CHAPTER 7 Biology of Meningiomas
INTRODUCTION
Meningiomas are important tumors biologically as well as clinically.1 Other chapters of this text discuss chromosomal changes and angiogenic molecules as part of their biology. This chapter presents information about growth factors, steroid hormones, and other molecules important for their growth and maintenance. This information may provide better understanding of the pathogenesis of meningiomas and also suggest novel treatment options. This chapter discusses:
GROWTH FACTORS AND THEIR RECEPTORS
Growth factors are naturally occurring proteins that play an important role in cellular growth and proliferation. An important early discovery in oncology was the realization that PDGF, a growth factor, was the product of the oncogenic virus v-sis through which the virus caused tumors by stimulating growth.2 Growth factors and their receptors important in meningiomas include PDGF, EGF, VEGF, and FGF.
Although genetic changes account for much of the growth derangement in meningiomas,3,4 these growth factors and other changes are also important. Methylation, for example, may keep certain genes biologically inactive.1 Studies from our laboratory several years ago assessing the clonality of meningiomas suggested that at least some meningiomas are polyclonal—that is, do not arise from the same parent cell, suggesting that environmental factors may also be important in their growth.5 Growth factors are almost certainly among those factors.2
Platelet-Derived Growth Factor
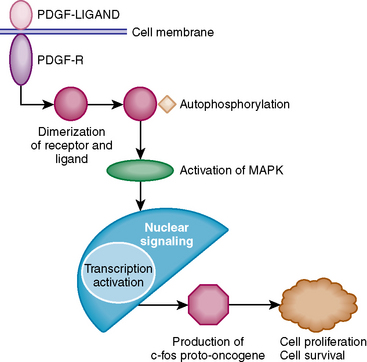
There are four different isoforms of PDGF: PDGF-A, PDGF-B, PDGF-C, and PDGF-D; the situation is made more complex by the fact that in human tumors these molecules dimerize as PDGF AA, PDGF BB, and PDGF AB to create active forms.6–8 These activate a cellular response through two different cell surface receptors, α and β. On activation by the ligand (PDGF-BB) the PDGF-β receptor dimerizes and undergoes autophosphorylation, leading to activation of signal transduction pathways (Fig. 7-1). Western blot analysis has shown the presence of mitogen-activated protein kinase (MAPK) and activated MAPK in cerebral meningiomas as a transducer of mitogenic signals of PDGF, contributing to the growth of these tumors. A downstream effect of this signal transduction is the regulation of the expression of genes such as the c-fos proto-oncogene that contributes to tumor formation.
Studies using Northern blot analysis, polymerase chain reaction (PCR), and immunohistochemistry have all demonstrated that meningiomas express transcripts for three members of the platelet-derived growth factor family: PDGF-A, PDGF-B, and PDGFR-B.6–8 Although both PDGF-A and PDGF-B are present in meningiomas, PDGF-BB is the major functional ligand as shown by induction of the c-fos proto-oncogene in meningioma cultures.9
Expression of PDGF isoforms and the appropriate receptor in some meningiomas suggests that they have an autocrine loop pathway for growth in which the ligand stimulates the receptor in the same cell. This hypothesis is supported by the presence of active c-sis/PDGF-2 proto-oncogene and PDGF-receptor gene and their protein products in meningioma cultures.9–11 The coexpression of mitogen and its receptor contributes to growth and maintenance of human human meningioma cells.12,13 These autocrine loops can be targeted by monoclonal antibodies and may possibly serve as future treatment options for meningiomas.14,15 Several drugs have been tested based on this concept. Schrell and colleagues used Suramine, a growth factor scavenger, to inhibit growth-factor-induced proliferation in meningioma cell culture. Suramine causes cell arrest in S and G2/M phase of the cell cycle. Trapidil is a drug that has an antagonistic action against PDGF and produces dose-dependent inhibition of cultured meningioma cells by blocking mitogenic stimulation.13 These and other PDGF antagonists such as Gleevec may be useful in treatment of recurrent or inoperable meningiomas.
Vascular Endothelial Growth Factor
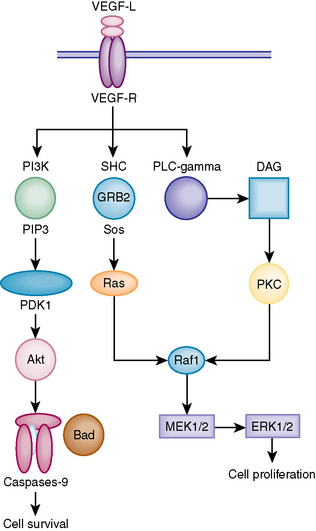
VEGF is an important proangiogenic growth factor that plays an important role in unregulated tumor growth by enhancing the growth of blood vessels into the tumor. Reverse transcriptase polymerase chain reaction (RT-PCR), enzyme-linked immunosorbent assay (ELISA), and immunohistochemistry have been used to analyze the expression of VEGF in meningiomas. These studies have shown an increased expression of VEGF isoforms and the mRNA stability factor HuR in meningiomas. Figure 7-2 describes the mechanism by which VEGF exerts its intracellular effect. Elevated levels of VEGF in meningiomas are associated with increased amount of peritumoral brain edema (PTBE) and higher histologic grades.16–18 Abe and Black demonstrated that peritumoral brain edema can be diffuse or localized.18 The diffuse type is that associated with leaky endothelial cells in the meningioma and is the most likely to be associated with aggressive growth.19
Preoperative embolization leads to hypoxic conditions in meningiomas which may enhance the expression of hypoxia inducible factor and mRNA stability factor which in turn increase the expression of VEGF in meningiomas.20,21 Levels of VEGF are up-regulated in meningioma tissue by both hypoxia inducible factor and HuR. Although no correlation is present between VEGF levels and histologic subtypes of meningiomas, enhanced expression of flt-1 bound VEGF is found in some meningiomas.22 One study showed no relationship between tumor recurrence and VEGF levels,23 but it appears that expression of VEGF and its receptors leads to a more aggressive meningioma. Antibodies to VEGF could be used as an important pharmacologic agent to treat high-grade meningiomas, and at the present time Avastin is being used for this purpose in some centers (Patrick Wen, Dana-Farber Brigham Women’s Cancer Center, personal communication).
Epidermal Growth Factor
EGF and its receptors are important in many types of systemic tumor including lung carcinoma. Several studies have shown the presence of activated EGF-R in meningioma cells.23,24 Activated EGF-R interacts with and phosphorylates Shc, an SH2 domain-containing adapter protein that transduces the mitogenic signals from EGF-R through activation of the Ras signaling pathway.25 Present data suggest that EGF-R regulates phospholipase C gamma 1 activity in meningioma cells, which is thought to have antiapoptotic activity in tumor cells.26 Levels of EGF-R diminish postoperatively depending on the extent of resection. Measurement of serum EGFR may possibly be used as a tool for follow up of patients after surgery and perhaps to detect recurrence.26 Immunohistochemical studies have shown that EGF-R immunoreactivity can be used as a predictor of short-term survival in meningioma patients. Lack of EGF-R in patients with an atypical meningioma is a strong indicator of short-term survival.27 EGFR antagonists may also play an important role in treating patients with recurrent or high-grade meningiomas.
Fibroblast Growth Factor
FGF has both angiogenic and mitogenic properties and may be involved in autonomous growth and tumorigenesis of meningioma cells. Immunohistochemical studies have shown the presence of basic FGF and FGF-R in human meningioma cells.28–30 The presence of both ligand and receptor again supports the hypothesis of an autocrine mechanism in meningiomas that can be used to formulate treatment.
FGF signaling is also important for the growth and migration of endothelial and tumor cells, and this property can be used to establish therapeutic strategies against meningioma cells. One of these efforts is arming oncolytic herpes simplex virus (HSV) with a dominant negative FGF-R. This helps to destroy both tumor cells and tumor endothelial cells in experimental models and may provide a future therapeutic possibility for meningiomas.31 A general problem with targeted therapies against this or other growth factors is that a specific meningioma may use several pathways, which makes single-targeted therapy less useful.
HORMONES AND MENINGIOMAS
There is substantial evidence that sex hormones may play an important role in meningioma growth. Meningiomas occur more often in women than in men: 65% of all intracranial and 85% of intraspinal meningiomas occur in women.1 Observation of increased growth of meningiomas in pregnancy and the luteal phase of the menstrual cycle along with regression in postpartum period strongly support the role of steroid sex hormones in meningioma epidemiology. Many studies have demonstrated the presence of steroid hormones in meningiomas, and therapeutic agents have been used as treatment options for meningiomas based on these findings.
Estrogen Receptors
There have been significant controversies regarding the functional importance of estrogen receptors in meningiomas. Early studies demonstrated a variable presence of estrogen receptors ranging from 30% to 80% by immunohistochemistry. Recent studies have shown both estrogen receptor A and estrogen receptor B (ER-A and ER-B) in meningiomas.32–34 These receptors may not be functional in all tumor cells, and the role they play in meningioma growth is still controversial. There may not be particular value in assessing estrogen receptor status in a given tumor, as therapy based on receptor expression is ineffective. Importantly, receptor are lost in atypical meningiomas and recurrent cases, so no therapies based on hormone receptors appear to be useful for the most difficult meningiomas we treat.35 In tissue culture, meningiomas quickly lose their receptor expression, so attempts at assessing drug response in cultured cells are not likely to be useful.
The issue of hormone replacement therapy and meningioma formation is a difficult one that has been addressed by a recent review article.36
Progesterone Receptors
Many immunohistochemical studies have shown the presence of progesterone receptors in meningiomas,37 and these receptors can be shown to be functional by a complex culture technique.38 Despite this, it has not been useful to target these receptors. Their use as therapeutic targets is limited in cases of atypical and anaplastic lesions because of scarcity or absence of these receptors, hampering the development of a medical treatment modality.
Progesterone receptor (Pg-R) expression appears to be associated with a more favorable prognosis in meningiomas. Diminished receptor expression is considered a poor prognostic indicator and is associated with higher rate of recurrence and more aggressive biologic behavior.39–42 The worsened clinical course of meningiomas with low or absent Pg-R is supported by molecular data providing the fact that Pg-R has an inverse relationship with anti-apoptotic factor bcl2 and ki-67 (MIB-1 clone) proliferation index. Pg-R negativity and higher ki-67 and bcl2 are considered predictive for recurrence in WHO grade 1 tumors that have been completely resected.43
Benign surgically nonresectable tumors with a high Pg-R status have been treated with antiprogesterone therapy with disappointing results. Clinical trials have suggested minor regression in meningiomas, especially in male and premenopausal patients after long-term therapy. Long-term treatments have been well tolerated with mild adverse effects including fatigue, hot flushes, gynecomastia/breast tenderness, skin rash, cessation of menses, decreased libido, endometrial hyperplasia, and polyps.44
Part of the reason patients show a varied response to hormonal therapy may be the fact that tumors express steroid receptors cofactors including steroid receptor coactivator (SRC-1) and transcription intermediary factor 2 (TIF 2); these cofactors modify the response of meningiomas to the hormonal therapy.45
Androgen Receptors
Carroll and colleagues studied the androgen receptor (AR) expression in cerebral meningiomas and concluded higher expression of these receptors in female patients as compared to men and evidence of histochemical staining in nuclear area, suggesting a possible role of ARs in gene expression.46 Lesch and colleagues demonstrated the positive correlation between ER immunoreactivity and AR binding activity, questioning the possible predictive role of AR and its implication for tumor therapy.47
OTHER RECEPTORS AND MOLECULES
Somatostatin Receptors
Somatostatin receptors are present in almost all meningiomas, suggesting a potential role for somatostatin receptors as radiodiagnostic, prognostic, and therapeutic agents. They suppress growth in vitro.48 The somatostatin receptors ssT2 are present on most meningiomas and may be useful for imaging for residual or recurrent tumors.49 Early clinical trials have shown an inhibitory effect of somatostatin analogues in treatment of recurrent meningiomas.50 It has also been observed that somatostatin analogues influence the production of VEGF in meningiomas, affecting peritumoral brain edema.51
Laboratory studies have shown that somatostatin stimulates meningioma growth through inhibition of adenylate cyclase in human cell culture; thus both in vitro and in vivo it may have important clinical implications.52,53
Dopamine Receptors
Dopamine receptors D1, D2, and prolactin receptors are present in human meningiomas, but only the D1 activated receptors are found through PCR studies.54 Although the role of dopamine in meningioma growth is not well defined, culture studies have shown an inhibitory effect of dopaminergic agonist like SKF-38393 in cultured meningioma cells.55,56
Endothelin
Endothelin is a peptide that acts through two types of receptors: ET-A and ET-B. It may be an important growth factor for meningiomas by inducing DNA synthesis and angiogenesis.57,58 A positive correlation is found between VEGF and ET-A levels in cerebral meningiomas.59
There is increased expression of ET-A in high-grade meningiomas, and based on this fact the selective ET-A antagonist BQ23 has been tried on surgically excised meningioma cells and cultured meningioma cells, showing growth inhibition in a dose-dependent manner.60,61 ET-1 receptor antagonists may therefore join the list of possible therapeutic agents for meningiomas.
Interferon and Meningiomas
Interferon-α (IFN-α) has been shown to have a therapeutic efficacy in patients with meningiomas who have postoperative residual masses, recurrent tumor, or primary inoperable tumors. A positron emission tomography (PET) scan with l-[11C]methionine is used to predict which patients are suitable for treatment.62
Long-term treatment trials have shown that the drug is oncostatic and potential therapeutic benefit can be achieved in selected patients.63,64 The possible mechanism of action of IFN-α is the inhibition of DNA synthesis in meningioma cells induced by PDGF and EGF.65
There are early reports that IFN-β may induce meningioma occurrence in patients treated for multiple sclerosis.66
Cytokines
Interleukin-1 (IL-1), IL-6, and oncostatic M have been observed to affect the activity of meningioma cells. It has been suggested that interleukin has an autocrine loop that has an inhibitory effect on meningioma growth. Recombinant cytokines may be a possible future treatment option for inoperable and recurrent meningiomas.66,67
[1] Zhu J., Frosch M.P., Busque L., Beggs A.H., Dashner K., Gilliand D.G., et al. Analysis of meningiomas by methylation and transcription based clonality assays. Cancer Res. 1995;55(17):3865-3872.
[2] Krisch M., Santarius T., Black P. Molecular biology of meningiomas and peripheral nerve sheath tumors. Concepts Neurosurg. 1997;8:126-145.
[3] Leon S., Zhu J., Black P.M. Genetic aberrations in human brain tumors. Neurosurgery. 1993;34:708-722.
[4] Black P.M., Carroll R., Zhang Z. The molecular biology of Hormones and Growth Factor Receptors on meningiomas. Acta Neurochir. 1996;65(Suppl.):50-53.
[5] Zhu J.J., Maruyama T., Jacoby L.B., Herman J.G., Gusell J.F., Black P.M., et al. Clonal analysis of a case of multiple meningiomas using multiple molecular genetic approaches: pathology case report. Neurosurgery. 1999;45(2):409-416.
[6] Black P.M., Carroll R., Glowacka D., Riley K., Dashner K. Platelet derived growth factor expression and stimulation in human meningiomas. J Neurosurg. 1994;81(3):388-393.
[7] Maxwell M., Galanopoulos T., Hedley-Whyte E.T., Black P.M., Antoniades H.N. Human meningiomas co-express platelet derived growth factor and PDGF-receptor gene and their protein products. Int J Cancer. 1990;46(1):16-21.
[8] Black P., Carroll R., Zhang J. The molecular biology of hormones and growth factor receptors in meningiomas. Acta Neurochir. 1996;65(Suppl.):50-53.
[9] Todo T., Adams E.F., Fahlbusch R., Dingermann T., Werner H. Autocrine stimulation of human meningioma cells by platelet derived growth factor. J Neurosurg. 1996;84(5):852-858. discussion 858–859
[10] Adams E.F., Todo T., Schrell U.M., Thierauf P., White M.C., Fahlbusch R. Autocrine control of human meningioma proliferation: secretion of platelet derived growth factor like molecules. Int J Cancer. 1991;49(3):398-402.
[11] Shamah S.M., Alberta J.A., Giannobile W.V., Guha A., Kwon YK., Carroll RS., et al. Detection of activated platelet derived growth factor receptor in human meningioma. Cancer Res. 1997;57:4141-4147.
[12] Yang S.U., Xu G.M. Expression of PDGF and its receptors as well as their relationship to proliferating activity and apoptosis of meningiomas in human meningiomas. J Clin Neurosci. 2001;8(Suppl. 1):49-53.
[13] Schrell U.M., Gauer S., Kiesewetter F., et al. Inhibition of proliferation of human cerebral meningioma cells by suramin, effect on cell growth, cell cycle phase, extracellular growth factor and PDGF-BB autocrine growth loop. J Neurosurg. 1995;82:600-607.
[14] Todo T.E., Adams F., Fahlbusch R. Inhibitory effect of trapidil on human meningiomas cells proliferation autocrine growth stimulation. J Neurosurg. 1993;78:463-469.
[15] Sakuma T., Nakagawa T., Ido K., Takeuchi H., Sato K., Kubota T. Expression of vascular endothelial growth factor-A and mRNA stability factor HuR in human meningiomas. J Neurooncol. 2008;88(2):143-155.
[16] Jensen R.L., Soleau S., Bhayani M.K., Christiansen D. Expression of hypoxia inducible factor 1 and correlation with preoperative embolization of meningiomas. J Neurosurg. 2002;97(3):658-667.
[17] Kalkanis S.N., Carroll R.S., Zhang J.P., Zamani A.A., Black P.M. Correlation of vascular endothelial growth factor messenger RNA expression with peritumoral vasogenic cerebral edema in meningiomas. J Neurosurg. 1996;85:1059-1101.
[18] Abe T., Black P.M., Ojemann R.G., Hedley-White E.T. Cerebral edema in intracranial meningiomas: evidence for local and diffuse patterns and factors associated with its occurrences. Surg Neurol. 1994;42:471-475.
[19] Park K., Kim J.H., Nam D.H., Lee J.I., Kim J.S., Hong S.C., et al. Vascular endothelial growth factor expression under ischemic stress in human meningiomas. Neurosci Lett. 2000;283(1):45-48.
[20] Christi C., Lechapt-Zalcman E., Adle-Biassette H., Nachev S., Gherrardi R.K. Vascular permeability factor /vacular endothelial growth factor (VPF/VEGF) and its receptor flt-1 in microcystic meningiomas. Acta Neuropathol. 1999;98(4):4.
[21] Majuri F., De Carol Model B., Esposito F., Capacitance P., Stressful V., Pettiness G., et al. Recurrence of meningiomas: predictive value of pathological features and hormonal and growth factors. J Neurooncol. 2007;82(1):63-68.
[22] Jones N.R., Rossi M.L., Gregorious M., Hughes J.T. Epidermal growth factor receptor in 72 meningiomas. Cancer. 1990;66(1):152-155.
[23] Carroll R.S., Black P.M., Zhang J., Kirsch M., Percec I., Lau N., et al. Expression and activation of epidermal growth factor receptor in meningiomas. J Neurosurg. 1997;87(2):315-323.
[24] Johnson M.D., Horiba M., Winnier A.R., Arteaga C.L. The epidermal growth factor receptor is associated with phospholipase C gamma 1 in meningiomas. Hum Pathol. 1994;25(2):146-153.
[25] Kong Y.G., Su C.B., Ren Z.Y., Wang R.Z. Measurement of epidermal growth factor receptor concentration in the pre- and postoperative serum in patients with meningiomas. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2002;24(4):427-429.
[26] Smith J.S., Lal A., Harmon-smoth M., Bollen A.W., McDermott M.W. Association between absence of epidermal growth factor immunoreactivity and poor prognosis in patients with atypical meningioma. J Neurosurg. 2007;106(6):1034-1040.
[27] Paulus W., Grothe C., Sensenbrenner M., Janet T., Baur I., Graf M., et al. Localization of basic fibroblastic growth factor, a mitogen and angiogenic factor, in human brain tumors. Acta Neuropathol. 1990;79(4):418-423.
[28] Ueba T., Takahashi J.A., Fukumoto M., Ohta M., Ito N., Oda Y., et al. Expression of fibroblastic growth factor receptor -1 in human glioma and meningioma tissues. Neurosurgery. 1994;34(2):221-225. discussion 225–226
[29] Todo T., Kondo T., Kirino T., Asai A., Adams E.F., Nakamura S., et al. Expression and growth stimulatory effect of fibroblast growth factor 9 in human brain tumors. Neurosurgery. 1998;43(4):337-346.
[30] Liu TC, Zhang T., Fukhuhara H., Kuroda T., Todo T., Canron X., et al. Dominant-negative fibroblast growth factor receptor expression enhances antitumoral potency of oncolytic herpes virus in neural tumors. Clin Cancer Res. 2006;12:6791-6799.
[31] Brandis A., Mirzai S., Tatagiba M., Walter G.F., Samoo M., Ostertag H. Immunohistochemical detection of female sex hormone receptors in meningiomas: correlation with clinical and histological features. Neurosurgery. 1993;33(22):212-217. discussion 217–218
[32] Carroll R., Zhang J., Black P.M. Expression of estrogen receptor alpha and beta in human meningiomas. J Neurooncol. 1999;42(2):109-116.
[33] Pravdenkova S., Al-Mefty O., Sawyer J., Husain M. Progesterone and estrogen receptors: opposing prognostic indicators in meningiomas. J Neurosurg. 2006;105(2):161-162. discussion 162
[34] Konstantinidou A.E., Korkolopoulo P., Mahera H., Kotsiakis X., Hranioti S., Eftychiadis C., et al. Hormone receptor in non-malignant meningiomas correlates with apoptosis, cell proliferation and recurrence-free survival. Histopathology. 2003;43(3):280-290.
[35] Hsu D.W., Efridt J.T., Hedley-Whyte E.T. Progesterone and estrogen receptor in meningiomas: prognostic considerations. J Neurosurg. 1997;86(1):113-120.
[36] Claus E.B., Black P.M., Bondy M.L., Calvocressi L., Schildkrut J.M., Wiemels J.L., et al. Exogenous hormone use and meningioma risk. Cancer. 2007;110:471-476.
[37] Carroll R., Glowacka D., Dashner K., Black P.M. Progesterone receptors in meningiomas. Cancer Res. 1993;53:1312-1316.
[38] Carroll R.S., Zhang J., Dashner K., Black P.M. Progesterone and glucocorticoid receptor activation in meningiomas. Neurosurgery. 1995;37:496-504.
[39] Piquer J., Cedar M., Lluch A., Barcia Salorio J.L., Garcia-Conde J. Correlation off female steroid hormone receptor with histologic feature in meningiomas. Acta Neurochir (Wien).. 1991;110(1–2):38-43.
[40] Kostron H., Daxenbichler G., Maier H. Steroid receptor in atypical histology as prognostic parameters in meningioma. Wien Klin Wochenschr. 1990;102(18):525-528.
[41] Maiuri F., De Carol Model B., Esposito F., Capacitance P., Stressful V., Pettinato G., et al. Recurrence of meningiomas: predictive value of pathological features and hormonal and growth factors. J Neurooncol. 2007;82(1):63-68.
[42] Metellus P., Nanni I., Dussert C., Trinkhaus M., Fuentes S., Chinot O., et al. Prognostic implications of biologic markers in intracranial meningiomas: 120 cases. Neurochirugie. 2008;54:750-756.
[43] Verheijen F.M., Donker G.H., Viera C.S., et al. Progesterone receptors, bcl-2 and bax expression in meningiomas. J Neurooncol. 2002;56(1):35-41.
[44] Grunberg S.M., Weiss M.H., Russell C.A., Spita I.M., Ahmadi J., Sandun A., et al. Long-term administration of mifepristone (RU486): clinical tolerance during extended treatment of meningiomas. Cancer Invest. 2006;24(8):727-733.
[45] Carroll R.S., Brown M., Zhang J., DiRenzo J., Font De Mora J., Black P.M. Expression of a subset of steroid receptor cofactor is associated with progesterone receptor expression in meningiomas. Clin Cancer Res. 2002;6(90):3570-3575.
[46] Carroll R.S., Zhang J., Dashner K., Sar M., Wilson E.M., Black P.M. Androgen receptor expression in meningiomas. J Neurosurg. 1995;82(3):453-460.
[47] Lesch K.P., Schott W., Engl F.G., Gross S., Thierauf P. Gonadal steroid receptors in meningiomas. J Neurol. 1987;234(5):328-333.
[48] Kunert-Radek J., Stepien H., Radek A., Pawlikoski M. Somatostatin suppression of meningioma cell proliferation in vitro. Acta Neurol Sci. 1987;75(6):434-436.
[49] Reubi J.C., Laissue J., Krenning E., Lamberts S.W. Somatostatin receptor in human cancer: incidence, characteristics, functional correlate and clinical implications. J Steroid Biochem Mol Biol. 1992;43(1–3):27-35.
[50] Chamberlain M.C., Glantz M.J., Fadul C.E. Recurrent meningioma: salvage therapy with long acting somatostatin analogue. Neurology. 2007;469(10):969-973.
[51] Pistolesi S., Fontanini G., Boldrini L., et al. The role of somatostatin in vasogenic meningioma associated brain edema. Tumori. 2003;89(2):136-140.
[52] Koper J.W., Markstein R., Kohler C., Kwekkeboom D.J., Avezzat C.J., Lamberts S.W., et al. Somatostatin inhibits activity of adenylate cyclase in cultured human meningioma cells and stimulates their growth. J Clin Endocrinol Metab. 1992;74(3):543-547.
[53] Prat R., Banzo J., Diaz F.Z. Somatostatin receptors in meningioma: diagnostic and therapeutic value. Rev Neurol. 1997;25(148):2002-2005.
[54] Carroll R.S., Schrell U.M., Zhang J., Dashner K., Nomikos P., Fahlbusch R., et al. Dopamine D1, dopamine D2, and prolactin receptor messenger ribonucleic acid expression by the polymerase chain reaction in human meningiomas. Neurosurgery. 1996;38(2):367-375.
[55] Schrell U.M., Nomikos P., Fahlbusch R. Presence of dopamine D1 and absence of Dopamine D2 receptor in human cerebral meningioma tissue. J Neurosurg. 1992;77(2):288-294.
[56] Schrell U.M., Fahlbusch R., Adams E.F., Nomikos P., Reif M. Growth of cultured human cerebral meningioma is inhibited by dopaminergic agents. Presence of high affinity dopamine-D1 receptors. J Clin Endocrinol Metab. 1990;77(6):1669-1671.
[57] Jimenez-Hakim E., El-Azouzi M., Black P.M. The effect of prolactin and bombesin on growth of meningioma derived cells in monolayer culture. J Neurooncol. 1993;16:185-190.
[58] Kitagawan N., Tsutsumi K., Niwa M., et al. Expression of a functional endothelin (ETA) receptor in human meningiomas. J Neurosurg. 1994;86(4):723-731.
[59] Bolderini L., Pisotolesi S., Grisfredi S., et al. Expression of endothelin 1 and its angiogenic role in meningiomas. Virchows Arch. 2006;449(5):546-553.
[60] Paggota U., Arzberger T., Hopfner U., et al. Expression and localization of endothelin-1 and endothelin receptor in human meningiomas. Evidence for a role in tumoral growth. J Clin Invest. 1995;96(4):2017-2025.
[61] Kitagawa N., Tsutsumi K., Niwa M., et al. A selective endothelin ETA antagonist, BQ-123, inhibits 125l-endothelin-1(125l-ET-1) binding to human meningiomas and antagonizes ET-1–induced proliferation of meningioma cells. Cell Mol Neurobiol. 1994;14(2):105-118.
[62] Muhr C., Gudjonsson O., Lilja A., Hartman M., Zhang Z.J., Langstrom B. Meningioma treated with interferon-alpha, evaluated with ((11) C)-L-methionine positron emission tomography. Clin Cancer Res. 2001;7(8):2269-2276.
[63] Kaba S.E., DeMonte F., Bruner J.M., Kyritsis A.P., Jaeckle K.A., Levin V., et al. The treatment of recurrent unresectable and malignant meningioma with interferon alpha-2B. Neurosurgery. 1997;40(2):271-275.
[64] Koper J.W., Zwarthoff E.C., Hagemeijer A., Braakman R., Avezaat C.J., Bergstrom M., et al. Inhibition of growth of meningioma cells by recombinant interferon-alpha. Eur J Cancer. 1991;27(4):416-419.
[65] Zhang Z.J., Muhr C., Wang J.L. Interferon-alpha inhibits the DNA synthesis induced by PDGF and EGF in cultured meningioma cells. Anticancer Res. 1996;16(2):717-723.
[66] Drevalagas A., Xinou E., Karacostas D., Parissis D., Karkavelas G., Milonas I. Meningioma growth and interferon beta-1b treated multiple sclerosis: co-incidence or relationship. Neuroradiology. 2005;47(7):516-519.
[67] Todo T., Adams E.F., Rafferty B., Fahlbusch R., Dingermann T., Werner H. Secretion of interleukin-6 by human meningioma cells: possible autocrine inhibitory regulation of neoplastic cell growth. J Neurosurg. 1994;81(3):394-401.