Biomechanics of Skin Flaps
Biomechanical Properties of Cutaneous Tissue
The mechanical properties of a material are described by the relationship that exists between a force applied to a specimen and the resultant deformation of the specimen as a function of time. In engineering, these mechanical properties are defined by the quantities stress (force per unit of original cross section) and strain (change in length divided by the original length). The stress/strain ratio thus measures the relationship between force and elongation by said force for a given cross-sectional area. The stress-strain relationship is independent of the dimensions of the specimen and is a property of the material itself. Uniform materials, such as titanium, have a linear stress-strain relationship. Their mechanical properties can be described by the proportionality constant (C = stress/strain). If the stress-strain relationship does not vary as a function of time, the material is said to be elastic.1
Nonlinearity
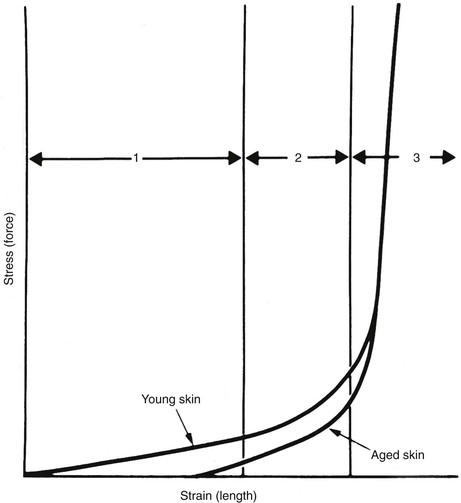
A typical stress-strain curve for isolated skin is shown in Figure 3-1. The shape of the curve is similar to stress-strain and force-advancement curves obtained from tests on skin flaps and other soft tissues.1 It is apparent from the graph that skin and skin flaps have nonlinear stress-strain relationships. The mechanical behavior can be divided into three separate regions: (1) an initial flat section in which considerable extension occurs with little force, (2) an intermediate section of rapid transition, and (3) a terminal section where little extension is possible despite great increases in applied force. Histologic examinations of the sequential stages of skin extension provide an explanation for the nonlinear nature of this graph. During initial deformation, randomly oriented collagen and elastic fibers are stretched in the direction of the applied force. Collagen fibers do not bear a significant burden until the bundle is completely straight in the direction of the applied force.1 As a result, there is little resistance to initial deformation, and the stress-strain relationship is nearly linear and elastic (region 1). As deformation progresses, additional collagen fibers are recruited into the load-carrying role and resistance rises. The low stress required for the high strain inherent in region 1 allows the small movements resulting from flexion and extension at joints, enabling freedom of movement and natural facial expression. Region 2 is the strain at which many collagen fibers transition from non–load carrying to a load-carrying role. At high-stress loads (region 3), virtually all the dermal collagen fibers are aligned in the direction of the applied force. At this point, no further deformation is possible because of the inextensible nature of fully oriented collagen. Wholly oriented collagen (region 3) preserves the structural integrity of skin by limiting deformation during accidental stresses.1 Raposio and Nordstrom2 studied the stress-strain curve of human scalp tissue during serial excision of the scalp and found scalp to be initially linear from 0 to 500 g, gradually reduced in compliance from 500 to 1500 g, and rapidly increasing in stiffness from 1500 to 5000 g.
Anisotropy
There are enormous individual variations in the extensibility of human skin—differences between the slim and obese, young and old, male and female. The shape of the force-advancement curve is further influenced by edema, inflammation, hormonal conditions, and body weight. It is the variation in skin tension within the same individual, however, that is the most interesting to the reconstructive surgeon. On the face, the skin is lax around the eyes and cheek, whereas it is taut on the nose, chin, and forehead. In each of these locations, there are directional (anisotropic) considerations for skin movement. In most regions of the body, there is skin tension in every direction, but the degree of tension is greatest parallel to the relaxed skin tension lines (RSTLs).3 Pierard and Lapiere4 showed that the presence of skin tension lines depends on the interaction between elastic fibers and collagen fibers and the anchorage of collagen fibers to each other. An incision made at a right angle to the RSTL will gape widely and is more likely to produce a widened or hypertrophic scar. The lines of maximal extensibility (LME) run perpendicular to the RSTLs and represent the direction in which closure can be performed with the least tension.5 Therefore, elliptical excisions should be performed parallel to the RSTLs to place the maximum closure tension parallel to the LME and local skin flaps should be designed so that the donor site closure is parallel to the LME.
RSTLs are not to be confused with Langer’s lines. Langer’s lines of cutaneous tension correspond to the orientation of forces that cause a circular puncture mark in the skin of a cadaver to distort into a fusiform shape. The orientation of Langer’s lines is often distinct from the creases formed in living subjects when the joints are placed in a relaxed position. RSTLs follow the longest and straightest furrows formed when skin is relaxed and are not visible features of the skin. Rather, RSTLs derive from the act of pinching the skin and observing the furrows and ridges that form.3 When skin tension is preserved at the time of biopsy, a small number of extracellular collagen and elastin bundles have been demonstrated to run parallel to the RSTLs.4
Viscoelasticity
When a material resists shear flow and strain in a linear fashion related to time in response to a force, it is said to be viscous. Elastic materials demonstrate immediate strain when a force is applied but return to their original state when the force is removed. Skin exhibits properties of both viscosity and elasticity (viscoelasticity). Skin behaves like an elastic substance only during initial deformation at low stress level (see Fig. 3-1, region 1). At higher stress loads, skin shows viscoelastic properties, and strain becomes a function of both load and time. Two time-dependent properties of skin that are routinely exploited by surgeons are creep and stress relaxation.
Creep refers to the increase in the length of skin compared with the original length when skin is placed under a constant stress (force per unit area). At high-stress loads, a modicum of creep can be achieved in a short time. A small increase in length (strain) occurs as compressed and straightened collagen fibers displace interstitial fluid that is loosely bound to the extracellular matrix (ground substance). When skin is stretched in air, it is possible to see this fluid collect on the undersurface.6 Time is required for fluid to move from one area to another, which explains the time dependence of the process. This mechanism partially explains the biomechanical and histologic changes that occur during tissue expansion, obesity, and pregnancy.
Mechanotransduction and Skin
The mechanical properties of skin including nonlinearity, anisotropy, and viscoelasticity are readily observed at a macroscopic level and exploited during reconstructive surgery. It stands to reason that the physical forces applied to skin at the macroscopic level would induce changes at the cellular level. Some of the first evidence of this at a cellular level was provided by Folkman and Moscona7 in 1978. The authors grew cells in culture in varying concentrations of poly(2-hydroxyethyl methacrylate) to control cell shape and, indirectly, cell tension and found that varying shape and tension resulted in differences in DNA synthesis and proliferation. These results suggested that cells sense changes in force and respond accordingly at the molecular level. The mechanism by which these mechanical forces lead to cellular responses came to be called mechanotransduction.
Direct Mechanotransduction
Direct mechanotransduction is the process by which a cell converts mechanical stimuli into biochemical signals. For a cell to convert a mechanical signal to a biochemical signal, it must be able to sense the signal. Ingber8 proposed the idea of “tensegrity,” in which a cell’s cytoskeletal framework is coupled to the extracellular matrix as well as to the cytoskeletal framework of nearby cells, creating a homeostasis of tension from which deviations might be recognized.
Cellular adhesion molecules, such as integrins, are key to tensegrity. Integrins couple the extracellular matrix to the actin cytoskeleton. This coupling allows integrins to transduce changes from external mechanical forces into the cell and vice versa. The activation of integrins, which are primarily in an off state in the cellular membrane, can modulate downstream signaling events controlling a wide variety of cellular events including apoptosis, proliferation, and gene expression, among others.9 Altered expression of integrins has been implicated in studies of wound healing and chronic wounds. For example, Hakkinen et al10 used in situ hybridization to investigate the expression of β6 integrin in chronic wounds, hypertrophic scars, and keloids in humans. The authors found that chronic wounds expressed high levels of β6 integrin, whereas keloids and hypertrophic scars did not demonstrate any expression. Furthermore, the authors engineered a homozygous transgenic mouse line that constitutively expressed β6 integrin in skin epithelium and found that a significant percentage (27%) of these mice spontaneously developed chronic wounds, suggesting that altered expression of integrins might be responsible in some cases of poor healing.
Whereas the mechanisms of mechanotransduction by integrins have been the best characterized, cells possess other means by which to translate mechanical forces into biochemical signals. Some cells possess mechanosensitive ion channels that respond to mechanical forces with either the activation or inactivation of an ion flux.11 One of the most important of these is the mechanosensitive calcium ion channel. Yano et al12 cultured human keratinocytes on flexible dishes that were stretched 20% and discovered that the stretching induced hyperproliferation as measured by BrdU incorporation secondary to the activation of extracellular signal–regulated kinases (ERK1/2). The possibility of a role for the mechanosensitive calcium-gated ion channel was suggested by almost complete inhibition of hyperproliferation when a calcium channel inhibitor was cultured with the cells.
Protein deformation is another mechanism of direct mechanotransduction.13 Protein deformation is the process by which mechanical forces induce conformational changes to a protein, which may unmask new binding motifs or, alternatively, mask previously accessible binding sites. For example, Tamada et al14 removed the cell membranes and soluble proteins from human embryonic kidney cells and demonstrated that stretching of the remaining cytoskeleton could activate the protein Rap1, which is important for the activation of the p38 mitogen-activated protein kinase cascade. Similarly, transforming growth factor β1 (TGF-β1) is an important mediator of inflammation and wound healing. TGF-β1 typically remains in an inactive state bound to latency-associated peptide in the large latent complex. The binding of integrins to the large latent complex can cause conformational changes in the large latent complex and dissociation of the latency-associated peptide from TGF-β1, which renders it active.15
Indirect Mechanotransduction
Indirect mechanotransduction involves a cell-sensing mechanical force leading to the release of proteins or other chemical messengers, which then act on nearby cells to mediate a variety of effects. One example of indirect mechanotransduction is the peripheral nervous system. The peripheral nervous system contains mechanosensitive and nociceptive sensory nerves that sense mechanical forces and effect changes on surrounding cells. The skin contains low-threshold mechanoreceptors, such as Meissner’s corpuscles, as well as high-threshold nociceptors, which sense pain as well as mechanical forces. The activation of nociceptors leads to the release of neuropeptides from afferent sensory nerve endings. The neuropeptides released from afferent sensory nerve endings include calcitonin gene–related peptide (CGRP), substance P (SP), somatostatin, and vasoactive intestinal peptide (VIP), among others. For example, Chin et al16 used a murine model of skin stretching in which skin was exposed to cyclic or continuous stretch for 4 hours and observed increased expression of both SP and CGRP.
The neuropeptides released by sensory nerve endings act on nearby cells, leading to vasodilation, cytokine production, and increased vascular permeability, among others.17 Keratinocytes possess receptors for SP, VIP, and CGRP.18 Activation of the receptor for SP on human and murine keratinocytes induces proliferation while promoting migration of fibroblasts.19,20
The release of neuropeptides in response to mechanical forces has also been implicated in inflammation and wound healing.21 SP and CGRP serve to initiate neurogenic inflammation by acting on mast cells.18 Binding of SP and CGRP to their receptors on mast cells leads to degranulation and histamine release with subsequent erythema, vasodilation, and capillary leakage. Additional effects of SP include stimulation of the release of tumor necrosis factor 1 from mast cells and interleukins 1 and 4 from keratinocytes and upregulation of intercellular adhesion molecules, which lead to increased binding of immune cells to endothelium and subsequent migration into the tissue. SP also induces endothelial cell proliferation, leading to neovascularization and angiogenesis important for wound healing.22 Deviations of mechanotransduction leading to prolific inflammation have been implicated in the development of keloids and hypertrophic scars.
Surgical Applications of the Biomechanics of Skin
Tissue Expansion
Physiologic tissue expansion occurs during rapid growth at puberty, during pregnancy, and at times of rapid weight gain. During both pregnancy and rapid growth, the time interval can be relatively short and skin thinning can occur as evidenced by the striae that sometimes form. Obesity causes an overall increase in skin surface area to occur, but the skin maintains a normal thickness in both the epidermis and dermis with a normal collagen content.23
The first clinical application of tissue expansion was published by Neumann24 in 1957 and then improved by Radovan.25 Although it is clinically useful, debate has centered on the biomechanical properties of the expanded skin. Preservation of epidermal thickness during tissue expansion has been verified by several authors.26,27 The underlying mechanism for the overall increase in skin surface area without thinning of the epidermis is increased epidermal mitotic activity and keratinocyte proliferation.28–30 The long-term effects of tissue expansion on the dermis are more difficult to discern. Unequivocally, dermal thickness is reduced both during tissue expansion and for an undefined period thereafter. What is not clear, however, is whether eventual restoration of the normal dermis occurs and by what mechanism. Johnson et al28,31 demonstrated in a porcine model that an increase in surface area of skin occurred during and after tissue expansion but that dermal thinning persisted for 36 weeks after completed expansion (Fig. 3-2). Clinical extrapolation of these data is difficult because the tissue expanders were left in place (Fig. 3-3). Dermal compensation in response to tissue expansion appears to be slow compared with the rapid response found in the epidermis. This discrepancy is found during pregnancy but not during chronic obesity, in which both dermal thickness and epidermal thickness are preserved.23 Expanded skin does demonstrate an increase in vascularity as well as in vasodilation, thought to account for the increased mean surviving lengths of expanded skin flaps compared with random skin flaps.26
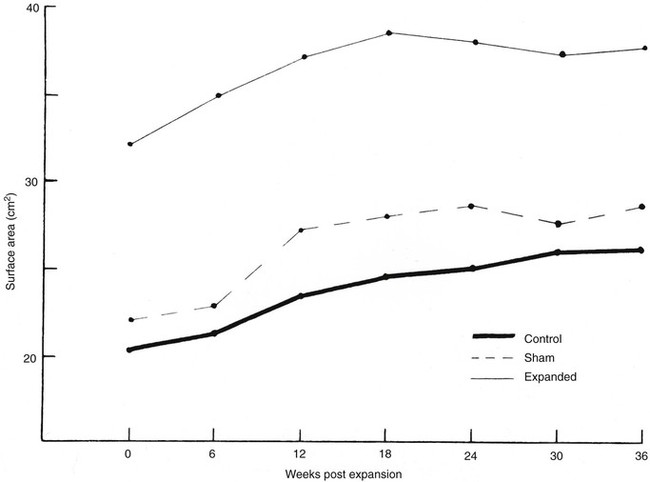
FIGURE 3-2 Increase in surface area seen with tissue expansion. Tissue expanders were left implanted during postexpansion period. (From Johnson PE, Kernahan DA, Bauer BS: Dermal and epidermal response to soft-tissue expansion in the pig. Plast Reconstr Surg 81:391, 1988.)
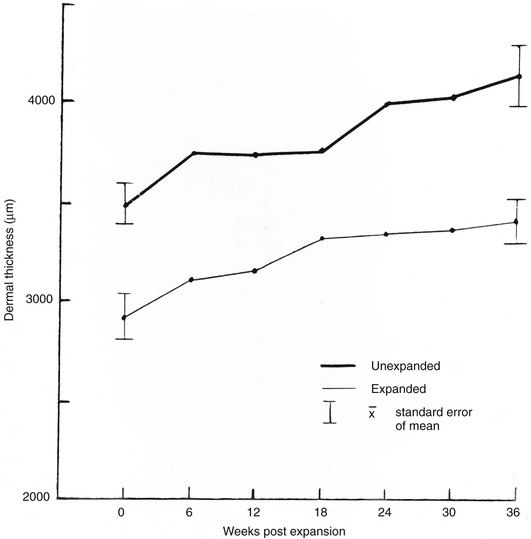
FIGURE 3-3 Dermal thickness after tissue expansion. Persistent thinning is noted 36 weeks after expansion. Tissue expanders were left implanted during postexpansion period. (From Johnson PE, Kernahan DA, Bauer BS: Dermal and epidermal response to soft-tissue expansion in the pig. Plast Reconstr Surg 81:391, 1988.)
Gibson and Kenedi6 first suggested that additional tissue for wound closure could be obtained by acutely stretching the skin. The clinical efficacy of intraoperative cyclic loading of skin was thought to derive primarily from the effects of increased undermining.32 However, others have shown that intraoperative tissue expansion increases skin compliance with decreased tension compared with simple undermining.33
Wound Tension and Blood Flow
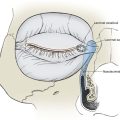
The effect of wound closure tension on blood flow has been studied by comparing tissue survival to wound closure tensions in animal models.6,34 A quantitative description of the relationship between blood flow and wound closure tension is seen in Figure 3-4. In this experimental pig model, the investigators created a random skin flap 3 cm wide and 6 cm long. Laser Doppler blood flow measurements were taken at the base of the flap (area 4) and at 2-cm intervals toward the distal tip (area 1). Tension was increased by 50-g increments to maximal tension. This experiment demonstrated that blood flow is inversely proportional to the distance from the base of the flap and that it decreases steadily with increasing tension until a critical value of 200 to 250 g is reached.35 On the basis of the supporting evidence in Figure 3-4, it seems fair to conclude that excess wound closure tension can result in necrosis, especially at the distal aspect of a flap. Larrabee et al35 explored this possibility by designing a study to measure the effect of tension on survival of 3-cm-wide flaps with a length of 0 to 9 cm (Fig. 3-5). Not surprisingly, all flaps longer than 6 cm underwent some necrosis and all flaps shorter than 1.5 cm remained viable, irrespective of wound closure tension. Flaps of intermediate length were sensitive to the effects of tension. Flaps closed with less than 250 g of tension tended to survive, and those closed under greater tension tended to have areas of necrosis.35 This study confirms the clinical observation that flaps with ample blood flow can withstand the extremes of tension. However, flaps subjected to excessive wound closure tension can necrose because of insufficient blood flow.
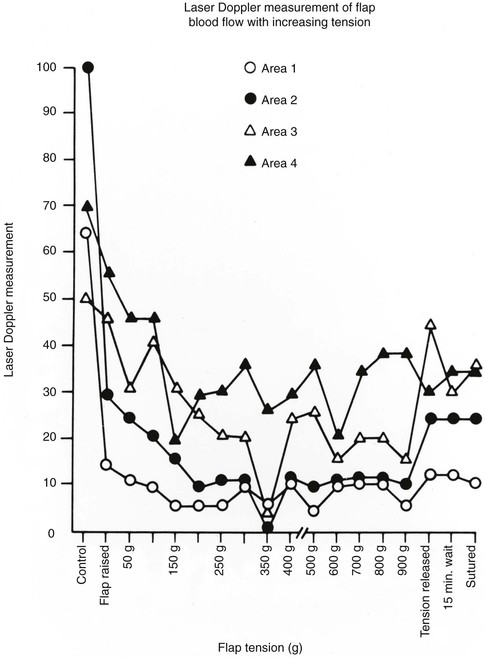
FIGURE 3-4 Relationship between tension and blood flow in 3-cm-wide and 6-cm-long random skin flap. Measurements were taken at 2-cm intervals from base of flap (area 4) to distal tip (area 1). Flow decreases steadily until critical value of 200 to 250 g is reached. (From Larrabee WF Jr, Holloway GA Jr, Sutton D: Wound tension and blood flow in skin flaps. Ann Otol Rhinol Laryngol 93:114, 1984.)
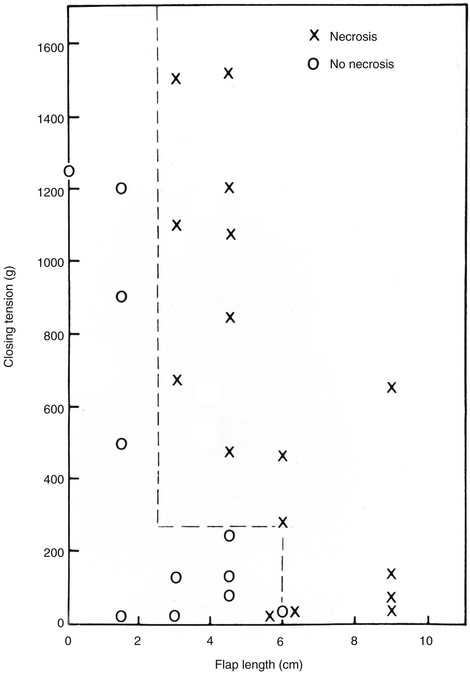
FIGURE 3-5 Effect of tension on survival of random patterned skin flaps. Flaps of intermediate length are sensitive to effects of tension. Flaps closed with less than 250 g of tension tended to survive, whereas flaps closed with greater tension underwent necrosis. (From Larrabee WF Jr, Holloway GA Jr, Sutton D: Wound tension and blood flow in skin flaps. Ann Otol Rhinol Laryngol 93:114, 1984.)
Flap Design and Undermining
Minor variations in flap design can alter the mechanical characteristics of cutaneous tissue and change the force necessary for advancement and wound closure. A properly designed skin flap will minimize wound closure tension and maintain critical blood flow. In like manner, a poorly designed flap risks injury to important nutrient vessels while securing little or no mechanical advantage. Many practical questions concerning proper flap design have already been addressed in the pig model. According to Larrabee and Sutton,36 the rectangular advancement flap should be designed with a length-to-width ratio of at least 1 : 1. After this point, an increase in the ratio results in a gradual decline in wound closure tension. Further increases in the length-to-width ratio are not always profitable and may risk necrosis of the distal portion of the flap. Advancement flaps should be designed with use of the minimal length-to-width ratio necessary to effect a low-tension closure. When facial flaps are used, a ratio between 1 : 1 and 2 : 1 is ideal; further lengthening is usually unnecessary. Similarly, animal models have shown little mechanical benefit of extending rotation flaps past 90°.36 Other considerations, such as skin redraping, may warrant the use of longer rotation flaps.
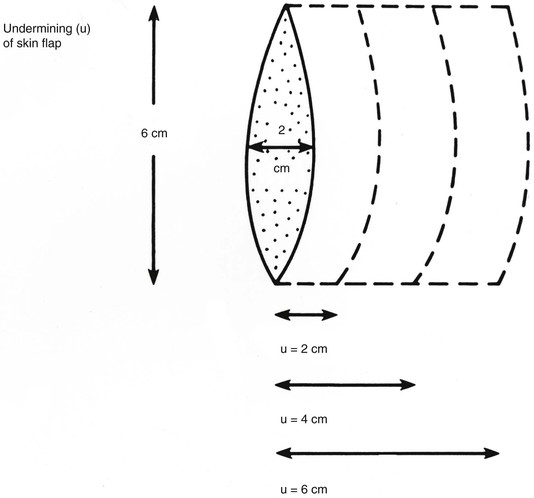
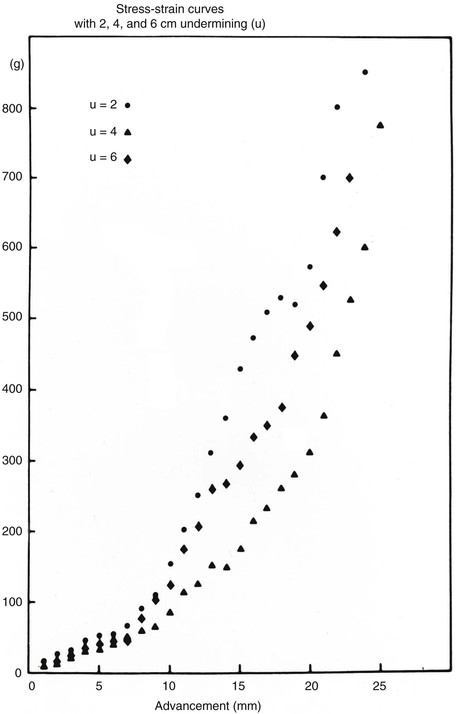
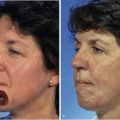
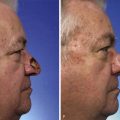
The forces that resist skin flap advancement are more complex than we have discussed thus far. The edge of a skin flap is advanced both to stretch the skin and to overcome resistance between the dermis and underlying tissues.37 The magnitude of this resistance (shearing force) varies considerably, depending on the site and age of the patient. Shearing forces can often be reduced by undermining in the subcutaneous plane. With undermining, vertical attachments between the dermis and tissue beneath the dermis are lysed, releasing the skin to slide unrestricted over the subcutaneous tissue. As undermining is increased, the origin of the shearing force is moved away from the edge of the flap and more free skin is available for low-resistance redraping (stretching). Most of the benefit from undermining is achieved within the first 1 to 2 cm. Undermining beyond a short distance risks injury to surrounding structures and may compromise blood flow. In broad-based flaps, extensive undermining has been shown to result in an unexplained increase in the force necessary for advancement (Figs. 3-6 and 3-7).37,38
References
1. Daly, CH, Odland, GF. Age-related changes in the mechanical properties of human skin. J Invest Dermatol. 1979; 73:84–87.
2. Raposio, E, Nordstrom, RE. Tension and flap advancement in the human scalp. Ann Plast Surg. 1997; 39:20–23.
3. Borges, AF. Relaxed skin tension lines. Dermatol Clin. 1989; 7:169–177.
4. Pierard, GE, Lapiere, CM. Microanatomy of the dermis in relation to relaxed skin tension lines and Langer’s lines. Am J Dermatopathol. 1987; 9:219–224.
5. Borges, AF. The rhombic flap. Plastic Reconstr Surg. 1981; 67:458–466.
6. Gibson, T, Kenedi, RM. Biomechanical properties of skin. Surg Clin North Am. 1967; 47:279–294.
7. Folkman, J, Moscona, A. Role of cell shape in growth control. Nature. 1978; 273:345–349.
8. Ingber, DE. Cellular mechanotransduction: putting all the pieces together again. FASEB J. 2006; 20:811–827.
9. Hynes, RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002; 110:673–687.
10. Hakkinen, L, Koivisto, L, Gardner, H, et al. Increased expression of β6-integrin in skin leads to spontaneous development of chronic wounds. Am J Pathol. 2004; 164:229–242.
11. Sachs, F, Morris, CE. Mechanosensitive ion channels in nonspecialized cells. Rev Physiol Biochem Pharmacol. 1998; 132:1–77.
12. Yano, S, Komine, M, Fujimoto, M, et al. Mechanical stretching in vitro regulates signal transduction pathways and cellular proliferation in human epidermal keratinocytes. J Invest Dermatol. 2004; 122:783–790.
13. Reichelt, J. Mechanotransduction of keratinocytes in culture and in the epidermis. Eur J Cell Biol. 2007; 86:807–816.
14. Tamada, M, Sheetz, MP, Sawada, Y. Activation of a signaling cascade by cytoskeleton stretch. Dev Cell. 2004; 7:709–718.
15. Wipff, PJ, Hinz, B. Myofibroblasts work best under stress. J Bodyw Mov Ther. 2009; 13:121–127.
16. Chin, MS, Lancerotto, L, Helm, DL, et al. Analysis of neuropeptides in stretched skin. Plast Reconstr Surg. 2009; 124:102–113.
17. Ogawa, R. Mechanobiology of scarring. Wound Repair Regen. 2011; 19(Suppl 1):s2–s9.
18. Peters, EM, Ericson, ME, Hosoi, J, et al. Neuropeptide control mechanisms in cutaneous biology: physiological and clinical significance. Journal Invest Dermatol. 2006; 126:1937–1947.
19. Scholzen, T, Armstrong, CA, Bunnett, NW, et al. Neuropeptides in the skin: interactions between the neuroendocrine and the skin immune systems. Exp Dermatol. 1998; 7:81–96.
20. Kahler, CM, Sitte, BA, Reinisch, N, et al. Stimulation of the chemotactic migration of human fibroblasts by substance P. Eur J Pharmacol. 1993; 249:281–286.
21. Akaishi, S, Ogawa, R, Hyakusoku, H. Keloid and hypertrophic scar: neurogenic inflammation hypotheses. Med Hypotheses. 2008; 71:32–38.
22. Steinhoff, M, Stander, S, Seeliger, S, et al. Modern aspects of cutaneous neurogenic inflammation. Arch Dermatol. 2003; 139:1479–1488.
23. Black, MM, Bottoms, E, Shuster, S. Skin collagen and thickness in simple obesity. Br Med J. 1971; 4:149–150.
24. Neumann, CG. The expansion of an area of skin by progressive distention of a subcutaneous balloon; use of the method for securing skin for subtotal reconstruction of the ear. Plast Reconstr Surg (1946). 1957; 19:124–130.
25. Radovan, C. Breast reconstruction after mastectomy using the temporary expander. Plast Reconstr Surg. 1982; 69:195–208.
26. Cherry, GW, Austad, E, Pasyk, K, et al. Increased survival and vascularity of random-pattern skin flaps elevated in controlled, expanded skin. Plast Reconstr Surg. 1983; 72:680–687.
27. Austad, ED, Pasyk, KA, McClatchey, KD, et al. Histomorphologic evaluation of guinea pig skin and soft tissue after controlled tissue expansion. Plast Reconstr Surg. 1982; 70:704–710.
28. Johnson, PE, Kernahan, DA, Bauer, BS. Dermal and epidermal response to soft-tissue expansion in the pig. Plast Reconstr Surg. 1988; 81:390–7.
29. Olenius, M, Dalsgaard, CJ, Wickman, M. Mitotic activity in expanded human skin. Plast Reconstr Surg. 1993; 91:213–216.
30. Austad, ED, Thomas, SB, Pasyk, K. Tissue expansion: dividend or loan? Plast Reconstr Surg. 1986; 78:63–67.
31. Johnson, PE, Kernahan, DA, Bauer, BS. Dermal and epidermal response to soft-tissue expansion in the pig. Plast Reconstr Surg. 1988; 81:390–397.
32. Mackay, DR, Saggers, GC, Kotwal, N, et al. Stretching skin: undermining is more important than intraoperative expansion. Plast Reconstr Surg. 1990; 86:722–730.
33. Raposio, E, Cella, A, Panarese, P, et al. Quantitative benefits provided by acute tissue expansion: a biomechanical study in human cadavers. Br J Plast Surg. 2000; 53:220–224.
34. Stell, PM. The effects of varying degrees of tension on the viability of skin flaps in pigs. Br J Plast Surg. 1980; 33:371–376.
35. Larrabee, WF Jr, Holloway, GA, Jr., Sutton, D. Wound tension and blood flow in skin flaps. Ann Otol Rhinol Laryngol. 1984; 93(Pt 1):112–115.
36. Larrabee, WF, Jr., Sutton, D. The biomechanics of advancement and rotation flaps. Laryngoscope. 1981; 91:726–734.
37. Cox, KW, Larrabee, W, Jr. A study of skin flap advancement as a function of undermining. Arch Otolaryngol. 1982; 108:151–155.
38. Larrabee, WF, Jr., Trachy, R, Sutton, D, et al. Rhomboid flap dynamics. Arch Otolaryngol. 1981; 107:755–757.