CHAPTER 34 Biologic Glenoid Resurfacing
Biologic resurfacing of the glenoid has been performed with various materials, including fascia lata autograft, fascia lata allograft, meniscal allograft, and Achilles tendon allograft.1 The main advantage of biologic glenoid resurfacing lies in providing a new articulating surface for the glenoid while avoiding potential late-term complications associated with polyethylene wear and failure. Consequently, consideration of biologic glenoid resurfacing is most reasonable in younger patients. Biologic glenoid resurfacing also allows resurfacing of localized glenoid articular cartilage lesions that may result from trauma in young patients. In our practice, we almost always combine biologic glenoid resurfacing with humeral head arthroplasty (hemiarthroplasty or surface replacement) because our patients with glenoid articular cartilage disease almost always have associated humeral head articular cartilage disease.
INDICATIONS AND CONTRAINDICATIONS
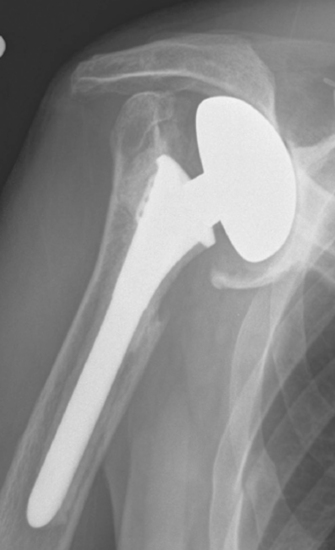
Indications in our practice for biologic glenoid resurfacing are limited to a young patient (<40 years old) with glenoid disease; in an older patient, the use of a prosthetic glenoid component would be indicated. We have performed biologic resurfacing most commonly in patients with a generalized arthritic condition (i.e., glenohumeral chondrolysis; Fig. 34-1) but have also used it for localized traumatic glenoid articular cartilage lesions. One situation in which we opt for a prosthetic glenoid component in a young patient is a diagnosis of juvenile rheumatoid arthritis.
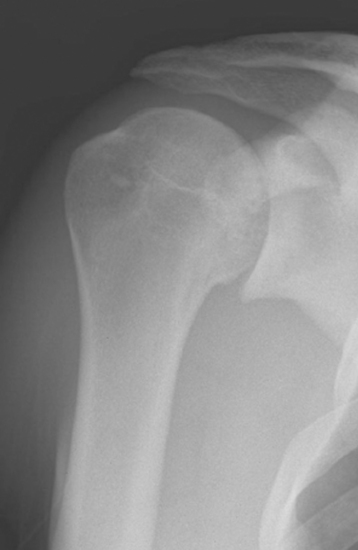
Figure 34-1 Radiograph of a young patient with glenohumeral joint chondrolysis after shoulder arthroscopy.
A rare situation in which we use biologic glenoid resurfacing is a revision case with a competent rotator cuff in which hemiarthroplasty has resulted in symptomatic glenoid erosion and insufficient bone exists to permit prosthetic resurfacing (Fig. 34-2). In these scenarios, biologic resurfacing represents an alternative to resection arthroplasty (see Chapter 35 for more on indications for revision shoulder arthroplasty). In most primary cases in older patients in whom unconstrained arthroplasty is indicated yet insufficient glenoid bone exists for prosthetic resurfacing, we generally perform isolated hemiarthroplasty because most of these patients obtain satisfactory pain relief and function (although these results are inferior to those obtained with total shoulder arthroplasty) without prosthetic or biologic glenoid resurfacing.
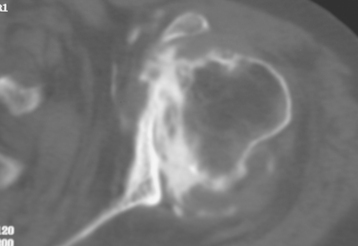
Contraindications to biologic glenoid resurfacing include the standard contraindications to unconstrained shoulder arthroplasty. Additionally, any situation in which the native glenoid is insufficient to allow anchorage of the biologic tissue is a contraindication to biologic resurfacing (Fig. 34-3).
TECHNIQUE FOR BIOLOGIC GLENOID RESURFACING
Autogenous Fascia Lata Harvest
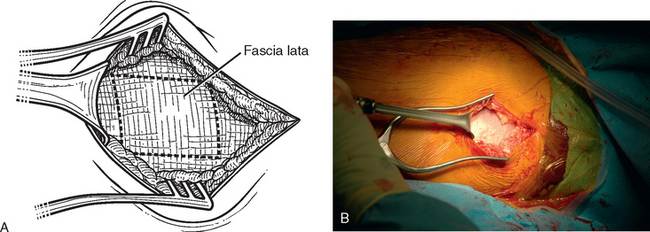
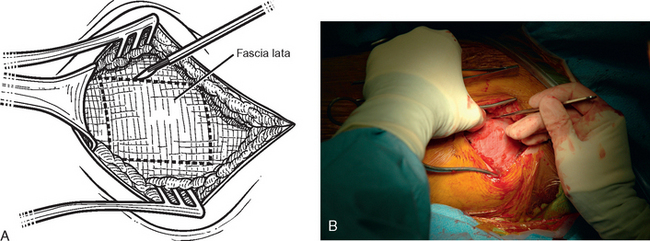
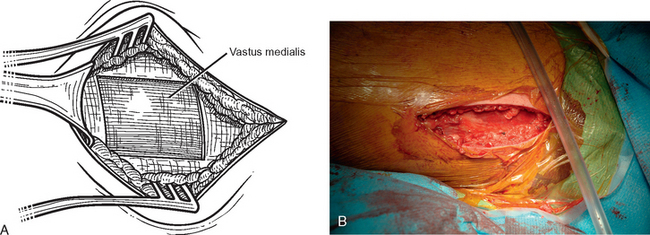
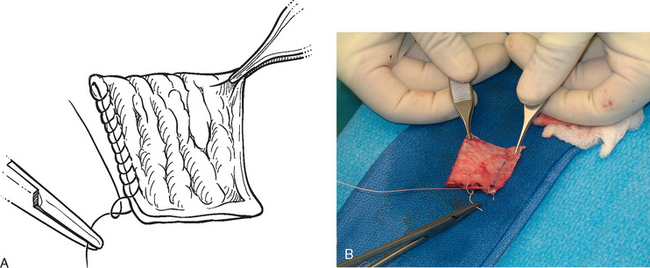
The operating room setup, anesthesia, patient positioning, skin preparation, surgical draping, and surgical approach are identical to that for other shoulder arthroplasties. Additionally, the contralateral lower extremity and hip region are prepared and draped (Fig. 34-4). Using the contralateral lower extremity permits an assistant to close the fascia lata harvest wound while the approach is made to the shoulder. An 8-cm incision is started 3 cm distal to the greater trochanter of the femur and extended distally along the longitudinal axis of the femur (Fig. 34-5). A needle tip electrocautery is used to obtain hemostasis and aids in dissection through the subcutaneous layer down to the fascia lata. The fascia lata is cleared of the overlying subcutaneous fat to complete the exposure (Fig. 34-6). A scalpel is used to harvest an 8-cm-long by 3-cm-wide segment of fascia lata (Fig. 34-7). The wound is irrigated; it is not necessary to close the residual defect in the fascia lata (Fig. 34-8). The subcutaneous tissue is closed with 2-0 absorbable braided suture in an interrupted technique. The skin is closed with 3-0 absorbable monofilament suture in a continuous running subcuticular technique. Steri-Strips and sterile dressings are applied. The fascia lata is folded to double its thickness. Braided 2-0 absorbable suture is used to suture the perimeter of the autograft (Fig. 34-9).
Implantation of the Fascia Lata Autograft
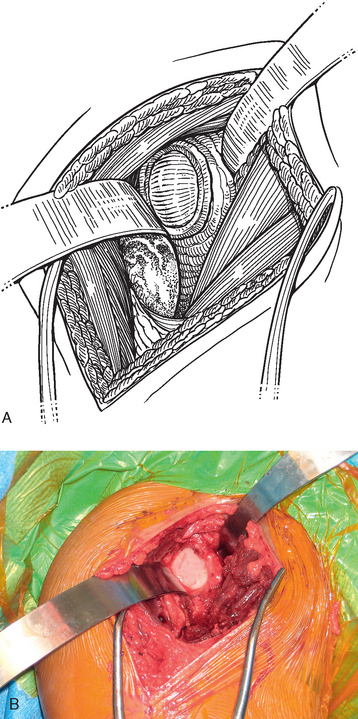
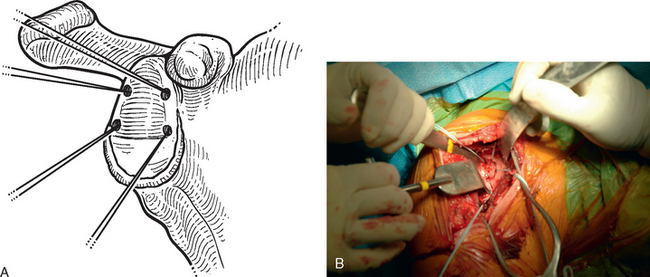
A standard deltopectoral approach is performed as described in Chapter 8. The subscapularis is treated as described in Chapter 9 if hemiarthroplasty is planned. If humeral head resurfacing is planned, the anterior humeral circumflex vessels are preserved as detailed in Chapter 33. The glenoid is exposed by releasing the inferior glenohumeral joint capsule from the glenoid neck (Fig. 34-10). The glenoid joint surface is prepared by removing any residual articular cartilage with a Cobb elevator or motorized burr, if necessary. Any nonconcentric wear is corrected with the burr or a reamer (see Chapter 12), if needed. Two options exist for graft fixation. If the glenoid labrum is intact circumferentially, the autograft can be sutured directly to the intact glenoid labrum. If the glenoid labrum is absent or diseased to the extent that graft fixation would be compromised, bioabsorbable suture anchor fixation is used to secure the graft (we use a 2.9-mm Bioraptor anchor, Smith Nephew, Inc., Andover, MA).
In patients with an intact glenoid labrum, no. 2 braided permanent sutures are placed through the labrum at the 12, 2, 4, 6, 8, and 10 o′clock positions (Fig. 34-11). These sutures are kept separate by tagging them with hemostats. The autograft is oriented so that the nonsutured side of the graft is superior. The glenoid side suture at each location is passed through the perimeter of the graft at each position (12, 2, 4, 6, 8, and 10 o′clock; Fig. 34-12). The graft is then passed down the sutures until it rests on the glenoid surface (Fig. 34-13). The sutures are tied sequentially to complete the resurfacing (Fig. 34-14). Alternatively, in patients with an insufficient labrum, a doubly loaded bioabsorbable suture anchor can be placed on the glenoid margin in each quadrant of the glenoid (a total of four anchors), and the same suturing process used (Fig. 34-15). When using anchor fixation, a 3.0-mm hole is predrilled at each location and the anchors placed via standard insertion technique.
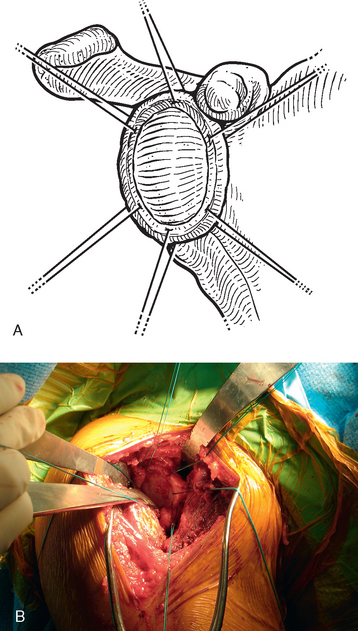
Figure 34-11 A and B, Sutures placed circumferentially around the labrum for use in fixation of the autogenous fascia lata.
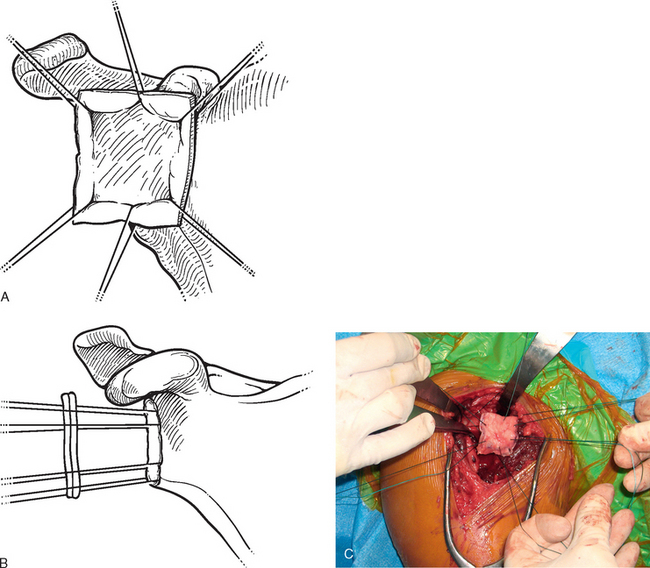
Figure 34-12 A to C, Sutures are passed through the autograft outside the wound to facilitate graft placement and fixation.
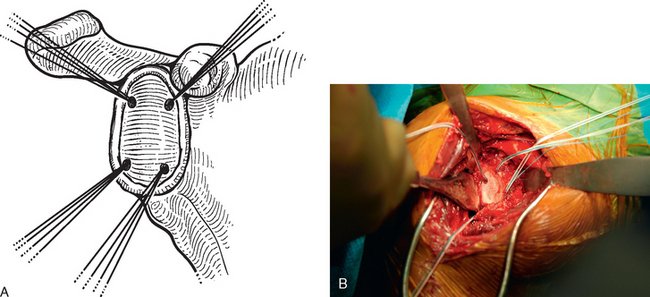
Figure 34-15 A and B, Suture anchor placement for biologic glenoid resurfacing in a patient with a deficient labrum.
We have also performed biologic glenoid resurfacing for incomplete defects of glenoid articular cartilage. In this circumstance, only the area of full-thickness cartilage loss is prepared with the Cobb elevator or motorized burr. The fascia lata autograft is prepared as previously described, except that it is trimmed to fit the glenoid articular cartilage defect (Fig. 34-16). In this circumstance it is often helpful to use suture anchors to fix the graft at the location immediately adjacent to the remaining intact glenoid articular cartilage (Fig. 34-17). Figure 34-18 shows the final construct after resurfacing of the superior half of the glenoid.