Beyond the central nervous system: neurovascular entrapment syndromes
BRADLEY W. STOCKERT, PT, PhD, LAURA J. KENNY, PT, OCS, FAAOMPT and PETER I. EDGELOW, PT, MS, DPT
Peripheral neuroanatomy
The PNS is generally regarded as the portion of the nervous system that lies outside the CNS (i.e., the brain and spinal cord).1,2 The major components of the PNS include motor, sensory, and autonomic neurons found in spinal, peripheral, and cranial nerves. Although this partitioning is valid from an anatomical perspective, it often leads to a lack of appreciation as to the truly continuous nature and integrative function of the nervous system as a whole. The concept that the entire nervous system is a continuous tissue tract reinforces the idea that limb and trunk movements can have a mechanical effect on the PNS and the CNS that is local and global.
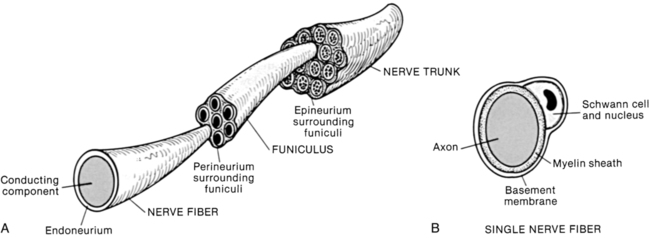
Three levels in the organization of a peripheral nerve have been described2,3 (Figure 18-1). At the innermost level the nerve fiber is the conducting component of a neuron (nerve cell). A connective tissue layer called the endoneurium surrounds each nerve fiber. The endoneurium surrounds the basement membrane of the neuron and plays an important role in maintaining fluid pressure within the endoneurial space. There are no lymphatic channels within the endoneurial space. The pressure within the endoneurial space increases with compression of the neuron.4
The second level of organization consists of a collection of many nerve fibers (a fascicle) surrounded by a layer of connective tissue called the perineurium.2,3 The perineurium acts as a selective barrier to diffusion and as such exerts significant control over the local movement of fluid and ions. This connective tissue layer acts like a pressurized container—that is, extrusion of the contents occurs if the membrane is cut. The compartment enclosed by the perineurium does not contain lymphatic channels.4 This may be a problem during inflammatory states when edema is present deep to the perineurium. The perineurium is the last connective tissue layer to rupture in tensile testing of peripheral nerves.5 The outermost connective tissue layer of a peripheral nerve is called the epineurium. The epineurium surrounds, protects, and enhances gliding between the fascicles. Lymphatic channels are found within the epineurial compartment.
All three connective tissue layers are interconnected—they are not separate and distinct, but continuous tissue layers.2,3 Each of the connective tissue layers contains free nerve endings from the nervi nervorum. As a result, all three connective tissue layers are a potential source of pain. In addition, all three layers are continuous with the homologous connective tissue layers of the CNS—for example, the dura mater and the epineurium.
Peripheral nerves are regularly subjected to compression and elongation (stretching), which have been shown to increase intraneural pressure. An increase in intraneural pressure decreases the diameter of the intrinsic blood vessels and results in a reduction in blood flow within the nerve. Compressive forces of 20 to 30 mm Hg have been shown to adversely affect intraneural blood flow,6 and compressive forces of 50 to 70 mm Hg have been shown to result in complete arrest of blood flow7 and cause damage to myelin and axons.6 A strain (elongation) of 6% to 8% has been shown to decrease intraneural blood flow by 50% to 70% in the sciatic nerve of rats.4,8,9 A strain of 15% in the sciatic nerve of a rabbit has been shown to result in complete arrest of blood flow,7 and the same strain produces an 80% reduction in blood flow in the rat sciatic nerve.9 Strains of 11% or greater are produced by some of the positions used in neurodynamic tests of the upper limb.6 Significant increases in intraneural pressure and concomitant decreases in intraneural blood flow have been shown to adversely affect neuronal conduction.7,10,11
The cytoplasm in cells moves and has thixotropic properties—that is, the viscosity of cytoplasm is lower when it is continuously moving.4 In neurons the movement of the cytoplasm from the cell body through the axons (anterograde movement) occurs at two speeds. Fast axoplasmic flow occurs at a rate of about 100 to 400 mm/day and is used to carry ion channels and neurotransmitters (i.e., materials required for normal impulse conduction) to the nerve terminals. Slow axoplasmic flow occurs at a rate of about 6 mm/day and is used to transport cytoskeleton proteins, neurofilaments, and other materials used to maintain the physical health of the cell. A third flow occurs in the opposite direction (retrograde) at a rate of about 200 mm/day. Retrograde transport carries unused substances and exogenous materials taken up at the terminus—for example, neurotrophic factors. The material carried back to the cell body by retrograde transport has been shown to influence activity in the cell nucleus.4
Compression raises intraneural pressure, which has a negative impact on the flow of cytoplasm.4 Anterograde and retrograde flow of axoplasm is impaired with 30 mm Hg compression on the nerve, hypoxia, or a strain of 11% or greater.12–14 Prolonged or intense exposure to compression can result in conduction abnormalities, endoneurial edema, fibrin deposition, demyelination, and axonal sprouting. Each of these events increases the likelihood of developing adhesions and abnormal impulse-generating sites (AIGSs).4 (The negative impact of AIGSs is discussed in the section on adaptive responses to pain.)
Mobility of the peripheral nervous system
Several types of tissues (e.g., bone, fascia, and muscle) surround peripheral nerves as they “travel” to target tissues. Peripheral nerves can be thought of as passing through a series of tissue tunnels composed of various biological materials. The composition of the tissue tunnel changes with the passage of the nerve from the vertebral column (an osseous tunnel) to the target tissue—for example, from an osseous tunnel to a soft tissue and/or fibro-osseous tunnel. A “mechanical interface” exists at the junction between the nerve and the material adjacent to the nerve that forms the tissue tunnel. Movement of the trunk and/or limbs can cause three types of movement to occur in the peripheral nerves: unfolding, sliding, and elongation.15
When there is little or no tension in a peripheral nerve, the axon typically contains undulations (folds). As tension is applied the axon will “unfold” so that the undulations disappear. “Sliding” can be defined as movement between the nerve and the surrounding tissues at the mechanical interface (extraneural movement). Sliding by itself does not cause significant elongation or tension to develop within the nerve, so intraneural pressure remains relatively unchanged. Ultrasound studies have shown that the median, ulnar, sciatic, and tibial nerves undergo extraneural movement (sliding) with movement of the upper and lower limbs, respectively.16–19
“Elongation” of the nerve occurs when tension is applied to a nerve and there is little or no unfolding and sliding at the mechanical interface. Elongation causes movement to occur between the neural elements and connective tissue layers (intraneural movement). Elongation decreases the diameter of the nerve, resulting in an increase in the intraneural tension and pressure.15 An increase in intraneural pressure has been shown to decrease the flow of blood and axoplasm, resulting in altered neural function (see previous section). Elongation within the median and ulnar nerves has been shown to occur with movements of the upper limb.6
Both extraneural and intraneural movements may occur simultaneously within a nerve, but they may not be uniformly distributed. When a body moves, some parts of the PNS will undergo primarily extraneural movement (sliding) with little or no development of tension while other areas undergo intraneural movement (elongation) that results in an increase in intraneural tension and pressure. As a consequence, some areas within a nerve slide, developing little or no tension, whereas other areas of the same nerve elongate significantly, increasing the amount of intraneural tension.6 In areas repeatedly exposed to high amounts of tension, for example, the median nerve at the wrist, the nerves are found to contain a higher-than-average amount of connective tissue.15
If one considers the entire nervous system as a continuous tissue tract, then the idea that movement and/or tension developed in one region of the nervous system can be distributed and dissipated throughout the entire nervous system becomes apparent.20,21 The inability of a component within the nervous system to dissipate and/or distribute movement and tension can lead to abnormal force development and lesions elsewhere in the continuous tissue tract.22
Peripheral nerve entrapment
Seddon’s classification of nerve injury is based upon mechanical trauma.23 Schaumberg2 modified this paradigm into an anatomically based scheme containing three classes of injury (Table 18-1). Injuries in class II and III are caused by macrotrauma that results in some disruption to the integrity of the nerve fiber. The following discussion of entrapment is focused on microtrauma in which there is no breach in the anatomical integrity of the nerve fiber (class I). Mechanical microtrauma resulting in nerve entrapment can occur with excessive or abnormal friction, compression, and/or tension (elongation).2
TABLE 18-1 
CLASSIFICATION OF ACUTE TRAUMATIC PERIPHERAL NERVE INJURY
| ANATOMICAL CLASSIFICATION | CLASS I | CLASS II | CLASS III |
| Previous nomenclature | Neuropraxia | Axonotmesis | Neurotmesis |
| Lesion | Reversible conduction block resulting from ischemia or demyelination | Axonal interruption but basal lamina remains intact | Nerve fiber and basal lamina interruption (complete nerve severance) |
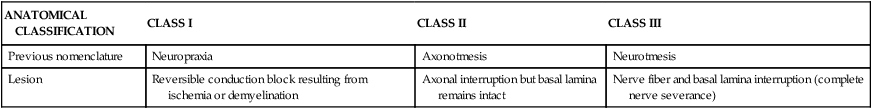
Modified from Schaumberg HH, Spencer PS, Thomas PK: Disorders of peripheral nerves, Philadelphia, 1983, FA Davis.
Tissue tunnels, peripheral nerves, and the mechanical interfaces between them are all vulnerable to mechanical microtrauma—that is, abnormal friction, compression, and/or tension.2,15 Some peripheral nerves are exposed to bony hard interfaces, for example, the lower cords of the brachial plexus at the first rib, which are potential sources of abnormal friction. Inflammation and swelling within a tissue tunnel can produce compression of a nerve, for example, the median nerve within the carpal tunnel. The point at which a nerve branches limits the amount of gliding (extraneural movement) available at that location and increases the amount of local intraneural tension developed with movement, for example, the tibial nerve in the popliteal fossa.2,15
Microtrauma can produce an intraneural lesion that causes a decrease in intraneural flow of blood and axoplasm, demyelination, and/or conduction defects.2,15 If the lesion occurs in the connective tissues of the nerve, there may be pain, inflammation, proliferation of fibroblasts, and scar formation (fibrosis). Ultrasound studies have shown that the median nerve in patients with carpal tunnel syndrome is enlarged approximately 30%.24 An intraneural scar decreases the compliance of the nerve and increases the amount of intraneural pressure and tension generated with elongation.21 Intraneural lesions can impair or completely block the ability of the nerve to conduct action potentials.2,15,25 Partial or complete conduction blocks can result in abnormal sensation, loss of motor function, autonomic dysfunction, and atrophy of target tissue, for example, muscle and/or skin.
Microtrauma can produce an extraneural lesion.2,15,21,25 The damage in an extraneural lesion occurs in the tissue surrounding the nerve or at the mechanical interface. Swelling within the tissue tunnel can produce compression of the nerve. Fibrosis can produce adhesions at the mechanical interface leading to a decrease in sliding of the nerve. A decrease in the ability of a nerve to slide within a tissue tunnel will result in an abnormal increase in intraneural tension and pressure as movement is imposed on the nerve. The increase in local intraneural tension can produce abnormal changes in the conduction of action potentials, and the tension will be distributed in an aberrant pattern throughout the continuous tract of the nervous system. The resultant abnormal distribution of tension predisposes the nervous system to the development of lesions at other sites.15
Friction, compression, and tension can produce microtrauma that results in intraneural and extraneural pathology.2,15 For example, fibrosis can produce a combined pathological state that results in a substantial reduction in the ability of a nerve to slide within the tissue tunnel and a substantial increase in intraneural tension during nerve elongation as the compliance of the nerve is decreased. Movement of the median nerve at the carpal tunnel has been shown to occur with movements of the upper limb.16–19 Longitudinal and transverse movements of the median nerve at the carpal tunnel have been shown to be reduced in the presence of microtrauma, that is, carpal tunnel syndrome,24,26 nonspecific arm pain,27,28 and whiplash injury.28
Intraneural and extraneural lesions result in an abnormal distribution of sliding and tension throughout the nervous system with movement of the trunk and/or limbs. The abnormal distribution of tension within a nerve increases the probability of a second lesion or abnormality developing within the nerve. This situation led Upton and McComas29 in 1973 to first use the term “double crush injury.” (This term should be considered a misnomer because a “crush” does not necessarily occur.) For example, entrapment of the median nerve at the carpal tunnel can cause the development of abnormal tension in cervical spinal nerves, resulting in a lesion at that site. Upton and McComas29 have shown that a lesion at the carpal tunnel increases the risk of having a second neural lesion in the cervical region.
Pathogenesis of neurovascular entrapment
Neurovascular entrapment can occur at any point along the continuous tract of the nervous system. The carpal tunnel, a common site of entrapment, has been studied and provides a framework of information regarding the pathogenesis of neurovascular entrapments. Sunderland30 has reasoned that a change in the normal pressure gradients within the carpal tunnel can lead to compression of the median nerve. In order to maintain homeostasis in the carpal tunnel and the median nerve, blood must flow into the tunnel, then into the nerve and back out of the tunnel. For normal blood flow to occur in the median nerve the blood pressure must be highest within the epineurial arterioles and becomes progressively lower in the capillaries and epineurial venules and lowest within the extraneural space of the carpal tunnel. Any increase in the pressure of a single compartment has the potential to disrupt the normal pressure gradients and impair the flow of blood within the compartments of the carpal tunnel and median nerve. Impaired intraneural blood flow can lead to localized hypoxia, edema, inflammation, and fibrosis.30
An increase in pressure within the carpal tunnel can occur for a variety of reasons, for example, synovial hyperplasia, thickening of tendons, venous congestion, inflammation, and/or edema. Venous blood flow within a nerve will be impaired and venous stasis will develop if pressure within the extraneural space of the carpal tunnel becomes greater than the pressure within the epineurial venules. Because blood pressure within venules is relatively low, partial occlusion of blood flow can begin to occur with pressures as low as 20 to 30 mm Hg.15,31
The pressure within the carpal tunnel is normally about 3 mm Hg with the wrist in a neutral position.4 The pressure can rise to over 30 mm Hg when the wrist is placed in 90 degrees of extension32 or with the functional task of using a computer mouse to drag or point at an object.33 Studies have shown that the pressure within the carpal tunnel in someone with carpal tunnel syndrome can be 30 mm Hg, or more, with the wrist in neutral and can increase to about 100 mm Hg when the wrist is in 90 degrees of flexion4,12,13 or extension.12 Compressive forces of 20 to 30 mm Hg have been shown to adversely affect intraneural blood flow,6 whereas compressive forces of 50 to 70 mm Hg have been shown to result in complete arrest of blood flow7 and cause demonstrable damage to myelin and axons.6 Motor and sensory abnormalities begin to manifest at about 40 mm Hg, and complete blockade of the median nerve has been shown to occur at 50 mm Hg.34 The pressure found in the carpal tunnel of people with carpal tunnel syndrome is clearly adequate to disrupt the normal flow of blood, axoplasm, and action potentials within the median nerve, causing severe impairment to normal nerve functions.
Sunderland30 proposed that venous congestion or stasis within the carpal tunnel will lead to localized hypoxia, edema, and fibrosis. Hypoxia causes capillary endothelial cells to deteriorate and local C fibers to secrete substance P and calcitonin gene–related peptide,4 which in turn cause mast cells to release histamine and serotonin.35,36 Together these chemical mediators augment the inflammatory state and cause the endothelial cells of capillaries to further deteriorate by becoming flatter, larger, and leakier, enhancing exudation and edema.
The set of circumstances described earlier may be referred to as a neurovascular entrapment syndrome, and it has the potential to cause the development of problems elsewhere in the system—that is, a double crush injury (see previous section). Upton and McComas29 studied 115 subjects with carpal tunnel syndrome or ulnar impingement at the elbow. They found that 81 of the 115 subjects also had evidence of a neural lesion at the neck. Because all nerves essentially travel within tissue tunnels, the potential exists for this scenario to occur elsewhere in the continuous tissue tract of the nervous system, for example, the capsule of the dorsal root ganglion and the thoracic outlet.5,15,37
Adaptive responses to pain
A thorough discussion of the pain associated with neurovascular entrapment is beyond the scope and intent of this chapter. The topic of pain management is discussed in Chapter 32 of this book. However, we would like to describe the development of hyperexcitable states and AIGSs in neurons as well as their role in the development of pain associated with neurovascular entrapment.
“Normal” or physiological pain occurs when peripheral nociceptors are subjected to a stimulus that is at or above the threshold for firing. “Abnormal” or pathological pain can occur when there is a change in the sensitivity (threshold) of the somatosensory system.38 Devor39 wrote that “the crucial pathophysiological process triggered by nerve injury is an increase in neuronal excitability.”
Neurons that become inflamed, hypoxic, and/or demyelinated can enter a hyperexcitable state.2,39–47 A neuron in a hyperexcitable state can begin to discharge spontaneously and/or develop a sustained rhythmic discharge after stimulation. In addition, hyperexcitable neurons can develop mechanosensitivity,41 chemosensitivity,4 and/or thermal sensitivity,45 all of which can result in the production of allodynia, a form of pathological pain.* These changes in the behavior of a nerve can occur in the absence of detectable degeneration.40–42 The changes in impulse generation and neuronal sensitivity are characteristics of an AIGS.4 A hyperexcitable state and an AIGS can develop with the mechanical microtrauma and inflammation often associated with peripheral nerve pathology, for example, compression, tension, and friction.2,39,48,49 A variety of chemical mediators have been implicated in the development of a hyperexcitable state in a neuron—for example, neurotrophins,50,51 histamine,52 and other inflammatory mediators,53 which are thought to act through changes in gene expression,45,50,51 changes in voltage gated sodium channel expression,45 and a reduction in anterograde axoplasmic transport.44,52
The dorsal root ganglion appears to play a significant role in the pain associated with peripheral nerve pathology.38,41 Mechanical microtrauma to and inflammation of peripheral nerves can cause the dorsal root ganglion to become hyperexcitable (sensitized).41 The change in sensitivity allows what were weak, subthreshold stimuli to evoke pain and suprathreshold stimuli to evoke exaggerated pain (hyperalgesia). In addition, the dorsal root ganglion can develop mechanosensitivity, chemosensitivity, and thermal sensitivity, resulting in allodynia.45 This change in sensitivity reflects a change in the physiology of the nerve and may be a component in the development of enhanced central sensitivity to pain and development of a chronic pain state.38
As noted previously, the PNS and CNS represent a continuous tissue tract. The pain and symptoms associated with musculoskeletal injury and/or peripheral nerve pathology can include changes that are the result of an alteration in the autonomic nervous system, which is considered part of the continuous tissue tract of the nervous system.2,54 For example, catecholamines do not normally elicit pain. However, if a nerve is injured or if there is local inflammation, the catecholamines can induce pain (chemosensitivity) and they can maintain or enhance pain in inflamed tissues.4
Some patients who are treated for musculoskeletal injuries have signs that may be related to autonomic dysreflexia.37 Wyke55 demonstrated that stimulation of nociceptors in spinal joints resulted in reflex changes in the cardiovascular, respiratory, and endocrine systems. Dysregulated breathing has been documented in patients with chronic pain.56 Patients with nonspecific arm pain have a reduced sympathetic vasoconstrictor response in the hand of the affected limb.57 Thermal asymmetry has been documented in the hands of patients with neurogenic thoracic outlet syndrome.58 Feinstein59,60 has shown that injecting saline solution into the thoracic paraspinal muscles caused pallor, diaphoresis, bradycardia, and a drop in the blood pressure. These cardiovascular and respiratory changes are often associated with an alteration in the output from the autonomic nervous system.37,54,61
In patients with cumulative trauma disorder (CTD), signs of abnormal autonomic nervous system output can include (1) vasomotor reflexes leading to cool, pale skin,57 (2) changes in the pattern of sweating (hypohidrosis and/or hyperhidrosis), (3) trophic changes in the skin, (4) hyperactive flexor withdrawal reflexes, and/or (5) paradoxical breathing patterns.37 Edgelow has described paradoxical breathing as the predominant use of the scalene muscles for ventilation during quiet breathing versus normal ventilation, which is predominantly a function of the diaphragm.37 Edgelow found that paradoxical breathing is present in most patients with CTD of the upper extremity.37 A better appreciation of the contribution of the autonomic nervous system to the pathology and symptoms present in some patients with neurovascular entrapment may enhance the effectiveness of their treatment.
Clinical examination and treatment of neurovascular entrapment
The Edgelow protocol for examination and treatment of neurovascular entrapment challenges the traditional musculoskeletal paradigm by placing the primary emphasis on the response of the neurovascular and neuromotor systems to injury.62–64 The standard musculoskeletal evaluation centered on a biomechanical model of the musculoskeletal and nervous systems is adequate for patients with straightforward symptoms that appear to be of biomechanical origin. However, a biomechanical approach is inappropriate for patients with severe or irritable signs and symptoms that may be neurological or vascular in origin. Patients with neurovascular entrapment often have severe, irritable symptoms. First a subjective evaluation is conducted in a patient with a potential neurovascular entrapment problem to determine how the objective examination should proceed. The history of the condition is discussed with the patient. Key components that should be discussed include history of trauma, repetitive activities, sustained static or tension postures, such as computer keyboard work, or physical activities performed with a high level of cognitive demand, as seen in a pianist. The history should include a discussion of general health, including any potentially relevant medical conditions (e.g., asthma, diabetes, hypothyroidism). Phase I of differential diagnosis (medical screening) should be completed to ensure that the patient is appropriate for evaluation and intervention. (See Chapter 7 on medical screening.)
A discussion of the patient’s symptoms and complaints should include questions that determine whether the neural or vascular system is a potential source of the problem. Symptoms relevant to the potential problem of neurovascular entrapment include complaints of fullness in the upper extremity; a feeling of swelling, tingling, pain, coldness, or numbness; or dropping things. In addition, the progression of the symptoms or complaints and the level of irritability should be determined. If pain is a major factor, then a functional pain questionnaire should be completed (see Chapter 32 on pain management). Motor changes of relevance to the potential problem of neurovascular entrapment include complaints of dropping things, weakness, or an inability to perform motor tasks that were done previously without difficulty. The level of neural irritability and the presence of peripheral or central sensitization should be determined by asking the patient what activities aggravate and ease the symptoms. When an extended period of time is required for symptoms to ease after provocation, irritability may be a cause. Sensitization is indicated when minor mechanical or normally nonnoxious stimuli, such as clothing on the skin, provoke pain. Vascular complaints relevant to the potential problem of neurovascular entrapment include complaints of fullness, swelling, abnormal skin color, or cool skin temperatures. A change in the vascular symptoms with a change in limb position is particularly significant.
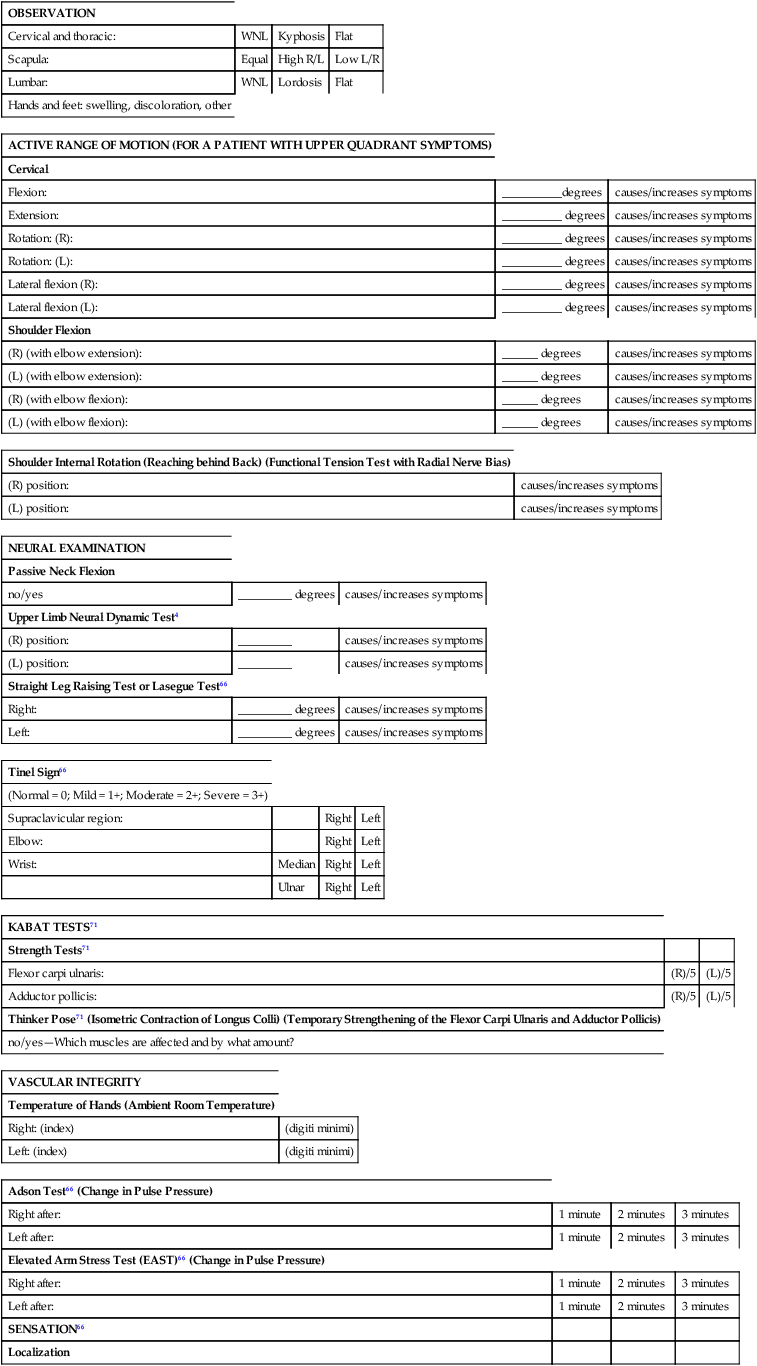
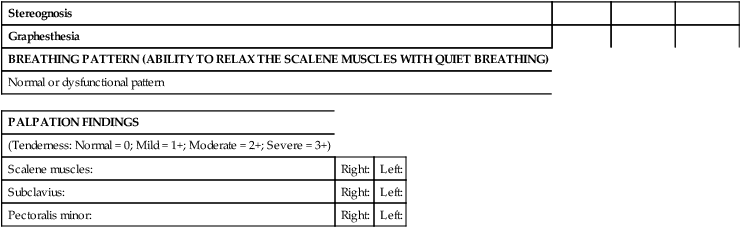
In a biomechanical evaluation model the therapist examines the quantity and quality of active movements and determines whether there is pain, spasm, or resistance at an end feel. In patients with neurovascular entrapment, this procedure may evoke a significant flare and worsening of symptoms. In patients with neurovascular entrapment the “feel” of involuntary muscle tension can be the first sign of abnormality in assessing movement. This tension is often subtle and may occur earlier in the range of motion than where traditional biomechanical symptoms or the end feel normally occurs.65 Moving into the range of motion to the initial onset of tension minimizes the risk of provoking adverse neurological or vascular consequences. In patients with suspected neurovascular entrapment who have symptoms suggestive of neural irritability and sensitization, the biomechanical examination and treatment techniques should be modified or deferred until the sensitivity and irritability of the nervous system are improved. See Table 18-2 for suggested modifications to a standard biomechanical evaluation.
TABLE 18-2 
SUGGESTED MODIFICATIONS TO A STANDARD BIOMECHANICAL EVALUATION
| OBSERVATION | |||
| Cervical and thoracic: | WNL | Kyphosis | Flat |
| Scapula: | Equal | High R/L | Low L/R |
| Lumbar: | WNL | Lordosis | Flat |
| Hands and feet: swelling, discoloration, other |
| ACTIVE RANGE OF MOTION (FOR A PATIENT WITH UPPER QUADRANT SYMPTOMS) | ||
| Cervical | ||
| Flexion: | __________degrees | causes/increases symptoms |
| Extension: | __________ degrees | causes/increases symptoms |
| Rotation: (R): | __________ degrees | causes/increases symptoms |
| Rotation: (L): | __________ degrees | causes/increases symptoms |
| Lateral flexion (R): | __________ degrees | causes/increases symptoms |
| Lateral flexion (L): | __________ degrees | causes/increases symptoms |
| Shoulder Flexion | ||
| (R) (with elbow extension): | ______ degrees | causes/increases symptoms |
| (L) (with elbow extension): | ______ degrees | causes/increases symptoms |
| (R) (with elbow flexion): | ______ degrees | causes/increases symptoms |
| (L) (with elbow flexion): | ______ degrees | causes/increases symptoms |
| Shoulder Internal Rotation (Reaching behind Back) (Functional Tension Test with Radial Nerve Bias) | |
| (R) position: | causes/increases symptoms |
| (L) position: | causes/increases symptoms |
| NEURAL EXAMINATION | ||
| Passive Neck Flexion | ||
| no/yes | _________ degrees | causes/increases symptoms |
| Upper Limb Neural Dynamic Test4 | ||
| (R) position: | _________ | causes/increases symptoms |
| (L) position: | _________ | causes/increases symptoms |
| Straight Leg Raising Test or Lasegue Test66 | ||
| Right: | _________ degrees | causes/increases symptoms |
| Left: | _________ degrees | causes/increases symptoms |
| Tinel Sign66 | |||
| (Normal = 0; Mild = 1+; Moderate = 2+; Severe = 3+) | |||
| Supraclavicular region: | Right | Left | |
| Elbow: | Right | Left | |
| Wrist: | Median | Right | Left |
| Ulnar | Right | Left |
| KABAT TESTS71 | ||
| Strength Tests71 | ||
| Flexor carpi ulnaris: | (R)/5 | (L)/5 |
| Adductor pollicis: | (R)/5 | (L)/5 |
| Thinker Pose71 (Isometric Contraction of Longus Colli) (Temporary Strengthening of the Flexor Carpi Ulnaris and Adductor Pollicis) | ||
| no/yes—Which muscles are affected and by what amount? |
| VASCULAR INTEGRITY | |
| Temperature of Hands (Ambient Room Temperature) | |
| Right: (index) | (digiti minimi) |
| Left: (index) | (digiti minimi) |
| Adson Test66 (Change in Pulse Pressure) | |||
| Right after: | 1 minute | 2 minutes | 3 minutes |
| Left after: | 1 minute | 2 minutes | 3 minutes |
| Elevated Arm Stress Test (EAST)66 (Change in Pulse Pressure) | |||
| Right after: | 1 minute | 2 minutes | 3 minutes |
| Left after: | 1 minute | 2 minutes | 3 minutes |
| SENSATION66 | |||
| Localization | |||
| Stereognosis | |||
| Graphesthesia | |||
| BREATHING PATTERN (ABILITY TO RELAX THE SCALENE MUSCLES WITH QUIET BREATHING) | |||
| Normal or dysfunctional pattern |
| PALPATION FINDINGS | ||
| (Tenderness: Normal = 0; Mild = 1+; Moderate = 2+; Severe = 3+) | ||
| Scalene muscles: | Right: | Left: |
| Subclavius: | Right: | Left: |
| Pectoralis minor: | Right: | Left: |
One component of the examination involves evaluating the integrity of the vascular system in the extremities. The hands or feet should be inspected for discoloration, and the skin temperature should be determined in each of the peripheral nerve territories present in the affected limb. Cool, cyanotic skin can be an indication of arterial insufficiency or sympathetic dysreflexia in the area, whereas swelling can be an indication of inflammation and venous or lymphatic insufficiency. An Adson test and the elevated arm stress test (EAST) can be used to evaluate vascular integrity by determining whether the pulse pressure decreases with a change in the position of the limb.66 The Adson test and EAST should be performed on both upper extremities, and the pulse pressure evaluated at 1, 2, and 3 minutes. These tests may be modified or deferred depending on the level of neural irritability found.
Sensory changes may be subtle and are not always accompanied by obvious motor dysfunction. The most common complaint with neurovascular entrapment of the upper extremity is “I drop things,” yet standard tests of strength, light touch, and two-point discrimination may have normal results. Therapists often think of this problem as motor until our standard tests fail to demonstrate motor dysfunction. Subtle changes in the somatosensory cortex can occur as a consequence of repetitive motions, particularly when performed under conditions of intense concentration or in the presence of pain.67–69 Byl observed severe degradation in the representation of the hand in the somatosensory cortex of owl monkeys that were trained in a behavior of rapid, active opening and closing of the hand under conditions of high cognitive drive.68 In addition, Byl67 found a significant difference in response on some sensory integration and praxis tests in human subjects with diagnoses of tendinitis and focal dystonia. Byl has postulated that similar changes can be identified in humans with repetitive strain injuries with the use of Jean Ayers’s tests of sensory localization, stereognosis, and graphesthesia.69
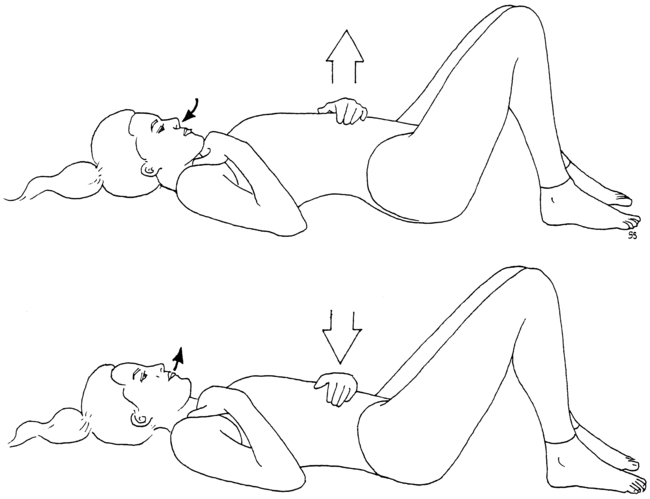
An assessment of the patient’s breathing pattern at rest and palpation of the subclavius, pectoralis minor, and scalene muscles should be performed. The normal breathing pattern at rest is primarily diaphragmatic (Figure 18-2). However, patients with neurovascular entrapment often demonstrate a breathing pattern at rest that relies predominantly on the scalene muscles. The scalene breathing pattern mechanically narrows the thoracic outlet area, thus potentially perpetuating a neurovascular entrapment syndrome in the area. The scalene breathing pattern may be a sign of protective posturing. Palpation is used to determine whether tenderness or tightness is present. Palpation of the subclavius, pectoralis minor, and scalene muscles is significant because of the relationship these muscles have with the subclavian vein, brachial plexus components, and subclavian artery, respectively. The results of the palpation should be correlated to the neurological and vascular changes found elsewhere in the extremity.
Neurovascular entrapment examination
Symptom patterns characteristic of neurovascular entrapment
Six common signs of neurovascular entrapment
1. Abnormal hand temperature within the following parameters:
a. Cold hands defined as in the 70° F range at rest and during activity at the target task
b. Asymmetry between the temperatures of the second digit and the fifth digit, with the fifth digit being colder.70
c. Asymmetry between hands in which there is an abnormal temperature cooling response to diaphragmatic breathing, aerobic walking, and repeated use of the upper extremities in an activity such as bouncing a gymnastic ball.
2. Abnormal breathing pattern: accessory, chest, or paradoxical rather than diaphragmatic.
3. Abnormal mobility and sensitivity of the nervous system: specifically the dura, the brachial plexus, or the sciatic nerve or sacral plexus.
4. Cardiovascular deconditioning: patient has a low level of endurance and is easily fatigued.
5. Sensory dysfunction of the hand at the cortical level: abnormal tactile localization, graphesthesia, and stereognosis.
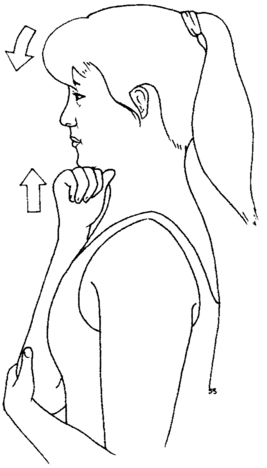
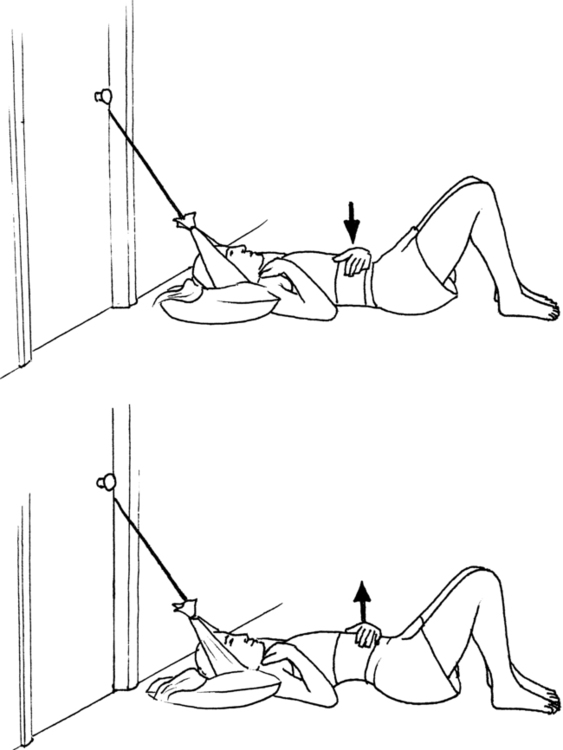
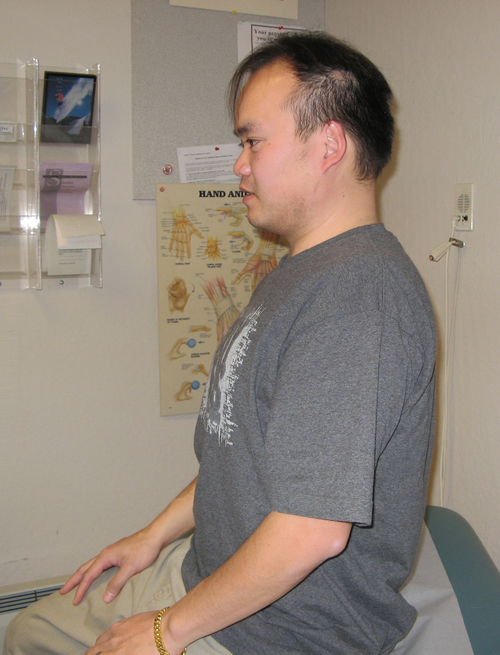
6. A positive Kabat71 sign: weakness of the flexor pollicis brevis in the shortened range of adduction that is unilateral and reversed with a gentle 30-second isometric contraction of the longus colli obtained with the “thinker pose” (Figure 18-3).
Neurovascular entrapment examination procedures
Neurodynamics of the upper extremity is assessed with the use of upper limb neural dynamic tests as described by Butler.4 Passive neck flexion is examined to assess dural sensitivity, whereas the straight leg raise test is used to assess the sensitivity of the sciatic nerve and sacral plexus. In addition to these passive neural dynamic tests performed by the examining therapist, the patient is taught an “arm self-test” to use as a self-assessment technique.
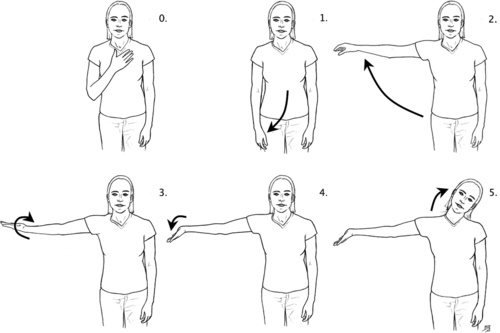
The arm self-test is performed by guiding the standing patient through a series of positions using the upper extremity (Figure 18-4). The sequence goes from position zero to five, with zero being the position of least general tension on the brachial plexus and five being a position of maximum elongation and general tension on the brachial plexus. Care must be taken to educate the patient to stop at the first sensation of tension and not linger with the arm in any self-test position that provokes symptoms. The test sequence begins in the zero position with the patient’s hand resting on the chest. In position one the patient’s arm is straight at the side; in position two the patient abducts the arm to shoulder height with the palm pronated. In position three the patient maintains abduction to shoulder height while supinating the forearm and hand; position four is performed by maintaining position three while extending the wrist. If the patient does not experience tension or pain in the previous positions, he or she can progress to position five by adding cervical lateral flexion away from the side being tested. Coppieters,72 in a cadaver study of nerve gliding, noted increased strain on the medial nerve in positions of wrist extension combined with elbow extension; thus position four of the arm self-test can be too provocative to use on the affected extremity in a patient with severe symptoms. (Please refer to the references at the end of this chapter for more information on neural dynamic tests.)
CNS sensory dysfunction of the hand (specifically tactile localization, graphesthesia, and stereognosis) is assessed by the methods of Byl.69
Hand strength is assessed by examining for the presence of a Kabat sign.71 The patient is instructed to hold the arm at the side with the elbow flexed to 90 degrees and fully supinated. The wrist is positioned in neutral flexion-extension with the fingers fully extended and the thumb in the shortened range of adduction and flexion (thumb in the plane of the palm). The distal phalanx of the thumb is held in full extension. This starting position inhibits the median innervated muscles of the palm and finger flexors. A manual muscle test is done to test the strength of flexor pollicis brevis and adductor pollicis in the shortened range. If there is a “giving way” at the metacarpophalangeal joint, then this is quantified using a “thumbometer,” an inexpensive device consisting of an eye drop bottle attached to a blood pressure cuff sphygmomanometer. Clinical experience demonstrates that after longus colli isometric contraction there is a strengthening of the affected muscles in the thumb. There will be a weakening effect on thumb strength if the patient has cervical instability during the performance of activities or exercises. This indicates the activity is too much for the patient at that time. If there is no effect on thumb strength then the patient is stable enough for the activity.
Neurovascular entrapment interventions
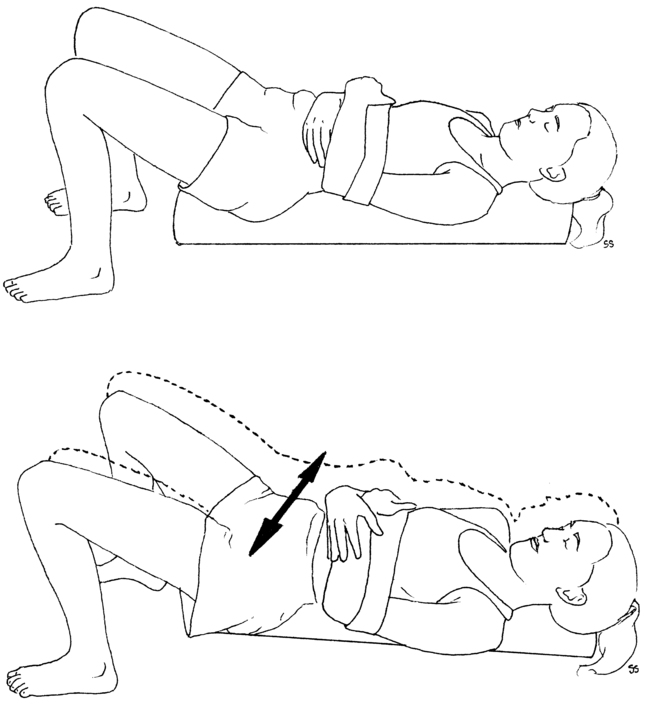
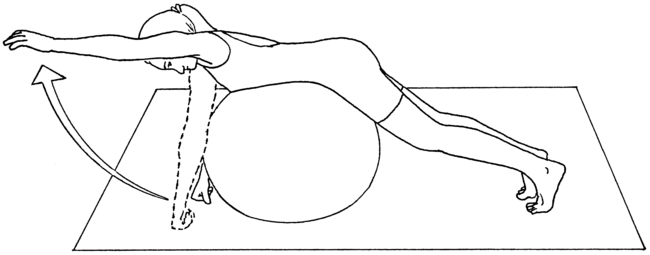
Treatment is begun using sensory motor integration with an emphasis on functional skills (e.g., breathing, balance, and hand function) in a manner that does not cause irritation of the patient’s condition. The patient is guided through a series of breathing exercises designed to improve the circulation to the extremities, calm the nervous system, and retrain the scalene muscles, if appropriate. The breathing exercises are progressed through the use of foam rollers (Figure 18-5). These are used to increase the mobility of the spine and rib cage. The breathing exercises are combined with functional movements of the trunk and extremities in a manner that mobilizes the nervous system. Once the patient is able to manage the symptoms, the treatment can progress to stabilization exercises with a gym ball (Figure 18-6). If the patient has vestibular, balance, or sensory integration deficits, then specific techniques for vestibular, balance, or sensory retraining would be added.
Core components of treatment
The reversible weakness of the thumb is addressed by strengthening the longus colli muscle. This is accomplished by a 30-second isometric contraction using the “thinker pose” (see Figure 18-3) and specific muscle reeducation for the longus colli with Jull’s protocol.73 The expected effect of the thinker pose is to reverse the identified weakness of the thumb. The patient is taught to minimize mechanical stress to the cervical and thoracic spine through instruction in body mechanics. An important concept is to train the patient to identify the coactivation position for stability of the neck and to visualize that position before moving the body away from the center of gravity or moving the arm. This method has the patient assume the thinker pose to stimulate the deep neck flexors to contract before the movement is performed.
The temperature, neurodynamic, and breathing dysfunctions are addressed by training the patient to perform relaxed diaphragmatic breathing with spinal motion (see Figure 18-2). The expected outcomes are to normalize hand temperature and increase the range of motion while decreasing sensitivity to the neurodynamic tests. Low cardiovascular endurance is addressed by having the patient begin a progressive aerobic conditioning program of walking. Because the examination identifies six signs of dysfunction, the goals of treatment are to normalize these six signs. Clinical experience teaches that as these signs improve there is a reduction in pain and an increase in function.
Role of the patient
Maximizing the treatment response requires a unique partnership between the physical therapist and the patient that facilitates the patient’s feeling of being in control. The feeling of being in control is thought to have a positive impact on the response to treatment. Patients are taught methods for self-assessment of the immediate effect of the treatment. They are taught to assess their responses to the core program on the basis of signs of hand strength and hand temperature and mobility and sensitivity of the nervous system. The patients are provided with self-assessment tools and treatment devices. The details of the exercise program are provided in a patient booklet, audiotapes, and a videotape, which can be found at www.edgelow.com.