© Springer Japan 2016
Yasuhiko Tomino (ed.)Pathogenesis and Treatment in IgA Nephropathy10.1007/978-4-431-55588-9_18
18. Beyond the Differences in Tonsillectomy in IgA Nephropathy: From Rationale To Indications in Patients
(1)
Division of Nephrology, Department of Internal Medicine, Juntendo University Faculty of Medicine, 2-1-1 Hongo, Bunkyo-ku, Tokyo 113-8421, Japan
(2)
Nephrology, Dialysis and Transplantation, Regina Margherita Children’s University Hospital, Piazza Polonia 94, 10126 Turin, Italy
Contributed equally
Abstract
In the special symposiums on IgA nephropathy (IgAN) (Symposium 3, IgAN Basic; Symposium 4, IgAN Clinical/KDIGO) at the last Asian Pacific Congress of Nephrology (APCN) 2014 in Tokyo, discussion by expert nephrologists from Asian and Western countries revealed how actual clinical practices in IgAN, including timing of renal biopsy and choices of treatments, are different, despite evidence-based guidelines. In particular, indication for tonsillectomy with or without steroid pulse therapy for IgAN patients is markedly different between Asian and European-American practices. The tonsillectomy is considered to be old-fashioned in Western countries, while it is still widely accepted in Asian countries such as Japan and China. The present chapter discusses rationale of tonsillectomy, with up-to-date understanding of IgAN pathogenesis, and summarizes the actual difference in the IgAN practice with respect to tonsillectomy with or without steroid pulse therapy, such as clinical stages at the intervention, based on some key papers from Asian and Western countries. In addition, we attempt to identify the medical and social causes behind these differences.
Keywords
TonsillectomySteroid pulse therapyMucosal immunityGalactose-deficient IgARandomized clinical trial
1st and 2nd authors equally contributed to this work.
18.1 Introduction
The hallmark of IgA nephropathy (IgAN), well known to every medical student, is macroscopic hematuria, which is coincident with or immediately following acute tonsillitis [1]. This presentation is very common; therefore, patients with recurrence of such episodes receive a likely diagnosis of IgAN even without a renal biopsy detecting IgA in the mesangial area. Tonsillectomy was traditionally considered the treatment of choice for patients with IgAN, and reduction in the frequency of episodes of macroscopic hematuria is commonly observed when recurrent bacterial tonsillitis is cured via tonsillectomy [2]. However, it became clear that repeated episodes of gross hematuria do not represent a sign of progression and not a risk factor of IgAN [3]. Relentless progression is frequently associated with persistent heavy hematuria, particularly proteinuria [4]. This finding suggests that less clinical benefit of tonsillectomy is observed in IgAN patients than that expected. On the other hand, in Japan, only 10 % of IgAN patients are detected by episodic macroscopic hematuria, while 70 % IgAN patients are detected by chance microscopic hematuria in annual screening via urinalysis. This clinical fact clearly indicates that hematuria is an initial and essential manifestation of IgAN. However, the degree of proteinuria presents a greater risk for progressive IgAN than that of hematuria [4, 5]. These observations are reasonable because glomerular injury events leading to hematuria may precede those leading to persistent proteinuria in IgAN. Previous epidemiological studies assessing risk factors for CKD [6, 7] further support the idea that hematuria precedes proteinuria. Therefore, it is not surprising that proteinuria is a stronger predictor of IgAN progression than episodic macroscopic hematuria [5].
In addition to causing a debate over the clinical outcome of tonsillectomy, these observations had different effects on clinical practice all over the world. In Western countries, such as Europe and the USA, tonsillectomy is considered to be old-fashioned, while it is still widely used in Japan and China.
18.2 Comparison of Different Clinical Practices for Tonsillectomy in IgA Nephropathy
In the special symposiums on IgAN at the last Asian Pacific Congress of Nephrology (APCN) 2014 in Tokyo (President: Prof. Yasuhiko Tomino), Asian and European nephrologists discussed the differences in clinical practice of IgAN treatment in their respective countries as well as up-to-date understanding of IgAN pathogenesis. This discussion revealed how actual clinical practices, including timing of renal biopsy and choices of treatments, are different, despite evidence-based guidelines. Indication for tonsillectomy [often associated with steroid pulse therapy] is markedly different between Asian and European-American practices. This debate was followed by a blog discussion launched by NDT-Educational. Here we summarize the actual difference in the IgAN practice with respect to tonsillectomy and attempt to identify the medical and social causes behind this difference.
18.3 The Rationale for Tonsillectomy in IgA Nephropathy Is Debated
One can speculate that tonsillectomy represents an easy means to eliminate a pathogen source. However, several studies suggest that abnormalities of the mucosal-associated lymphoid tissue (MALT) are critical for the development of IgAN, with infections representing the simple role of triggering an event [1]. Experimental IgAN can be produced in animals after abrogation of the natural process of mucosal tolerance, which favors the host defense against pathogens [8]. In this context, IgAN is likely to develop because of a failure of mucosal antigen elimination and altered IgA synthesis, leading to the production of nephritogenic IgA. On the other hand, studies with experimental IgAN also demonstrated that chronic mucosal infection is not required for nephritogenic IgA production [9]. Moreover, transient mucosal activation of a pattern recognition receptor (PRR), such as Toll-like receptor (TLR), by pathogen-associated molecular patterns in IgAN-prone mice is sufficient to exacerbate this disease with rapid serum elevation of IgA [9, 10]; this suggests that preexisting mucosal B-cell clones produce nephritogenic IgA. Palatine tonsils have a unique cellular composition in the reticulated subepithelium, which is ideal for productive antigen sampling. One of the most important characteristics of the palatine tonsils is that very rich B-cell lymphoid follicles at the subepithelial space foster the development of memory B cells and plasma cells. This is very different from other tonsils in Waldeyer’s ring. Japanese nephrologists and otolaryngologists are aware that the beneficial effect of tonsillectomy in IgAN patients is independent of the size of the palatine tonsils and the presence of abscesses. Therefore, if the responsible B cells producing nephritogenic IgA are localized in the palatine tonsils, tonsillectomy may abrogate mucosal antigen encounters to such B cells, even if not chronic, leading to acute elevation of nephritogenic serum IgA [9, 10] and their clonal expansion [11].
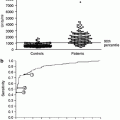
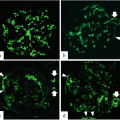
It is now accepted that galactose-deficient IgA1 (GdIgA1) and GdIA1 and immune complexes (IC) with endogenous antiglycan antibodies are essential nephritogenic molecules initiating IgAN [12, 13]. Although there is no study clearly demonstrating the type of mucosal B cells involved, the recent studies revealed that the palatine tonsils are, at least in part, delivery sources of GdIgA1 under abnormal cytokine conditions [14–18]. Total IgA is decreased by 10 % on average after tonsillectomy alone in IgAN patients; because patients who showed tonsillar activation of innate immunity and a large decrease of serum IgA after the tonsillectomy had a better clinical outcome, it was thought that the palatine tonsils may be the major delivery source of nephritogenic IgA [19]. One recent study directly demonstrated that tonsillectomy alone rapidly decreased serum levels of GdIgA1 in patients who showed rapid improvement of hematuria after this therapy [20].
However, when considering tonsillectomy for reducing MALT surface, we should consider that tonsils represent only 0.5 m2 of the entire 400 m2 of the total mucosal surface in humans. In a recent study [21], IgAN patients who underwent tonsillectomy showed a long-term reduction, but not normalization, of GdIgA1, signs of persistently activated MALT, ongoing oxidative stress, and increased expression of TLR4. The ligand of TLR4 is lipopolysaccharide (LPS), produced by gram-negative intestinal bacteria. Notably, a correlation was found between progressive cases of IgAN and genetic polymorphism of the membrane receptor for LPS (CD14–159). A renewed interest toward intestinal immunity has been recently raised by a genetic-wide association study (GWAS), showing a strong association between IgAN and genes of intestinal MALT response [22]. These recent data, together with past reports on the role of dietary antigens in IgAN [23], tend to limit the extent of the rationale for tonsillectomy in patients with IgAN.
In contrast, other studies further supported tonsillectomy in nephropathy because serum levels of IgA and GdIgA1 were found to be correlated with tonsillar TLR9 overexpression [19, 20], and the TLR9 genotype was strongly associated with histological severity of IgAN [9]. Patients who did not show a decrease in GdIgA1 after tonsillectomy did so after the first additional steroid pulse therapy with improvement of hematuria, indicating GdIgA1 production outside the tonsils [20]. This finding suggests that some parts of the responsible cells producing GdIgA1 in such patients may be disseminated to MALT other than the tonsils and other lymphoid organs such as the bone marrow or spleen [24, 25]. It is known that tonsillectomy with steroid pulse therapy (TSP) leads to a better clinical outcome than tonsillectomy alone [26]. These clinical and experimental findings suggest that additional steroid pulse therapy on tonsillectomy may target these disseminated extratonsillar responsible cells [27].
18.4 The Clinical Effects of Tonsillectomy in IgA Nephropathy Are Difficult to Assess Using Randomized Controlled Trials
In the absence of large series studies or randomized clinical trials (RCTs), nephrologists in Western countries tend to consider tonsillectomy in IgAN as a procedure to be performed only in patients with macroscopic hematuria and clinically evident recurrent tonsillitis. However, in eastern countries tonsillectomy continues to be regularly performed. In 2003, a breakthrough report by Xie et al. from Japan resurrected the interest for tonsillectomy in IgAN because for the first time, the procedure was associated with a better outcome at long-term follow-up [28]. A Japanese retrospective analysis of 118 patients reported a significant effect of tonsillectomy on survival from dialysis.
Here, the scientific conflict regarding the benefits of tonsillectomy between Western and Asian countries began. In Europe, retrospective analyses of single medical centers showed negative results in tonsillectomy [29, 30]. However, reports from Japan showed a benefit of tonsillectomy, particularly with steroid pulse therapy versus steroid therapy alone [31–33]. A benefit of tonsillectomy was also shown in IgAN recurrent in grafted kidneys while receiving a standard immunosuppressive regimen [34].
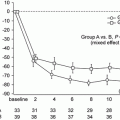
A recent RCT including 72 cases of IgAN in Japan was conducted. The patients received methylprednisolone pulse alone or in combination with tonsillectomy; some positive effects in reducing proteinuria at 1-year follow-up were observed [35]. However, this RCT did not provide data on the benefits on renal function decline and a longer follow-up is needed.
Due to these conflicting reports, the Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guidelines did not recommend the tonsillectomy as a therapeutic approach for IgAN [36].
Conflicting research findings highlight the differences between the European and American perceptions of tonsillectomy as a treatment for IgAN. The European-American practice, which limits tonsillectomy to cases with an obvious focal infection, is in clear disagreement with the common indication in Asia to perform tonsillectomy upon diagnosis of IgAN.
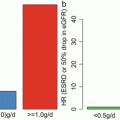
Why is there an underlying regional scientific conflict regarding the benefits of tonsillectomy? Although this conflict is based on the discrepancy between the abovementioned two European retrospective studies [29, 30] and some Asian studies [28, 31, 32], the clinical stages of the IgAN patients in these studies appear to be different. Rasche et al. included patients with relatively advanced stages: 55 % of the participants had hypertension, 35 % had elevated serum creatinine (>150 mmol/L), 62 % had heavy proteinuria (>1.5 g/day), and 25 % of the participants reached ESRD within 2.3 years after tonsillectomy [29]. Although Piccoli et al. selected only CKD stage 1 and 2 patients with tonsillectomy, they analyzed the efficacy of the tonsillectomy in both IgAN and non-IgAN patients [30].
Meanwhile, Xie et al. examined 118 Japanese patients in a moderate stage: 38.1 % had <0.5 g/day proteinuria and the mean serum creatinine was 1.07 mg/dl; in addition, a better outcome for tonsillectomy during the long-term follow-up was observed [28]. A recent study by Maeda et al. included a relatively early stage of IgAN patients; they demonstrated the efficacy of tonsillectomy adjusted for the known risk factors including blood pressure, proteinuria, and histological findings [31]. Therefore, perceived discrepancies in efficacy between Europe and Asia may partly be because of the clinical stage of IgAN at the time of intervention [5].
18.5 Current Clinical Practice in Western and Eastern Worlds with Respect to Indication for Tonsillectomy
In Europe and the USA, the guidelines for tonsillectomy provided by Ears, Nose, and Throat (ENT) International Societies do not recommend tonsillectomy in patients who do not present with recurrent tonsillitis, including fever, pharyngeal or tonsillar erythema, enlarged tonsils, tonsillar exudate, cervical adenopathy, and supportive microbiological test results. Moreover, watchful waiting is recommended for patients with recurrent throat infections if they have had fewer than seven episodes in the previous year. At least 12 months of observation is recommended before considering tonsillectomy. No specific benefit is supported by nephrology guidelines; therefore, tonsillectomy in a patient with IgAN without clinical signs could be questionable in case of complications, even malpractice.
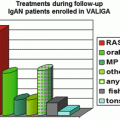
Following Hotta’s 2001 report [32], tonsillectomy in Japan is frequently performed in combination with steroid pulse therapy. According to a nationwide survey in more than 350 Japanese teaching hospitals, TSP is now performed in approximately 65 % of the center hospitals [37], with remission of hematuria or proteinuria in more than 50 % patients. These uncontrolled clinical data represent the basis for TSP as the standard therapy for IgAN in Japan.
The majority of Japanese IgAN patients are less than 40 years old. 70 % of the patients were diagnosed at early stage of this disease [38] through chance hematuria in screening urinalysis. Although the KDIGO guidelines recommend renin-angiotensin system blockade (RASB) agents as the first choice of treatment for IgAN patients [39], Japanese medical guidelines do not permit the use of RASB in IgAN patients without hypertension. These guidelines in addition to teratogenic side effects further nourish a hesitation for RASB usage in normotensive young females with IgAN in common Japanese medical practice.
The difficultly in prescribing RASB along with well-demonstrated benefits of TSP are factors that favor tonsillectomy in early phases of IgAN in Japan, making it difficult to justify multicenter RCTs. However, a large, nationwide prospective cohort study in Japan began in 2008 and will provide some much needed clarity to this matter.
18.6 Conclusion
Tonsillectomy as monotherapy in IgAN has only a small possibility to be proven beneficial by RCT. It would not be feasible for opposite reasons in the two parts of the world.
In Europe and Asia, no ethical committee would permit a potentially invasive surgical intervention, which is not recommended by ENT or nephrology guidelines. No ENT specialist could be convinced to participate without a strong, evidence-based recommendation.
On the opposite side, in Japan, social medicine does not support RASB in normotensive young IgAN patients. Medical practitioners and patients generally believe that tonsillectomy and TSP are safe and beneficial. Tonsillectomy/TSP is the standard of practice; therefore, it would be not easy to conduct an RCT.
The abovementioned differences in clinical practice of tonsillectomy in IgAN provoke a still-passionate discussion at international meetings about this procedure in patients with IgAN. The interest remains strong and we do not abandon the hope to find a scientific solution.
Acknowledgments
We would like to thank all participants in the special symposiums of IgAN in APCN 2014: Prof. Kawamura T, Yuzawa Y, Narita I, Katafuchi R, Yasuda T, Yasuda Y, Yamamoto R, Hirano K, Goto S, Suzuki H, Li PKT, Liu ZH, Chae DW, Zhang H, Barratt J, Novak J, Coppo R, Mustonen J, Glassock R, the Asian Pacific Society of Nephrology (APSN) president Prof. Harris D, and the president of Japanese Society of Nephrology (JSN) Prof. Matsuo S. We also thank NDT-Educational for the possibility to expand the discussion on tonsillectomy in IgAN refining the material for the preparation of this manuscript.
Conflict of Interest The authors declare that they have no conflict of interest.
References
1.
Schena FP, Coppo R. IgA nephropathies. In: Davison AM, Ritz E, Cameron JS, Winearls C, editors. Oxford textbook of clinical nephrology. 3rd ed. Oxford: Oxford University Press; 2005.
2.
3.
Coppo R, D’Amico G. Factors predicting progression of IgA nephropathies. J Nephrol. 2005;18:503–12.PubMed
4.
5.
Suzuki Y, Suzuki H, Yasutake J, Tomino Y. Paradigm shift in activity assessment of IgA nephropathy – optimizing the next generation of diagnostic and therapeutic maneuvers via glycan-targeting. Expert Opin Biol Ther. 2015;20:1–11.
6.
7.
8.
9.
Suzuki H, Suzuki Y, Narita I, Aizawa M, Kihara M, Yamanaka T, et al. Toll-like receptor 9 affects severity of IgA nephropathy. J Am Soc Nephrol. 2008;19:2384–95.CrossRefPubMedPubMedCentral
10.
Kajiyama T, Suzuki Y, Kihara M, Suzuki H, Horikoshi S, Tomino Y. Different pathological roles of toll-like receptor 9 on mucosal B cells and dendritic cells in murine IgA nephropathy. Clin Dev Immunol. 2011;2011:819646.CrossRefPubMedPubMedCentral
11.
12.
Novak J, Moldoveanu Z, Julian BA, Raska M, Wyatt RJ, Suzuki Y, et al. Aberrant glycosylation of IgA1 and anti-glycan antibodies in IgA nephropathy: role of mucosal immune system. Adv Otorhinolaryngol. 2011;72:60–3.PubMed
13.
Suzuki H, Kiryluk K, Novak J, Moldoveanu Z, Herr AB, Renfrow MB, et al. The pathophysiology of IgA nephropathy. J Am Soc Nephrol. 2011;22(10):1795–803.CrossRefPubMedPubMedCentral
14.
15.
16.
17.
He L, Peng Y, Liu H, Yin W, Chen X, Peng X, et al. Activation of the interleukin-4/signal transducer and activator of transcription 6 signaling pathway and homeodomain-interacting protein kinase 2 production by tonsillar mononuclear cells in IgA nephropathy. Am J Nephrol. 2013;38(4):321–32.CrossRefPubMed
18.
Suzuki H, Raska M, Yamada K, Moldoveanu Z, Julian BA, Wyatt RJ, et al. Cytokines alter IgA1 O-glycosylation by dysregulating C1GalT1 and ST6GalNAc-II enzymes. J Biol Chem. 2014;289:5330–9.CrossRefPubMedPubMedCentral
19.
20.
Nakata J, Suzuki Y, Suzuki H, Sato D, Kano T, Yanagawa H, et al. Changes in nephritogenic serum galactose-deficient IgA1 in IgA nephropathy following tonsillectomy and steroid therapy. PLoS One. 2014;21:9.
21.
Vergano L, Loiacono E, Albera R, Coppo R, Camilla R, Peruzzi L, et al. Can tonsillectomy modify the innate and adaptive immunity pathways involved in IgA nephropathy? J Nephrol. 2015;28(1):51–8.
22.
Kiryluk K, Li Y, Scolari F, Sanna-Cherchi S, Choi M, Verbitsky M, et al. Discovery of new risk loci for IgA nephropathy implicates genes involved in immunity against intestinal pathogens. Nat Genet. 2014;46:1187–96.CrossRefPubMedPubMedCentral
23.
24.
Suzuki Y, Tomino Y. The mucosa-bone-marrow axis in IgA nephropathy. Contrib Nephrol. 2007;157:70–9.PubMed
25.
26.
Kawaguchi T, Ieiri N, Yamazaki S, Hayashino Y, Gillespie B, Miyazaki M, et al. Clinical effectiveness of steroid pulse therapy combined with tonsillectomy in patients with immunoglobulin A nephropathy presenting glomerular haematuria and minimal proteinuria. Nephrology. 2010;15:116–23.CrossRefPubMed
27.
Suzuki Y, Suzuki H, Nakata J, Sato D, Kajiyama T, Watanabe T, et al. Pathological role of tonsillar B cells in IgA nephropathy. Clin Dev Immunol. 2011;2011:639074.CrossRefPubMedPubMedCentral
28.
29.
Rasche FM, Schwarz A, Keller F. Tonsillectomy does not prevent a progressive course in IgA nephropathy. Clin Nephrol. 1999;51:147–52.PubMed
30.
31.
32.
33.
Miura N, Imai H, Kikuchi S, Hayashi S, Endoh M, Kawamura T, et al. Tonsillectomy and steroid pulse (TSP) therapy for patients with IgA nephropathy: a nationwide survey of TSP therapy in Japan and an analysis of the predictive factors for resistance to TSP therapy. Clin Exp Nephrol. 2009;13:460–6.CrossRefPubMed
34.
35.
Kawamura T, Yoshimura M, Miyazaki Y, Okamoto H, Kimura K, Hirano K, et al. A multicenter randomized controlled trial of tonsillectomy combined with steroid pulse therapy in patients with immunoglobulin A nephropathy. Nephrol Dial Transplant. 2014;29(8):1546–53.CrossRefPubMedPubMedCentral
36.
KDIGO clinical practice guideline for glomerulonephritis. Kidney Int Suppl 2012;2:218–20.
37.
Matsuzaki K, Suzuki Y, Nakata J, Sakamoto N, Horikoshi S, Kawamura T, et al. Nationwide survey on current treatments for IgA nephropathy in Japan. Clin Exp Nephrol. 2013;17:827–33.CrossRefPubMedPubMedCentral
38.