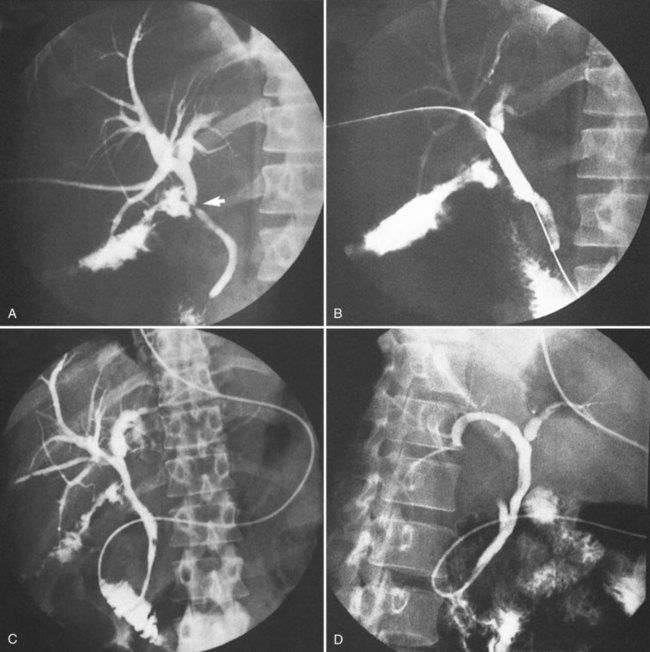
Chapter 42B Biliary fistulae
Internal Biliary Fistulae
Incidence and Etiology
Whether occurring as a consequence of calculous biliary tract disease, trauma, neoplasm, or congenital anomalies, internal biliary fistulae are uncommon. Estimates of incidence are crude, gleaned only from many small series, usually with fewer than 50 patients. If all types of internal biliary fistula are included, calculous biliary tract disease accounts for 90%; peptic ulcer disease, 6%; and neoplasm, trauma, parasitic infection, and congenital anomalies make up the remaining 4% (Piedad & Wels, 1972).
Overall, 1% to 3% of patients with cholesterol cholethiasis in Western countries develop biliary-enteric fistula, with a female/male ratio of 3 : 1. In 11,808 cases of nonmalignant biliary tract disease encountered at New York Hospital/Cornell Medical Center during the years 1932 through 1978, the incidence of biliary-enteric fistula was 0.9% with a male/female ratio of 2.3 : 1 (Glenn et al, 1981). A large series from Greece (Lygidakis, 1981) showed an incidence of 2%, and in Native Americans, the incidence is 3.2% (Zwemer et al, 1979). In Japan, where bilirubin stones and primary intraductal disease predominate, the incidence of fistula is between 13% and 18% with a slight male predominance (Urakami & Kishi, 1978). The type of fistula noted in this group of patients usually involves the ductal system rather than the gallbladder. The pathogenic sequence of events for calculous biliary tract disease has been well described by Glenn and Mannix (1957). It consists of pressure necrosis and erosion of part of the biliary tract wall into an adjacent structure to which it has become adherent in the course of repeated bouts of inflammation, often with distal biliary tract obstruction. The likelihood of the branches of the hepatobiliary tree to become inflamed and anatomic proximity to adjacent hollow viscera largely determine the relative incidence of the different types of spontaneous biliary-enteric fistulae secondary to calculous disease.
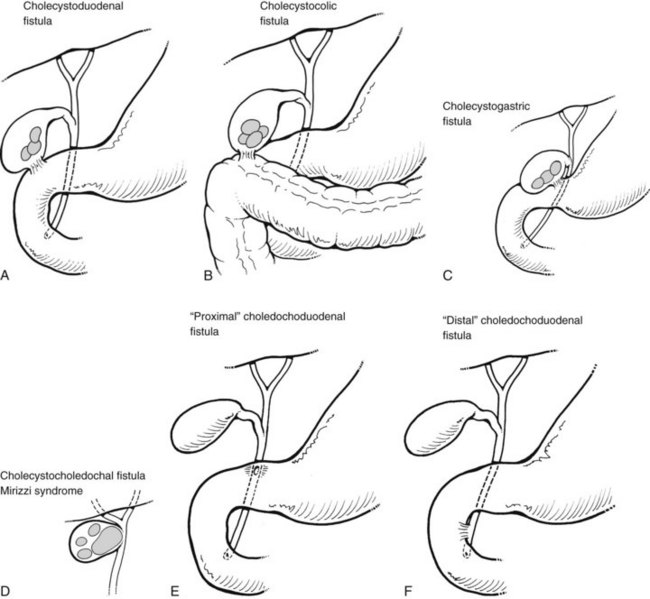
The various types of biliary-enteric fistulae can best be subclassified from an anatomic point of view, by the names of the principal organs involved (Fig. 42B.1). Some names for a particular fistula between two structures vary in the literature (e.g., bronchobiliary vs. biliobronchial) but do not connote differences in etiology or pathogenesis.
Fistulae Involving the Gallbladder
In Western countries, where cholesterol cholelithiasis abounds, the gallbladder is most often the site of severe inflammation and obstruction. Cholecystenteric fistulae constitute 70% to 85% of all biliary fistulae reported in the world literature up to 1982 (Rau et al, 1980; Safaie-Shirazi et al, 1973). Of these, 55% to 75% are cholecystoduodenal, 15% to 30% are cholecystocolic, and 2% to 5% are cholecystogastric (see Fig. 42B.1). Multiple fistulae (e.g., cholecystoduodenocolic) are very rare (Shocket et al, 1970). Of the 23 cases reported up to 1978, 21 were secondary to gallstone disease, and one case of each was due to duodenal ulcer and a primary carcinoma of the gallbladder (Morris et al, 1978).
Gallstone ileus, a dramatic clinical presentation of a cholecystenteric fistula, is reported in 8% to 20% of large series of patients with biliary-enteric fistulae (Clavien et al, 1990; Heuman et al, 1980; Kasahara et al, 1980; LeBlanc et al, 1983; Rau et al, 1980; Safaie-Shirazi et al, 1973; VanLandingham & Broders, 1982). As a cause of intestinal obstruction alone, gallstone ileus accounts for 1% to 4% of cases, although in patients older than 65 years, gallstone ileus may account for 25% of cases of small bowel obstruction, regardless of a history of prior abdominal surgery (Clavien et al, 1990; Reisner & Cohen, 1994; Rodriguez-Hermosa et al, 2001; Lassandro et al, 2004; Kirchmayr et al, 2005; Chou et al, 2007).
Although most fistulae between the gallbladder and intestinal tract become obvious preoperatively or intraoperatively, cholecystocholedochal fistulae are insidious and may not be appreciated even at surgery. These biliobiliary fistulae develop between the ampulla of the gallbladder or cystic duct and the proximal common hepatic or common bile duct (CBD; see Fig. 42B.1D). In either instance, the mechanism of formation is the same: pressure necrosis into the common duct by a large solitary calculus impacted in either the ampulla of the gallbladder or the intramural portion of the cystic duct. Cholecystocholedochal fistula has been estimated to occur in 1% to 6% of biliary operations for calculous disease (Corlette & Bismuth, 1975). Although this estimate seems high and is not corroborated by other reports, an awareness of the possibility of cholecystocholedochal fistula is important and may help avoid damage to the common duct at operation.
Fistulae Involving the Common Bile Duct, Cystic Duct Remnant, and Other Extrahepatic Ducts
Choledochoduodenal fistulae are classified as either proximal or distal (see Fig. 42B.1E and 42B.1F). A proximal choledochoduodenal fistula is the principal form of abnormal communication between the CBD and adjacent structures and represents 4% to 20% of all biliary-enteric fistulae. At one time, 80% were caused by peptic ulcer erosion from the first portion of the duodenum into the proximal CBD. This cause is now much less common as a result of the development of effective medical therapy with antacid drugs. Other, less common causes of choledochoduodenal fistula include cholelithiasis, operative trauma, duodenal diverticula, echinococcal infection, Crohn disease, and neoplasms of the stomach, distal bile duct, ampullary region, and duodenum (Feller et al, 1980; Sarr et al, 1981; Kuroki et al, 2005). Since the beginning of the acquired immunodeficiency syndrome (AIDS) epidemic, there have only been three known case reports of choledochoduodenal fistula caused by AIDS/human immunodeficiency virus–associated tuberculous infection (Patino et al, 2003).
Distal choledochoduodenal fistulae connect to the duodenum in the distal 2 cm of the CBD, and the fistula opening can be seen during percutaneous transhepatic cholangiography (PTC) and endoscopic retrograde cholangiopancreatography (ERCP). The incidence of distal choledochoduodenal fistula secondary to cholelithiasis or operative trauma is variable in different parts of the world. With the development of PTC and ERCP studies of pathologic anatomy (see Chapters 18, 27, and 28), it is becoming apparent that many patients with minor or major biliary-digestive complaints and gallstone disease may have a distal choledochoduodenal or parapapillary choledochoduodenal fistula. Large series from Argentina showed the incidence of parapapillary choledochoduodenal fistula to be 0.7%. Jorge and colleagues (1991) reviewed 2012 ERCP studies from 1976 through 1989, and only 14 cases were found: two cases were from stone disease and the other 12 were from iatrogenic causes.
In Japan, where there is a high incidence of primary intrahepatic calculous biliary tract disease, Tanaka and Ikeda (1983) reported a 5.3% incidence of parapapillary fistula in ERCP studies of 1500 patients. The male/female ratio was roughly equal, in keeping with the Japanese sex distribution of gallstone disease. Ikeda and Okada (1975) classified these as type I fistula, characterized by a small fistula opening on the longitudinal fold of the duodenum just proximal to the papilla, probably caused by penetration of a small calculus through the intramural portion of the common duct into the duodenum, and type II fistula, a larger opening of the duodenum wall adjacent to the longitudinal fold, probably caused by a relatively large stone eroding from the extramural portion of a greatly dilated common duct into the duodenum.
In Turkey, Karincaoglu and colleagues (2003a) reported an incidence rate of 4.9% on review of 1347 ERCP studies in 841 patients. This study also found an intriguing association between peripapillary fistula with common duct stones and complications of cholangitis. The incidence of parapapillary choledochoduodenal fistula is probably less in Western Europe, the United Kingdom, and the United States, and perhaps a greater proportion of these fistulae are not due to spontaneous gallstone disease itself but rather to iatrogenic surgical injury or other instrumental damage to the distal common duct in operations directed against choledocholithiasis. Hunt and Blumgart (1980) and Tytgat and colleagues (1979) published their experience of these fistulous complications of biliary tract surgery.
Unusual choledochal fistulae are mentioned only briefly in this context. Spontaneous fistula formation between the common duct and the colon has been recorded only four times in the English literature (Bose & Sastry, 1983; Rawas et al, 1987; Guitron-Cantu et al, 2001; Anees et al, 2008). We are also aware of one case of a choledochocolonic fistula that developed after blunt abdominal trauma (Benson et al, 2001) and another that developed as a complication from diverticulitis (Blanco-Benavides & Rodriguez-Jerkov, 1992). Peptic ulcer disease has rarely produced fistulae between the common duct, duodenum, and pancreas (Aitken et al, 1986). We have observed one case and are aware of several other cases of severe pancreatitis or pancreatic cancer producing a fistulous communication between pancreatic pseudocysts or necrotic pancreatic cavities and the CBD (DeVanna et al, 1983; Ellenbogen et al, 1981; Lebovics et al, 1990; Miller et al, 1988). Chandar and Hookman (1980) reported one case of GI hemorrhage resulting from a choledochocolonic fistula that developed from a cystic duct remnant 28 years after cholecystectomy, and it was associated with a benign stricture in the ampullary segment of the CBD. More recently, a bile duct stricture associated with a choledochocolonic fistula was reported following an uncomplicated open cholecystectomy four months prior (Munene et al, 2006). Woods and colleagues (1992) reviewed four cases of cystic duct remnant fistulization to the GI tract.
Fistulae Involving the Intrahepatic Ducts, Liver, and Lung
Thoracobiliary and bronchobiliary fistulae are quite rare (Boyd, 1977; Chan et al, 1984; Cleve & Correa, 1958); the former refers to communications between the biliary tree and the pleural cavity and the latter to communications between the bile ducts and the bronchial tree. The three major categories of bronchobiliary fistula are 1) those resulting from infection, especially parasitic, or those acquired, such as from iatrogenic injury as a result of biliary tract surgery or, less commonly, from gastric and pancreatic operations; 2) traumatic fistula; and 3) congenital fistula. The many diverse causes of acquired bronchobiliary fistulae have been well referenced by Sane and colleagues (1971) and more recently by Gugenheim and colleagues (1988). The two main factors responsible for the development of bronchobiliary fistulas are “mechanical” biliary obstruction and infection. Worldwide, the principal cause of bronchobiliary fistula in adults is parasitic disease of the liver, either echinococcal or amebic abscess (see Chapters 67 and 68). In developed countries, iatrogenic injury to the biliary ductal system is the most frequent cause of these fistulae.
The hallmark symptom of a bronchobiliary fistula is biliptysis in conjunction with other pulmonary complaints, jaundice, cholangitis, and external biliary fistula or subphrenic abscess. Radiologic confirmation of the diagnosis is possible by a variety of methods. In the presence of an external fistula, injection of contrast solution (fistulogram) is the most facile and direct approach. PTC and ERCP are equally effective in their ability to show the fistula and have the advantage of offering an avenue for potential therapeutic intervention. The choice of one or the other of these techniques depends on the presence or absence of dilated ducts and the expertise available. Bronchobiliary fistulae also have been shown by cholescintigraphy (Gunlemez et al, 2009; Santra et al, 2009; Uramoto et al, 2008; Andalkar et al, 2004; Savitch et al, 1983; Velchik et al, 1991). Computed tomography (CT) and magnetic resonance imaging cholangiopancreatography (MRCP) have been used to assess bronchobiliary fistulae (see Chapters 16 and 17). These imaging techniques, although helpful in assessing the upper abdomen, rarely visualize the fistula tract (Oettl et al, 1999; Yeatman et al, 2004; Kuroki et al, 2005; Ragozzino et al, 2005).
The surgical treatment of parasitic disease of the liver is discussed elsewhere in this book (see Chapters 67 and 68). In large series of surgically treated cases of hepatic echinococcal disease in Greece and Turkey, only 2% were complicated by rupture into the lung or bronchi (Alestig et al, 1972). Amebic abscess of the liver has been reported in association with bronchobiliary fistula in 8% of cases (Razemon et al, 1963). These complications of parasitic disease are more likely to be suspected in patients from Mediterranean countries, North Africa, Mexico, and some of the southern border states of the United States. The successful treatment of these fistulae depends on the use of appropriate surgical drainage or resection in conjunction with appropriate drug therapy.
The incidence of bronchobiliary fistula as a consequence of surgically treated calculous or neoplastic disease of the hepatobiliary tract and adjacent structures has been decreasing, as patients are operated on earlier in the course of their disease and by better-trained surgeons (Ramesh et al, 1991; Warren et al, 1983). Then again, modern surgical techniques and equipment have contributed to a new cause of biliary fistulae: reports of bronchobiliary fistula caused by new liver tumor ablative therapies, such as radiofrequency ablation (RFA), are beginning to emerge (Kim et al, 2005; Tran et al, 2007; Yoon et al, 2009; see Chapter 85A, Chapter 85B, Chapter 85C, Chapter 85D ). In all these case reports, the fistula responded to conservative treatments focused on biliary drainage.
The incidence of bile duct injury in the course of cholecystectomy has increased with the advent of laparoscopic removal of the calculous gallbladder (Davidoff et al, 1992). Most of the injuries are repaired at or shortly after their occurrence. Bronchobiliary fistulae secondary to bile duct injury are a late consequence of the event in patients with neglected or recurrent strictures after unsuccessful operations. Less frequently, gastric or pancreatic procedures may result in injury to the extrahepatic bile ducts.
Untreated choledocholithiasis complicated by repeated episodes of bile duct obstruction and cholangitis is another, albeit infrequent, cause of bronchobiliary fistula. Brem and colleagues (1990) reported the successful treatment of an 87-year-old patient with this clinical situation by endoscopic papillotomy alone. Indeed, bronchobiliary fistulae should be treated first by biliary decompression, either by endoscopic papillotomy or by transampullary drainage to reduce the fistula tract pressure. Some patients with a persistent fistula or infection will require debridement by thoracotomy.
Posttraumatic thoracobiliary fistulae are extremely rare. Although penetrating or blunt trauma to the abdomen and chest is a common enough civilian and wartime occurrence, the number of fistulae resulting has remained small, often because their initial surgical treatment has been excellent, and because no obstruction to proper bile flow existed (Oparah & Mandal, 1978). Ivatury and colleagues (1984) reported on three cases and reviewed 32 previously reported patients. This series included 20 thoracobiliary and 15 bronchobiliary fistulae. Twenty-six patients sustained penetrating injuries, including 16 with gunshot wounds, 6 with stab wounds, and 4 in which the weapon was not specified. Seven patients were admitted because of blunt trauma, and in an additional four patients, the mechanism of injury was not documented. All of the patients in this report had thoracoabdominal injuries, and the causes of the fistulae were nonoperative therapy, missed or overlooked injury to the diaphragm, inadequate drainage, and subphrenic abscess. All patients had pleural effusion, and bile was aspirated by thoracentesis. Successful treatment frequently required pulmonary decortication, repair of the diaphragm, and adequate drainage above and below the diaphragm. More recent publications support the notion that inadequate drainage caused posttraumatic bronchobiliary fistulae (Navsaria et al, 2002; Eryigit et al, 2007; Ball et al, 2009).
Neuhauser and colleagues (1952) first described congenital bronchobiliary fistula in an infant with a tract communicating between the right main stem bronchus and the hepatic duct that passed through the posterior mediastinum. The diagnosis was made antemortem, but the infant died before surgical therapy. Tommasoni and colleagues (2000) described two newborn infants who were promptly diagnosed and successfully treated surgically. These authors emphasized the importance of a high index of suspicion for early diagnosis and recognition of the very high incidence (approximately 36%) of coexisting hypoplasia or biliary atresia. Therefore, all of these patients need laparotomy and thoracotomy for successful treatment (Tommasoni et al, 2000). The fistulous tract in the posterior mediastinum adjacent to the esophagus should also be excised to avoid repeat thoractomy (Gunlemez et al, 2009). Yamaguchi and colleagues (1990) reported one adult patient with a congenital bronchobiliary fistula and summarized the data of 16 previously reported cases, four of them adults. Interestingly, all adults were younger than 32 years (range, 22 to 32 years). The only congentital bronchobiliary fistula in an elderly person was reported recently by Uramoto (2008).
On microscopic examination, the proximal portion of the fistula in most patients resembles the bronchus, and the distal segment resembles the esophagus; the embryologic explanations for this anomaly are conjectural (Dyon et al, 1978; Sane et al, 1971). Transthoracic excision of the fistula and surgical correction of associated biliary anomolies is usually curative.
Diagnostic Tests
Sputum analysis for bilirubin and viable scolices or membranes can be used as laboratory evidence to establish the diagnosis of bronchobiliary fistulae secondary to echinococcosis (Gerazounis et al, 2002). Although no specific serologic tests for biliary-enteric fistula are available, in the evaluation and management of an elderly, high-risk, or critically ill patient with a symptomatic biliary fistula, tests of liver function, electrolytes, and blood count are useful.
Plain and Contrast Radiographs
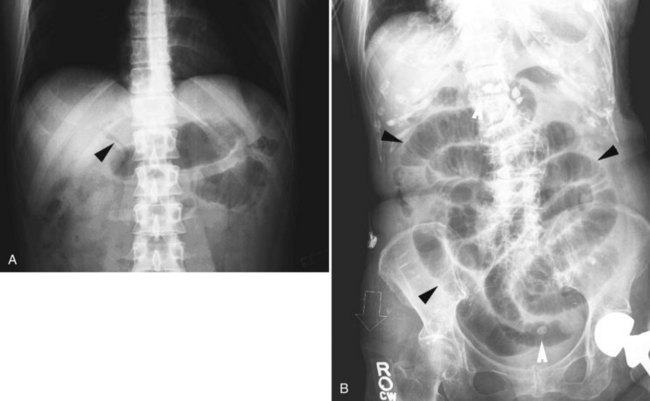
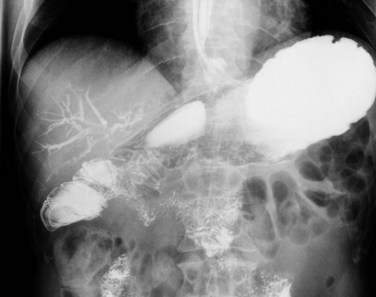
Pneumobilia, the presence of air in the biliary tree, may be noted on plain films of 30% to 50% of patients with gallstone ileus (Fig. 42B.2A). Other, less common causes of pneumobilia include an incompetent sphincter of Oddi, emphysematous cholecystitis, suppurative cholangitis, and prior biliary-enteric bypass surgery. Hricak and Vander Molen (1978) suggested that the low incidence of pneumobilia in gallstone ileus is due to the cystic duct obstruction that led to the acute cholecystitis and perforation into an adjacent viscus; the obstruction also prevents retrograde passage of air into the extrahepatic and intrahepatic bile ducts.
Other classic radiographic signs of gallstone ileus are visualization of a calcified gallstone in the peritoneal cavity a distance away from the gallbladder (Fig. 42B.2B), change on repeat films in the position of a previously observed calcification, and a change in the level of mechanical intestinal obstruction—the so-called tumbling obstruction (Day & Marks, 1975; VanLandingham & Broders, 1982). However, only 30% of gallstones are sufficiently calcified to be radiopaque.
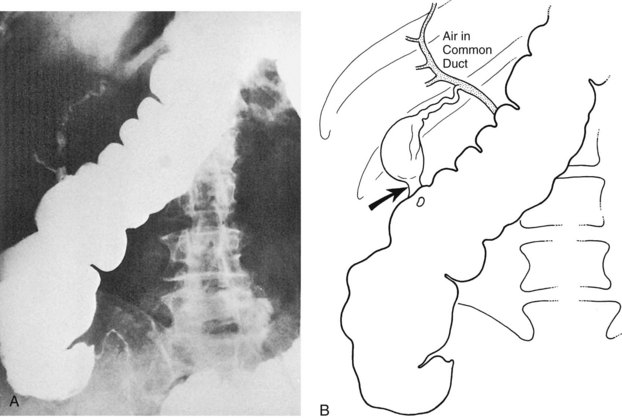
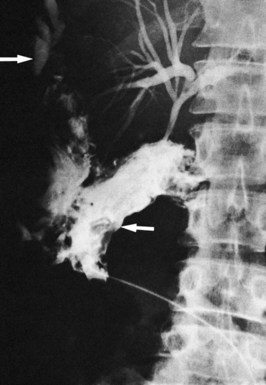
A barium meal or upper GI series shows reflux of contrast material into the fistula in 40% of cholecystoduodenal communications and 75% of choledochoduodenal fistulae of peptic ulcer origin (Balthazar & Gurkin, 1976; Kourias & Chouliaras, 1964). If the plain film and barium swallow are used in concert, more than 60% of biliary enteric fistulae are correctly diagnosed preoperatively (see Fig. 42B.2A; Balthazar & Schechter, 1975). A negative upper GI series in the presence of pneumobilia is an indication for a barium enema, which discloses more than 95% of cholecystocolic fistulae. CT scans with contrast material, endoscopy, radionuclide imaging, and sonography are also useful in the diagnosis of biliary fistulae (Becker et al, 1984); and more recently, MRCP has yielded valuable information.
Endoscopy and Iodinated Dye Studies
Since 1970, numerous reports have appeared in the literature supporting the efficacy of ERCP in documenting surgical or spontaneous biliary fistulae (Al Nakib et al, 1982; Tanaka & Ikeda, 1983; Tytgat et al, 1979; Van Linda & Rosson, 1984). Endoscopically, the alimentary side of a fistula can be visualized directly, be it gastric (Stempfle & Diamantopoulos, 1976), duodenal, or colic; the fistula itself or the ampulla of Vater can be cannulated to obtain a high-quality radiograph of the communicating biliary anatomy (Moreira et al, 1984; Watkins et al, 1975; Chatzoulis et al, 2007). Similarly, the largely unappreciated and frequently asymptomatic parapapillary choledochoduodenal fistula has been found to be quite common (Hunt & Blumgart, 1980; Karincaoglu et al, 2003b; Tanaka & Ikeda, 1983; Kuroki et al, 2005). There have been far fewer reports of the role of PTC in showing biliary fistulae, but PTC has been helpful in the diagnosis and treatment of bronchobiliary fistula in association with echinococcal disease of the liver and dilated intrahepatic ducts (e.g., cholecystocholedochal fistula; Cornud et al, 1981; Mannella et al, 1999).
Radionuclide Imaging (See Chapter 15)
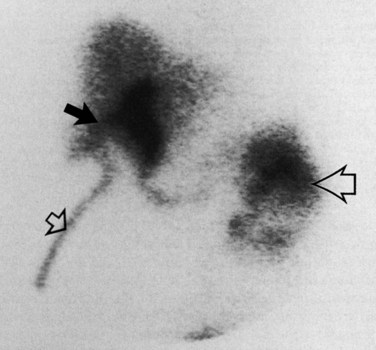
With the introduction of the imidoacetic acid agents bound to technetium (p-isopropylacetanilido iminodiacetic acid [PIPIDA] and hepatobiliary iminodiacetic acid [HIDA]), radionuclide scans have rapidly become the preferred method of outlining the normal and pathologic anatomy of the extrahepatic bile passages. These relatively noninvasive procedures do not rely on hepatocellular concentration of bile or on short critical time-flow periods for satisfactory visualization of the biliary tree. Prolonged (24 hours) accumulation of radioactivity, measured by patient scanning or quantitative isotopic counts of body secretions (e.g., sputum), has been used to show very small or intermittent fistulae from the biliary tract to the respiratory passages (Fig. 42B.3) and the colon (Benson et al, 2001; Santra et al, 2009; Uramoto et al, 2008; Andalkar et al, 2004; Bretland, 1983; Edell et al, 1981; Henderson et al, 1981; Savitch et al, 1983; Taillefer et al, 1983).
Sonography
Sonography is a useful noninvasive diagnostic aid in the preoperative evaluation of a patient with a suspected biliary fistula (Porta et al, 1981; Renner et al, 1982) and even gallstone ileus (Davies et al, 1991; Pedersen et al, 1988; see Chapter 13). Although the radionuclide scan may readily show a fistula, the sonogram indicates the presence of calculi in the gallbladder; common duct stones; and inflammatory, cystic, or infiltrative disease of the liver and pancreas (Griffin et al, 1983). Sonography can readily detect pneumobilia, indicating a high likelihood of a biliary-enteric fistula. In gallstone ileus, sonography can detect an ileal stone not seen on plain films and is increasingly being used by emergency room physicians (Zironi et al, 2007). Ripolles and colleagues (2001) reported that 22 of 23 patients who had undergone surgery for gallstone ileus were found on sonogram to have pneumobilia. This information is valuable in the decision-making process, such as to evaluate the need for or advisability of cholecystectomy and dismantling of a fistula at the time of operation for gallstone ileus in an elderly or high-risk patient. This information may be obtained more rapidly and safely, and probably with equivalent accuracy, by a preoperative sonogram rather than by intraoperative manipulations about the mass of inflamed tissue in the right upper quadrant. Ultrasound (US) has been used to document the persistence or closure of biliary enteric fistulae after initial emergent surgical or combined endoscopic lithotripter treatment of gallstone ileus, and it has aided the decision for or against further surgery (Clavien et al, 1990).
Computed Tomography and Magnetic Resonance Imaging Cholangiopancreatography (See Chapters 16 and 17)
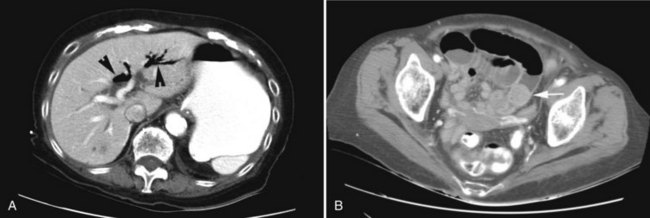
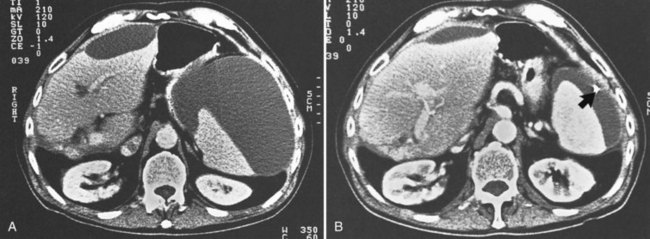
CT and MRCP are better able to show a moderately calcified ectopic gallstone, air in the biliary tree, the presence of additional stones, and a cholecystoduodenal fistula (Swift & Spencer, 1998). CT is useful also for estimating the size of the impacted stone and determining the site of obstruction for planning the operative approach (Fig. 42B.4). Indeed, new multidector CT scanners that use multiplanar or three-dimensional volume-rendering reconstruction will further improve the diagnostic role of CT in detecting fistulae. Already, modern MRI and MRCP scans with improved image resolution and reduced motion artifact have been shown to accuarately detect a small fistulous tract (Chatzoulis et al, 2007). Although such scanning may disclose fusion of the biliary tract to an adjacent viscus, it may not always precisely show a fistula; but MR scanning should be used to show other important pathologic changes, such as distal common duct stones or other obstructive processes, the presence of a subphrenic abscess, pleural effusion, or parasitic disease of the liver (Porta et al, 1981). Scanning also can be used to verify and localize contrast extravasation after interventional procedures, such as PTC or ERCP, or after contrast injection of an existing controlled external fistula (Fig. 42B.5).
Specific Clinical Presentations and Treatment
Gallstone Ileus
The classic plain abdominal film triad of small bowel obstruction, pneumobilia, and ectopic gallstone is considered pathognomonic of gallstone ileus (Rigler et al, 1941); however, the triad is encountered in only 30% to 35% of cases (Balthazar & Schechter, 1978). Pneumobilia is often not appreciated even in retrospect. If biliary-enteric fistula is suspected, and a barium meal is administered, reflux of barium into the biliary tree yields a correct preoperative diagnosis in 60% of patients (Fig. 42B.6). Calculi large enough to obstruct the intestine usually do so in the last 50 cm of ileum, although sometimes also in the jejunum or duodenum and rarely in the sigmoid colon. Such calculi are usually larger than 2.5 cm; if partially calcified, they are readily apparent on a plain radiographs (see Fig. 42B.2B).
Abdominal ultrasound, as mentioned earlier, often establishes the diagnosis of gallstone ileus or provides other information relevant to the diagnosis (Clavien et al, 1990; Davies et al, 1991; Pedersen et al, 1988; Ripolles et al, 2001; Zironi et al, 2007), revealing pneumobilia; the site of the fistula; additional stones still in the gallbladder, or more importantly, in the common duct; and occasionally the location of an ectopic calculus. The diagnositic superiority of CT over abdominal plain films and sonography for gallstone ileus is now well established (Ayantunde & Agarwal, 2007; Lassandro et al, 2005; Loren et al, 1994; Reimann et al, 2004; Swift & Spencer, 1998). In fact, the clinical significance of Rigler’s triad has been revitalized by CT, because all three findings are more consistantly detected (approximately 78%) by this method (Kirchmayr et al, 2005; Ayantunde & Agarwal, 2007; Chou et al, 2007).
The clinical presentation of gallstone ileus has not changed in the past 40 years. In patients with intestinal obstruction, preoperative diagnostic accuracy is approximately 75% (Glenn et al, 1981). Preoperative diagnostic accuracy has contributed to an improved outcome of therapy (Deitz et al, 1986). The increased use of CT in patients with symptoms of intestinal obstruction has led to additional improvement in the diagnostic accuracy of gallstone ileus and has resulted in a decrease in the high mortality rate encountered with this disease (Delabrousse et al, 2000; Lassandro et al, 2004; Reimann et al, 2004; Ayantunde & Agarwal, 2007; Chou et al, 2007).
The terminal ileum is the site of obstruction in 70% of cases (see Figs. 42B.2B and 42B.4B). Unless the obstructed segment is ischemic or has perforated and requires a small bowel resection, the obstructing calculus can be manipulated proximally to a healthy section of bowel, where a safe enterotomy and stone removal may be executed. Jejunal impaction, often by stones larger than 4 cm, occurs approximately 15% of the time, and enterotomy may be made at the site or just proximal to it. Duodenal obstruction, usually in the bulb, is known as Bouveret syndrome (Argyropoulos et al, 1979; Bhama et al, 2002; Cooper & Kucharski, 1978; Koulaouzidis & Moschos 2007; Frattaroli et al, 1997; Maglinte et al, 1987; Thomas et al, 1976), which occurs in 10% of patients and may be handled by duodenostomy or pyloroplasty. It occasionally may be possible to manipulate the stone back into the stomach and remove it via gastrotomy. Rarely, a gastroenterostomy is necessary to protect a duodenotomy or severely traumatized duodenum at the site of impaction.
Bouveret syndrome and other cases of gallstone impaction high in the jejunum have been managed successfully by a combination of endoscopy and electrohydraulic lithotripsy (Fujita et al, 1992; Holl et al, 1989; Moriai et al, 1991; Sackmann et al, 1991). We are aware of one case report of a failed endoscopic extraction that led to spontaneous uneventful passage of the stone (Wagholikar & Ibrarullah, 2004). In rare instances, the sigmoid colon is the site of obstruction of a calculus that has managed to pass through the terminal ileum or enter the colon via a cholecystocolic fistula (Anseline, 1981; Clavien et al, 1990). Almost invariably, some other pathologic process, such as diverticulitis, has produced an area of colonic narrowing. A colostomy is necessary to decompress the proximal bowel. If the impacted stone cannot be manipulated proximally to a transverse colostomy, consideration should be given to exteriorization of the impacted segment with a Hartmann closure of the bowel distal to it (Milsom & MacKeigan, 1985).
Open exploration and enterolithotomy has always been the standard surgical approach for the treatment of classical gallstone ileus. It not only allows removal of the obstructing stone but also permits careful palpation of the entire bowel and gallbladder region, to determine whether other gallstones are in transit more proximally or if any still reside in the diseased gallbladder (see Fig. 42B.2B). These calculi may be poised for passage through the fistula, possibly to induce a recurrent episode of gallstone ileus, a phenomenon estimated to occur in 5% of cases; however, by simple maneuvers, this can be prevented (Clavien et al, 1990; Haq et al, 1981; Levin & Shapiro, 1980). More recently, some authors have described laparoscopic or laparoscopic-assisted treatment for patients with gallstone ileus (Allen et al, 2003; Malvaux et al, 2002; Moberg & Montgomery, 2007; Owera et al, 2008). Because of the limited number of laparoscopically treated patients, it is unclear wherther this approach will reduce the associated high mortality and morbidity rates. Therefore it is our opinion that an open surgical approach should remain the standard of practice and that laparoscopic treatment needs further evaluation.
Considerable debate in the surgical literature surrounds whether cholecystectomy or common duct exploration or both, with dismantling and closure of the cholecystenteric fistula, should accompany enterotomy and relief of the obstruction or await a second operation (Kirchmayr et al, 2005; Muthukumarasamy et al, 2008; Zuegel et al, 1997). Historical data of published reports from the years 1953 through 1993 (Reisner & Cohen, 1994) showed a lower mortality rate of 11.7% in the enterolithotomy-alone group compared with 16.7% for patients who underwent a one-stage operation. Several published reports indicated that operative mortality is lower in these critically ill, elderly patients when only the gallstone obstruction is relieved (Muthukumarasamy et al, 2008; Tan YM et al, 2004; Heuman et al, 1980; Kasahara et al, 1980; VanLandingham & Broders, 1982). This has lead to the general agreement that enterolithotomy alone should be done for fragile patients with significant comorbidities and that the single-stage procedure should be reserved for young, fit, and low-risk patients.
Although some centers have recently published impressively low perioperative mortality rates (0%) for surgical treatment of gallstone ileus, other centers still report rates in the traditional high range (23%; Muthukumarasamy et al, 2008; Tan et al, 2004; Ayantunde & Agarwal, 2007). In a case series from the United Kingdom (Anyantunde & Agarwal, 2007), five deaths occurred in 22 patients; four of the five deaths were patients treated with enterolithomy alone. Seven of 19 patients from YM Tan and colleagues’ (2004) series were clinically unstable and underwent enterolithotomy alone; on long-term follow-up, these patients did not have further complications.
Indeed, careful follow-up of patients treated with enterolithotomy alone indicates that one third to one half will have minimal or no symptoms after relief of the gallstone ileus, and no further treatment will be necessary (Clavien et al, 1990; van Hillo et al, 1987; Tan YM et al, 2004). If a patient has jaundice and intestinal obstruction at the outset, common duct exploration, or at least tube decompression of the common duct, may also be necessary as a lifesaving procedure. For patients who continue to be symptomatic after relief of gallstone ileus, careful preoperative evaluation and bowel preparation can precede a later operation, adding to its safety.
Because common duct stones are found in 40% of patients with biliary-enteric fistula, it is likely that patients who continue to be symptomatic would require endoscopic or open surgical removal of their common duct stones in addition to cholecystectomy and repair of the fistula. As mentioned, operative mortality rate slowly declined to 15% to 25% in large reported series until the early 1970s. Further reductions in operative mortality rates have been possible by limiting treatment to enterotomy and stone removal alone without further surgical therapy in high-risk patients (Ayantunde & Agarwal, 2007; Glenn et al, 1981; Muthukumarasamy et al, 2008; Rodriguez-Sanjuan et al, 1997; Tan YM et al, 2004).
Cholecystoduodenal Fistulae
Most cholecystoduodenal fistulae do not result in gallstone ileus (see Fig. 42B.1A). Rather they are asymptomatic or occur in association with the usual digestive complaints consistent with gastric or biliary tract disease. They may be found during an upper GI barium study or under less welcome circumstances, such as at the time of abdominal surgery for an unrelated problem.
If an asymptomatic or mildly symptomatic cholecystenteric fistula is diagnosed preoperatively, many of the management decisions regarding gallstone ileus discussed previously may apply. Elective surgery may never be necessary in a completely asymptomatic individual, and surgery may present an unfavorable risk-to-benefit ratio in an elderly, minimally symptomatic patient. Along with dismantling of the fistula and common duct exploration, other alternatives to cholecystectomy must be considered, such as a period of expectant management with careful observation, endoscopic papillotomy and stone extraction with the gallbladder left in situ, or interval cholecystectomy if symptoms of pain or cholangitis persist after endoscopic biliary surgery. In a relatively healthy patient younger than 70 years, we believe that open surgical extirpation of the gallbladder, closure of the fistula, and treatment of any common duct pathology promise the best long-term therapeutic result, although some authors advocate laparoscopic treatment of preoperatively diagnosed biliary-enteric fistulae (Chikamori et al, 2001; Crouch & Kuhnke, 2000; Kwon & Inui, 2007; Lee et al, 2004; Rohatgi & Singh, 2006).
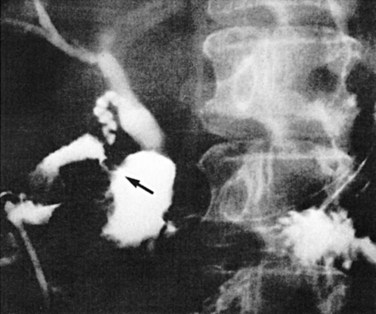
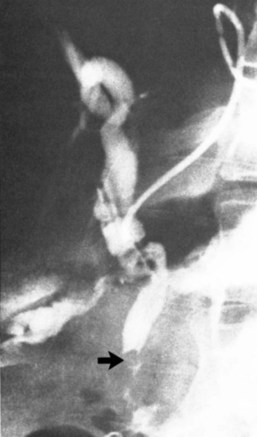
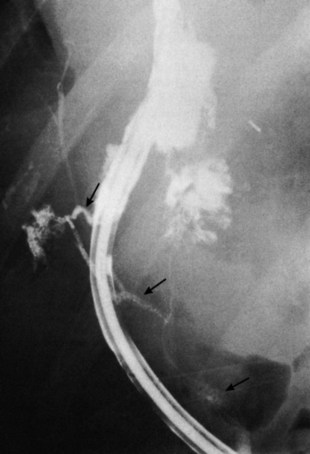
If an incidental cholecystoduodenal fistula is discovered at the time of surgery, the major intraoperative decision revolves around the patient’s need for, and ability to tolerate, additional surgical manipulations. If the patient is judged an unsound risk, or if the biliary tract pathology is not believed to be pertinent to the major indication for operation, nothing need be done. Rarely, a cholecystostomy (Fig. 42B.7) and extraction of large calculi can be accomplished with little additional risk. Usually, the gallbladder is shrunken and barely palpable, however, because it is stuck to the duodenum. If the patient can tolerate additional surgery, and sufficient indication exists to proceed at the present operation, rather than at a subsequent one, an attempt should be made to show the biliary-enteric anatomy and state of the common duct by an operative cholangiogram before proceeding with a definitive procedure.
Cholecystocolic Fistulae and Choleric Enteropathy
A cholecystocolonic fistula may develop acutely in patients with long-standing mild or moderately symptomatic biliary tract disease and may be heralded by a sudden change in bowel habits with multiple, loose stools and the development of fever, chills, and other signs of cholangitis from colonic bacterial reflux into the biliary tract. Many elderly patients either weather or ignore these symptoms without seeking medical attention, and some develop signs and symptoms that may incriminate the entire GI tract. Increased stool frequency persists, particularly after ingestion of food, and bouts of fever and malaise subside. Other characteristic symptoms then appear, such as eructation, nausea, weight loss, and increasing diarrhea and steatorrhea. These latter symptoms precede the onset of choleric enteropathy, a dramatic complication of cholecystocolonic fistula. This enteropathy also is seen in other major disturbances of bile acid metabolism, such as with major ileal resection or blind loop syndrome (Brandt, 1984).
Choleric enteropathy comprises of a wide spectrum of anatomic, physiologic, and biochemical changes produced by a significant alteration of the enterohepatic circulation of bile acids. The malabsorption syndrome secondary to cholecystocolonic fistula was clinically documented first by Augur and Gracie (1970) and has since been studied by others (Rau et al, 1980). Ordinarily, 95% of bile acids are passed down the jejunum, aiding in fat and cholesterol absorption, before being largely reabsorbed in the terminal ileum as part of an efficent enterohepatic circulation. Two or three cycles of the bile acid pool per meal occur, with further metabolism of bile acids in the colon and very little lost. With a cholecystocolonic fistula, however, a large part of all of the primary bile acid pool is shunted directly into the colon, resulting in a high luminal concentration of bile acids. In the colon, the primary bile acids undergo deconjugation and dehydroxylation by fecal bacteria, and this increased concentration of bile acids causes a water secretory diarrhea.
Interestingly, only the two natural bile acids with two α-hydroxy groups, chenodeoxycholic acid and deoxycholic acid, induce colonic secretion (Keely et al, 2007). Depending on the amount of bile still passing via the common duct into the small bowel, fat absorption is affected, which over time may result in fatty acid diarrhea. More immediately, however, colonic secretion of water and electrolytes is maximally stimulated by the dihydroxy bile acids. Chenodeoxycholic acid gains access to the colon in large quantities, and massively increased amounts of deoxycholic acid are formed by bacterial dehydroxylation of cholic acid. The primary bile acids and their metabolites are no longer actively absorbed and are lost from the body, eventually causing a chronically lowered bile salt pool beyond the liver’s capacity to synthesize new bile acids to replete it. At this point, even with a partial shunt to the colon, the bile acid concentration still normally passing down the common duct and through the small intestine may be too small to effect micellar solubilization of dietary fat. In contrast to a blind loop syndrome, in which bacterial overgrowth contributes to the choleric enteropathy and can be largely corrected by antibiotics, at least temporarily, cholecystocolonic fistula does not respond adequately to antibiotics. Until the fistula is dismantled, massive shunting of bile acids to the intestine persists and promotes continued watery diarrhea, diminished bile salt pool, and a variable degree of fat malabsorption.
Cholangitis seems to be a more prominent feature of cholecystocolonic fistula, when the fistula is narrow and prone to intermittent obstruction of bile flow (Edell et al, 1981; Safaie-Shirazi et al, 1973), whereas diarrhea with a lesser incidence of fever and chills is more common with a wide-open fistula (Lygidakis, 1981); we are also aware of one case report of a patient with a cholecystocolonic fistula who was seen with massive lower GI hemorrhage (Kunasani et al, 2003). In any instance, a fistula to the colon is more likely than other biliary-enteric fistulae to have associated cholangitis; if it does, the serum levels of bilirubin, alanine aminotransferase, and γ-glutamyltransferase may be slightly elevated and may direct attention to the biliary and upper digestive tract.
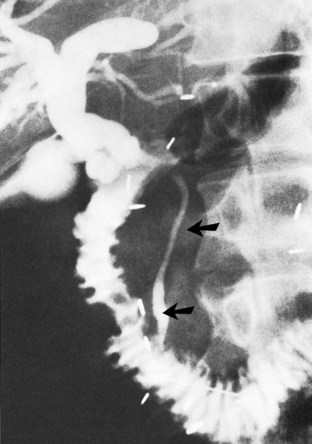
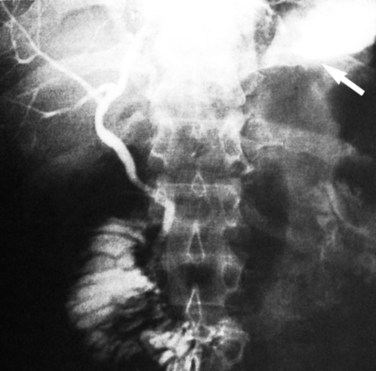
Because of the unusual initial complaints, full investigations for malabsorption that include upper GI studies, GI sonography, and jejunal biopsy may be undertaken but usually do not clarify the diagnosis. A pathognomonic triad consisting of pneumobilia, chronic diarrhea, and vitamin K malabsorption was recently suggested by Savvidou and colleagues (2009) to aid in the diagnosis of cholecystocolonic fistula. Diversion of bile acids into the colon results in malabsorption of fat and fat-soluble vitamins. In particular, vitamin K deficiency can be clinically significant and is easily detected by a prolonged prothrombin time (PT), and it can be corrected by parenteral supplementation (Savvidou et al, 2009). Although the condition of bile acid malabsorption can be easily diagnosed by the selenium-75-homocholic acid taurine (SeHCAT) test, this study is time consuming and is not widely available (Pattni & Walters, 2009). Plain abdominal radiographs have been reported to reveal air in the biliary tree in only 50% of cases, however, the diagnosis becomes evident only if a barium enema examination is done, preferably with air contrast, because barium (Fig. 42B.8) and air fill the gallbladder and extrahepatic bile ducts. In a recent report, a barium enema showing contrast filling of the biliary tract was used to diagnose a patient with a rare choledochocolonic fistula causing severe diarrhea (Anees et al, 2008). Failure of even a barium enema to show a cholecystocolonic fistula has been reported, but this is quite rare. In such cases, the cholecystocolic fistula is diagnosed by ERCP (Arvanitidis et al, 2004; Schoeters et al, 2002).
There is little controversy about the appropriate treatment for cholecystocolic fistulae. Except in the most extenuating circumstances, the fistulae should be dismantled because of the ever-present risk of sepsis. If the diagnosis can be made preoperatively, suitable mechanical and antibiotic bowel preparation reduces the chance of infectious complications of surgery and aids primary closure of the large bowel. Cholecystectomy and, if indicated, common duct exploration should be done at the same time. Centers with significant expertise in laparoscopic surgery have reported successful treatment of preoperatively diagnosed cholecystocolonic fistulae by this method (Chatzoulis et al, 2007; Gentileschi et al, 1995; Hida et al, 1999; Lee et al, 2004; Wang et al, 2006). Regardless of the choice of surgical treatment, preoperative ERCP with sphincterotomy and stone extraction may obviate the need to explore the CBD and, in rare cases, might result in spontaneous closure of the fistula, obviating surgery altogether (Marshall et al, 1990).
Cholecystocholedochal Fistula, Including Mirizzi Syndrome
The pathogenesis of cholecystobiliary fistula is similar to the mechanism of fistula formation between the gallbladder and other adjacent segments of the alimentary tract: the offending calculus remains impacted in the ampulla of the gallbladder or cystic duct, and the resultant inflammation causes adherence and then perforation into the adjacent structure (see Fig. 42B.1D) In 1948, Mirizzi described the clinical picture in detail and called it functional hepatic syndrome. It is now commonly referred to as Mirizzi syndrome (see Chapters 29 and 43). The mechanism of jaundice was postulated by Mirizzi to be due to spasm of the hepatic sphincter secondary to inflammation in the region of the cystic duct junction with the common hepatic duct (Mirizzi, 1948); however, extensive histologic studies have failed to disclose a “sphincter” in the common hepatic duct. The large size of the stone and the acute cholecystitis with marked pressure necrosis and inflammatory reaction at the site of stone intrusion into the common hepatic duct combine to produce jaundice with variable components of extrinsic compression and intrinsic calculous blockage of bile flow.
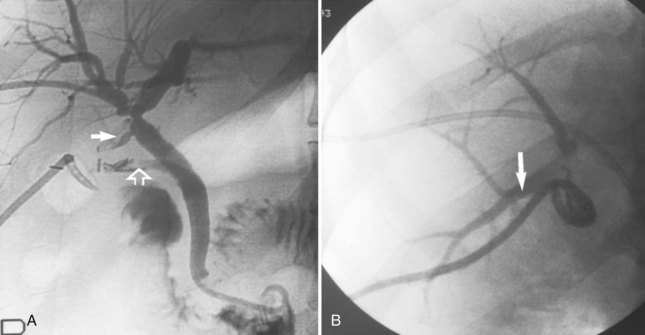
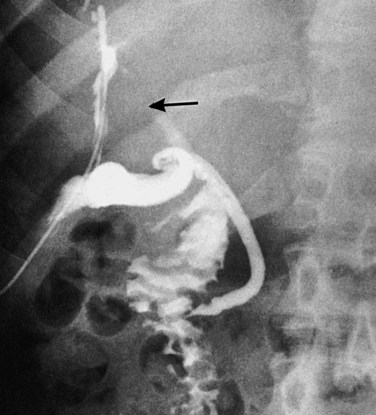
The early phase of this cholecystobiliary fistula presumably exists when a large gallstone is impacted in the ampulla of the gallbladder or in the intramural segment of the cystic duct, which often courses parallel to the common duct. The jaundice that occurs as a consequence of this pathologic anatomy is clinically indistinguishable from choledocholithiasis. McSherry and colleagues (1982) suggested that this classic picture (Fig. 42B.9) of Mirizzi’s “functional hepatic syndrome” be subclassified as type 1. With progression of the disease, extrusion of the stone into the common hepatic duct may occur, and a fistula may form between the gallbladder and the hepatic duct. This is classified as type 2 (see Fig. 42B.1D), which is characteristically diagnosed intraoperatively by observing a gush of bile on removal of the impacted stone, indicating a biliobiliary fistula. Csendes and colleagues (1989) further subclassified type 2 patients into three categories based on the percentage of the wall of the common duct that was eroded by the offending calculus. In this subclassification, type II is a fistula involving less than one third of the circumference of the bile duct, type III involves two thirds, and type IV is a fistula with complete bile duct destruction. This classification scheme was recently updated to include a type V, which describes cholecystoenteric fistula, with or without gallstone ileus, coexisting with any of the other types (Csendes et al, 2007).
The large and solitary nature of the ampullary and cystic duct stones may be a hint to the preoperative diagnosis. The classic findings on ultrasonography include dilation of the biliary system that includes the common hepatic duct above the gallbladder neck, a stone impacted in the gallbladder, and an abrupt change in the caliber of the common duct below the stone (Hayek et al, 1988). Because most of these patients present with jaundice or some abnormalities on LFTs, a preoperative ERCP or PTC frequently is performed. These imaging studies permit a precise delineation of the condition, markedly altering surgical strategy. Cornud and others (1981) showed the appearance of biliobiliary fistulae at PTC, whereas Heil and Belohlavek (1978) relied on ERCP. In the six patients documented by McSherry and colleagues (1982), PTC and ERCP were equally effective in elucidating the cause of common duct obstruction. Although MRCP has been shown to diagnose Mirizzi syndrome preoperatively, it is limited, because it rarely shows the fistula and does not afford therapeutic stenting (Rappeport, 2002).
Biliobiliary fistulae have been referred to as a “trap” in the surgery of cholelithiasis (Corlette & Bismuth, 1975). This term is appropriate, because the presence of such a fistula frequently is not recognized until the time of surgery, often not soon enough to prevent injury to the common duct in an attempt to dissect the ampulla of the gallbladder or the cystic duct. Lygidakis (1981) emphasized the technical problems in operating on these patients. Other authors (Baer et al, 1990; Dewar et al, 1990, 1991; Yip et al, 1992) emphasized the importance of preoperative direct cholangiography. In patients with jaundice, biliary drainage may be necessary in the preoperative management.
If a large adherent mass about the gallbladder and CBD is encountered at surgery, operative cholangiography, although difficult, may delineate the presence of true choledocholithiasis or of the impacted stone without fistula (Mirizzi type 1) or the biliary fistula itself (Mirizzi type 2). In any event, it is unwise to attempt complete cholecystectomy; it is much better to open the gallbladder, remove the stones, and directly assess the presence or absence of a fistula. A portion of the gallbladder wall is sent for frozen section histology to exclude concomitant malignancy, but choledochotomy is seldom necessary, because multiple stones are infrequent in this disease. For Mirizzi type 2 disease, direct choledochotomy may be done, but the obstructing stone usually can be removed via the opened gallbladder. The gallbladder may be dissected 1 to 2 cm proximal to the fistula, between the ampulla and the common hepatic duct, at which point it is transected and sutured closed. T-tube drainage of the common duct may be achieved through the longitudinal choledochotomy, but this is often unnecessary. Alternatively, the opened gallbladder may be anastomosed to the duodenum as described by Hadjis and colleagues (1987) and later advocated by Baer and others (1990) and Mishra and others (1990). Stenosis of the biliary tree often resolves spontaneously in the postoperative period, and choledochotomy is seldom indicated.
Since the introduction of laparoscopic approaches to surgery of the gallbladder and the bile ducts, several cases and small series of laparoscopic treatment of Mirizzi syndrome have been reported (Bagia et al, 2001; Binnie et al, 1992; Chowbey et al, 2000; Paul et al, 1992; Rust et al, 1991; Schafer et al, 2003; Silecchia et al, 1995; Vezakis et al, 2000; Yeh et al, 2003; Rohatgi & Singh, 2006; Kwon & Inui, 2007). To address the utility of laparoscopic treatment of Mirizzi syndrome, Antoniou and colleagues (2010) recently conducted a comprehensive systematic review of the literature on this topic, a query confined to the English and German literature. Because of the strict study criteria, the authors identified only 10 of 66 articles for comparison in the years between 1989 and 2008. The total number of patients treated by laparoscopy was 124, of which 73 (59%) were sucessfully completeted. Conversion to open operation was necessary in 51 (41%) patients. Interestingly, patients from studies reporting a high preoperative diagnosis rate (>80%) had a significantly lower risk for conversion. The main reasons for conversion included technical failure as a result of dense adhesions in the triangle of Calot, unclear anatomy, and unsuccessful stone retrieval. The analysis showed a complication rate of 16%, with residual stones and bile duct injury the most common complications. The authors concluded, and we agree, that laparoscopic treatment of Mirizzi syndome cannot be recommended as a standard procedure because of the high failure rate (Antoniou et al, 2010).
Paul and others (1992) described successful laparoscopic treatment of Mirizzi syndrome by adhering to a luminal approach to the problem, as advocated by Dewar and associates (1991). This approach includes preoperative ERCP and placement of a biliary stent, followed by use of a flexible choledochoscope introduced into the cystic duct high from the open neck of the gallbladder. Stone extraction from the dilated cystic duct via the choledochoscope may avoid carrying the dissection too far down and injuring the common duct. Although such successful laparoscopic approaches to Mirizzi syndrome have been reported, we believe an open approach should be used when the diagnosis is made preoperatively, or a procedure should be converted to open when encountered during laparoscopy. Open technique affords the use of tactile senses, allowing easier removal of the impacted stone and sampling of the gallbladder wall to exclude a coexisting carcinoma (see Chapter 49); an open approach also minimizes the risk of bile duct injury.
Proximal Choledochoduodenal Fistulae Secondary to Peptic Ulcer Disease
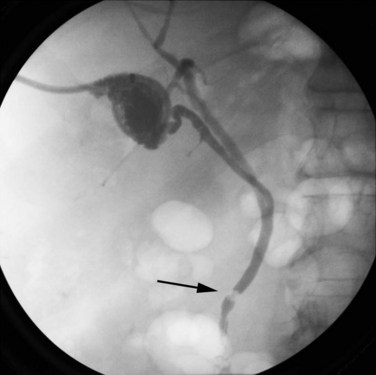
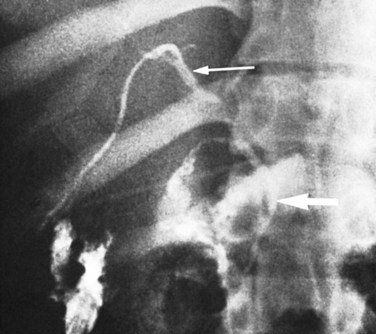
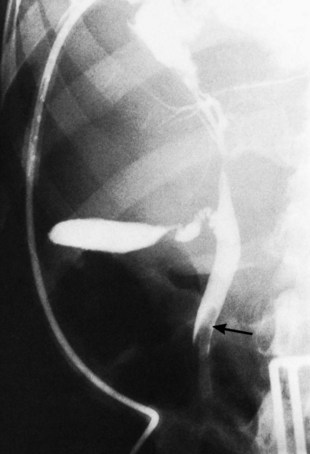
Patients with proximal choledochoduodenal fistulae secondary to peptic ulcer disease may be asymptomatic, or they may present with GI complaints suggesting peptic ulcer (see Fig. 42B.1E). Biliary tract symptoms are usually absent, and these patients generally do not have associated cholelithiasis. In rare instances, cholangitis, jaundice, or abnormal LFTs are part of the clinical picture and indicate concomitant biliary tract infection and obstruction. The diagnosis may be suggested by pneumobilia in 15% to 60% of patients. More often, the diagnosis is made as contrast material from a barium meal refluxes up the common duct to outline a normal-sized, functioning gallbladder. Endoscopy with direct visualization of the ulcer and fistula and ERCP are the best studies to confirm the diagnosis and evaluate the extent of disease (Fig. 42B.10).
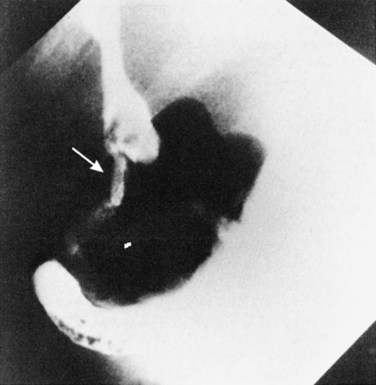
FIGURE 42B.10 Choledochoduodenal fistula secondary to gallstone disease shown by endoscopic retrograde cholangiopancreatography.
Given the paucity of reported experiences with choledochoduodenal fistula (Kourias & Chouliaras, 1964) and long-standing differences of opinion about the correct approach to peptic ulcer disease, it is not surprising that, until recently, treatment recommendations have been controversial. Excellent discussions of the natural history, clinical presentation, and management of these fistulae have been reported by Constant and Turcotte (1968), Feller and colleagues (1980), and Sarr and colleagues (1981). Most authors now agree that treatment should be directed at the ulcer diathesis and not at the biliary tract or the fistula itself. Medical management with proton-pump inhibitors and therapy for Helicobacter pylori is often sufficient to control the ulcer disease and even bring closure of the fistula.
More recent high success rates of medical management for peptic ulcer disease have resulted in a drastic reduction in the number of case reports of patients with proximal choledochoduodenal fistulae compared with years before 1990 (H’ng & Yim, 2003; Jaballah et al, 2001; Wong et al, 2004). Although choledochoduodenal fistula as a result of peptic ulcer is now extremely rare, clinicians should remain aware of this clinical entity, especially in symptomatic patients with refactory peptic ulcer disease. In such patients, surgery is indicated to prevent major complications such as hemorrhage and death (La Greca et al, 2008). Otherwise, surgery is not generally indicated for severe ulcer disease, except in the presence of uncontrollable hemorrhage, free perforation, obstruction, or intractability.
Distal Parapapillary Choledochoduodenal Fistula
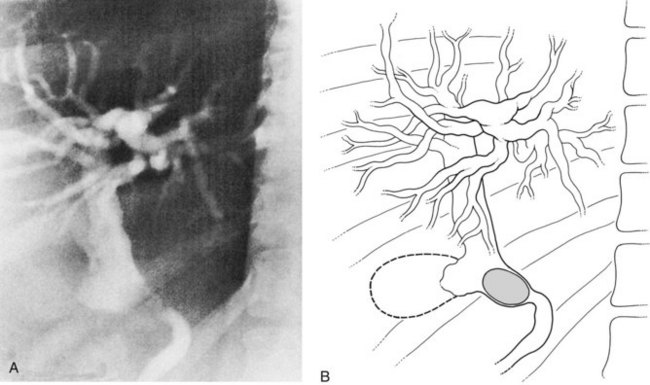
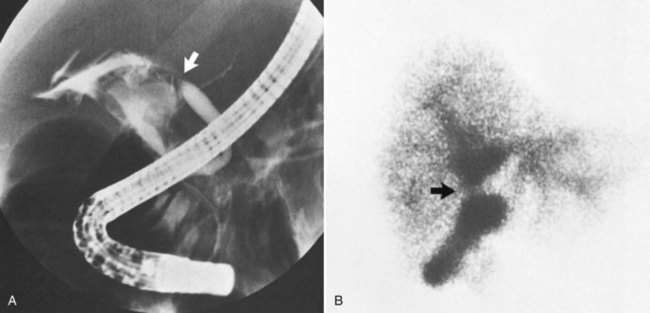
Whether caused by spontaneous gallstone erosion or by iatrogenic damage to the distal common duct, parapapillary fistulae have a clinical presentation similar to that of other advanced calculous biliary tract diseases. In the series reported by Tanaka and Ikeda (1983), the following observations were made: a history of biliary symptoms longer than 10 years’ duration in 46%; pain and/or jaundice in 88% and 69%, respectively; prior biliary surgery in 54%; air or barium in the biliary tree in 41%; cholelithiasis in 71%; and choledocholithiasis in 38%. The anatomic diagnosis rests on meticulous endoscopic observation and expertise in the technique of ERCP (Fig. 42B.11; see Fig. 42B.1F). In Japan, the incidence of primary intrahepatic stone disease is reported to be 17% to 30% (Tanaka & Ikeda, 1983; see Chapter 39). Particularly in Japanese patients with parapapillary fistulae, careful and complete evaluation of the intrahepatic ductal system is important.
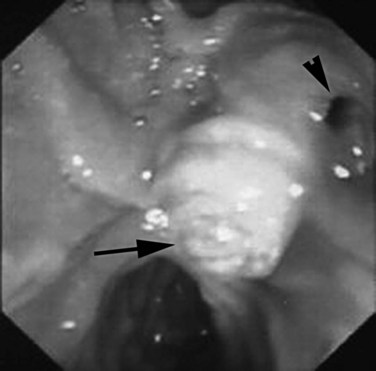
FIGURE 42B.11 Duodenoscopy showing the papilla of Vater (arrow) and a distal or peripapillary choledochoduodenal fistula (arrowhead).
(Courtesy Dr. Melih Karincaglu.)
The management of these biliary fistulae is still evolving, and a variety of endoscopic techniques have been advocated (Osnes & Kahrs, 1977; Ramsey et al, 1992; Schapira & Khawaja, 1982; Urakami & Kishi, 1978; Van Linda & Rosson, 1984). These techniques involve using endoscopic sphincterotomy to widen the choledochal fistula enough to permit free drainage or to create a common channel between the fistula and natural ampullary orifice. When necessary, open surgical procedures, such as hepaticodochojejunostomy, have been used (Hunt & Blumgart, 1980).
Fistula to the Hepatic Veins or Portal Veins
Biliary-venous fistula is a rare occurrence that results in bilhemia, a condition that can be dangerous (see Chapter 105), and such a fistula can also be associated with interventional transhepatic maneuvers. When a large caliber intrahepatic bile duct is transected, the creation of a transjugular intrahepatic portosystemic stent-shunt (TIPSS) may be complicated by a biliary-venous fistula. The resulting biliary leak plays an important role in the stenosis and occlusion of the portosystemic shunt (Jalan et al, 1996).
External Biliary Fistulae
Etiology and Prevention
Fistulae After Cholecystostomy
The first open cholecystotomy was done by Bobbs in 1867 (Sparkman, 1967), but open cholecystostomy is infrequently performed today. Historically, the procedure was used in high-risk patients with acute cholecystitis. In selected cases, cholecystostomy also was used as the first step of a two-stage operation for obstructive jaundice secondary to malignancy (see Chapter 62A, Chapter 62B ). Percutaneous cholecystostomy, a less invasive procedure that requires only local anesthesia, is currently the procedure of choice in high-risk elderly patients (Byrne et al, 2003; Ito et al, 2004; Winbladh et al, 2009). Percutaneous placement of a catheter into the lumen of the gallbladder can be performed and can even be done under US guidance at the bedside of critically ill patients (see Chapters 28 and 32). A persistent fistula denotes distal biliary obstruction (e.g., a retained gallstone lodged within a Hartmann pouch).
Biliary Fistulae After Invasive Radiologic Procedures
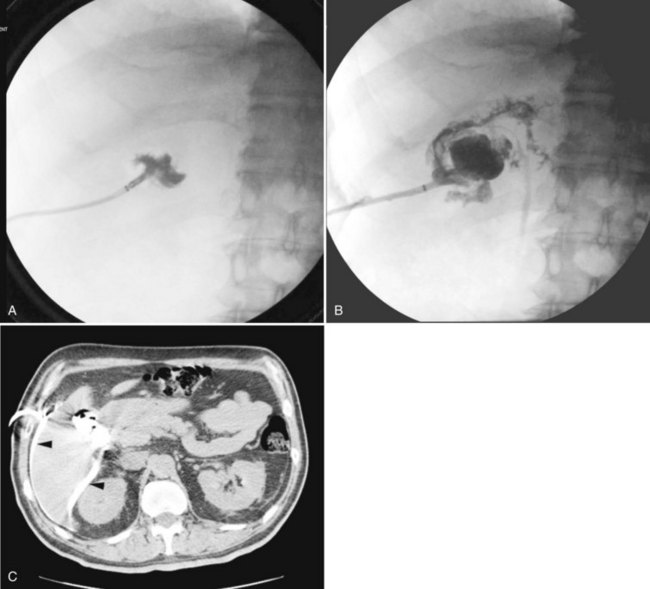
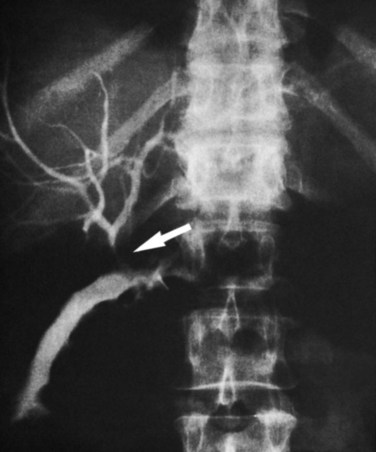
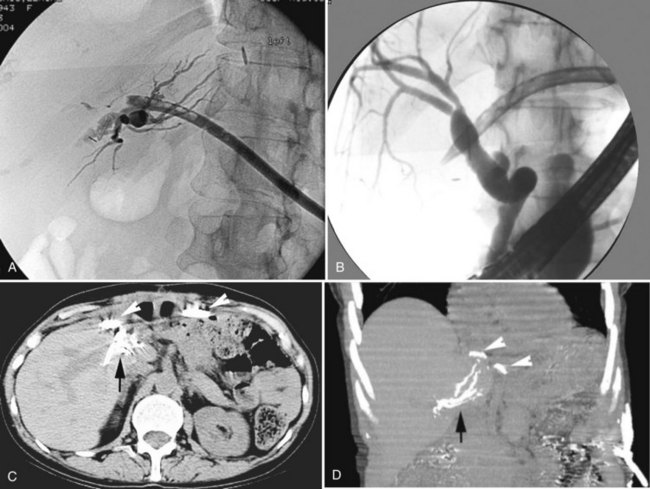
Biliary leak and fistulae may follow any invasive radiologic procedure involving the hepatobiliary system. Usually these are due to distal biliary tract obstruction, a result of either a retained bile duct stone or an unrecognized malignancy (Fig. 42B.12). Published rates for biliary leak after interventional radiologic procedures vary significantly and depend on patient selection and individual practitioner experience. Complications result from suboptimal puncture location, incorrect selection of biliary catheter size, and inadequate postprocedural tube care (Winick et al, 2001). Burke and colleagues (2003) classified bile leakage, sepsis, and infected biloma as major complications that occur at rates of 2% after PTC and biliary drainage procedures (see Fig. 42B.5). The risk of an internal fistula after radiologic intervention resulting in bilhemia was referred to earlier.
Fistulae After Abdominal Operations
Laparoscopic Cholecystectomy
Laparoscopic cholecystectomy is currently the standard procedure for symptomatic cholelithiasis and for all forms of cholecystitis, including acute cholecystitis, and it is performed even in instances of gangrenous cholecystitis (Kiviluoto et al, 1998; Gharaibeh et al, 2002; Borzellino et al, 2008). However, laparoscopic cholecystectomy is associated with an increased incidence of bile duct injuries, including fistulae, which are reported in 1.3% to 5.5% of cases (see Chapter 34; Adamsen et al, 1997; Kum et al, 1996). Under these difficult circumstances, the procedure should be performed, or at least supervised, by an experienced surgeon. A high conversion rate of 40% in instances of gangrenous cholecystitis should be expected (Eldar et al, 1998; Gharaibeh et al, 2002; Borzellino et al, 2008).
As mentioned previously, the operative treatment of Mirizzi syndrome now includes a laparoscopic option. In accordance with others, however, we believe that when the presence of Mirizzi syndrome, particularly type 2, is suspected or realized, safety demands that the laparoscopic procedure be converted to open laparotomy (Moser et al, 1993; Yeh et al, 2003; Antoniou et al, 2010).
Small amounts of bile leakage may occur occasionally in the immediate postoperative period after inadvertent damage to a subvesical duct (Viikari, 1960), which is present in normal subjects in 20% to 50% of cases (Kune & Sali, 1980). Removal of an intrahepatic gallbladder also may cause damage to tiny bile ducts in the liver around the gallbladder fossa.
Most ductal injuries are not recognized during the initial laparoscopic cholecystectomy (Lillemoe et al, 1997) or in the immediate postoperative period, mainly because drains usually are not left in the abdomen, and the patient typically leaves the hospital within 24 hours. The resulting uncontrolled biliary fistula becomes evident within days or sometimes weeks after the operation, with the clinical presentation of abdominal pain, fever, jaundice, and the demonstration of an intraabdominal fluid collection that produces bile on puncture.
Compared with open cholecystectomy, laparoscopic cholecystectomy is associated with an increased rate of bile duct injury (see Chapter 42A; Davidoff et al, 1992; Waage & Nilsson, 2006). Such injuries are common to most reported series of laparoscopic cholecystectomy and may reach an incidence of 0.9%. Reviews by Strasberg and colleagues (2001) and Vecchio and colleagues (1998) that encompass more than 100,000 patients found the incidence of major bile duct injury to be around 0.5%, and this incidence has reached a “steady state” (Walsh et al, 1998; Wherry et al, 1996; Waage & Nilsson, 2006). A large retrospective cohort analysis of nearly 1.6 million Medicare patients in the United States undergoing laparoscopic cholecystectomy from 1992 through 1999 confirmed the incidence rate of bile duct injury at 0.5% (Flum et al, 2003), and a Swedish population-based study of more than 150,000 patients showed a similar bile duct injury rate of 0.4% (Waage & Nilsson, 2006).
The mechanisms resulting in bile duct injuries are now well recognized (see Chapter 42A). The most common injury is caused by misidentification of the common duct for the cystic duct, resulting in complete transection of the common duct, often with some portion of the biliary tree (Fig. 42B.13). A traction injury results from inadvertent lateral traction of the gallbladder and “tenting” of the cystic duct–common duct junction. In this instance, the clip intended for the cystic duct may occlude the CBD, or a portion of the CBD may be removed between clips. An unrecognized anomalous biliary system—in particular, a cystic duct that empties directly into the right hepatic duct or a low-entry right hepatic sectoral duct—may result in similar damage (Fig. 42B.14; see Chapters 1B and 42A; Davidoff et al, 1992; Lillemoe et al, 1997). Other, less common mechanisms include thermal injury resulting from excessive use of the cautery or laser and the application of excessive clips to control bleeding in the triangle of Calot.
The role of operative cholangiography (see Chapter 21) in the prevention of bile duct injuries remains controversial despite two large, population-based studies demonstrating a protective effect (Flum et al, 2003; Waage & Nilsson, 2006). Intraoperative cholangiography (IOC) may supply information regarding the presence of unsuspected choledocholithiasis and unexpected anomalous anatomy, and its routine use is recommended by several authors (Fletcher et al, 1999; Flum et al, 2003; Kohn et al, 2004; Stuart et al, 1998; Waage & Nilsson, 2006). The use of IOC has not increased; in reports of laparoscopic cholecystectomy in the United States, IOC was performed in about 40% of cases (Flum et al, 2003; MacFadyen et al, 1998). In a population-based study by Flum and colleagues (2003), the rate of bile duct injury was found to be significantly higher when IOC was not used (0.39% vs. 0.58%). The more recent study by Waage and Nilsson (2006) showed that IOC reduced the risk of bile duct injury by 37%.
Before these reports, evidence that IOC prevented major bile duct injury did not exist (Wright & Wellwood, 1998). In a national survey of American surgeons performing laparoscopic cholecystectomy, Archer and colleagues (2001) found that IOC was helpful for intraoperative detection of bile duct injury. Interestingly, of the 1600 surgeons surveyed in the United States, one third had experienced this complication. A single institutional study by Kohn and associates (2004) examined the use of routine versus selective cholangiography among surgeons performing laparoscopic cholecystectomy. This study found that surgeons performing selective cholangiography were less likely to attempt IOC, even if indications arose resulting in significantly worse adverse events. Regardless of surgeon preference for routine or selective IOC, we recommend a low threshold for early conversion to laparotomy, if the ductal anatomy remains unclear or for any other concern. IOC remains the gold standard to define biliary anatomy, but it cannot prevent all bile duct injuries; and in some rare cases, it may even be the cause.
Open Cholecystectomy
The occurrence of unexpected biliary fistulae after cholecystectomy almost always indicates operative injury to a major bile duct. Such fistulae may arise from damage to the CBD (Fig. 42B.15) or to an anomalous sectoral right hepatic duct (see Chapter 42A). A 0.21% incidence of bile duct injuries was found in a study of 42,474 patients who underwent an open cholecystectomy (Roslyn et al, 1993). Bile duct injury is recognized at the time of cholecystectomy in only a few patients; in approximately 25% to 40% of patients with unrecognized bile duct injury, the injury becomes apparent only when the presence of a biliary fistula is recognized (Andren-Sandberg et al, 1985a, 1985b). In the remaining patients, the injury is recognized only later, when a biliary stricture develops (Blumgart et al, 1984). The recognition of biliary ductal anomalies and prevention of biliary injury at cholecystectomy are discussed in detail in Chapters 1B and 42A. Inadequately ligated or sloughed ligatures on the cystic duct are rarely responsible for biliary fistulae, and for this reason, transfixion suturing of the cystic stump is recommended. However, the presence of an unrecognized significant distal obstruction may be followed by a blowout of the cystic duct stump, resulting in a biliary fistula or bile peritonitis.
Cholecystectomy is sometimes performed under difficult circumstances, as in the presence of a gangrenous gallbladder associated with fibrosis and inflammation in the region of the triangle of Calot. In these instances, proper identification and transfixion of the cystic stump may not be possible, and the patient is left with a temporary biliary fistula (Fig. 42B.16). As described previously, type 2 Mirizzi syndrome may pose significant technical difficulties, and specific surgical techniques have been devised to deal with this situation (Baer et al, 1990).
Common Duct Exploration
The classic open common duct exploration is now less frequently performed, having been replaced by laparoscopic and endoscopic techniques (see Chapter 35; Csendes et al, 1998). Whether it occurs after open or laparoscopic exploration of the CBD or persists after removal of a T-tube, a biliary fistula is almost always due to a residual common duct gallstone (Fig. 42B.17). It is essential to perform cholangiography and rule out the presence of retained stones before removal of a T-tube or biliary stent.
Less commonly, an overlooked malignant distal obstruction is the causative factor (Fig. 42B.18). In addition, the passage of metal bougies through the papilla during common duct exploration may result in the creation of false tract, which may result in a choledochoduodenal fistula and may cause jaundice, ascending cholangitis, and acute or chronic recurrent pancreatitis. Treatment may be by endoscopic papillotomy or sphincterotomy, joining the fistulous orifice with the papillary opening (Chung & Roberts-Thomson, 2000; Jorge et al, 1991; Karincaoglu et al, 2003b), but a surgical approach may be necessary (Hunt & Blumgart, 1980).
Biliary-Intestinal Anastomosis
A major biliary fistula after biliary-intestinal anastomosis occasionally occurs, although this is uncommon. Anastomoses created well below the hilus, such as choledochoduodenostomy or choledochojejunostomy, are rarely associated with fistulae (Parrilla et al, 1991), whereas a biliary fistula after hepaticojejunostomy is more common, with a reported incidence of 0.4% to 8% (de Castro et al, 2005). When fistulae do occur, a technical error must be suspected, such as suture line disruption or failure to incorporate a significant bile duct within the anastomosis. Failure of the surgeon to appreciate ductal anatomy is particularly likely to occur in the hilar region, where the mode of confluence of the right and left ducts and caudate lobe ducts is extremely variable (see Chapter 1B). In these instances, the fistula becomes evident immediately after surgery. Meticulous technique with mucosa-to-mucosa anastomosis (see Chapter 29) prevents most such leaks. Alternatively, suture line disruption also may be caused by local factors, such as abscess or ischemic necrosis of the bile duct or bowel wall, and such fistulae may become evident days after surgery. It is important to ascertain whether the fistula is purely biliary or whether it also contains duodenal or pancreatic juice or both.
Fistula After Liver Injury, Liver Resection, and Liver Transplantation
Liver Injury
Liver injury (see Chapter 102) may be followed by the formation of biliary fistulae. Such fistulae may occur in association with damage to the liver (Fig. 42B.19) and the bile ducts or may follow sequestration and infection of areas of liver necrosis. Blunt or penetrating grade III or IV liver trauma may be complicated by bile collections and biliary fistulae in 0.5% to 14% of patients (Glaser et al, 1994; Goldman et al, 2003; Gourgiotis et al, 2007; Shahrudin & Noori, 1997; Vassiliu et al, 2004; Wahl et al, 2005). Emergency partial hepatectomy for major liver trauma may result in injury to the bile ducts at the confluence with an early biliary leak and, later, a biliary stricture (Bismuth et al, 1986). Complete transection of the CBD requires immediate hepaticojejunostomy, whereas lacerations of the main biliary channel may be sutured after placement of a T-tube.
Although nonoperative management of blunt liver injury is now firmly established as standard therapy for hemodynamically stable patients (Malhotra et al, 2000; Pachter & Hofstetter, 1995; Christmas et al, 2005), caution should be exercised, because this approach can be hazardous sometimes and demands close observation to avoid delayed surgical treatment of potentially fatal complications (Goldman et al, 2003). Most posttraumatic biliary fistulae can be expected to heal spontaneously (Vassiliu et al, 2004); however, in a case series of 32 patients treated nonoperatively, Carrillo and colleagues (1999) found severe biliary complications in up to 85% of patients with a liver abbreviated injury score (AIS) of grade IV or higher. A study specifically examining the diagnosis and management of bile leaks after liver injury by Wahl and colleagues (2005) confirmed that patients with liver injuries higher than grade IV are more likely to develop bile leaks, especially if treated by arterial embolization. These authors proposed a treatment algorithm for blunt liver injury associated with a bile leak, which includes laparoscopic washout for a diffuse leak. Laparoscopic evacuation and lavage as a new treatment option for bile collections has also been advocated by others to prevent the inflammatory sequelae of bile peritonitis (Franklin et al, 2007). Persistent biliary fistulae may occur from a segment of the liver isolated by the injury. Management of this situation is difficult, particularly when the fistula is associated with a distal stricture. Rarely, the fistula can be identified at operation and oversewn. Alternatively, a well-developed fibrous fistulous tract may be anastomosed to a jejunal loop or to the gallbladder (see Fig. 42B.19; Smith et al, 1982).
Biliovenous fistula with subsequent leakage of bile into the venous system (bilhemia) is a rare but serious complication of liver trauma. After the formation of a necrotic cavity within the liver, an open connection between a vein and an intrahepatic bile duct may occur, allowing bile to leak into the venous circulation (see Chapter 105; Haberlik et al, 1992). Rarely, transjugular intrahepatic portosystemic shunting (TIPS; see Chapter 76E) has been reported to be a direct cause of bilihemia (Jawaid et al, 2003; Singh et al, 2007; Singal et al, 2009).
Liver Resection
The incidence of bile leak after hepatic resection ranges from 1.7% to 12% in large series (Lo et al, 1998; Capussotti et al, 2006; Nagano et al, 2003; Vigano et al, 2008; Tanaka et al, 2002; Yamashita et al, 2001; see Chapter 25, Chapter 90A, Chapter 90B, Chapter 90C, Chapter 90D, Chapter 90E, Chapter 90F ). In most patients, bile leak resolves spontaneously, but sometimes a persistent fistula requires reoperation (Honore et al, 2009). Liver resection done for tumor (see Chapter 90A, Chapter 90B, Chapter 90C, Chapter 90D, Chapter 90E, Chapter 90F ) may be followed by biliary fistula, which may result from inadequate ligation of the bile ducts at the cut liver surface (Thompson et al, 1983) or from failure to secure the bile ducts at the hilus (Fig. 42B.20). This failure is more likely after right hepatectomy, in which the anatomy of the right sectoral ducts is variable in the hilar region. Extended left hepatic lobectomy also has been associated with a high incidence of biliary fistula (see Chapter 90A, Chapter 90B, Chapter 90C, Chapter 90D, Chapter 90E, Chapter 90F ; Starzl et al, 1982). In Japan, Yamashita and colleagues (2001) reported a biliary fistula rate of 4% (31 of 781) after hepatic resection. Their analysis identified operative procedures exposing the major Glisson capsule and including the hilum—anterior sectorectomy, central hepatectomy, and caudate resections—to be high-risk operations for development of postoperative bile leakage. Other factors that may contribute to a persistent bile fistula may include underlying cirrhosis or chronic hepatitis that impair wound healing (Tanaka et al, 2002). Once a posthepatecomy biliary fistula is established, if the drainage output exceeds 100 mL per day 10 days after diagnosis, interventional or endoscopic procedures should be considered to promote definitive closure (Vigano et al, 2008).
Operative injury to the biliary tract that is likely to result in fistula is more common after resection of lesions involving or close to the hilar structures (Fig. 42B.21). It also is more likely to occur after resection of lesions involving the caudate lobe ducts, because the anatomy of these ducts is significantly variable in the hilar region. Using major hepatic resection, resection of cholangiocarcinoma combined with biliary-enteric reconstruction may be complicated by a biliary fistula originating from the caudate lobe ducts or from the biliary-enteric anastomosis (see Chapter 90A, Chapter 90B, Chapter 90C, Chapter 90D, Chapter 90E, Chapter 90F ). Hepatic cryotherapy (see Chapter 85B), which may be used at the cut liver surface after hepatectomy or for ablation of deep intrahepatic lesions, may also be complicated by biliary fistula (Sarantou et al, 1998). It may be prudent to leave a drain in situ after this procedure.
Liver Transplantation
Biliary leaks and fistulae are a continuing source of morbidity and mortality after liver transplantation. Pathogenesis usually is related to technical and vascular considerations and, in particular, to hepatic artery thrombosis (see Chapter 100).
Gastrectomy
Injury to the bile duct may occur during gastrectomy, particularly when the pyloric region or the first part of the duodenum is grossly distorted and inflamed (Florence et al, 1981). Such an injury becomes apparent either as a biliary fistula or at a later stage with the development of a stricture (see Chapter 42A).
Surgery for Hydatid Disease
Surgery is an important treatment option for patients with hydatid disease (Dziri et al, 2004). Operative options include pericystectomy and hepatic resection or simply unroofing of the cyst and treatment of the residual cavity. Patients treated by these methods are prone to all the known complications associated with liver resection. Hydatid disease of the liver (see Chapter 68) is associated with biliary involvement by the disease in approximately 10% of instances (Erguney et al, 1991). An expanding liver cyst results in compression and stretching of adjacent liver tissue, including the bile ducts. It may erode into a stretched bile duct, with the establishment of continuity between the cyst cavity and the biliary system. Hydatid material may enter into the biliary tree, or bile may leak into the cyst. As with other hepatobiliary conditions, a laparoscopic surgical management option for hepatic hydatid disease has been introduced, but it is limited to those few centers where the disease is endemic (Duttaroy et al, 2008; Palanivelu et al, 2006; Sharma et al, 2009).
Biliary fistula may develop after operation for hydatid disease in certain situations. First, a communication between the cyst cavity and the biliary system is missed at operation and is not directly secured (Fig. 42B.22). It is prudent to drain all cyst cavities, particularly those of multiloculated hydatid cysts, to ensure that if a biliary fistula were to develop, it would be controlled. Unless a distal obstruction is present, these fistulae usually close spontaneously. Second, and rarely, the presence of hydatid material within the biliary tract produces biliary ductal obstruction (Fig. 42B.23) that results in a persistent biliary fistula that is relieved only when the hydatid material passes or is removed. Removal is achieved by exploration of the CBD with or without a bypass procedure (Ozmen et al, 1992) or by endoscopic methods (Iscan & Duren, 1991).
Assessment of the biliary tree, preferably by endoscopic cholangiography, should be performed before surgery in patients with a history of jaundice or cholangitis or in the presence of a large cyst located centrally and abutting the hilar structures. Kayaalp and colleagues (2003) in a series of 113 patients showed that the location of the hydatid cyst near the hilum is a risk factor for the development of a cystobiliary communication and cavity-related complications. When a cystobiliary communication is shown, the biliary system should be cleared of all debris and cyst remnants, and endoscopic sphincterotomy should be performed before surgical intervention (Kornaros & Aboul-Nour, 1996). Percutaneous treatment of hydatid cysts is associated with a 10% incidence of biliary fistula (Men et al, 1999). Such fistulae usually close spontaneously after any biliary distal obstruction is relieved.
Clinical Presentation
In uncontrolled biliary fistula, biliary drainage is inadequate, resulting in an intraabdominal bilious collection. Because the bile is usually or soon becomes infected, the presentation is mostly of a subphrenic or subhepatic abscess or generalized peritonitis. The situation may be complicated further by cholangitis with or without intrahepatic abscess and septicemia that demands urgent treatment. In some patients with sterile bile, huge volumes may accumulate within the peritoneal cavity with minimal clinical findings apart from a distended abdomen (Fig. 42B.24). A high index of suspicion is important, because minimal abdominal symptoms and slightly deranged LFTs may be the only indicators of biliary damage. The presence of skin excoriation and digestion implies that activated digestive enzymes are present in the fistula effluent, because bile alone contains no digestive enzymes.
Pathologic Consequences of External Biliary Fistulae
Total biliary loss for short periods (≤3 weeks) may not result in a serious depletion of electrolytes and fluid, because the body is able to compensate for this loss. Long-term total external biliary fistula results in fluid and electrolyte disturbances if replacement therapy is not instituted. Sodium loss is usually greater than chloride loss, leading to metabolic acidosis (Cass et al, 1955). The serum potassium level is initially lowered, but the accompanying fluid loss may lead to a decrease in plasma volume, low-output renal failure, and hyperkalemia (Knochel et al, 1962). Absence of bile from the GI tract causes interference in the absorption of fat-soluble vitamins A, D, and K. Vitamin A and D deficiencies are associated with long-term total biliary fistula and are rarely seen today, whereas vitamin K deficiency is evident earlier and can be easily diagnosed by a prolonged PT. Other adverse effects of total biliary loss are disruption of intestinal barrier function and bacterial translocation (Slocum et al, 1992). These findings are supported by an observation that bile replacement restored intestinal barrier function in patients with malignant obstruction undergoing external biliary drainage (Kamiya et al, 2004). Even in the short term, patients with an external biliary fistula feel unwell, weak, and lethargic. In advanced and neglected cases, caloric and protein malnutrition results in gradual weight loss, whereas the electrolyte changes may result in stupor and vasomotor collapse.
Diagnostic Procedures and Initial Treatment
The presence of a biliary fistula may first become apparent at reoperation. More commonly, and particularly after laparoscopic cholecystectomy, the biliary fistula becomes apparent at ERCP or after percutaneous drainage of a perihepatic collection. Reoperation is usually required for peritonitis or for drainage of an intraabdominal collection that is not amenable to percutaneous treatment. Such collections may be a complication from a previous surgery on the hepatobiliary system, the pancreas, or, rarely, the stomach or duodenum. Rarely, they may follow a spontaneously occurring pathologic process, such as hemorrhagic pancreatitis or rupture of a liver cyst. When the presence of an uncontrolled biliary fistula has been realized, initial management demands conversion of the fistula into a controlled one, usually by means of tube drainage. No attempt at definitive repair should be made at this early stage, because the involved bile ducts are collapsed, friable, and usually embedded within a severe local inflammatory reaction; it is virtually impossible to expose healthy bile ducts for any form of long-lasting definitive repair, and any attempt to do so is bound to fail and renders further operation more difficult (see Chapter 42A; Czerniak et al, 1988).
Alternatively, percutaneous CT- or US-guided drainage of a subphrenic or infrahepatic fluid collection may be followed by the establishment of an external biliary fistula. It is important to ensure adequate drainage and to see that the fistula is well controlled. Biliary drainage is ideally carried out using a sealed drainage-bag system (Blenkharn et al, 1981). Drainage initially should be done using a low-pressure, closed-suction system, which is valuable in reducing the cavity of the intraabdominal bile collection or in reducing the abscess of a fistula tract. Improvement of the clinical picture and repeated US or CT studies eventually should show proper positioning of the drain and reveal no residual collection or abscess. HIDA scintigraphy (see Chapter 15) and tubography are helpful in this respect (see Fig. 42B.18).
After the fistula is controlled, conservative treatment is instituted, the patient is nourished, deficits of electrolytes and vitamins (mostly vitamin K) are corrected, and infection is treated. It is important to know whether the biliary fistula contains bile only or whether it also contains duodenal, pancreatic, or intestinal juice; when the latter is present, appropriate measures to protect the skin should be taken. Parenteral nutrition is an essential element in the management of significant duodenal and pancreatic fistulae, in which total prohibition of oral intake is important to allow healing to occur. It has been shown that treatment with somatostatin can reduce bile secretion significantly (Nyberg, 1990; Sahin et al, 1999), and somatostatin has been used successfully in the management of pancreatic and small intestine fistulae (Draus et al, 2006); it may also be valuable in the management of combined biliary-pancreatic-duodenal fistulae. However, the benefit of somatostatin therapy in promoting closure of biliary fistulae arising entirely from the biliary tree remains unproven (Hesse et al, 2002). We are aware of three case reports that describe successful use of octreotide, a somatostatin analogue, in the treatment of bronchobiliary fistulae (Ong et al, 2004; Petrolati et al, 2009; Singh et al, 2002).
Fistulography is a simple and effective means of determining whether biliary drainage is adequate and whether a fistulous cavity has converted to a fistulous tract. The site and underlying cause of the biliary fistula often can be clearly shown by fistulography (Fig. 42B.25; see Figs. 42B.13, 42B.14, 42B.19, 42B.21, and 42B.23). PTC is used when the findings of fistulography are equivocal, and the intrahepatic biliary tract, the right system in particular, is not fully shown. Iatrogenic damage to a right sectoral hepatic duct is an example of this problem, and PTC is the only diagnostic modality to yield accurate definition.
ERCP is a useful diagnostic and therapeutic tool where there is a continuity of the extrahepatic biliary system (Davids et al, 1992), particularly after laparoscopic cholecystectomy (Agarwal et al, 2006) and after liver transplantation (Sherman et al, 1993). The value of ERCP is limited in fistulae arising at the hilus of the liver and resulting from iatrogenic bile duct injury (see Chapters 18 and 27).
HIDA scintigraphy, as mentioned earlier (see Chapter 15), is a useful noninvasive method of evaluating liver function and bile secretion (Holbrook et al, 1991). Although it may not supply accurate anatomic details, information such as the presence of a fistula, its origin (liver or extrahepatic biliary system; see Figs. 42B.3 and 42B.24), and adequacy of its drainage (controlled or uncontrolled; Figs. 42B.26 and 42B.27; McPherson et al, 1984) can be clearly obtained.
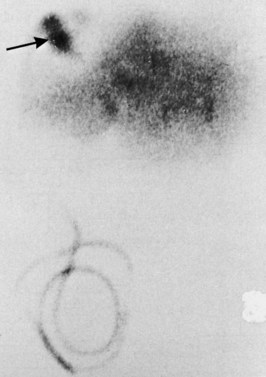
FIGURE 42B.26 Hepatobiliary iminodiacetic acid scan showing a fistula issuing from the liver surface (arrow). This is the same patient as is Figure 42B.23. The drainage tube also is shown.
(From McPherson GA, et al, 1984: The role of HIDA scanning in the assessment of external biliary fistulae. Surg Gastroenterol 3:77-80.)
MRCP and CT allow excellent assessment in hepatic biliary, arterial, and portal venous anatomy. In particular, use of CT after fistulography can help verify maturation of a fistula tract and define perihepatic bile collections or isolated liver segments (see Figs. 42B.5 and 42B.20).
Treatment
The principles of management of postoperative biliary fistulae are essentially the same regardless of the initial surgical procedure; these principles are outlined in Figure 42B.28 and are modified slightly according to the initial surgical procedure and the individual patient. ERCP and endoscopic techniques play an integral and indispensable role in the management of biliary fistulae. The individual treatment plan should be made and agreed on jointly by all members of the team involved in the treatment of the patient.
As stated earlier, it is important to establish a controlled fistula; this can be achieved initially by US- or CT-guided drainage of the abscess or collection. Operation is indicated when nonoperative measures are not suitable, such as in a patient with diffuse bile peritonitis, a septic patient with an intraabdominal abscess too large to be drained by the percutaneous route, or a patient with necrotic material and debris within the abscess. Early surgery also is indicated when percutaneous drainage has failed. During the operation, the temptation to attempt primary repair at this stage must be resisted; only good drainage should be established. Occasionally, drainage of large amounts of bile with significant fluid and electrolyte loss may necessitate early operation, when the external fistula may be converted to an internal fistulojejunostomy using a mobilized and approximated Roux-en-Y jejunal loop (see Fig. 42B.19; Smith et al, 1982). In this procedure, the drainage tube is led from the fistula origin through the jejunal loop to the exterior; this allows control, but it creates an almost certain necessity to reoperate for restenosis at a later stage.
It is important to identify the presence or absence of biliary-enteric bile flow, which differentiates a total biliary fistula from a partial fistula with some residual biliary-enteric continuity. Subsequent management is influenced significantly, because operation is usually unavoidable in the former, and a nonoperative approach may be successful in the latter. A total biliary fistula rarely closes spontaneously when an internal fistula develops between the divided upper duct and the gut (Collins & Gorey, 1984).
When biliary-enteric continuity is present, and bile flow is not obstructed distal to the origin of the fistula, a prolonged period of conservative treatment is indicated, because spontaneous closure of the fistula is common. Fistula closure may be facilitated by temporary placement of a stent across the fistulous opening in the bile duct, excluding bile flow through the fistula. This method may be attempted where the CBD is intact above the fistula origin, and the defect in the common duct is not too large. Stenting may be achieved by endoscopic placement of an endoprosthesis or a nasobiliary tube with its tip above the origin of the fistula. When a short period of stenting is anticipated, nasobiliary intubation is preferred, because it enables follow-up cholangiography, damage to the papilla is minimal, and a second endoscopic procedure is avoided (Barthel & Scheider, 1995; Grala et al, 2009). Using this method, some fistulae close within 2 weeks (Toriumi et al, 1989). It is also useful in patients with liver transplant who have undergone duct-to-duct biliary reconstruction. In patients with concomitant CBD stones or stricture, a papillotomy is indicated. Nasobiliary tubes are cumbersome for the patient, and they may occasionally dislodge; if used for prolonged periods, they may enhance metabolic acidosis resulting from bile loss.
Endoscopic treatment of fistulae with intact biliary-enteric flow by sphincterotomy, stenting, or both is effective in most patients (Agarwal et al, 2006; Rauws & Gouma, 2004). In most instances, a biliary endoprosthesis is used and is left in place for several weeks, until the fistula closes. Closure is verified by HIDA scan before removal of the stent. If standard endoscopic therapy fails, an alternative endoscopic technique using n-butyl-2-cyanoacrylate glue occlusion of the fistula has been shown to be effective and helps avoid the need for surgery (Seewald et al, 2004).
Some authors recommend endoscopic sphincterotomy alone with the intention of reducing the pressure gradient between the biliary system and the duodenum (Ponchon et al, 1989). This procedure is unnecessary, because the fistula will close if no distal obstruction is present. Sphincterotomy may also be associated with short-term and long-term septic and obstructive complications (Kracht et al, 1986), therefore it should be avoided unless specifically indicated (e.g., in patients with a papillary stricture). It also has been shown that stenting is more effective than sphincterotomy alone in the resolution of biliary fistulae (Marks et al, 1998). As stated earlier, we believe that although endoscopic approaches are useful, they generally should be delayed for days to a few weeks to allow spontaneous closure of the fistula, which obviates the need for further intervention and its attendant complications.
After an obstruction distal to the fistula has been diagnosed, it should be dealt with, because until it has been managed, the fistula will not close spontaneously. Obstruction is usually caused by a retained stone or a stricture: retained stones usually are removed by endoscopic means, and relief of a benign stricture can be achieved using balloon dilation, applied either endoscopically or by interventional radiology after PTC. The patient is treated conservatively and expectantly for fistula closure or for restricture, and fistula closure can be facilitated by temporary stenting (Fig. 42B.29), if the location and the size of the defect in the bile duct are suitable, and restricture is treated operatively.
The use of temporary stenting is limited in highly complex hilar fistulae combined with stricture, particularly fistulae with separation of a right and left ductal system. Some authors place an endoprosthesis across the stricture and leave it for months (Ponchon et al, 1989), even for more than 1 year, with periodic replacements (Davids et al, 1992). This practice is not only unnecessary but is often harmful, resulting in obstruction and septic complications; the endoprosthesis should be removed when the fistula has closed.
After closure of the fistula, patients are followed up carefully with regular LFTs and HIDA scans to detect early signs of the development of a biliary stricture, which may take months and sometimes years. Stricture is most likely to occur where a stricture has already been present or where a fistula is associated with an injury to a major bile duct (Blumgart et al, 1984). The proposed plan may involve relatively prolonged management, but it improves the chances of a successful and long-lasting bile duct repair, especially after injury at cholecystectomy. In this situation, early and untimely surgical attempts at definitive repair carry a high risk of biliary leak and anastomotic stricture.
Management of the rare patient with excluded or “orphaned” segemental bile ducts following hepatectomy is extremely challenging. This complication results from unrecognized anatomic biliary variations (see Chapter 1B), or when normal anatomic planes are ignored during hepatic transection. The end result is that a portion of liver tissue remains well perfused but entirely disconnected from the biliary tract (see Fig 42B.20). Multiple interventional treatments have been described, the majority consisting of sclerotic agents that are injected directly into the fistula tract. These substances include tetracycline, ethanol, and acetic acid, (Shaw et al, 1989; Shimizu et al, 2006; Park et al, 2005). Other agents such as fibrin glue, thrombin, and n-butyl cyanoacrylate have been used with variable success (Saad & Darcy, 2008; Vu et al, 2006). We are aware of one successful case report of a patient who was treated by percutaneous intraductal laser ablation after failing other treatments (Eicher et al, 2008). When these nonoperative treatments fail, repeat liver resection is necessary and usually definitive (Honore et al, 2009). The authors have successfully used ablation of the liver tissue containing the orphaned biliary segement to treat persistent biliary fistulae.
Adamsen S, et al. Bile duct injury during laparoscopic cholecystectomy: a prospective nationwide series. J Am Coll Surg. 1997;184:571-578.
Agarwal N, et al. Endoscopic management of postoperative bile leaks. Hepatobiliary Pancreat Dis Int. 2006;5:273-277.
Aitken RJ, et al. Choledochopancreatoduodenal fistula caused by duodenal ulceration: a case report. S Afr Med J. 1986;69:707-708.
Alestig K, et al. Biliobronchial fistula secondary to echinococcal abscess of the liver. Acta Chir Scand. 1972;138:90-94.
Allen JW, et al. Totally laparoscopic management of gallstone ileus. Surg Endosc. 2003;17:352.
Al Nakib B, et al. Choledochoduodenal fistula due to tuberculosis. Endoscopy. 1982;14:64-65.
Andalkar L, et al. Bronchobiliary fistula as a complication of liver metastases: diagnosis by HIDA scan. Clin Nucl Med. 2004;29:289-291.
Andren-Sandberg A, et al. Accidental lesions of the common bile duct at cholecystectomy. II. Results of treatment. Ann Surg. 1985;201:452-455.
Andren-Sandberg A, et al. Accidental lesions of the common bile duct at cholecystectomy: pre- and perioperative factors of importance. Ann Surg. 1985;201:328-332.
Anseline P. Colonic gall-stone ileus. Postgrad Med J. 1981;57:62-65.
Antoniou SA, Antoniou GA, Makridis C. Laparoscopic treatment of Mirizzi syndrome: a systematic review. Surg Endosc. 2010;24:33-39.
Archer SB, et al. Bile duct injury during laparoscopic cholecystectomy: results of a national survey. Ann Surg. 2001;234:549-559.
Argyropoulos GD, et al. Gallstone perforation and obstruction of the duodenal bulb. Arch Surg. 1979;114:333-335.
Arvanitidis D, et al. Cholecystocolic fistula demonstrated by endoscopic retrograde cholangiopancreatography. Postgrad Med J. 2004;80:526.
Augur NAJr, Gracie WAJr. Cholecystocolonic fistula associated with malabsorption. Am J Gastroenterol. 1970;53:558-563.
Ayantunde AA, Agarwal A. Gallstone ileus: diagnosis and management. World J Surg. 2007;31:1292-1297.
Baer HU, et al. Management of the Mirizzi syndrome and the surgical implications of cholecystcholedochal fistula. Br J Surg. 1990;77:743-745.
Bagia JS, et al. Mirizzi syndrome: an extra hazard for laparoscopic surgery. Aust N Z J Surg. 2001;71:394-397.
Ball CG, et al. Importance of liver drainage in biliary-bronchopleural fistula resulting from thoracoabdominal gunshot injury. Can J Surg. 2009;52:E12-E13.
Balthazar EJ, Gurkin S. Cholecystoenteric fistulas: significance and radiographic diagnosis. Am J Gastroenterol. 1976;65:168-173.
Balthazar EJ, Schechter LS. Gallstone ileus: the importance of contrast examinations in the roentgenographic diagnosis. Am J Roentgenol Radium Ther Nucl Med. 1975;125:374-379.
Balthazar EJ, Schechter LS. Air in gallbladder: a frequent finding in gallstone ileus. Am J Roentgenol. 1978;131:219-222.
Barthel J, Scheider D. Advantages of sphincterotomy and nasobiliary tube drainage in the treatment of cystic duct stump leak complicating laparoscopic cholecystectomy. Am J Gastroenterol. 1995;90:1322-1324.
Becker CD, et al. Preoperative diagnosis of the Mirizzi syndrome: limitations of sonography and computed tomography. Am J Roentgenol. 1984;143:591-596.
Benson AJ, et al. Biliary-colonic fistula diagnosed via hepatobiliary scintigraphy. Clin Nucl Med. 2001;26:150-151.
Bhama JK, et al. Bouveret’s syndrome. Surgery. 2002;132:104-105.
Binnie NR, et al. Mirizzi syndrome managed by endoscopic stenting and laparoscopic cholecystectomy. Br J Surg. 1992;79:647.
Bismuth H, et al. Liver injuries: the late cases. Clin Surg Int. 1986;12:139-145.
Blanco-Benavides R, Rodriguez-Jerkov J. Sigmoid-biliary fistula: a rare complication of colonic diverticulitis. Am J Gastroenterol. 1992;87:810-811.
Blenkharn JI, et al. An improved system for external biliary drainage. Lancet. 1981;2:781-782.
Blumgart LH, et al. Benign bile duct stricture following cholecystectomy: critical factors in management. Br J Surg. 1984;71:836-843.
Borzellino G, et al. Laparoscopic cholecystectomy for severe acute cholecystitis: a meta-analysis of results. Surg Endosc. 2008;22:8-15.
Bose SM, Sastry RA. Agenesis of gallbladder with choledocho-colonic fistula. Am J Gastroenterol. 1983;78:34-35.
Boyd DP. Bronchobiliary and bronchopleural fistulas. Ann Thorac Surg. 1977;24:481-487.
Brandt LJ. Gallbladder and biliary tree. In: Brandt, LJ, editor. Gastrointestinal Disorders of the Elderly. New York: Raven Press; 1984:578.
Brem H, et al. The use of endoscopy to treat bronchobiliary fistula caused by choledocholithiasis. Gastroenterology. 1990;98:490-492.
Bretland PM. Biliary-bronchial fistula due to old hydatid cyst demonstrated with Tc-HIDA. Br J Radiol. 1983;56:757-759.
Burke DR, et al. Quality improvement guidelines for percutaneous transhepatic cholangiography and biliary drainage. J Vasc Interv Radiol. 2003;14(9 Pt 2):S243-S246.
Byrne MF, et al. Percutaneous cholecystostomy in patients with acute cholecystitis: experience of 45 patients at a US referral center. J Am Coll Surg. 2003;197:206-211.
Capussotti L, et al. Bile leakage and liver resection: where is the risk? Arch Surg. 2006;141:690-694.
Carrillo EH, et al. Interventional techniques are useful adjuncts in nonoperative management of hepatic injuries. J Trauma. 1999;46:619-622.
Cass MH, et al. Electrolyte losses with biliary fistula: the post-choledochostomy acidotic syndrome. Med J Aust. 1955;42:165-169.
Chan YT, et al. Congenital bronchobiliary fistula associated with biliary atresia. Br J Surg. 1984;71:240-241.
Chandar VP, Hookman P. Choledochocolonic fistula through a cystic duct remnant: a case report. Am J Gastroenterol. 1980;74:179-181.
Chatzoulis G, et al. Mirizzi syndrome type IV associated with cholecystocolic fistula: a very rare condition—report of a case. BMC Surg. 2007;7:6.
Chaudhary A, et al. Reoperative surgery for postcholecystectomy bile duct injuries. Dig Surg. 2002;19:22-27.
Chikamori F, et al. Laparoscopic cholecystofistulectomy for preoperatively diagnosed cholecystoduodenal fistula. J Gastroenterol. 2001;36:125-128.
Chou JW, et al. Gallstone ileus: report of two cases and review of the literature. World J Gastroenterol. 2007;13:1295-1298.
Chowbey PK, et al. The management of Mirizzi syndrome in the laparoscopic era. Surg Laparosc Endosc Percutan Tech. 2000;10:11-14.
Christmas AB, et al. Selective management of blunt hepatic injuries, including nonoperative management, is a safe and effective strategy. Surgery. 2005;138:606-610.
Chung S, Roberts-Thomson IC. Gastrointestinal: choledochoduodenal fistula. J Gastroenterol Hepatol. 2000;15:93.
Clavien PA, et al. Gallstone ileus. Br J Surg. 1990;77:737-742.
Cleve EA, Correa JL. Bronchobiliary fistulas secondary to amebic abscess of liver. Gastroenterology. 1958;34:320-324.
Collins PG, Gorey TF. Iatrogenic biliary stricture: presentation and management. Br J Surg. 1984;71:980-982.
Constant E, Turcotte JG. Choledochoduodenal fistula: the natural history and management of an unusual complication of peptic ulcer disease. Ann Surg. 1968;167:220-228.
Cooper RA, Kucharski P. Ectopic gallstone as a cause of gastric outlet obstruction. Am J Gastroenterol. 1978;70:175-178.
Corlette MB, Bismuth H. Biliobiliary fistula: a trap in the surgery of cholelithiasis. Arch Surg. 1975;110:377-383.
Cornud F, et al. Mirizzi syndrome and biliobiliary fistulas: roentgenologic appearance. Gastrointest Radiol. 1981;6:265-268.
Crouch DS, Kuhnke M. Laparoscopic repair of cholecystoduodenal fistula: report of two cases. J Laparoendosc Adv Surg Tech A. 2000;10:223-226.
Csendes A, et al. Mirizzi syndrome and cholecystobiliary fistula: a unifying classification. Br J Surg. 1989;76:1139-1143.
Csendes A, et al. Present role of classic open choledochostomy in the surgical treatment of patients with common bile duct stones. World J Surg. 1998;22:1167-1170.
Csendes A, Munoz C, Alban M. Sindrome de Mirizzi—fistula colecistobiliar, una nueva clasificacion. Rev Chil Cir. 2007;59(Suppl):63-64.
Czerniak A, et al. The management of fistulas of the biliary tract after injury to the bile duct during cholecystectomy. Surg Gynecol Obstet. 1988;167:33-38.
Davidoff AM, et al. Mechanisms of major biliary injury during laparoscopic cholecystectomy. Ann Surg. 1992;215:196-202.
Davids PH, et al. Postoperative bile leakage: endoscopic management. Gut. 1992;33:1118-1122.
Davies RJ, et al. Case report: ultrasound in the diagnosis of gallstone ileus. Clin Radiol. 1991;43:282-284.
Day EA, Marks C. Gallstone ileus: review of the literature and presentation of thirty-four new cases. Am J Surg. 1975;129:552-558.
de Castro SM, et al. Incidence and management of biliary leakage after hepaticojejunostomy. J Gastrointest Surg. 2005;9:1163-1171.
Deitz DM, et al. Improving the outcome in gallstone ileus. Am J Surg. 1986;151:572-576.
Delabrousse E, et al. Gallstone ileus: CT findings. Eur Radiol. 2000;10:938-940.
DeVanna T, et al. Fistulous communication of pseudocyst to the common bile duct: a complication of pancreatitis. Pediatr Radiol. 1983;13:344-345.
Dewar G, et al. Operative strategy in Mirizzi syndrome. Surg Gynecol Obstet. 1990;171:157-159.
Dewar G, et al. Management of the Mirizzi syndrome and the surgical implications of cholecystcholedochal fistula. Br J Surg. 1991;78:378.
Draus JMJr, et al. Enterocutaneous fistula: are treatments improving? Surgery. 2006;140:570-576.
Duttaroy DD, et al. Giant hepatic hydatid cyst with sub-fascial extension treated by open minimally invasive surgery: a case report. J Med Case Reports. 2008;2:26.
Dyon JF, et al. Congenital tracheobiliary fistula: report of a case with choledocal hypoplasia [in French]. Chir Pediatr. 1978;19:189-195.
Dziri C, et al. Treatment of hydatid cyst of the liver: where is the evidence? World J Surg. 2004;28:731-736.
Edell SL, et al. Cholescintigraphic diagnosis of cholecystocolic fistula. Clin Nucl Med. 1981;6:303-304.
Eicher CA, et al. Laser ablation of a biliary duct for treatment of a persistent biliary-cutaneous fistula. J Vasc Interv Radiol. 2008;19:294-297.
Eldar S, et al. Laparoscopic cholecystectomy for the various types of gallbladder inflammation: a prospective trial. Surg Laparosc Endosc. 1998;8:200-207.
Ellenbogen KA, et al. Fistulous communication of a pseudocyst with the common bile duct: demonstration by endoscopic retrograde cholangiopancreatography. Johns Hopkins Med J. 1981;149:110-111.
Erguney S, et al. Complicated hydatid cysts of the liver [in French]. Ann Chir. 1991;45:584-589.
Eryigit H, et al. Management of acquired bronchodilatory fistula: 3 case reports on a literature review. J Cardiothorac Surg. 2007;2:52.
Feller ER, et al. Observations on management of choledochoduodenal fistula due to penetrating peptic ulcer. Gastroenterology. 1980;78:126-131.
Fletcher DR, et al. Complications of cholecystectomy: risks of the laparoscopic approach and protective effects of operative cholangiography: a population-based study. Ann Surg. 1999;229:449-457.
Florence MG, et al. Ampullary disconnection during the course of biliary and duodenal surgery. Am J Surg. 1981;142:100-105.
Flum DR, et al. Bile duct injury during cholecystectomy and survival in Medicare beneficiaries. JAMA. 2003;290:2168-2173.
Franklin GA, et al. Prevention of bile peritonitis by laparoscopic evacuation and lavage after nonoperative treatment of liver injuries. Am Surg. 2007;73:611-616.
Frattaroli FM, et al. Bouveret’s syndrome: case report and review of the literature. Hepatogastroenterology. 1997;44:1019-1022.
Fujita N, et al. Gallstone ileus treated by electrohydraulic lithotripsy. Gastrointest Endosc. 1992;38:617-619.
Gajraj H. Gallstone ileus. Br J Surg. 1991;78:378.
Gentileschi P, et al. Laparoscopic approach to cholecystocolic fistula: report of a case. J Laparoendosc Surg. 1995;5:413-417.
Gerazounis M, et al. Bronchobiliary fistulae due to echinococcosis. Eur J Cardiothorac Surg. 2002;22:306-308.
Gharaibeh KI, et al. Effect of timing of surgery, type of inflammation, and sex on outcome of laparoscopic cholecystectomy for acute cholecystitis. J Laparoendosc Adv Surg Tech A. 2002;12:193-198.
Glaser K, et al. Traumatic bilhemia. Surgery. 1994;116:24-27.
Glenn F, Mannix HJr. Biliary enteric fistula. Surg Gynecol Obstet. 1957;105:693-705.
Glenn F, et al. Biliary enteric fistula. Surg Gynecol Obstet. 1981;153:527-531.
Goldman R, et al. Delayed celiotomy for the treatment of bile leak, compartment syndrome, and other hazards of nonoperative management of blunt liver injury. Am J Surg. 2003;185:492-497.
Gourgiotis S, et al. Operative and nonoperative management of blunt hepatic trauma in adults: a single-center report. J Hepatobiliary Pancreat Surg. 2007;14:387-391.
Grala P, Skrzywanek P, Sowier A. Biliary fistulas resulting from blunt hepatic injury treated by endoscopic diversion of the bile flow. Acta Chir Belg. 2009;109:47-51.
Griffin J, et al. Hepatic amoebic abscess communicating with the biliary tree. Br J Radiol. 1983;56:887-890.
Gugenheim J, et al. Bronchobiliary fistulas in adults. Ann Surg. 1988;207:90-94.
Guitron-Cantu A, et al. Choledochocolonic fistula secondary to primary choledocholithiasis. Gastrointest Endosc. 2001;54:227.
Gunlemez A, et al. Surgical experience in a baby with congenital broncho-biliary fistula. Ann Thorac Surg. 2009;87:318-320.
Haberlik A, et al. Biliovenous fistula in children after blunt liver trauma: proposal for a simple surgical treatment. J Pediatr Surg. 1992;27:1203-1206.
Hadjis NS, et al. Mirizzi syndrome associated with liver atrophy: diagnostic and management considerations. J Hepatol. 1987;4:245-249.
Haq AU, et al. Recurrent gall-stone ileus. Br J Radiol. 1981;54:1000-1001.
Hayek T, et al. Preoperative diagnosis of Mirizzi syndrome. Am J Med Sci. 1988;296:74-75.
Heil T, Belohlavek D. The Mirizzi syndrome as a special form of obstructive icterus [in German]. Chirurg. 1978;49:57-59.
Henderson RW, et al. Gastrobiliary fistula: pre- and postoperative assessment with 99mTc-PIPIDA. Am J Roentgenol. 1981;137:163-165.
Hesse U, et al. Role of somatostatin-14 and its analogues in the management of gastrointestinal fistulae: clinical data. Gut. 2002;49(Suppl IV):iv11-iv20.
Heuman R, et al. Gallstone ileus: an analysis of 20 patients. World J Surg. 1980;4:595-598.
Hida Y, et al. Laparoscopic treatment of cholecystocolonic fistula: report of a case preoperatively diagnosed by barium enema. Surg Laparosc Endosc Percutan Tech. 1999;9:217-219.
H’ng MW, Yim HB. Spontaneous choledochoduodenal fistula secondary to long-standing ulcer disease. Singapore Med J. 2003;44:205-207.
Holbrook RF, et al. Biliary patency imaging after endoscopic retrograde sphincterotomy with gallbladder in situ: clinical impact of nonvisualization. Arch Surg. 1991;126:738-741.
Holl J, et al. Shock-wave therapy of gastric outlet syndrome caused by a gallstone. Gastroenterology. 1989;97:472-474.
Honore C, et al. Management of excluded segmental bile duct leakage following liver resection. HPB (Oxford). 2009;11:364-369.
Hricak H, Vander Molen RL. The radiology corner: duodenocolonic fistula with gallstone ileus. Am J Gastroenterol. 1978;69:711-715.
Hunt DR, Blumgart LH. Iatrogenic choledochoduodenal fistula: an unsuspected cause of post-cholecystectomy symptoms. Br J Surg. 1980;67:10-13.
Ikeda S, Okada Y. Classification of choledochoduodenal fistula diagnosed by duodenal fiberscopy and its etiological significance. Gastroenterology. 1975;69:130-137.
Iscan M, Duren M. Endoscopic sphincterotomy in the management of postoperative complications of hepatic hydatid disease. Endoscopy. 1991;23:282-283.
Ito K, et al. Percutaneous cholecystostomy versus gallbladder aspiration for acute cholecystitis: a prospective randomized controlled trial. Am J Roentgenol. 2004;183:193-196.
Ivatury RR, et al. Post-traumatic thoracobiliary fistula. J Trauma. 1984;24:438-442.
Jaballah S, et al. Choledochoduodenal fistula due to duodenal peptic ulcer. Dig Dis Sci. 2001;46:2475-2479.
Jalan R, et al. Transjugular intrahepatic portosystemic stent-shunt (TIPSS) occlusion and the role of biliary venous fistulae. J Hepatol. 1996;24:169-176.
Jawaid Q, et al. Biliary-venous fistula complicating transjugular intrahepatic portosystemic shunt presenting with recurrent bacteremia, jaundice, anemia and fever. Am J Transplant. 2003;3:1604-1607.
Jorge A, et al. Choledochoduodenal fistulas. Endoscopy. 1991;23:76-78.
Kamiya S, et al. The value of bile replacement during external biliary drainage: an analysis of intestinal permeability, integrity, and microflora. Ann Surg. 2004;239:510-517.
Karincaoglu M, et al. Association of peripapillary fistula with common bile duct stones and cholangitis. Aust N Z J Surg. 2003;73:884-886.
Karincaoglu M, et al. Common bile duct diameters after endoscopic sphincterotomy in patients with common bile duct stones: ultrasonographic evaluation. Abdom Imaging. 2003;28:531-535.
Kasahara Y, et al. Gallstone ileus: review of 112 patients in the Japanese literature. Am J Surg. 1980;140:437-440.
Kayaalp C, et al. Distribution of hydatid cysts into the liver with reference to cystobiliary communications and cavity-related complications. Am J Surg. 2003;185:175-179.
Keely SJ, et al. Bile acid-induced secretion in polarized monolayers of T84 colonic epithelial cells: structure–activity relationships. Am J Physiol Gastrointest Liver Physiol. 2007;292:G290-G297.
Kim YS, et al. Bronchobiliary fistula after radiofrequency thermal ablation of hepatic tumor. J Vasc Interv Radiol. 2005;16:407-410.
Kirchmayr W, et al. Gallstone ileus: rare and still controversial. Aust N Z J Surg. 2005;75:234-238.
Kiviluoto T, et al. Randomised trial of laparoscopic versus open cholecystectomy for acute and gangrenous cholecystitis. Lancet. 1998;351:321-325.
Knochel JP, et al. External biliary fistula: a study of electrolyte derangements and secondary cardiovascular and renal abnormalities. Surgery. 1962;51:746-754.
Kohn A, et al. Indicated cholangiography in patients operated on by routine versus selective cholangiographers. Am Surg. 2004;70:203-206.
Kornaros SE, Aboul-Nour TA. Frank intrabiliary rupture of hydatid hepatic cyst: diagnosis and treatment. J Am Coll Surg. 1996;183:466-470.
Koulaouzidis A, Moschos J. Bouveret’s syndrome: narrative review. Ann Hepatol. 2007;6:89-91.
Kourias BG, Chouliaras A. Spontaneous gastrointestinal biliary fistula complicating duodenal ulcer. Surg Gynecol Obstet. 1964;119:1013-1018.
Kozarek RA, et al. Endoscopic treatment of biliary injury in the era of laparoscopic cholecystectomy. Gastrointest Endosc. 1994;40:10-16.
Kracht M, et al. Cholangitis after endoscopic sphincterotomy in patients with stricture of the biliary duct. Surg Gynecol Obstet. 1986;163:324-326.
Kum CK, et al. Laparoscopic cholecystectomy for acute cholecystitis: is it really safe? World J Surg. 1996;20:43-48.
Kunasani R, et al. Cholecystocolonic fistula presenting as massive lower GI hemorrhage. Gastrointest Endosc. 2003;58:142-144.
Kune GA, Sali A. The Practice of Biliary Surgery. Boston: Blackwell Scientific; 1980.
Kuroki T, et al. Parapapillary choledochoduodenal fistula associated with cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 2005;12:143-146.
Kwon AH, Inui H. Preoperative diagnosis and efficacy of laparoscopic procedures in the treatment of Mirizzi syndrome. J Am Coll Surg. 2007;204:409-415.
La Greca G, et al. [Complicated duodeno-biliary fistula in bleeding duodenal ulcer: case report an literature review]. Ann Ital Chir (in Italian). 2008;79:57-61.
Lassandro F, et al. Gallstone ileus analysis of radiological findings in 27 patients. Eur J Radiol. 2004;50:23-29.
Lassandro F, et al. Role of helical CT in diagnosis of gallstone ileus and related conditions. Am J Roentgenol. 2005;185:1159-1165.
LeBlanc KA, et al. Spontaneous biliary enteric fistulas. South Med J. 1983;76:1249-1252.
Lebovics E, et al. Pancreaticobiliary fistula and obstructive jaundice complicating 1251 interstitial implants for pancreatic cancer: endoscopic diagnosis and management. Gastrointest Endosc. 1990;36:610-611.
Lee JH, et al. Laparoscopic repair of various types of biliary-enteric fistula: three cases. Surg Endosc. 2004;18:349.
Levin B, Shapiro RA. Recurrent enteric gallstone obstruction. Gastrointest Radiol. 1980;5:151-153.
Lillemoe KD, et al. Major bile duct injuries during laparoscopic cholecystectomy: follow-up after combined surgical and radiologic management. Ann Surg. 1997;225:459-468.
Lillemoe KD, et al. Postoperative bile duct strictures: management and outcome in the 1990s. Ann Surg. 2000;232:430-441.
Lo CM, et al. Biliary complications after hepatic resection: risk factors, management, and outcome. Arch Surg. 1998;133:156-161.
Loren I, et al. Gallstone ileus demonstrated by CT. J Comput Assist Tomogr. 1994;18:262-265.
Lygidakis NJ. Spontaneous internal biliary fistulae: early surgery for prevention, radical surgery for cure—a report of 75 cases. Med Chir Dig. 1981;10:695-699.
MacFadyen BVJr, et al. Bile duct injury after laparoscopic cholecystectomy: the United States experience. Surg Endosc. 1998;12:315-321.
Maglinte DD, et al. Sonography of Bouveret’s syndrome. J Ultrasound Med. 1987;6:675-677.
Malhotra AK, et al. Blunt hepatic injury: a paradigm shift from operative to nonoperative management in the 1990s. Ann Surg. 2000;231:804-813.
Malvaux P, et al. Laparoscopic treatment of a gastric outlet obstruction caused by a gallstone (Bouveret’s syndrome). Surg Endosc. 2002;16:1108-1109.
Mannella P, et al. Interventional radiology in percutaneous management of bile duct obstruction: biliary drainage through a spontaneous common hepatic duct–duodenal fistula. Clin Imaging. 1999;23:103-106.
Marks JM, et al. Biliary stenting is more effective than sphincterotomy in the resolution of biliary leaks. Surg Endosc. 1998;12:327-330.
Marshall T, et al. Endoscopic treatment of biliary enteric fistula. Br Med J. 1990;300:1176.
McPherson GA, et al. The role of HIDA scanning in the assessment of external biliary fistulae. Surg Gastroenterol. 1984;3:77-80.
McSherry CK, et al. The Mirizzi syndrome: suggested classification and surgical therapy. Surg Gastroenterol. 1982;1:219-225.
Men S, et al. Percutaneous treatment of hepatic hydatid cysts: an alternative to surgery. Am J Roentgenol. 1999;172:83-89.
Miller BM, et al. Intrapancreatic communication of bile and pancreatic ducts secondary to pancreatic necrosis. Arch Surg. 1988;123:1000-1003.
Milsom JW, MacKeigan JM. Gallstone obstruction of the colon: report of two cases and review of management. Dis Colon Rectum. 1985;28:367-370.
Mirizzi PL. Sindrome del conducto hepatico. J Int Chir. 1948;8:731-732.
Mishra MC, et al. Biliobiliary fistula: preoperative diagnosis and management implications. Surgery. 1990;108:835-839.
Moberg AC, Montgomery A. Laparoscopically assisted or open enterolithotomy for gallstone ileus. Br J Surg. 2007;94:53-57.
Moreira VF, et al. Endoscopic retrograde cholangiography (ERCP) and complicated hepatic hydatid cyst in the biliary tree. Endoscopy. 1984;16:124-126.
Moriai T, et al. Successful removal of massive intragastric gallstones by endoscopic electrohydraulic lithotripsy and mechanical lithotripsy. Am J Gastroenterol. 1991;86:627-629.
Morris SJ, et al. Cholecystoduodenocolic fistula secondary to carcinoma of the gallbladder. Am J Dig Dis. 1978;23:849-852.
Moser JJ, et al. [Mirizzi syndrome—a contraindication for laparoscopic surgery]. Helv Chir Acta. 1993;59:577-580.
Munene G, et al. Biliary-colonic fistula: a case report and literature review. Am Surg. 2006;72:347-350.
Muthukumarasamy G, et al. Gallstone ileus: surgical strategies and clinical outcome. J Dig Dis. 2008;9:156-161.
Nagano Y, et al. Risk factors and management of bile leakage after hepatic resection. World J Surg. 2003;27:695-698.
Navsaria PH, Adams S, Nicol AJ. Traumatic thoracobiliary fistulae: a case report with a review of the current management options. Injury. 2002;33:639-643.
Neuhauser EB, et al. Congenital direct communication between biliary system and respiratory tract. AMA Am J Dis Child. 1952;83:654-659.
Nyberg B. Bile secretion in man: the effects of somatostatin, vasoactive intestinal peptide and secretin. Acta Chir Scand Suppl. 1990;557:1-40.
Oettl C, et al. Bronchobiliary fistula after hemihepatectomy: cholangiopancreaticography, computed tomography and magnetic resonance cholangiography findings. Eur J Radiol. 1999;32:211-215.
Ong M, et al. Octreotide in bronchobiliary fistula management. Ann Thorac Surg. 2004;78:1512-1513.
Oparah SS, Mandal AK. Traumatic thoracobiliary (pleurobiliary and bronchobiliary) fistulas: clinical and review study. J Trauma. 1978;18:539-544.
Osnes M, Kahrs T. Endoscopic choledochoduodenostomy for choledocholithiasis through choledochoduodenal fistula. Endoscopy. 1977;9:162-165.
Owera A, Low J, Ammori BJ. Laparoscopic enterolithotomy for gallstone ileus. Surg Laparosc Endosc Percutan Tech. 2008;18:450-452.
Ozmen V, et al. Surgical treatment of hepatic hydatid disease. Can J Surg. 1992;35:423-427.
Pachter HL, Hofstetter SR. The current status of nonoperative management of adult blunt hepatic injuries. Am J Surg. 1995;169:442-454.
Palanivelu C, et al. Palanivelu hydatid system for safe and efficacious laparoscopic management of hepatic hydatid disease. Surg Endosc. 2006;20:1909-1913.
Park JH, et al. Acetic acid sclerotherapy for treatment of a biliary leak from an isolated bile duct after hepatic surgery. J Vasc Interv Radiol. 2005;16:885-888.
Parrilla P, et al. Long-term results of choledochoduodenostomy in the treatment of choledocholithiasis: assessment of 225 cases. Br J Surg. 1991;78:470-472.
Patino C, et al. Bile duct-duodenal fistula caused by AIDS/HIV–associated tuberculosis. Rev Hosp Clin Fac Med Sao Paulo. 2003;58:223-226.
Pattni S, Walters JR. Recent advances in the understanding of bile acid malabsorption. Br Med Bull. 2009;92:79-93.
Paul MG, et al. Laparoscopic surgery in the treatment of Mirizzi’s syndrome. J Laparoendosc Surg. 1992;2:157-163.
Pedersen PR, et al. Value of ultrasonography in the diagnosis of gallstone ileus. Radiologe. 1988;28:479-480.
Petrolati A, et al. Management of biliobronchial fistula with octreotide: a case report. Am J Gastroenterol. 2009;104:2638-2639.
Piedad OH, Wels PB. Spontaneous internal biliary fistula, obstructive and nonobstructive types: twenty-year review of 55 cases. Ann Surg. 1972;175:75-80.
Ponchon T, et al. Endoscopic treatment of biliary tract fistulas. Gastrointest Endosc. 1989;35:490-498.
Porta E, et al. Contribution of CT and ultrasound to the preoperative diagnosis of biliobronchial fistula caused by echinococcosis of the liver. J Comput Tomogr. 1981;5:349-350.
Ragozzino A, et al. Bronchobiliary fistula evaluated with magnetic resonance imaging. Acta Radiol. 2005;46:452-454.
Ramesh GN, et al. Successful treatment of post-operative pleurobiliary fistula by endoscopic technique. Gastrointest Endosc. 1991;37:574-576.
Ramsey WH, et al. Choledocho-duodenal fistula: tailoring the fistulotomy using a needle knife papillotome. Gastrointest Endosc. 1992;38:190-192.
Rappeport ED. [Mirizzi’s syndrome-diagnosed preoperatively by MR scanning]. Ugeskr Laeger. 2002;165:50-51.
Rau WS, et al. Spontaneous cholecystocolonic fistula: a model situation for bile acid diarrhea and fatty acid diarrhea as a consequence of a disturbed enterohepatic circulation of bile acids. Hepatogastroenterology. 1980;27:231-237.
Rauws EA, Gouma DJ. Endoscopic and surgical management of bile duct injury after laparoscopic cholecystectomy. Best Pract Res Clin Gastroenterol. 2004;18:829-846.
Rawas MM, Yee AC, Ho CS. Choledochocolonic fistula—a rare complication of cholangiocarcinoma. Can Assoc Radiol J. 1987;38:139-140.
Razemon P, et al. [Amebic liver abscesses fistulized into the bronchi]. Lille Chir. 1963;18:201-207.
Reimann AJ, et al. Atypical cases of gallstone ileus evaluated with multidetector computed tomography. J Comput Assist Tomogr. 2004;28:523-527.
Reisner RM, Cohen JR. Gallstone ileus: a review of 1001 reported cases. Am Surg. 1994;60:441-446.
Renner W, et al. Ultrasound demonstration of a non-calcified gallstone in the distal ileum causing small-bowel obstruction. Radiology. 1982;144:884.
Rigler L, et al. Gallstone obstruction; pathogenesis and roentgen manifestations. JAMA. 1941;117:1753-1759.
Ripolles T, et al. Gallstone ileus: increased diagnostic sensitivity by combining plain film and ultrasound. Abdom Imaging. 2001;26:401-405.
Rodriguez-Hermosa JI, et al. Gallstone ileus: results of analysis of a series of 40 patients. Gastroenterol Hepatol. 2001;24:489-494.
Rodriguez-Sanjuan JC, et al. Cholecystectomy and fistula closure versus enterolithotomy alone in gallstone ileus. Br J Surg. 1997;84:634-637.
Rohatgi A, Singh KK. Mirizzi syndrome: laparoscopic management by subtotal cholecystectomy. Surg Endosc. 2006;20:1477-1481.
Rosenberg L, Brown RA. Sandostatin in the management of nonendocrine gastrointestinal and pancreatic disorders: a preliminary study. Can J Surg. 1991;34:223-229.
Roslyn JJ, et al. Open cholecystectomy: a contemporary analysis of 42,474 patients. Ann Surg. 1993;218:129-137.
Rust KR, et al. Mirizzi’s syndrome: a contraindication to coelioscopic cholecystectomy. J Laparoendosc Surg. 1991;1:133-137.
Saad WE, Darcy MD. Percutaneous management of biliary leaks: biliary embosclerosis and ablation. Tech Vasc Interv Radiol. 2008;11:111-119.
Sackmann M, et al. Gallstone ileus successfully treated by shock-wave lithotripsy. Dig Dis Sci. 1991;36:1794-1795.
Safaie-Shirazi S, et al. Spontaneous enterobiliary fistulas. Surg Gynecol Obstet. 1973;137:769-772.
Sahin M, et al. Effect of octreotide (Sandostatin 201-995) on bile flow and bile components. Dig Dis Sci. 1999;44:181-185.
Sane SM, et al. Congenital bronchobiliary fistula. Surgery. 1971;69:599-608.
Santra A, et al, 2009: Traumatic bronchobiliary fistula diagnosed by 99mTc-mebrofenin hepatobiliary scintigraphy. Nucl Med Commun 30:652-653.
Sarantou T, et al. Complications of hepatic cryosurgery. Semin Surg Oncol. 1998;14:156-162.
Sarr MG, et al. Choledochoduodenal fistula: an unusual complication of duodenal ulcer disease. Am J Surg. 1981;141:736-740.
Savitch I, et al. Demonstration of a biliary-bronchial fistula using Tc-99m p-butyl IDA imaging. Clin Nucl Med. 1983;8:139-140.
Savvidou S, et al. Pneumobilia, chronic diarrhea, vitamin K malabsorption: a pathognomonic triad for cholecystocolonic fistulas. World J Gastroenterol. 2009;15:4077-4082.
Schafer M, et al. Incidence and management of Mirizzi syndrome during laparoscopic cholecystectomy. Surg Endosc. 2003;17:1186-1190.
Schapira L, Khawaja FI. Endoscopic fistulo-sphincterotomy: an alternative method of sphincterotomy using a new sphincterotome. Endoscopy. 1982;14:58-60.
Schoeters P, et al. A cholecystocolic fistula demonstrated by endoscopic retrograde cholangiopancreatography. Endoscopy. 2002;34:595.
Seewald S, et al. Endoscopic sealing of pancreatic fistula by using N-butyl-2-cyanoacrylate. Gastrointest Endosc. 2004;59:463-470.
Shahrudin MD, Noori SM. Biloma and biliary fistula associated with hepatorrhaphy for liver injury. Hepatogastroenterology. 1997;44:519-521.
Sharma D, et al. Laparoscopy for liver hydatid disease: where do we stand today? Surg Laparosc Endosc Percutan Tech. 2009;19:419-423.
Shaw DW, et al. Use of tetracycline for sclerosis of a biliary-cutaneous fistula. Am J Roentgenol. 1989;153:65-66.
Sherman S, et al. Endoscopic management of biliary fistulas complicating liver transplantation and other hepatobiliary operations. Ann Surg. 1993;218:167-175.
Shimizu T, et al. Postoperative bile leakage managed successfully by intrahepatic biliary ablation with ethanol. World J Gastroenterol. 2006;12:3450-3452.
Shocket E, et al. Cholecysto-duodeno-colic fistula with gallstone ileus. Arch Surg. 1970;101:523-526.
Silecchia G, et al. Minimally invasive approach in Mirizzi’s syndrome. J Laparoendosc Surg. 1995;5:151-156.
Singal AK, et al. Bilhemia after trans-jugular intra-hepatic porto-systemic shunt and its management with biliary decompression. World J Gastroenterol. 2009;15:3681-3683.
Singh B, et al. Conservative management of thoracobiliary fistula. Ann Thorac Surg. 2002;73:1088-1091.
Singh V, et al. Endoscopic management of traumatic hepatobiliary injuries. J Gastroenterol Hepatol. 2007;22:1205-1209.
Slocum MM, et al. Absence of intestinal bile promotes bacterial translocation. Am Surg. 1992;58:305-310.
Smith EE, et al. The management of post-traumatic intrahepatic cutaneous biliary fistulas. Br J Surg. 1982;69:317-318.
Sparkman RS. Bobbs’ centennial: the first cholecystotomy. Surgery. 1967;61:965-971.
Starzl TE, et al. Left hepatic trisegmentectomy. Surg Gynecol Obstet. 1982;155:21-27.
Stempfle B, Diamantopoulos G. [Spontaneous cholecysto-gastric fistula with massive gastrointestinal bleeding: endoscopic diagnosis and concrement extraction]. Fortschr Med. 1976;94:444-447.
Stewart L, Way LW. Bile duct injuries during laparoscopic cholecystectomy: factors that influence the results of treatment. Arch Surg. 1995;130:1123-1128.
Strasberg SM, et al. Results of a new strategy for reconstruction of biliary injuries having an isolated right-sided component. J Gastrointest Surg. 2001;5:266-274.
Stuart SA, et al. Routine intraoperative laparoscopic cholangiography. Am J Surg. 1998;176:632-637.
Swift SE, Spencer JA. Gallstone ileus: CT findings. Clin Radiol. 1998;53:451-454.
Taillefer R, et al. Demonstration of a bronchobiliary fistula by 99mTc-HIDA cholescintigraphy. Eur J Nucl Med. 1983;8:37-39.
Tan KY, et al. Mirizzi syndrome: noteworthy aspects of a retrospective study in one center. Aust N Z J Surg. 2004;74:833-837.
Tan YM, et al. A comparison of two surgical strategies for the emergency treatment of gallstone ileus. Singapore Med J. 2004;45:69-72.
Tanaka M, Ikeda S. Parapapillary choledochoduodenal fistula: an analysis of 83 consecutive patients diagnosed at ERCP. Gastrointest Endosc. 1983;29:89-93.
Tanaka S, et al. Incidence and management of bile leakage after hepatic resection for malignant hepatic tumors. J Am Coll Surg. 2002;195:484-489.
Thomas TL, et al. Gallstone obstruction and perforation of the duodenal bulb. Br J Surg. 1976;63:131-132.
Thompson HH, et al. Major hepatic resection: a 25-year experience. Ann Surg. 1983;197:375-388.
Tommasoni N, et al. Congenital tracheobiliary fistula. Pediatr Pulmonol. 2000;30:149-152.
Toriumi D, et al. Transnasal biliary drainage for treatment of common bile duct leakage and bile peritonitis. Dig Dis Sci. 1989;34:315-319.
Tran T, et al. Successful endoscopic management of bronchobiliary fistula due to radiofrequency ablation. Dig Dis Sci. 2007;52:3178-3180.
Tytgat GN, et al. Common duct complications of choledocholithiasis revealed by ERCP. Gastrointest Endosc. 1979;25:63-66.
Urakami Y, Kishi S. Endoscopic fistulotomy (EFT) for parapapillary choledochoduodenal fistula. Endoscopy. 1978;10:289-294.
Uramoto H, et al. Congenital bronchobiliary fistula in a 65-year-old woman. Intern Med. 2008;47:1367-1370.
van Hillo M, et al. Gallstone obstruction of the intestine: an analysis of ten patients and a review of the literature. Surgery. 1987;101:273-276.
VanLandingham SB, Broders CW. Gallstone ileus. Surg Clin North Am. 1982;62:241-247.
Van Linda BM, Rosson RS. Choledochoduodenal fistula and choledocholithiasis: treatment by endoscopic enlargement of the choledochoduodenal fistula. J Clin Gastroenterol. 1984;6:321-324.
Vassiliu P, et al. A prospective study of post-traumatic biliary and pancreatic fistuli: the role of expectant management. Injury. 2004;35:223-227.
Vecchio R, et al. Laparoscopic cholecystectomy: an analysis on 114,005 cases of United States series. Int Surg. 1998;83:215-219.
Velchik MG, et al. Bronchobiliary fistula detected by cholescintigraphy. J Nucl Med. 1991;32:136-138.
Vezakis A, et al. Laparoscopic treatment of Mirizzi syndrome. Surg Laparosc Endosc Percutan Tech. 2000;10:15-18.
Vigano L, et al. Bile leak after hepatectomy: predictive factors of spontaneous healing. Am J Surg. 2008;196:195-200.
Viikari SJ. Operative injuries to the bile ducts. Acta Chir Scand. 1960;119(Suppl):83-92.
Vu DN, Strub WM, Nguyen PM. Biliary duct ablation with N-butyl cyanoacrylate. J Vasc Interv Radiol. 2006;17:63-69.
Waage A, Nilsson M. Iatrogenic bile duct injury: a population-based study of 152,776 cholecystectomies in the Swedish Inpatient Registry. Arch Surg. 2006;141:1207-1213.
Wagholikar GD, Ibrarullah M. Bouveret’s syndrome—an unusual cause of spontaneous resolution of gastric outlet obstruction. Indian J Gastroenterol. 2004;23:109-110.
Wahl WL, et al. Diagnosis and management of bile leaks after blunt liver injury. Surgery. 2005;138:742-747.
Walsh RM, et al. Trends in bile duct injuries from laparoscopic cholecystectomy. J Gastrointest Surg. 1998;2:458-462.
Wang WK, Yeh CN, Jan YY. Successful laparoscopic management for cholecystoenteric fistula. World J Gastroenterol. 2006;12:772-775.
Warren KW, et al. Surgical treatment of bronchobiliary fistulas. Surg Gynecol Obstet. 1983;157:351-356.
Watkins L, et al. Biliary-bronchial fistula demonstrated by endoscopic retrograde cholangiography. Can Med Assoc J. 1975;113:868-874.
Wherry DC, et al. An external audit of laparoscopic cholecystectomy in the steady state performed in medical treatment facilities of the Department of Defense. Ann Surg. 1996;224:145-154.
Winbladh A, et al. Systematic review of cholecystostomy as a treatment option in acute cholecystitis. HPB (Oxford). 2009;11:183-193.
Winick AB, et al. Complications of percutaneous transhepatic biliary interventions. Tech Vasc Interv Radiol. 2001;4:200-206.
Wong WM, Hu WH, Lai KC. Images of interest—hepatobiliary and pancreatic: choledochoduodenal fistula secondary to duodenal ulcer disease. J Gastroenterol Hepatol. 2004;19:829.
Woods MS, et al. Cystic duct remnant fistulization to the gastrointestinal tract. Surgery. 1992;111:101-104.
Wright KD, Wellwood JM. Bile duct injury during laparoscopic cholecystectomy without operative cholangiography. Br J Surg. 1998;85:191-194.
Yamaguchi M, et al. Congenital bronchobiliary fistula in adults. South Med J. 1990;83:851-852.
Yamashita Y, et al. Bile leakage after hepatic resection. Ann Surg. 2001;233:45-50.
Yeatman CF, et al. Bronchobiliary fistula after liver transplantation. J Comput Assist Tomogr. 2004;28:717-720.
Yeh CN, et al. Laparoscopic treatment for Mirizzi syndrome. Surg Endosc. 2003;17:1573-1578.
Yip AW, et al. Type II Mirizzi syndrome: diagnosis by endoscopic retrograde cholangiopancreatography. J R Coll Surg Edinb. 1992;37:49-50.
Yoon DH, et al. Percutaneous management of a bronchobiliary fistula after radiofrequency ablation in a patient with hepatocellular carcinoma. Korean J Radiol. 2009;10:411-415.
Zironi G, Modolon C, Cavazza M. Emergency ultrasound and gallstone ileus. Eur J Emerg Med. 2007;14:94-96.
Zuegel N, et al. Advantages of one-stage repair in case of gallstone ileus. Hepatogastroenterology. 1997;44:59-62.
Zwemer FL, et al. Biliary enteric fistulas: management of 47 cases in Native Americans. Am J Surg. 1979;138:301-304.