CHAPTER 1 Biology of Healing and Tissue Repair
ROTATOR CUFF PATHOLOGY
Natural History of Rotator Cuff Tears
In the diagnosis and treatment of rotator cuff pathology, it is important to understand when tears occur and how they progress. Using ultrasound and magnetic resonance imaging (MRI), there is strong evidence to show that the incidence of rotator cuff tears increases with age. When ultrasound was used, asymptomatic subjects demonstrated tear prevalence of 13% in their sixth decade, which increased to 51% in their ninth decade; there was an overall tear prevalence of 23% in subjects older than 50 years.1 Using MRI as an imaging tool, the prevalence of asymptomatic tears was found to be 4% in subjects younger than 40 years, dramatically increasing to 40% in subjects older than 50 years.2
Yamaguchi and colleagues3 have investigated not only the prevalence of rotator cuff tears, but also how these tears progress. They used ultrasound to screen patients presenting with unilateral shoulder pain and found that 40% of these patients had full-thickness tears in their contralateral asymptomatic shoulder, of which 51% became symptomatic within 3 years and none decreased in size. They observed a risk for tear and symptom progression with time. In a further study, they found a strong correlation between the prevalence of rotator cuff tears and age, while demonstrating that larger tears were more symptomatic.4 The symptomatic tears were 30% larger than asymptomatic tears. Based on these data, this group has recommended routine ultrasound follow-up for patients treated nonoperatively.
Rotator Cuff Degeneration
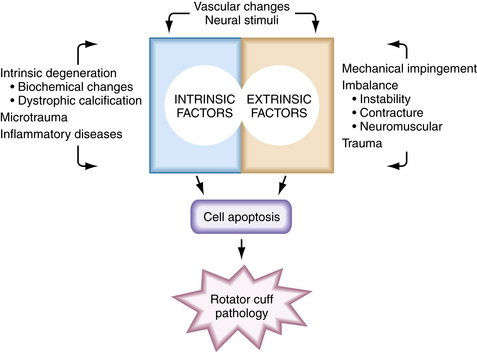
Intrinsic Factors
There is histologic and molecular evidence of intrinsic tendon degenerative changes associated with tendinopathy. Evaluation of tissue taken from torn rotator cuffs at the time of repair demonstrates histologic degenerative changes. Hashimoto and associates5 have described characteristics of age-related degeneration, including thinned and disoriented collagen fibers with chondroid metaplasia, myxoid and hyaline degeneration, calcification, fatty infiltration, and vascular proliferation. Biochemical alterations in the rotator cuff tendon have also been demonstrated with aging. In the aged tendon, the more mechanically resistant type II collagen is replaced with type III collagen, and there is increased tendon calcification and microtearing at the tendon-bone insertion.6 Additionally, elevated levels of alpha–smooth muscle actin (SMA) have been found at the edges of torn human rotator cuff tendons.7 SMA has been postulated to be involved in tendon retraction and is stimulated by the cytokine transforming growth factor beta 1 (TGF-β1).
Microtrauma is another hypothesized cause of intrinsic degeneration. It is theorized that repetitive tendon overload leads to small cuff injuries and, ultimately, full-thickness tears. This theory is consistent with increased prevalence of articular-sided supraspinatus tears, because higher tensions have been shown to occur on the articular half of this tendon. Soslowsky and coworkers8 have proposed a rat model for this repetitive degeneration. They found that rats exposed to repetitive exercise demonstrate signs of rotator cuff tendinopathy similar to those seen in human degenerative tendons.
Extrinsic Factors
Extrinsic factors contributing to rotator cuff pathology include anatomic, environmental, and demographic factors. Neer and Poppen8A first introduced the theory of mechanical impingement on the rotator cuff. Based on intraoperative observations, they hypothesized that bursal-sided lesions were likely caused by the anterior acromion. Correspondingly, Bigliani8B defined acromial morphologies that were more associated with rotator cuff tears. These acromial “spurs” were initially thought to be congenital, but studies have demonstrated an age-acquired progression that may be traction-related.9
In rotator cuff tendinopathy, acromial impingement does not act in isolation, because it has been shown that acromioplasty does not prevent later rotator cuff tears.10 However, Ko and colleagues have provided evidence that bursal-sided tears are likely caused by impingement, whereas articular-sided tears are derived from other degenerative processes.11 They recommended performing acromioplasty with bursal-sided tearing, but only if there is obvious spurring with isolated articular-sided tears.
Impingement that is attributed to functional or static instability has been termed secondary impingement. This imbalance can be to the result of neurologic factors, capsular laxity or contracture, or a combination of factors. Other extrinsic factors include overuse; some have found an increased incidence of tendinopathy in dominant extremities.4
Vascularity
Vascular changes have long been postulated to play an important role in degeneration, but this remains controversial. Historically, several groups have looked at the histology in the critical zone medial to the tendon-bone attachment to evaluate the vascularity and its potential role in tendinopathy. In a more recent study, Brooks and associates12 evaluated this area and found decreased vascularity within 15 mm of the rotator cuff insertion in both the supraspinatus and infraspinatus. They concluded that other factors are responsible for tendinopathy, because they believed that tendinopathy is more prevalent in the supraspinatus than the infraspinatus. However, they did not suggest the possibility that vascularity may be part of a multifactorial process.
More recently, Rudzki and coworkers13 used contrast-enhanced ultrasound to show a statistically significant decrease in supraspinatus vascularity at the critical zone in asymptomatic subjects older than 40 years with intact rotator cuffs. This was consistently most pronounced at the articular surface of the tendon as it approaches the medial footprint. Biberthaler and colleagues,14 in another human in vivo model, used polarized imaging to measure functional capillary density at the time of arthroscopy and found quantitatively decreased vascularity at the edge of degenerative rotator cuff lesions. These results suggest the possibility of a vascular contribution to the pathogenesis of rotator cuff tendinopathy that occurs with aging.
Neuropeptides
The possibility of neuropeptides contributing to tendinopathy has recently been introduced. Glutamate, substance P, and serotonin are neuropeptides that have been investigated in tendinopathy. Increased expression of glutamate signaling proteins has been demonstrated in rat supraspinatus tendons subjected to overuse. Additionally, tenocytes exposed to glutamate have increased rates of apoptosis.15 Substance P has been shown to be elevated in the bursa of diseased rotator cuff tendons.16
Tendinopathy as a Multifactorial Process
Rotator cuff tendinopathy is likely caused by multiple factors and it would be shortsighted to imply that there is only one cause. Soslowsky and associates17 have investigated the overlap of intrinsic and extrinsic factors by using the aforementioned rat tendon overuse model, along with a model of extrinsic compression. This study demonstrated that tendon injury created through the combination of their microtrauma model and their extrinsic compression model was significantly greater than either in isolation.
Apoptosis
Apoptosis has been shown to be associated with rotator cuff disease. Yuan and coworkers18 have found that apoptotic cell proportion is increased from 13% in controls to 34% at the edge of rotator cuff tears in humans. They have also shown increased tenocyte production of apoptotic mediators in response to in vitro oxidative stress. Additionally, they found an upregulation of the antioxidant peroxiredoxin 5 (PRDX5) in degenerative rotator cuff tendons. Interestingly, PRDX5 in vitro has been shown to decrease apoptosis and increase collagen synthesis. Heat shock proteins are other cell-protective molecules associated with apoptosis in rotator cuff tendinopathy.19
The mechanism whereby oxidative and other stress factors induce tenocyte apoptosis has not been fully established. However, the protein-activating enzyme c-Jun N-terminal protein kinase (JNK) has been implicated. JNK has been shown to activate transcription factors in the apoptotic pathway and to be linked with matrix metalloprotease-1 (MMP-1) in torn human supraspinatus tendons.20 Data suggest that stress-induced JNK may activate MMP-1, which in turn may be responsible for apoptotic tissue degeneration. These molecules may become potential therapeutic targets. Figure 1-1 summarizes the degenerative factors discussed in this section.
BIOLOGIC PROCESS OF ROTATOR CUFF HEALING
Anatomy of the Tendon-Bone Insertion and Rotator Cuff Healing
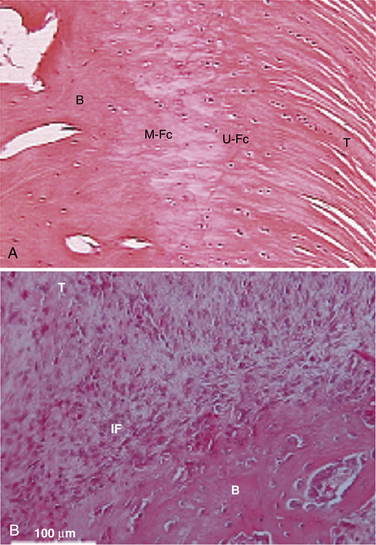
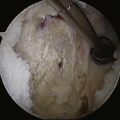
Traditionally, we have evaluated rotator cuff healing by investigating tendon-bone healing. The normal anatomy of the tendon-bone interface consists of a four-zone transition, including tendon, fibrocartilage, calcified fibrocartilage, and bone.21,22 The histiologic reconstitution of this bone-tendon interface in rotator cuff repairs has been shown to occur by reactive scar formation, which fails to recreate the native interface (Fig. 1-2). Preservation of the native transitional zone is clinically important to mitigate changes in mechanical properties between tendon and bone and to protect our repairs, because the mechanical properties of fibrous scar are less robust.23,24 Thus, creating a healing environment for scarless healing may be the next biologic hurdle to improve repair strength and decrease failure rate.
It has been postulated that the influx of macrophages and subsequent cytokine production leads to formation of the fibrous scar tissue at the tendon-bone insertion site. This hypothesis is derived from the study of fetal wounds in which tissue regeneration occurs, rather than scar formation. Through the study of knockout mice, it has been shown that healing rates are not affected by the absence of macrophages and neutrophils, suggesting that the initial inflammatory response may not be necessary to healing.25 Hays and colleagues26 used an anterior cruciate ligament (ACL) reconstruction model in macrophage-depleted rats and showed improved biomechanical and morphologic healing properties at the tendon-bone interface, likely as a result of the lack of cytokine-producing macrophages.
Origins of the Rotator Cuff Healing Response
Uhthoff and associates27 have studied which tissues are responsible for rotator cuff healing in a rabbit model 2 weeks after an experimental rotator cuff repair. They found no cellular or vascular healing at the tendon stump, but did find increased cellularity and vascularity in the cancellous bone and bursa. They concluded that it is likely better to avoid significant débridement of the tendon stump for reparative tissue volume and to attempt to preserve subacromial bursa.
Hirose and coworkers28 have researched rotator cuff healing in rabbits and found that reparative cells are derived from the bursal-sided epitenon, without evidence of the continuity of reparative tissue to the subacromial bursa. This is consistent with clinical data that showed comparable results in arthroscopic rotator cuff repair with bursectomy and in open surgery in which the bursa is spared.29 Lo and associates30 found upregulation of collagen and proteoglycans involved with healing at the bursa and rotator cuff tear margins in patients undergoing rotator cuff repairs and concluded that both the tendon and bursa contribute to healing.
Biologic Factors in Rotator Cuff Healing
Inflammatory Response.
The inflammatory response is the first phase of rotator cuff healing. Within a few days of cuff repair, proinflammatory neutrophils and recruited phagocytic macrophages are seen at the tendon-bone interface. Within 7 to 10 days, resident macrophages and lymphocytes are present in a proregenerative response.21,31 Cytokines are the mediators of early healing and are released and stimulated by these cells.
Cytokines.
Cytokines are molecules that modulate and influence cell recruitment, proliferation, differentiation, and matrix synthesis. Cytokines involved in bone and tendon healing include TGF-β, interleukins (ILs), bone morphogenic proteins (BMPs), insulin-like growth factors (IGFs), vascular endothelial growth factors (VEGFs), and platelet-derived growth factors (PDGFs). The interplay of these factors in tendon healing is complicated. Their expression in rotator cuff healing seems to peak from about 1 to 2 weeks, and can be upregulated for weeks before returning to baseline by 16 weeks.21
Cytokines have been shown to be an integral part of rotator cuff healing; transgenic knockout mice studies have demonstrated that IL-4 and IL-6 are required modulators of the healing response.32 The cytokine PDGF-β has also been shown to augment healing in a rat rotator cuff repair model through restoration of the normal crimp pattern and collagen bundle alignment that were not seen in nonaugmented repairs.33
Bone Morphogenic Proteins
Special categories of cytokines are tissue inductive molecules, such as BMPs, now ubiquitous in the orthopedic literature. These molecules, particularly BMPs 2 through 7, have been shown to be osteoinductive. Because bone-tendon healing depends on bone ingrowth, it is reasonable that the addition of BMP may affect healing. Rodeo and colleagues34 looked at this possibility, in a sheep model, by supplementing rotator cuff repairs with BMP-2 through BMP-7, along with other cytokines. They demonstrated increased reparative tissue in the augmented repairs with increased failure loads. However, when the reparative tissue was normalized to tissue volume, failure loads were identical, which implies that the supplemental healing was quantitative and not qualitative.
Other BMPs, such as BMP-12 and BMP-14 molecules, are more specific to tendon insertion sites, and induce embryologic tendon and fibrocartilage formation. Using a sheep model, with rotator cuff repairs augmented with recombinant human BMP-12 in a collagen or hyaluronan sponge, repairs were two to three times stronger in load to failure and stiffness at 8 weeks. Histologic examination yielded more advanced healing in the BMP-12 group, although it was still qualitatively inferior to the native tendon-bone interface. These data suggest that BMPs may have the potential to supplement repairs through accelerated healing and rehabilitation.35 In knockout mice studies, BMP-14 has been shown to be essential in the modulation of tendon healing.36 Additionally, in human full-thickness rotator cuff tears, BMP-14 mRNA was found in high concentrations at the bursal side of the torn edge of the tendon in a similar regional distribution as type I collagen, implying a roll in the reparative process.37
Matrix Metalloproteases.
MMPs are catabolic enzymes that appear to be involved in apoptosis and the remodeling phase of rotator cuff healing. Tissue inhibitors of metalloproteinases (TIMPs) are endogenous molecules know to inhibit MMPs. Choi and associates38 have explored the spontaneous healing of full-thickness rotator cuff tears in rabbits. Their data demonstrated MMP-2 expression and activation at both edges of the torn tendon during healing, with reparative tissue encroaching from the bursal side. TIMP-1 and -2 were also expressed at the cut tendon edges, as well as in the reparative tissue.
Collagen and Extracellular Matrix Molecules.
Collagen and matrix proteins are the structural components of tendon healing. Tendons consist primarily of collagen, specifically tendons, which are 95% type I collagen. Other collagen in tendons include types II, V, and VI. Proteoglycans, negatively charged macromolecules, and other matrix molecules make up the other components of tendons that contribute their unique mechanical properties. These molecules are regulated during the initial week to months of healing and are involved in rotator cuff healing and remodeling.21,39
In both rat and human rotator cuff tendon repair studies, increased collagen expression has been demonstrated; specifically, type I collagen was elevated in both species. Additionally, specific proteoglycans were suppressed and stimulated. Decorin was decreased, whereas aggrecan was increased across both species.30,39 These data demonstrate that regulation of extracellular matrix molecules is an integral part of rotator cuff healing.
Vascularity in Rotator Cuff Healing.
The role of vascularity is unclear in rotator cuff healing, not unlike the role of vascularity in tendinopathy. Capillary proliferation has been shown to occur in rat rotator cuff repair healing at 3 days and peak at 10 days.21 Fealy and coworkers40 have investigated vascularity after rotator cuff repair clinically using sonography. They found a robust vascular response in the repaired tendon, which predictably decreased over 6 months. The response was most prevalent at the peritendinous region and lowest at the bony attachment site. Vascular scores did not differ in repairs with or without defects.
Stem Cells and Transcription Factors.
As noted, rotator cuff repairs do not restore the native tendon-bone interface, which likely compromises repairs. Understanding tendon development and the role of stem cells may provide clues into how to obtain healing without reactive scar formation. Stem cells have the unique properties of self-renewal and pluripotency, Implantation of mesenchymal stem cells has been shown to accelerate early histologic remodeling of tendon-bone healing in a rat model.41 In a rabbit ACL model, stem cells have also been shown to increase allograft osteointegration with a more native tendon-bone interface and improve load to failure testing of this interface while decreasing stiffness.42
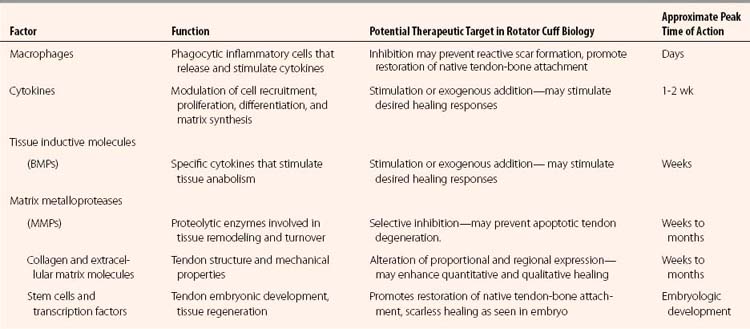
Until recently, little was known about the development and growth of tendons. We are elucidating factors essential in tendon development, which will hopefully lead to techniques to achieve scarless healing and recreate the native tendon attachment. The transcription factor scleraxis has been shown be expressed throughout tendon differentiation. It is upregulated by fibroblast growth factor 4 (FGF-4) and has been shown to regulate the transmembrane glycoprotein tenomodulin positively, which has been associated with tendon proliferation and maturation.43 Myostatin, or growth differentiation factor 8 (GDF-8), is another protein that has been shown to be essential for the development of normal tendon in knockout mice, as well as being able to stimulate type I collagen production.44 A summary of the biologic molecules discussed in this section is presented in Table 1-1.
Tendon-Tendon Healing.
There is a dearth of literature on tendon-tendon healing in the rotator cuff because most investigations of rotator cuff healing have focused on tendon-bone healing. This is unfortunate, because tendon-tendon healing becomes more relevant with increased understanding of tear geometry and the use of techniques such as margin convergence. Nobuhara and colleagues45 have reported on a series or rotator cuff repairs and found that 33% of those in the tendon-tendon group had exertional pain, compared with 18% of the tendon-bone group. Burkhart and associates’ clinical review of cases46 found no difference between margin convergence repairs and tendon-bone repairs when the geometry of the tear was appropriately considered to recreate native anatomy. Hamada and colleagues47 quantified labeled mRNA of alpha 1 type 1 procollagen as a marker of newly synthesized type I collagen and found that tendons likely have an intrinsic ability to heal. These studies suggest that there is likely tendon-tendon healing potential, but it is probably not as robust as in tendon-bone healing.
Mechanical Factors in Rotator Cuff Healing
Double-Row Repair
Recent studies have demonstrated conflicting data on whether or not double-row repairs are more biomechanically stable than single-row repairs. However, these studies do not account for the potential biologic healing benefits of restoring the native anatomic footprint and increasing the contact area and force over this area. A recent study has attempted to compare biologic healing of double-row and single-row repairs in a rabbit model.48 The results demonstrated increased load to failure in the double row and histologic evidence of denser tendon-bone healing in the double-row group at 8 weeks.
To date, no studies have demonstrated a significant difference in clinical outcome between double- and single-row repairs, despite some evidence of increased repair integrity in double-row repairs. Park and associates49 have reported no clinical benefit of double-row repairs in aggregate; however, when their results were stratified by tear size, the clinical outcomes were improved for larger tears in the double-row repair group. Further investigation is needed to definitively answer whether double row-repairs improve clinical results and under what circumstances this technique should be applied.
Mechanical Stimuli
Mechanical stimulation through loading can alter the properties of healing tendons and has been investigated in a rotator cuff healing model in rats.37 Through controlling postoperative activity, it was seen that immobilized shoulders had increased structural, compositional, and viscoelastic properties with respect to exercised shoulders. This is somewhat contrary to the dogma of the importance of early motion in rotator cuff healing, and it provides insight into an area not fully elucidated.
EXOGENOUS SOURCES THAT AFFECT ROTATOR CUFF BIOLOGY
Pharmacologic Agents
Nonsteroidal Anti-inflammatory Drugs
Recent studies have demonstrated that nonsteroidal anti-inflammatory drugs (NSAIDs), both nonselective and cyclooxygenase-2 (COX-2) inhibitors, inhibit tendon-bone healing in a rat rotator cuff repair model.50 The NSAID-treated tendons had a less robust and more disorganized reparative response, with decreased mechanical failure loads. These data have not been substantiated in humans, but are relevant because these medications are commonly prescribed for postoperative pain.
Nicotine and Tobacco
The ill effects of smoking have been ubiquitously documented throughout medicine. It is not surprising that nonsmokers undergoing rotator cuff repair have been shown to have statistically significant pain improvement and improved functional outcomes compared with smokers.51 In a rat supraspinatus rotator cuff tendon repair model, nicotine caused healing delays, with early decreased biomechanical properties and a prolonged increased inflammatory response.52 Perioperative smoking cessation is recommended for all those undergoing surgery, but particularly in tendon healing procedures.
Steroids
Surgeons and primary care physicians have effectively used local steroid injections as an adjuvant to help alleviate the pain of rotator cuff pathology. However, steroids have been shown to inhibit healing and degrade tissues. Meta-analysis has suggested that subacromial corticosteroid injections are generally safe and have a small, short-lived benefit likely superior to that of NSAIDs.53 Safety was studied in a rat model and no tendon damage was seen with a series of three weekly injections, but there was damage consistent with inflammation, collagen breakdown, and necrosis with five injections given every other week.54 These data suggest that corticosteroids need to be used judiciously, but can be used safely.
Anabolic steroids have become more prominently used by athletes and by those attempting to combat the effects of aging. These drugs have been shown to increase the incidence of tendon rupture through increased muscle contraction force and altered tendon morphology, which can stiffen tendons.55 However, there may be potential therapeutic benefits to these drugs that should not be overlooked. There is in vitro evidence that bioartificially engineered human supraspinatus tendons treated with nalondrone have more organized and complete tendon remodeling, as well as increased ultimate stress and strain.56 Additionally, anabolic steroids prescribed in a controlled setting may have a benefit in supplementing postoperative muscle rehabilitation through the prevention of muscle atrophy during rotator cuff immobilization or stimulation of muscle healing.
Topical Nitrates
Topical glyceryl trinitrate, used for years in the treatment of angina, has been investigated for its role in the treatment of rotator cuff tendinopathy. Its mechanism of action is through production of nitric oxide (NO), which is a short-lived free radical with several biologic functions. A recent double-blinded randomized controlled clinical trial has demonstrated significantly improved pain scores, motion, strength, and impingement symptoms in patients who used glyceryl trinitrate patches over placebo.57 Additionally, NO synthase is upregulated in tendon healing, and its inhibition has been shown to inhibit tendon healing.58 Although the mechanism of action is unclear, NO likely has a role in the healing process.
Insulin
It has been shown that insulin-dependent diabetics undergoing rotator cuff repairs obtain clinical improvement, but not as much as seen in controls. They also have higher complication and infection risk.59 These findings may be more reflective of the disease process of diabetes mellitus rather than the use of exogenous insulin, but are important to consider when treating diabetic patients. A summary of these pharmacologic factors is presented in Table 1-2.
TABLE 1-2 Pharmacologic Agents that May Affect Rotator Cuff Healing and Biology
| Pharmacologic Agent | Affect on Rotator Cuff Healing | Evidence |
|---|---|---|
| NSAIDs (nonselective and COX-2 inhibitors) | Inhibits tendon-bone healing | Rat rotator cuff model, not substantiated in humans |
| Nicotine, tobacco | Decreased functional outcomes, pain scores | Clinical evidence in humans |
| Delayed healing, decreased biomechanical properties | Rat rotator cuff model | |
| Corticosteroids | No significant adverse effects on healing with judicious use | Rat rotator cuff models, clinical observation |
| Clinical efficacy in treatment likely relatively small | Clinical trials in humans | |
| Anabolic steroids | Evidence of increased incidence of tendon rupture caused by morphologic changes and increased muscular force | Clinical observation |
| May enhance remodeling in healing | In vitro tendons | |
| May prevent muscle atrophy in rehabilitation and enhance muscle healing | Theoretical | |
| Topical nitrates (glyceryl trinatrate patch) | Improved functional and clinical outcomes with symptoms of tendinopathy | Double-blinded randomized controlled trial in humans |
| Insulin | Increased complication and infectious risk in patients with insulin-dependent diabetes mellitus, attenuated clinical improvement | Clinical review |
Other Exogenous Sources
Extracellular Matrix Patches
In spite of the fact that these commercially available scaffolds have a similar biochemical composition as tendon, they have been shown to have inferior biomechanical properties.60 However, in a recent cadaveric study, Barber and coworkers61 have demonstrated that supraspinatus rotator cuff repairs augmented with human dermal allograft have increased failure strengths.
Animal studies initially showed promising results with porcine small intestine submucosa. However, recent clinical trials with porcine small intestine submucosal scaffolds have demonstrated, at best, equivocal clinical results when compared with nonaugmented repairs. Additionally, early postoperative reactions required additional surgical treatment—in one series, 4 out of 10 patients and, in another, 4 out of 25 patients.62,63 Based on many recent studies, it is not recommended to augment rotator cuff tears with porcine small intestine submucosa.64
Better results have been seen with acellular dermal matrices. The clinical efficacy has not been definitively established with respect to nonaugmented repairs; however, they have been shown to be safe.65 Recently, Ide and colleagues66 evaluated the efficacy of human acellular dermal matrix graft in a rat rotator cuff tendon model. They found that augmented reconstructions had a more histologically and biomechanically superior result than defects without augmentation. Further study is needed to assess the efficacy of these products more clearly.
Energy Stimulation
Low-intensity pulsed ultrasound (LIPUS) in a partial patellectomy rabbit model has been shown to augment histologic and mechanical tendon-bone healing, with associated increased VEGF expression.67 In a similar model, extracorporeal shock wave therapy (ECSWT) has also been shown to improve tendon-bone healing, with increased osteogenesis and regeneration of the fibrocartilage zone.68 Further investigation of these modalities is needed to determine their efficacy in the clinical setting.
Growth Factor Aggregates
During rotator cuff healing, there is a complex temporal release of multiple factors. Enhancement of healing is not as simple as placing growth factors at the reparative site; it is dependent on dose, timing, carrier, and environmental interactions. This was experimentally demonstrated with an exogenous fibrin clot placed in a rat rotator cuff defect model and evaluated at 3, 6, and 12 weeks. No effect was seen with the addition of the fibrin clot except at 3 weeks, when the supplemented tendons demonstrated decreased biomechanical strength, implying inhibition of healing.69 With this information, we should be discerning when choosing to supplement our repairs with growth factor concentrates.
Platelet-rich plasma (PRP) is a specific aggregate that has a theoretical benefit of delivering growth factors to augment rotator cuff repairs biologically. Autologous blood is centrifuged to create PRP and then mixed with thrombin or thrombin-like material to create a workable matrix that can be sutured into a repair site. Commercially available systems are available that use proprietary methods to avoid immediate cytokine release. No studies have demonstrated the clinical efficacy of PRP in the augmentation of tendon repairs, but studies are underway to determine this. One recent study has shown PRP to be clinically safe.70
Other Exogenous Factors
Currently, there is a significant amount of development and investigation into exogenous interventions that may improve the biologic healing of the rotator cuff. There is evidence that hyaluronan can selectively downregulate mRNA for type III collagen without affecting type I collagen, which can lead to decreased adhesions and scar formation.71 Our group is currently investigating the role of pulsed radiofrequency and butyric acid–impregnated sutures. These developments must all be objectively reviewed before they are clinically implemented.
ROTATOR CUFF BIOLOGY IN THE CLINICAL SETTING
Timing of Rotator Cuff Repair
Tendon Quality
Tendon changes have been demonstrated in chronic rotator cuff tears. In a rat model, Galatz and associates72 have shown inferior viscoelastic tendon properties and persistent bone loss in repairs delayed 3 weeks. In human full-thickness rotator cuff tears persisting for more than 4 months, Hamada and coworkers47 have shown decreased type I collagen synthesis at the tendon tear site.
Tendon properties have also been shown to degrade more in larger tears, relative to smaller tears. Mathews and colleagues73 have compared rotator cuff tissue taken at the time of repair, and stratified their results to tear size. They found histologic differences more conducive to biologic healing in smaller rotator cuff tears, and concluded that tears should be repaired earlier than later. This group also found lower cellular activity in torn tendons, most pronounced at the edge of the tear and in larger tears.74
Muscle Atrophy
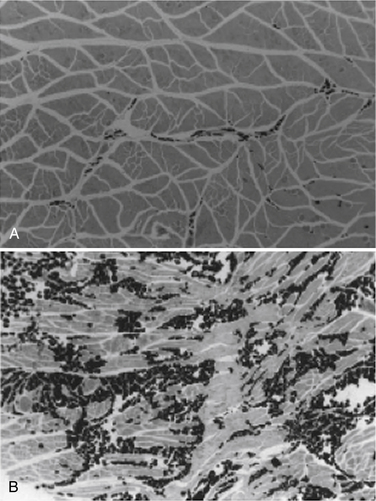
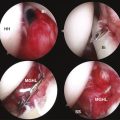
With complete tendon detachment, skeletal muscle is altered, with the development of atrophy and fatty muscular infiltration (Fig. 1-3). These muscle changes are clinically relevant because the extent of the changes has been shown to affect the success of repair and the clinical outcome.75 These muscle changes are likely irreversible and the degree of these changes appears to depend on the chronicity of the tear and its size.76
Gerber and associates,76 in a sheep model, have demonstrated that muscle changes are proportional to the amount of musculotendinous retraction. Additionally, they did not observe changes in muscle fiber composition nor muscle fiber degeneration. Their results indicate that successful repair may partially reverse muscular atrophy but not fatty infiltration. In the clinical setting, it was also shown that successful repair did not reverse preoperative muscular atrophy, and increased fatty muscle infiltration after repair was noted. In spite of this, good clinical results were obtained.77 Rubino and coworkers,78 in a rabbit model, demonstrated that muscle changes were seen at 6 weeks after muscle detachment. These changes also did not prevent successful repair, but they also were not reversible. Successful repair did prevent progression of these muscular changes.
Even though muscle changes are irreversible, Burkhart and colleagues,79 in a clinical review, demonstrated good clinical results, even with the presence of fatty changes. They found that patients with extensive (>50%) fatty changes undergoing rotator cuff repairs still had significant functional and clinical improvement, but much less clinical improvement was seen in patients with more than 75% fatty changes.
Results of Delayed Repairs
These studies suggest that rotator cuffs should be repaired earlier to prevent muscle and tendon changes. Additionally, over time, tear size increases and the ability to obtain successful repairs in larger tears diminishes.3 The success of the repair of chronic rotator cuff tears has been evaluated in a sheep model.80 Tears repaired immediately and at 6,and 18 weeks were reviewed. It was found that tendons repaired at 6 weeks had a rapid recovery of muscle function and tendon elasticity when compared with a more delayed repair at 18 weeks. The authors concluded that there is likely a point of no return in rotator cuff injury in which the muscle-tendon unit elasticity does not return to its native state.
Clinically, Bassett and Cofield reviewed a retrospective series and have reported that tears repaired within the first 3 weeks of an acute injury have greater return of motion than those repaired from 3 to 6 weeks and those repaired from 6 to 12 weeks.81 However, in studies by Romeo and associates82 and Burkhart and coworkers,46 the time from injury to repair was not found to be a significant factor. This question has not been definitively answered clinically. Data and a priori knowledge suggest that the earlier tears are repaired, the better the chance for a successful outcome, but it is not known at what point this becomes clinically relevant.
Healing Time
Gerber and colleagues,22 in a sheep infraspinatus tendon repair model, evaluated repair strength at various time points. At 6 weeks, failure strength was 30% of a normal intact infraspinatus tendon, at 3 months it was 52% of normal, and at 6 months it was 81% of normal. It was noted that regardless of repair technique, unprotected repairs fail at an exceedingly high rate. It was suggested that in the clinical setting, postoperative protection from tension overload with a device such as an abduction splint is likely important to attain successful healing of massive cuff tears.
Several other studies have considered healing rates and repair strength in other animal models (Table 1-3).28,48,83–85 It is difficult to interpret these data accurately, because healing rates are not equivalent in animals and humans. However, along with empirical clinical experiences, they provide rough guidelines for designing patients’ rehabilitation protocols.
TABLE 1-3 Animal Model Data for Time and Strength of Rotator Cuff Healing
| Model | Extent of Healing (%) | Time |
|---|---|---|
| Sheep22 : failure strength (% of normal) |
Clinical Importance of Rotator Cuff Healing
The question of whether it is clinically relevant if rotator cuff repairs heal is a humbling yet pertinent question. The literature has demonstrated that rotator cuffs do not always heal completely after surgical repair. Follow-up imaging has demonstrated a wide range of residual tears after repair, with an incidence from 13% to more than 94%.68,78,86,88 Most data suggest age and size of the tear as negative prognostic factors for complete healing.
In the classic study by Harryman and associates,86 patients who underwent rotator cuff repairs demonstrated improved functional outcomes dependent on repair integrity, with the size of the recurrent defect correlating to functional loss. They also found that older patients with larger tears had a greater prevalence of recurrent defects. Additionally, tears involving only the supraspinatus tendon had an 80% chance of maintaining an intact repair, whereas more than 50% of repairs involving more than the supraspinatus demonstrated a recurrent defect.
Boileau and coworkers89 have demonstrated similar results in chronic full-thickness tears of the supraspinatus repaired arthroscopically. Fully healed tears had increased functional results with improved strength. Factors that negatively affected healing included age and sagittal plane extension of the tear. Functional gains in strength were solely dependent on the integrity of the repair.
Even though these studies demonstrate improved functional outcomes with intact rotator cuff repairs, the integrity of the repair does not necessarily compromise patient pain relief and satisfaction. Jost and colleagues90 have demonstrated decreased pain and improved strength in a series of their open repairs that had reruputured. This led them to advocate attempted repair in all cuff tears, despite concern for rerupture. Galatz and associates88 similarly reported on their experience with arthroscopic repair of large and massive tears and found recurrent tears in 17 of 18 patients. Despite this, these patients experienced improvement in function and pain relief. However, the results deteriorated significantly between the 12- and 24-month follow-ups. Why patients clinically improve, even if their repair has failed, is unclear—it may be the result of débridement and decompression, addressing biceps pathology, or the benefit of a partial repair.91
SUMMARY
There are many unanswered questions in the complex biology of rotator cuff healing. However, there is a tremendous opportunity for investigation and improvement in how rotator cuff tears are treated. Ultimately, it is essential to be biologically aware when approaching the treatment and repair of rotator cuff tears.
1. Tempelhof S, Rupp S, Seil R. Age-related prevalence of rotator cuff tears in asymptomatic shoulders. J Shoulder Elbow Surg. 1999;8:296-299.
2. Sher JS, Uribe JW, Posada A, et al. Abnormal findings on magnetic resonance images of asymptomatic shoulders. J Bone Joint Surg Am. 1995;77:10-15.
3. Yamaguchi K, Tetro AM, Blam O, et al Natural history of asymptomatic rotator cuff tears: a longitudinal analysis of asymptomatic tears detected sonographically. J Shoulder Elbow Surg, 10; 2001:199-203.
4. Yamaguchi K, Ditsios K, Middleton W, et al. The demographic and morphological features of rotator cuff a comparison of asymptomatic and symptomatic shoulders. J Bone Joint Surg. 2006;88:1699-1704.
5. Hashimoto T, Nobuhara K, Hamada T Pathologic evidence of degeneration as a primary cause of rotator cuff tear. Clin Orthop Relat Res (415); 2003:111-120.
6. Kumagai J, Sarkar K, Uhthoff HK The collagen types in the attachment zone of rotator cuff tendons in the elderly: an immunohistochemical study. J Rheumatol, 21; 1994:2096-2100.
7. Premdas J, Tang JB, Warner JP, et al. The presence of smooth muscle actin in fibroblasts in the torn human rotator cuff. J Orthop Res. 2001;19:221-228.
8. Soslowsky LJ, Thomopoulos S, Tun S, et al Overuse activity injures the supraspinatus tendon in an animal model: a histologic and biomechanical study. J Shoulder Elbow Surg, 9; 2000:79-84.
8A. Neer CSII, Poppen NK. Supraspinatus outlet. Orthop Trans. 1987;11:234.
8B. Bigliani LU, Morrison DS, April EW. The morphology of the acromion and its relationship to rotator cuff tears. Orthop Trans. 1986;10:228.
9. Shah NN, Bayliss NC, Malcolm A Shape of the acromion: Congenital or acquired—a macroscopic, radiographic, and microscopic study of acromion. J Shoulder and Elbow Surg, 10; 2001:309-316.
10. Hyvonen P, Lohi S, Jalovaara P Open acromioplasty does not prevent the progression of an impingement syndrome to a tear: nine-year follow up of 96 cases. J Bone Joint Surg Br, 80; 1998:813-816.
11. Ko JY, Huang CC, Chen WJ, et al Pathogenesis of partial tear of the rotator cuff: a clinical and pathologic study. J Shoulder Elbow Surg, 15; 2006:271-278.
12. Brooks CH, Revell WJ, Heatley FW. A quantitative histological study of the vascularity of the rotator cuff tendon. J Bone Joint Surg Br. 1992;74:151-153.
13. Rudzki JR, Adler RS, Warren RF, et al Contrast-enhanced ultrasound characterization of the vascularity of the rotator cuff tendon: age- and activity-related changes in the intact asymptomatic rotator cuff. J Shoulder Elbow Surg, 17(Suppl); 2008:96S-100S.
14. Biberthaler P, Wiedemann E, Nerlich A, et al. Microcirculation associated with degenerative rotator cuff lesions. In vivo assessment with orthogonal polarization spectral imaging during arthroscopy of the shoulder. J Bone Joint Surg Am. 2003;85:475-480.
15. Molloy TJ, Kemp MW, Wang Y, Murrell GA Microarray analysis of the tendinopathic rat supraspinatus tendon: glutamate signaling and its potential role in tendon degeneration. J Appl Physiol, 101; 2006:1702-1709.
16. Gotoh M, Hamada K, Yamakawa H, et al. Increased substance P in subacromial bursa and shoulder pain in rotator cuff diseases. J Orthop Res. 1998;16:618-621.
17. Soslowsky LJ, Thomopoulos S, Esmail A, et al Rotator cuff tendinosis in an animal model: role of extrinsic and overuse factors. Ann Biomed Eng, 30; 2002:1057-1063.
18. Yuan J, Murrell GA, Trickett A, et al. Overexpression of antioxidant enzyme peroxiredoxin 5 protects human tendon cells against apoptosis and loss of cellular function during oxidative stress. Biochim Biophys Acta.. 2004;23; 1693:37-45.
19. Millar NL, Wei AQ, Molloy TJ, et al Heat shock protein and apoptosis in supraspinatus tendinopathy. Clin Orthop Relat Res (466); 2008:1569-1576.
20. Gotoh M, Hamada K, Yamakawa H, et al. Interleukin-1-induced glenohumeral synovitis and shoulder pain in rotator cuff diseases. J Orthop Res. 2002;20:1365-1371.
21. Galatz LM, Sandell LJ, Rothermich SY, et al. Characteristics of the rat supraspinatus tendon during tendon-to-bone healing after acute injury. J Orthop Res. 2006;24:541-550.
22. Gerber C, Schneeberger AG, Perren SM, Nyffeler RW. Experimental rotator cuff repair. A preliminary study. J Bone Joint Surg Am. 1999;81:1281-1290.
23. Thomopoulos S, Williams GR, Soslowsky LJ Tendon to bone healing: differences in biomechanical, structural, and compositional properties due to a range of activity levels. J Biomech Eng, 125; 2003:106-113.
24. Kovacevic D, Rodeo SA. Biological augmentation of rotator cuff tendon repair. Clin Orthop Relat Res. 2008;466:622-633.
25. Martin P, D’Souza D, Martin J, et al. Wound healing in the PU.1 null mouse—tissue repair is not dependent on inflammatory cells. Curr Biol. 2003;13:1122-1128.
26. Hays PL, Kawamura S, Deng XH, et al. The role of macrophages in early healing of a tendon graft in a bone tunnel. J Bone Joint Surg Am. 2008;90:565-579.
27. Uhthoff HK, Sano H, Trudel G, et al. Early reactions after reimplantation of the tendon of supraspinatus into bone. J Bone Joint Surg Br. 2000;82:1072-1076.
28. Hirose K, Kondo S, Choi HR, et al. Spontaneous healing of a supraspinatus tendon tear in rabbits. Arch Orthop Trauma Surg. 2004;124:374-377.
29. Gartsman GM Arthroscopic rotator cuff repair. Clin Orthop Relat Res (390); 2001:95-106.
30. Lo IK, Boorman R, Marchuk L, et al. Matrix molecule mRNA levels in the bursa and rotator cuff of patients with full-thickness rotator cuff tears. Arthroscopy. 2005;21:645-651.
31. Kawamura S, Ying L, Kim HJ, et al. Macrophages accumulate in the early phase of tendon-bone healing. J Orthop Res. 2005;23:1425-1432.
32. Lin TW, Cardenas L, Glaser DL, Soslowsky LJ. Tendon healing in interleukin-4 and interleukin-6 knockout mice. J Biomech. 2006;39:61-69.
33. Uggen JC, Dines J, Uggen CW, et al. Tendon gene therapy modulates the local repair environment in the shoulder. J Am Osteopath Assoc. 2005;105:20-21.
34. Rodeo SA, Potter HG, Kawamura S, et al. Biologic augmentation of rotator cuff tendon-healing with use of a mixture of osteoinductive growth factors. J Bone Joint Surg Am. 2007;89:2485-2497.
35. Seeherman HJ, Archambault JM, Rodeo SA, et al. rhBMP-12 accelerates healing of rotator cuff repairs in a sheep model. J Bone Joint Surg Am. 2008;90:2206-2219.
36. Chhabra A, Tsou D, Clark RT, et al. GDF-5 deficiency in mice delays Achilles tendon healing. J Orthop Res. 2003;21:826-835.
37. Nakase T, Sugamoto K, Miyamoto T, et al Activation of cartilage-derived morphogenetic protein-1 in torn rotator cuff. Clin Orthop Relat Res (399); 2002:140-145.
38. Choi HR, Kondo S, Hirose K, et al. Expression and enzymatic activity of MMP-2 during healing process of the acute supraspinatus tendon tear in rabbits. J Orthop Res. 2002;20:927-933.
39. Thomopoulos S, Hattersley G, Rosen V, et al The localized expression of extracellular matrix components in healing tendon insertion sites: an in situ hybridization study. J Orthop Res, 20; 2002:454-463.
40. Fealy S, Adler RS, Drakos MC, et al. Patterns of vascular and anatomical response after rotator cuff repair. Am J Sports Med. 2006;34:120-127.
41. Ju YJ, Muneta T, Yoshimura H, et al. Synovial mesenchymal stem cells accelerate early remodeling of tendon-bone healing. Cell Tissue Res. 2008;332:469-478.
42. Soon MY, Hassan A, Hui JH, et al An analysis of soft tissue allograft anterior cruciate ligament reconstruction in a rabbit model: a short-term study of the use of mesenchymal stem cells to enhance tendon osteointegration. Am J Sports Med, 35; 2007:962-971.
43. Shukunami C, Takimoto A, Oro M, Hiraki Y. Scleraxis positively regulates the expression of tenomodulin, a differentiation marker of tenocytes. Dev Biol. 2006;298:234-247.
44. Mendias CL, Bakhurin KI, Faulkner JA. Tendons of myostatin-deficient mice are small, brittle, and hypocellular. Proc Natl Acad Sci U S A. 2008;105:388-393.
45. Nobuhara K, Hata Y, Komai M. Surgical procedure and results of repair of massive tears of the rotator cuff. Clin Orthop Rel Res. 1994;304:54-59.
46. Burkhart SS, Danaceau SM, Pearce CEJr Arthroscopic rotator cuff repair: analysis of results by tear size and by repair technique-margin convergence versus direct tendon-to-bone repair. Arthroscopy, 17; 2001:905-912.
47. Hamada K, Tomonaga A, Gotoh M, et al Intrinsic healing capacity and tearing process of torn supraspinatus tendons: in situ hybridization study of alpha 1 (I) procollagen mRNA. J Orthop Res, 15; 1997:24-32.
48. Ozbaydar M, Elhassan B, Esenyel C, et al A comparison of single-versus double-row suture anchor techniques in a simulated repair of the rotator cuff: an experimental study in rabbits. J Bone Joint Surg Br, 90; 2008:1386-1391.
49. Park JY, Lhee SH, Choi JH, et al. Comparison of the clinical outcomes of single- and double-row repairs in rotator cuff tears. Am J Sports Med. 2008;36:1310-1317.
50. Cohen DB, Kawamura S, Ehteshami JR, Rodeo SA. Indomethacin and celecoxib impair rotator cuff tendon-to-bone healing. Am J Sports Med. 2006;34:362-369.
51. Mallon WJ, Misamore G, Snead DS, Denton P. The impact of preoperative smoking habits on the results of rotator cuff repair. J Shoulder Elbow Surg. 2004;13:129-132.
52. Galatz LM, Silva MJ, Rothermich SY, et al. Nicotine delays tendon-to-bone healing in a rat shoulder model. J Bone Joint Surg Am. 2006;88:2027-2034.
53. Arroll B, Goodyear-Smith F Corticosteroid injections for painful shoulder: a meta-analysis. Br J Gen Pract, 55; 2005:224-228.
54. Tillander B, Franzén LE, Karlsson MH, Norlin R Effect of steroid injections on the rotator cuff: an experimental study in rats. J Shoulder Elbow Surg, 8; 1999:271-274.
55. Inhofe PD, Grana WA, Egle D, et al. The effects of anabolic steroids on rat tendon. An ultrastructural, biomechanical, and biochemical analysis. Am J Sports Med. 1995;23:227-232.
56. Triantafillopoulos IK, Banes AJ, Bowman KFJr, et al. Nandrolone decanoate and load increase remodeling and strength in human supraspinatus bioartificial tendons. Am J Sports Med. 2004;32:934-943.
57. Paoloni JA, Appleyard RC, Nelson J, Murrell GA Topical glyceryl trinitrate application in the treatment of chronic supraspinatus tendinopathy: a randomized, double-blinded, placebo-controlled clinical trial. Am J Sports Med, 33; 2005:806-813.
58. Murrell GA, Szabo C, Hannafin JA, et al. Modulation of tendon healing by nitric oxide. Inflamm Res. 1997;46:19-27.
59. Chen AL, Shapiro JA, Ahn AK, et al. Rotator cuff repair in patients with type I diabetes mellitus. J Shoulder Elbow Surg. 2003;12:416-421.
60. Derwin KA, Baker AR, Spragg RK, et al. Commercial extracellular matrix scaffolds for rotator cuff tendon repair. Biomechanical, biochemical, and cellular properties. J Bone Joint Surg. 2006;88:2665-2672.
61. Barber FA, Dockery WD. Long-term absorption of poly-l-lactic acid interference screws. Arthroscopy. 2006;22:820-826.
62. Malcarney HL, Bonar F, Murrell GA Early inflammatory reaction after rotator cuff repair with a porcine small intestine submucosal implant: a report of 4 cases. Am J Sports Med, 33; 2005:907-911.
63. Walton JR, Bowman NK, Khatib Y, et al Restore orthobiologic implant: not recommended for augmentation of rotator cuff repairs. J Bone Joint Surg Am, 89; 2007:786-791.
64. Iannotti JP, Codsi MJ, Kwon YW, et al. Porcine small intestine submucosa augmentation of surgical repair of chronic two-tendon rotator cuff tears. A randomized, controlled trial. J Bone Joint Surg Am. 2006;88:1238-1244.
65. Badhe SP, Lawrence TM, Smith FD, Lunn PG. An assessment of porcine dermal xenograft as an augmentation graft in the treatment of extensive rotator cuff tears. J Shoulder Elbow Surg. 2008;17(Suppl):35S-39S.
66. Ide J, Kikukawa K, Hirose J, et al. Reconstruction of large rotator-cuff tears with acellular dermal matrix grafts in rats. J Shoulder Elbow Surg. 2009;18:288-295.
67. Lu H, Qin L, Cheung W, et al. Low-intensity pulsed ultrasound accelerated bone-tendon junction healing through regulation of vascular endothelial growth factor expression and cartilage formation. Ultrasound Med Biol. 2008;34:1248-1260.
68. Wang L, Qin L, Lu HB, et al. Extracorporeal shock wave therapy in treatment of delayed bone-tendon healing. Am J Sports Med. 2008;36:340-347.
69. Thomopoulos S, Soslowsky LJ, Flanagan CL, et al. The effect of fibrin clot on healing rat supraspinatus tendon defects. J Shoulder Elbow Surg. 2002;11:239-247.
70. Randelli PS, Arrigoni P, Cabitza P, et al. Autologous platelet rich plasma for arthroscopic rotator cuff repair. A pilot study. Disabil Rehabil. 2008;30:1584-1589.
71. Yamada T, Gotoh M, Nakama K, et al Effects of hyaluronan on cell proliferation and mRNA expression of procollagens alpha 1 (I) and alpha 1 (III) in tendon-derived fibroblasts from patients with rotator cuff disease: an in vitro study. Am J Sports Med, 35; 2007:1870-1876.
72. Galatz LM, Rothermich SY, Zaegel M, et al. Delayed repair of tendon to bone injuries leads to decreased biomechanical properties and bone loss. J Orthop Res. 2005;23:1441-1447.
73. Matthews T, Hand G, Rees J, et al Pathology of the torn rotator cuff tendon: reduction in potential for repair as tear size increases. J Bone Joint Surg Br, 88; 2006:489-495.
74. Matthews T, Smith R, Peach, Urban, Carr In vivo measurement of tissue metabolism in tendons of the rotator cuff: Implications For Surgical Management. J Bone Joint Surg Br, 89; 2007:633-638.
75. Goutallier D, Postel JM, Gleyze P, et al. Influence of cuff muscle fatty degeneration on anatomic and functional outcomes after simple suture of full-thickness tears. J Shoulder Elbow Surg. 2003;12:550-554.
76. Gerber C, Meyer DC, Schneeberger AG, et al Effect of tendon release and delayed repair on the structure of the muscles of the rotator cuff: an experimental study in sheep. J Bone Joint Surg Am, 86; 2004:1973-1982.
77. Fuchs B, Gilbart MK, Hodler J, Gerber C. Clinical and structural results of open repair of an isolated one-tendon tear of the rotator cuff. J Bone Joint Surg Am. 2006;88:309-316.
78. Rubino LJ, Sprott DC, Stills HFJr, Crosby LA. Fatty infiltration does not progress after rotator cuff repair in a rabbit model. Arthroscopy. 2008;24:936-940.
79. Burkhart SS, Barth JRH, Richards DP, et al. Arthroscopic repair of massive rotator cuff tears with stage 3 and 4 fatty degeneration. Arthroscopy. 2007;23:347-354.
80. Coleman SH, Fealy S, Ehteshami JR, et al. Chronic rotator cuff injury and repair model in sheep. J Bone Joint Surg Am. 2003;85:2391-2402.
81. Bassett RW, Cofield RH Acute tears of the rotator cuff. The timing of surgical repair. Clin Orthop Relat Res. (175); 1983:18-24.
82. Romeo AA, Hang DW, Bach BRJr, Shott S Repair of full-thickness rotator cuff tears. Gender, age, and other factors affecting outcome. Clin Orthop Relat Res. (367); 1999:243-255.
83. St. Pierre P, Olson EJ, Elliott JJ, et al. Tendon-healing to cortical bone compared with healing to a cancellous trough. A biomechanical and histological evaluation in goats. J Bone Joint Surg Am. 1995;77:1858-1866.
84. Miyahara H, Takagishi K, Arita C, et al A morphologic and biomechanical study on the healing of the repaired rotator cuff insertion in dogs: a preliminary report. Post M, Morrey BF, Hawkins RJ, editors. Surgery of the Shoulder. St. Louis: Mosby; 1990:224-227.
85. Uhtoff HK, Seki M, Backman DS, et al Tensile strength of the supraspinatus after reimplantation into bony trough: an experimental study in rabbits. J Shoulder Elbow Surg, 11; 2002:504-509.
86. Harryman DT, Mack LA, Wang KY, et al. Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73:982-989.
87. Gerber C, Fuchs B, Hodler J. The results of repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2000;82:505-515.
88. Galatz LM, Ball CM, Teffey SA, et al. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg. 2004;86:219-224.
89. Boileau P, Brassart N, Watkinson DJ, et al Arthroscopic repair of full-thickness tears of the supraspinatus: Does the tendon really heal. J Bone Joint Surg, 87; 2005:1229-1240.
90. Jost B, Pfirrmann CW, Gerber C. Clinical outcome after structural failure of rotator cuff repairs. J Bone Joint Surg Am. 2000;82:304-314.
91. Burkhart SS Partial repair of massive rotator cuff tears: the evolution of a concept. Orthop Clin North Am, 28; 1997:125-132.