Such redistribution or remodeling is predicted to directly affect longitudinal resistance and reduce conduction velocity along the long axis of the myocyte. To further complicate matters, the insertion of more gap junctions laterally has the potential to create arrhythmias.40 Studies using a canine heart failure model found a strong correlation between reduced Cx43 expression in vivo and reentrant ventricular arrhythmias.40 A number of studies have also found the phosphorylation state of Cx43 to be altered in ischemia and in nonischemic heart failure.41 Phosphorylation state might also trigger anatomic remodeling in response to ischemia or other pathophysiologic challenges to the heart. Clearly, gap junction channels along with many other membrane channels participate in electrical and anatomic remodeling in response to ischemic conditions. Which one dominates in response to ischemia is most likely disease dependent.
Atrial arrhythmias are also associated with electrical remodeling and changes in connexin distribution. Of particular interest is Cx40, in which abnormal expression results in an increased tendency toward atrial fibrillation.42
A number of studies have found strong correlates between mutations in Cx43 and disease state, with the most clearly understood being oculodentodigital dysplasia (ODDD), in which a number of mutations in Cx43 have been identified.43 Interestingly, patients with ODDD do not display any cardiac anomalies. Mutation in adhesion molecules is another way to affect connexin distribution within the intercalated disc. Naxos disease arises because of a mutation within the adhesion molecule plakoglobin. This mutant form does not traffic to the intercalated disc properly.44 As a result, Cx43 remodeling occurs with lesser junctional complexes being formed, culminating in an arrhythmogenic cardiomyopathy.
Summary
Gap junction channels are poorly selective intercellular channels that allow the movement of ions, solutes, second messengers, and even microRNAs and siRNAs from cell to cell exclusive of the extracellular milieu. Gap junctions participate in the propagation of the cardiac action potential and are expressed differentially to affect pacing rate, AV node delay, and rapid conduction in Purkinje fibers, ventricular, and atrial myocardium. The evidence is overwhelmingly clear that the cardiac connexins are essential to normal cardiac rhythm and are involved in the response to disease processes, such as the phenomenon of lateralization. Mutations in cardiac connexins resulting in or causing cardiac dysfunction must still be considered correlative rather than causative; they have not yet been unequivocally demonstrated to cause heart disease,45 unlike Cx26, where the mutations have been shown to cause deafness.43 For ischemic disease states, it appears that cardiac connexins are best considered as part of the effect rather than the cause. Again, the best example is remodeling associated with ischemia. In one sense, remodeling in the form of increased abundance of connexin along the lateral surfaces is most logically considered as an attempt to circumvent a damaged intercalated disc rather than a precipitating causal event. There is a risk of creating a reentrant arrhythmia with lateralization, but it is a better alternative than significant or complete loss of longitudinal conductivity because of a dysfunctional gap junction–mediated communication at the intercalated disc.
References
1. Yao, JA, Gutstein, DE, Liu, F, et al. Cell coupling between ventricular myocyte pairs from connexin43-deficient murine hearts. Circulation Research. 2003; 93(8):736–743.
2. Severs, NJ, Bruce, AF, Dupont, E, et al. Remodelling of gap junctions and connexin expression in diseased myocardium. Cardiovasc Res. 2008; 80(1):9–19.
3. Maeda, S, Nakagawa, S, Suga, M, et al. Structure of the connexin 26 gap junction channel at 3. 5 A resolution. Nature. 2009; 458(7238):597–602.
4. Cheng, A, Yeager, M. Bootstrap resampling for voxel-wise variance analysis of three-dimensional density maps derived by image analysis of two-dimensional crystals. J Struct Biol. 2007; 158(1):19–32.
5. Kovacs, JA, Baker, KA, Altenberg, GA, et al. Molecular modeling and mutagenesis of gap junction channels. Prog Biophys Mol Biol. 2007; 94(1-2):15–28.
6. Prochnow, N, Hoffmann, S, Dermietzel, R, et al. Replacement of a single cysteine in the fourth transmembrane region of zebrafish pannexin 1 alters hemichannel gating behavior. Exp Brain Res. 2009; 199(3-4):255–264.
7. Rackauskas, M, Neverauskas, V, Skeberdis, VA. Diversity and properties of connexin gap junction channels. Medicina (Kaunas). 2010; 46(1):1–12.
8. Duffy, HS, Fort, AG, Spray, DC. Cardiac connexins: genes to nexus. Adv Cardiol. 42(1-17), 2006.
9. Jansen, JA, van Veen, TA, de Bakker, JM, et al. Cardiac connexins and impulse propagation. J Mol Cell Cardiol. 2010; 48(1):76–82.
10. Bukauskas, FF, Verselis, VK. Gap junction channel gating. Biochim Biophys Acta. 2004; 1662(1-2):42–60.
11. Vogel, R, Valiunas, V, Weingart, R. Subconductance states of Cx30 gap junction channels: data from transfected HeLa cells versus data from a mathematical model. Biophys J. 2006; 91(6):2337–2348.
12. Brink, PR, Ramanan, SV, Christ, GJ. Human connexin 43 gap junction channel gating: evidence for mode shifts and/or heterogeneity. Am J Physiol. 1996; 271(1 Pt 1):C321–31.
13. Ramanan, SV, Valiunas, V, Brink, PR. Non-stationary fluctuation analysis of macroscopic gap junction channel records. J Membr Biol. 2005; 205(2):81–88.
14. Valiunas, V, Weingart, R, Brink, PR. Formation of heterotypic gap junction channels by connexins 40 and 43. Circulation Research. 2000; 86(2):E42–9.
15. Desplantez, T, Halliday, D, Dupont, E, et al. Cardiac connexins Cx43 and Cx45: formation of diverse gap junction channels with diverse electrical properties. Pflugers Arch. 2004; 448(4):363–375.
16. Bukauskas, FF, Jordan, K, Bukauskiene, A, et al. Clustering of connexin 43-enhanced green fluorescent protein gap junction channels and functional coupling in living cells. Pro Natl Acad Sci U S A. 2000; 97(6):2556–2561.
17. Kjenseth, A, Fykerud, T, Rivedal, E, et al. Regulation of gap junction intercellular communication by the ubiquitin system. Cell Signal. 2010; 22(9):1267–1273.
18. Kanaporis, G, Brink, PR, Valiunas, V. Gap junction permeability: selectivity for anionic and cationic probes. Am J Physiol Cell Physiol. 2011; 300(3):C600–9.
19. Valiunas, V, Beyer, EC, Brink, PR. Cardiac gap junction channels show quantitative differences in selectivity. Circ Res. 2002; 91(2):104–111.
20. Kanaporis, G, Mese, G, et al. Gap junction channels exhibit connexin-specific permeability to cyclic nucleotides. J Gen Physiol. 2008; 2600:293–305.
21. Valiunas, V, Polosina, YY, Miller, H, et al. Connexin-specific cell-to-cell transfer of short interfering RNA by gap junctions. J Physiol. 2005; 568(Pt 2):459–468.
22. Alig, J, Marger, L, Mesirca, P, et al. Control of heart rate by cAMP sensitivity of HCN channels. Proc Natl Acad Sci U S A. 2009; 106(29):12189–12194.
23. Chen, C, Nakamura, T, Koutalos, Y. Cyclic AMP diffusion coefficient in frog olfactory cilia. Biophys J. 1999; 76(5):2861–2867.
24. Hasan, W, Jama, A, Donohue, T, et al. Sympathetic hyperinnervation and inflammatory cell NGF synthesis following myocardial infarction in rats. Brain Res. 2006; 1124(1):142–154.
25. Gommans, WM, Berezikov, E. Controlling miRNA regulation in disease. Methods Mol Biol. 822(1-18), 2012.
26. Jia, Z, Bien, H, Shiferaw, Y, et al. Cardiac cellular coupling and the spread of early instabilities in intracellular Ca2+. Biophys J. 2012; 102(6):1294–1302.
27. Greener, ID, Monfredi, O, Inada, S, et al. Molecular architecture of the human specialised atrioventricular conduction axis. J Mol Cell Cardiol. 2011; 50(4):642–651.
28. Nof, E, Antzelevitch, C, Glikson, M. The Contribution of HCN4 to normal sinus node function in humans and animal models. Pacing Clin Electrophysiol. 2010; 33(1):100–106.
29. Valiunas, V, Kanaporis, G, Valiuniene, L, et al. Coupling an HCN2-expressing cell to a myocyte creates a two-cell pacing unit. J Physiol. 2009; 587(Pt 21):5211–5226.
30. Monastyrskaya, K, Tschumi, F, Babiychuk, EB, et al. Annexins sense changes in intracellular pH during hypoxia. Biochem J. 2008; 409(1):65–75.
31. Vaughan-Jones, RD, Spitzer, KW, Swietach, P. Intracellular pH regulation in heart. J Mol Cell Cardiol. 2009; 46(3):318–331.
32. Gu, H, Ek-Vitorin, JF, Taffet, SM, et al. Coexpression of connexins 40 and 43 enhances the pH sensitivity of gap junctions: a model for synergistic interactions among connexins. Circ Res. 2000; 86(10):E98–E103.
33. Xu, Q, Kopp, RF, Chen, Y, et al. Gating of connexin 43 gap junctions by a cytoplasmic loop calmodulin binding domain. Am J Physiol Cell Physiol. 2012; 302(10):C1548–56.
34. Decrock, E, Vinken, M, Bol, M, et al. Calcium and connexin-based intercellular communication, a deadly catch? Cell Calcium. 2011; 50(3):310–321.
35. Bodendiek, SB, Raman, G. Connexin modulators and their potential targets under the magnifying glass. Curr Med Chem. 2010; 17(34):4191–4230.
36. Desplantez, T, Verma, V, Leybaert, L, et al. Gap26, a connexin mimetic peptide, inhibits currents carried by connexin43 hemichannels and gap junction channels. Pharmacol Res. 2012; 65(5):546–552.
37. Falk, MM, Baker, SM, Gumpert, AM, et al. Gap junction turnover is achieved by the internalization of small endocytic double-membrane vesicles. Mol Biol Cell. 2009; 20(14):3342–3352.
38. Rhett, JM, Jourdan, J, Gourdie, RG. Connexin 43 connexon to gap junction transition is regulated by zonula occludens-1. Mol Biol Cell. 2011; 22(9):1516–1528.
39. Hawat, G, Benderdour, M, Rousseau, G, et al. Connexin 43 mimetic peptide Gap26 confers protection to intact heart against myocardial ischemia injury. Pflugers Arch. 2010; 460(3):583–592.
40. Wit, AL, Peters, NS. The role of gap junctions in the arrhythmias of ischemia and infarction. Heart Rhythm. 2012; 9(2):308–311.
41. Schulz, R, Heusch, G. Connexin43 and ischemic preconditioning. Adv Cardiol. 2006; 42:213–227.
42. Chaldoupi, SM, Loh, P, Hauer, RN, et al. The role of connexin40 in atrial fibrillation. Cardiovasc Res. 2009; 84(1):15–23.
43. Pfenniger, A, Wohlwend, A, Kwak, BR. Mutations in connexin genes and disease. Eur J Clin Invest. 2011; 41(1):103–116.
44. Saffitz, JE. Arrhythmogenic cardiomyopathy: advances in diagnosis and disease pathogenesis. Circulation. 2011; 124(15):e390–2.
45. Makita, N, Seki, A, Sumitomo, N, et al. A connexin40 mutation associated with a malignant variant of progressive familial heart block type I. Circ Arrhythm Electrophysiol. 2012; 5(1):163–172.
46. Valiunas, V, Gemel, J, Brink, PR, et al. Gap junction channels formed by coexpressed connexin40 and connexin43. Am J Physiol Heart Circ Physiol. 2001; 281(4):H1675–H1689.
47. Desplantez, T, Dupont, E, Severs, NJ, et al. Gap junction channels and cardiac impulse propagation. J Membr Biol. 2007; 218(1-3):13–28.
48. Bukauskas, FF, Elfgang, C, Willecke, K, et al. Biophysical properties of gap junction channels formed by mouse connexin40 in induced pairs of transfected human HeLa cells. Biophys J. 1995; 68(6):2289–2298.
49. Beblo, DA, Wang, HZ, Beyer, EC, et al. Unique conductance, gating, and selective permeability properties of gap junction channels formed by connexin40. Circ Res. 1995; 77(4):813–822.
50. Valiunas, V. Biophysical properties of connexin-45 gap junction hemichannels studied in vertebrate cells. J Gen Physiol. 2002; 119(2):147–164.
51. Elenes, S, Martinez, AD, Delmar, M, et al. Heterotypic docking of Cx43 and Cx45 connexons blocks fast voltage gating of Cx43. Biophys J. 2001; 81(3):1406–1418.
52. Sakai, R, Elfgang, C, Vogel, R, et al. The electrical behaviour of rat connexin46 gap junction channels expressed in transfected HeLa cells. Pflugers Arch. 2003; 446(6):714–727.
53. Brink, PR, Cronin, K, Banach, K, et al. Evidence for heteromeric gap junction channels formed from rat connexin43 and human connexin37. Am J Physiol. 1997; 273(4 Pt 1):C1386–96.
54. Rackauskas, M, Kreuzberg, MM, Pranevicius, M, et al. Gating properties of heterotypic gap junction channels formed of connexins 40, 43, and 45. Biophys J. 2007; 92(6):1952–1965.
55. Makowski, L, Caspar, DL, Phillips, WC, et al. Gap junction structures. VI. Variation and conservation in connexon conformation and packing. Biophys J. 1984; 45(1):208–218.