Chapter 26 Benign prostatic hypertrophy
AETIOLOGY AND EPIDEMIOLOGY
While benign prostatic hypertrophy of the prostate (BPH) (or more accurately benign prostatic hyperplasia) and erectile dysfunction (ED) are typically seen in the ageing male, these can occur at any age. Current understanding of the disease involves imbalanced states of androgens (particularly dihydrotestosterone [DHT]), oestrogens, insulin and insulin-like growth factor (IGF), and detrusor dysfunction of the bladder neck.1 The chief complaint of patients with BPH is urinary frequency, urgency, nocturia, decreased and intermittent force of stream and the sensation of incomplete emptying of the bladder, although these symptoms are neither specific or necessary for the diagnosis of BPH and do not correlate with the extent or degree of hypertrophy of the prostate.1 One study suggests that 20% of men in their 40s and 90% of men in their 70s have varying degrees of BPH,2 although incidence rates for age groups vary depending on population demographics (some suggest 50% of men older than 60, and 80% of men over 80 years).3 Increasing age is the predominant factor associated with prevalence of BPH. Likewise, the incidence and prevalence of ED are also linked to age, with United States of America data suggesting a tripling of the incidence occurring between the ages of 40 and 70 years, and up to 66% of men having suffered from some erectile dysfunction (mild to complete) by the age of 70 years.4 Worldwide estimates suggest up to 330 million men could be affected by this disorder,5 although criticism exists pertaining to the over-medicalisation and resulting prescription rates for drugs providing only symptomatic relief for some age-associated disorders like ED.6 Conversely, both ED and BPH may suffer from under-diagnosis due to the symptomatic nature and psychological stigma associated with each of these diseases.7
Previously, diagnosis of BPH was thought to involve solely lower urinary tract symptoms (LUTS) syndrome; however, recent evidence suggests that LUTS is often due to anatomical disorders (detrusor muscle dysfunction)9 and that symptoms of BPH do not correlate well with prostate size. However, an enlarged prostate could contribute to detrusor dysfunction and urinary retention, often termed ‘LUTS-BPH’. Clinicians should note that not all men with BPH have LUT symptoms and LUTS is not specific or exclusive to BPH.
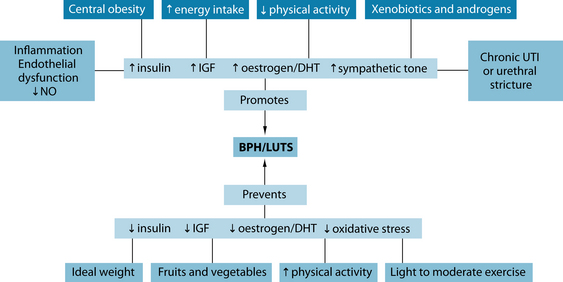
There is little certainty surrounding the exact pathogenesis of BPH. It is characterised by periurethral prostate tissue proliferation, which is indicated by an increase in the number (not size) of epithelial and stromal cells.10 This has been posited to be a relatively normal (or at least highly pervasive) process of ageing, often linked to the prostate tissue response to increase in androgens (dihydrotestosterone [DHT]) that occurs in the ageing male. Environmental exposure to hormone disruptors like xenoestrogens may result in a cascade of events that influence the development of dysfunctional regulation of testosterone, 17β-oestradiol and DHT (see Figure 26.1).10 The enzymes aromatase and 5-alpha reductase are involved in hormone regulation and these are often targets of both conventional as well as naturopathic treatment. Other evidence suggests that increased exposure to growth factors (insulin and insulin-like growth factor (IGF)) initiates the onset of hyperproliferation, ultimately leading to decreased urinary and vascular flow and an increased risk for ED, LUTS, prostatitis and prostate cancer, all of which can be associated disorders.1 As the prostate enlarges, it causes discomfort in the groin and increased pressure on the bladder, leading to variable urine flow rates, frequent urination, nocturia and a sense of incomplete voiding. With chronicity or severity of enlargement, blockage of the urethra can occur, increasing the risk for urinary tract infections, bladder stones and possible kidney damage.
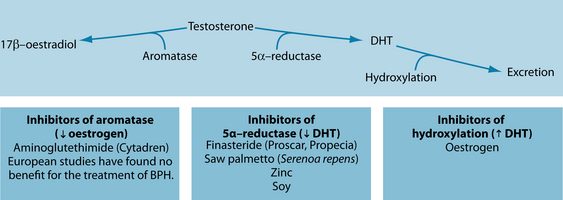
Figure 26.1 Hormonal factors in BPH
Source: Rakel D, ed. Integrative medicine. 2nd edn. Philadelphia: Saunders, 2007.
In 2001 a landmark paper suggested a link between ED, BPH, cardiovascular disease and depression in the ageing male,11 supporting earlier suggestions linking some of these disorders to vascular function.12 Since then, further evidence has confirmed a strong correlation among these disorders, with significantly higher prevalence rates of any of the listed conditions when a comorbidity from any of the other disorders exists. The correlation is particularly strong between BPH and ED, possibly due to a shared vasculogenic pathophysiology, revolving around atherosclerosis and endothelial dysfunction or injury, in production of nitric oxide (NO).13 Other factors associated with cardiovascular disease and/or depression (such as central obesity, increased circulating levels of insulin, diabetes, decreased physical activity, hypertension and increased oxidative stress) are also implicated in the state that promotes the hyperproliferationof prostatic tissue and decreased functioning of circuitry relating to achieving an erection.14,15
RISK FACTORS1,3,16
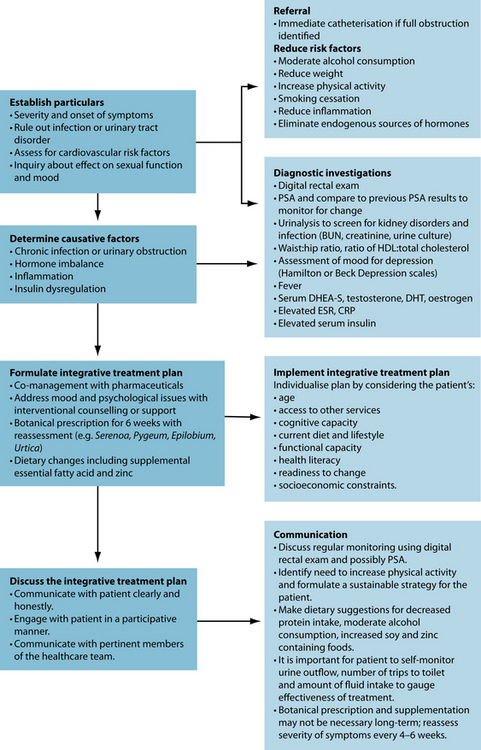
Several factors may promote BPH/LUTS, whereas other influences may have a protective effect (see Figure 26.2). The following is a list of potential risk factors and indicators of BPH:
CONVENTIONAL TREATMENT
Conventional treatment of BPH varies often begins with ‘watchful waiting’ if the symptoms are mild. Alpha-blockers (alfuzosin and tamsulosin) are often selected for decreasing sympathetic tone with minimal change in blood pressure to give LUT symptom relief and are often employed in those without significantly raised levels of PSA.1,16 5-alpha reductase inhibitors (finasteride or dutasteride) inhibit the conversion of testosterone to DHT and are the most frequently prescribed treatment, sometimes in conjunction with alpha-blockers with greater success. If symptoms are severe, there is a long history of smoking or risk factors for cancer, or PSA levels are relatively high, surgical removal of the prostate (transurethral resection of the prostate or TURP) may be indicated.
KEY TREATMENT PROTOCOLS
NATUROPATHIC TREATMENT AIMS
Lifestyle and exercise modification
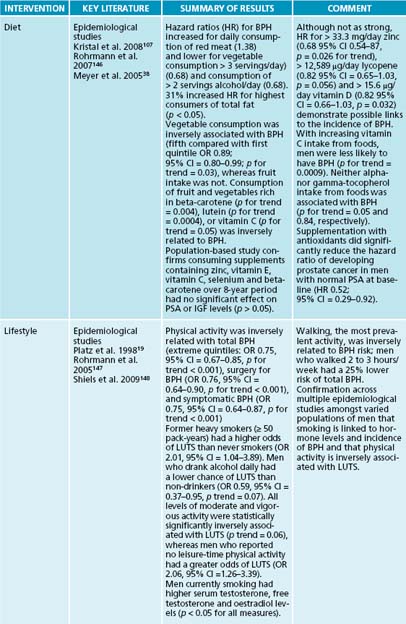
As mentioned above, lifestyle modification is an important protocol in treating BPH and ED. Observational studies indicate that there is a positive association between abdominal obesity and BPH,17,18 and an inverse association between physical activity and BPH.19,20 Diets high in fats or in calories may encourage abdominal obesity and sympathetic nervous system activity, both of which can increase the risk of BPH. Sympathetic nervous system activation (which is the ‘fight or flight’ arm of the autonomic nervous system) may cause the prostate smooth muscle to contract, resulting in a worsening of lower urinary tract symptoms. Studies suggest that there is no increased risk of BPH with higher caloric intake when accompanied by increased physical activity.18 With respect to ED, weight loss, physical activity, smoking cessation and decreased sympathetic activity (relaxation) have also been suggested as beneficial treatments.21 Dietary sources of caffeine and other diuretics should be reduced, and fluid intake monitored to reduce nocturia and urinary output to the bladder from the kidneys.
Three potential mechanisms for physical activity and decreased risk for BPH and ED may be proposed:
Nutritional hormonal modulation (5-alpha reductase, aromatase, dihydrotestosterone and oestrogen)
Clinicians should consider dietary strategies to modulate hormones, hydroxylation of DHT and maintain healthy cholesterol and cardiovascular states; these strategies include avoiding sources of pesticides,22 increasing fruit consumption, increasing the intake of zinc and essential fatty acid, decreasing butter consumption, avoiding margarine,23 decreasing coffee consumption24 and keeping cholesterol levels below 200 mg/dL.25
Inconclusive evidence exists regarding protein intake and BPH. Some research demonstrates that a high-protein diet (total calories made up of 44% protein, 35% carbohydrate and 21% fat) can inhibit 5-alpha reductase, while a low-protein diet (10% protein, 70% carbohydrate and 20% fat) may stimulate the enzyme.26 Contrary to this evidence, a large 8-year observational study of 3523 men with BPH suggests that total protein intake is positively associated with BPH, with the association being slightly stronger for animal protein intake than for vegetable protein intake.18 A second epidemiological study based in China also revealed a correlation between higher animal intake and the incidence of BPH (91.1% in those eating high animal diets as opposed to 11.8% in those not eating animal protein).27
Authors of these studies suggest that a high protein intake may result in a greater osmolar load, which may be one possible mechanism for its detrimental effects on symptoms of BPH. This will increase urinary output and thus impose undue extra burden on an already partially obstructed or strained elimination system.18 Consistent with an anti-inflammatory approach, a diet high in quality, plant-derived and coldwater fish-based protein sources in moderate amounts seems prudent, given the current evidence.
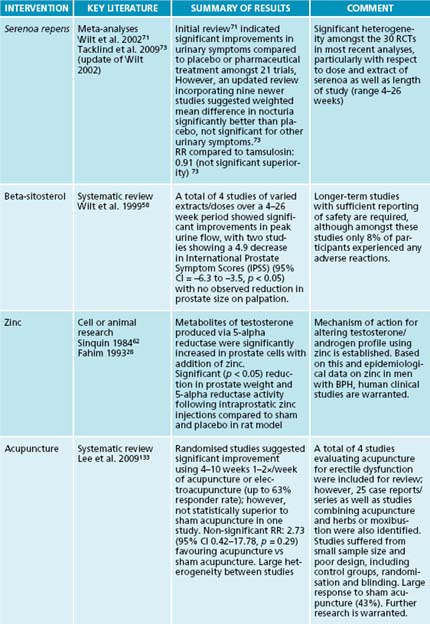
Zinc supplementation has reduced the size of the prostate (confirmed by rectal palpation, radiography and endoscopy) and reduced overall symptomatology in the majority of patients with BPH in studies conducted in the 1970s as well as recently.28 The clinical efficacy of zinc is probably due to its critical involvement in many aspects of androgen metabolism. High amounts of oestrogen can inhibit intestinal uptake of zinc, whereas elevated androgens increase absorption. Because oestrogen levels are increased in men with BPH, zinc uptake may be low, suggesting a higher functional need for this nutrient and begging the need for additional supplementation.29,30 However, one case-control study suggested a positive association between zinc levels and BPH.31 This deviation from other research may be partially explained by dietary confounding factors.
The relationship between zinc levels and 5-alpha reductase activity in hyperplastic prostates is inconsistent; however, zinc has been shown to inhibit the activity of 5-alpha reductase, and decreases the conversion of testosterone to DHT.32–35 Zinc can also inhibit specific binding of androgens to the cytosol and nuclear androgen receptors present in high amounts in prostatic tissue.32 These mechanisms suggest that zinc has more of a preventative role by maintaining a nutritional status that discourages prostate hypertrophy. No interventional studies investigating the use of zinc for BPH exist, although observational studies suggest it has a safe and effective role in diminishing the size of the prostate, particularly for prostate cancer, although one study suggests it can increase urinary symptoms.36–40
Zinc is also involved in the regulation of prolactin secretion, leading to increased uptake of testosterone and subsequent rise in DHT in the prostate.41–43 Natural (from beer, stress and tryptophan)44,45 and pharmaceutical prolactin antagonists (bromocriptine)46 have been shown to reduce many of the symptoms of prostatic hyperplasia. However, this strategy can have severe side effects, particularly pertaining to cardiovascular and neurological systems, including severe changes in mood.47,46
A 100 g serving of soybeans or soy products contains 90 mg of beta-sitosterol, which has been shown to decrease cholesterol; this is a potential mechanism for soy’s effect on BPH. The cholesterol-lowering effects of phytosterols are well documented,48,49 while also showing an effect on treating BPH.9 One double-blind study50 in 200 men using beta-sitosterol (20 mg) or placebo three times daily demonstrated an increase in the maximum urine flow rate from a baseline of 9.9 mL/s to 15.2 mL/s and a decrease in mean residual urinary volume of 30.4 mL from 65.8 mL in the beta-sitosterol group. No changes were observed in the placebo group. An increased consumption of soy and soy foods is associated with a decrease in the risk of prostate cancer,51,52 which is associated with the incidence of BPH. Phytoestrogens in soy (isoflavonoids, genistein and daidzein), as well as Trifolium pratense are implicated due to their effect on oestrogen receptors51,53 and inhibition of 5-alpha reductase.54 Other sources of beta-sitosterols such as Hypoxis hemerocallidea may also be of benefit, although many of these studies are lacking in the rigour of reporting including dose and method of extraction as well as adverse-event reporting.55 Clinical use of these substances may be most appropriate for men with BPH in the ‘watchful waiting’ stage of the condition56 with clinically effecting doses of beta-sitosterol ranging from 60–130 mg daily.57,58
One study59 suggested that 60 mg twice daily of the dried, ground flowers of Opuntia spp. over 2 to 6 months was effective at reducing urinary urgency and the sensation of a full bladder, although this remains to be repeated in other studies and stands as preliminary evidence.
The diet should be as free as possible from environmental toxins such as pesticides and other contaminants because many of these compounds (such as dioxin, polyhalogenated biphenyls, hexachlorobenzene and dibenzofurans) increase 5-alpha reduction of steroids.26 Diethylstilbestrol produces changes in rat prostates histologically similar to BPH.60 While cadmium is a known antagonist of zinc and can influence testosterone levels through effects on the activity of 5-alpha reductase, its concentration in, and effects on, the prostate are unclear. Several studies have produced conflicting results.61,62
Herbal hormonal modulation (5-alpha reductase, aromatase, dihydrotestosterone and oestrogen)
During the 1990s, botanical medicine in Germany and Australia were considered ‘first-line’ treatments for BPH and accounted for greater than 90% of all drugs in the medical management of BPH.63 In Italy, plant extracts accounted for roughly 50% of all medications prescribed for BPH, while alpha-blockers and 5-alpha reductase inhibitors account for only 5.1% and 4.8%, respectively.64
Clinical success with any of the botanical treatments of BPH may be determined by the degree of obstruction as indicated by the residual urine content.1,22 For mild LUTS symptoms and residual urine levels less than 50 mL, the results are usually excellent. For levels between 50 and 100 mL, the results are usually quite good. Residual urine levels greater than 100 mL will be more difficult to produce significant improvements in the customary 4- to 6-week period without co-management using other therapies including pharmaceuticals, and with botanical medicine adding little to the overall reduction in symptoms within a 4–6 week period. One small (n = 40) but well-designed clinic-based research study investigating the open use of phytotherapy for LUTS/BPH demonstrated a statistically significant superiority of phytotherapy use (as both adjuvant to pharmacological treatment or as a stand-alone) over use of alpha-blockers to reduce urinary retention, and improve erectile and ejaculatory dysfunction.65 Adulteration and/or contamination in herbal products for erectile dysfunction is relatively widespread66,67 particularly in ‘commercial’ natural health products in some countries. As with usual prescriptions, clinicians should ensure that patients are accessing high quality products.
The liposterolic extract of the fruit of Serenoa repens, native to Florida in the USA, has been shown to significantly improve the signs and symptoms of BPH in numerous clinical studies. The mechanism of action is postulated to be related to inhibition of DHT binding to both the cytosolic and the nuclear androgen receptors, inhibition of 5-alpha reductase, interference with intraprostatic oestrogen receptors and an anti-inflammatory effect.10 As a result of this multitude of effects, positive results have been produced in numerous double-blind clinical studies. However, different extracts of S. repens (permixon) may have more of an anti-inflammatory mechanism of action in modulating BPH;63,66–70 clinicians should be mindful of these differences in therapeutic action from the myriad of extraction methods used in natural health products and the multifaceted effects of herbal medicines, particularly S. repens.68
Synthesis research, including systematic reviews of clinical trials, has previously reported excellent results using S. repens.71,72 One examination of 21 randomised, controlled trials involving a total of 3139 men (including 18 double-blind trials) demonstrated that men treated with S. repens experienced decreased urinary tract symptom scores, less nocturia, better urinary tract symptom self-rating scores and peak urine flow improvements compared with men receiving placebo.71 This analysis also showed that, matched up with men receiving the DHT inhibitor finasteride (Proscar), men treated with S. repens had similar improvements in urinary tract symptom scores and peak urine. A related review also reported that adverse effects due to S. repens were mild and infrequent with erectile dysfunction appearing more frequently with finasteride (4.9%) than with S. repens (1.1%).72 However, a recent update to this Cochrane review, which included nine new studies comparing S. repens to either placebo or finasteride, demonstrated no significant benefit for treating any symptoms of BPH except nocturia.73 The large variability in patient population, severity of disease and extraction or source of S. repens, as demonstrated by a lack of heterogeneity between studies and wide confidence intervals for outcomes, highlights some of the challenges in herbal medicine research when applying research results to individuals. At the same time, these results indicate that clinical success in using serenoa for BPH is variable, with the greatest likelihood for improvement occurring in men with mild to moderate symptoms receiving treatment within the first 4–6 weeks of onset.
Overall, it can be stated that some men with mild to moderate BPH experience some improvement in symptoms during the first 4 to 6 weeks of therapy using S. repens. All major symptoms of BPH are improved, especially nocturia. And despite an efficacy profile similar to finasteride for mild to moderate severity, serenoa has a lower risk profile and is less expensive.74 The most common side effect is gastrointestinal distress, and this is easily remedied by taking S. repens with food.
Cernilton®, a proprietary combination of water-soluble and acetone-soluble extract of Secale cereale, has been used to treat prostatitis and BPH for years in Europe.75,76 Several short clinical studies and one systematic review suggest it to be beneficial in the treatment of BPH,77–83 with patients reporting moderate improvement. Patients who respond typically have reductions of nocturia and diurnal frequency of around 70%, as well as significant reductions in residual urine volume. The extract has been shown to exert some anti-inflammatory action and produce a contractile effect on the bladder while simultaneously relaxing the urethra.84
In one study, the clinical efficacy of Cernilton® in the treatment of symptomatic BPH was examined over a 1-year period.76 Seventy-nine males of an average age of 68 years (range 62 to 89), with a mean baseline prostatic volume of 33.2 cm2, were administered 63 mg Cernilton® pollen extract twice daily for 12 weeks. Average urine maximum flow rate increased from 5.1 to 6 mL/s. Average flow rate increased from 9.3 to 11 mL/s. Residual urine volume decreased from 54.2 mL to less than 30 mL. Urgency and discomfort improved by 76.9% with dysuria, incomplete emptying, intermittency, delayed or prolonged voiding all having 60–70% improvement. Nocturia improved by 56.8%. Overall, 85% of the test subjects experienced benefit, 11% reporting ‘excellent’, 39% reporting ‘good’, 35% reporting ‘satisfactory’ and 15% reporting ‘poor’ as a description of their outcome.
A systematic review conducted in 2000 compiling two placebo-controlled studies, two comparative trials (both lasting 12 to 24 weeks) and three double-blind studies of 444 men showed that, although Cernilton® did not improve urinary flow rates, residual volume or prostate size compared with placebo or the comparative study agents, it did improve self-rated urinary symptom scores and reduced nocturia compared with placebo.81 Again, this review highlights some of the variability in evidence for the use of this herbal extract with the chances of clinical success in using this as a stand-alone treatment remaining unclear.
Extracts from the bark of Pygeum africanum used in research studies are fat-soluble sterols and fatty acids. Virtually all of the research on pygeum has featured a pygeum extract standardised to contain 14% triterpenes including beta-sitosterol and 0.5% n-docosanol. The suggested therapeutic action has been suggested to be due, in part, to the inhibition of growth factors EGF, bFGF and IGF-I, which are responsible for the prostatic overgrowth.85,86
Clinical trials totalling more than 600 patients have demonstrated pygeum extract to be effective in reducing the symptoms and clinical signs of BPH, especially in early cases.86,87 The research studies suggest pygeum having a superiority in objective parameters, especially urine flow rate and residual urine content, over saw palmetto. However, as the two extracts have somewhat overlapping mechanisms of actions, they can be used in combination.
Lepidium meyenii (maca) is a root belonging to the brassica family traditionally known for its aphrodisiac properties in both men and women.88 A recent randomised, double-blind placebo-controlled trial demonstrated a subjective improvement on the International Index of Erectile Function (IIEF-5) and Satisfaction Profile (SAT-P) using 2400 mg/day of dry extract of maca compared to placebo.89 Although both groups improved significantly from baseline self-rated psychological performance scores, there was a statistically significant superiority in the maca group in physical and social performance. This represents a small but statistically significant clinical effect from using this dose of maca in men with mild erectile dysfunction. Two other randomised, placebo-controlled studies with limited information suggest that 1.5 and 3 g/day dose of maca can improve erectile dysfunction without altering testosterone levels in men with this disorder.90,91 Further research is necessary to better determine the mechanism of action, the clinical relevance and optimal dose and constituents in maca for use in erectile dysfunction. No clinical evidence exists for the use of maca for treating BPH.
Extracts of Urtica dioica have also been shown to be effective in the treatment of BPH, although less research evidence exists compared to other botanical medicines. A number of long-term, double-blind studies have shown it to be more effective than a placebo, with no adverse effects reported.92,93 A randomised, multicentre, double-blind study in 431 patients using extracts of both serenoa and U. dioica found clinical benefit equal to that of finasteride,94 with a larger, second study lasting 24 weeks confirming its benefit in reducing residual urine and improving peak flow.92 A similar therapeutic action between urtica and serenoa extracts appears to exist in that each interact with binding of DHT to cytosolic and nuclear receptors.95 Other in vitro studies also show that lignans found in U. dioica may modulate hormonal effects due to their affinity for sex hormone-binding globulin.96
In vitro studies of Epilobium spp. suggest steroid receptor and aromatase inhibition activity as well as potent cyclo-oxygenase-2 (COX-2) and antioxidant potential, so this herb could theoretically be used in BPH.97,98 Other cell-line research supports this and elucidates the antihyperplastic and potential anticancer action of Epilobium parviflorum extract (oenothein B) as a potent inhibitor of DNA synthesis in a human prostate cell line. Aqueous extracts have also demonstrated plausible COX-2 action,99 although ethanolic extracts appear to have the most potential inhibitory action on this enzyme linked to inflammatory pathways.100 These results have yet to be duplicated in clinical trials.
Inflammation
While limited, some studies suggest that the inflammatory process including the production of cytokines (interleukin 1 and 6, and tissue necrosis factor alpha) increases prostatic tissue proliferation.63,75 Other research links the presence of platelet-derived growth factor (PDGF) (produced during the inflammatory processes) and androgens with the incidence of BPH and hyperproliferation of prostate cells.101,102 In these studies, DHT failed to produce a mitogenic response in prostate cells without the presence of PDGF.
In a large, Italian, case-control study essential fatty acids (EFA) consisting of linoleic, linolenic and arachidonic acids have resulted in significant improvement for many BPH patients.103 All 19 subjects in an uncontrolled study showed diminution of residual urine, with 12 of the 19 having no residual urine by the end of several weeks of treatment. Prostatic and seminal lipid levels and ratios are often abnormal,104 and supplementation may be addressing an underlying EFA deficiency. Cell-line research suggests differences in essential fatty acid composition in hyperplastic or cancerous prostate cells.105,106 Because of concern regarding increased oxidation of unsaturated acids,18 a basic antioxidant regimen should be employed when taking EFAs.
The role of tomatoes, tomato sauce and lycopene in BPH is not clear; however, epidemiological and population-based studies suggest that their consumption is linked to decreased incidence of BPH,106 a reduction in PSA levels,107 and decreased progression of BPH to prostate cancer.109 The therapeutic action of lycopene in this disorder may be due to its potent antioxidant properties.
Sympathetic nervous system hyperactivity
Reduction of urethral stricture and relaxation of smooth muscles is aided by parasympathetic stimulation or parasympathetic mimetic agents.110 Reduction in sympathetic nervous activity and/or parasympathetic stimulation may aid in LUTS symptom relief, as evidenced by some studies suggesting that a combination of glycine, alanine and glutamic acid (in the form of two 6-grain capsules administered three times daily for 2 weeks and one capsule three times daily thereafter) relieved many BPH symptoms. Nocturia was relieved or reduced in 95%, urgency reduced in 81%, frequency reduced in 73% and delayed micturition alleviated in 70% in one study111 with similar results reported in other controlled studies.112 The mechanism of action is unknown. These amino acids may act as inhibitory neurotransmitters, providing symptom relief and reducing the feelings of a full bladder, or providing an overall nervine function that can positively affect urine flow in men suffering from BPH.113
While no clinical evidence supports the use of hops (Humulus lupulus), the antiproliferative effects of flavonoids on prostate cancer cell lines, as well as its nervine and parasympathetic action, may warrant further research and clinical benefit.68,114 Other nervine and antispasmodic botanical medicines may have a potential supportive role in managing BPH; examples are Scutellaria lateriflora and Passiflora incarnata. However, no studies have presently been conducted to assess this potential.
Vascular functioning
Chronic ischaemia can result in thickening of the prostate and growth of tissue in response to an altered endothelial growth factor resulting from the diminished blood supply.115 Atherosclerosis and diminished blood supply appear to be potential root causes in BPH and ED. Improving vascular function via herbal and nutrient interventions (discussed in the ED section), managing cholesterol and moderating alcohol consumption, in addition to dietary strategies to address cardiovascular risk, will possibly prove beneficial (see Section 3 on the cardiovascular system).
In addition to epidemiological studies suggesting a relationship between higher cholesterol levels and cardiovascular disease with higher incidence of BPH and ED, the breakdown products of cholesterol itself can also be cytotoxic and carcinogenic and have been shown to accumulate in hyperplastic or cancerous prostate tissue.104
Hypocholesterolaemic (statin) drugs have been shown to have a favourable influence on BPH possibly by limiting subsequent formation of epoxycholesterols (metabolic by-products of cholesterol).116 As discussed above, the regulation of cardiovascular risk factors (such as elevated LDL and total cholesterol) and decreased HDL may help in the holistic prevention and treatment of BPH.
Although only beer has been associated with increasing prolactin levels, higher overall alcohol intake may be associated with BPH. In a large Hawaiian study involving 6581 men, an alcohol intake of at least 740 mL/month was directly correlated with the diagnosis of BPH,31 with a stronger association for beer, wine and sake compared with distilled spirits. While this indicates a relationship in incidence, a smaller study suggested that men waiting for TURP surgery were more likely to avoid the surgical procedure (having remission of symptoms) with higher consumption of alcohol.24 This study also described the correlation of higher rates of BPH in men with coronary disease. Although these studies are apparently contradictory in relationship to alcohol, it is possible that in men at higher risk for coronary artery disease due to higher levels of low-density lipoproteins, alcohol may play an overall protective role by reducing these lipoproteins.
INTEGRATIVE MEDICAL CONSIDERATIONS
The severity of the symptoms will dictate whether surgery or catheterisation is necessary for acute urinary obstruction. Counselling may be necessary to address the psychological issues of sexual dysfunction. Progression of BPH to prostate cancer is of concern and should be monitored appropriately using regular DRE with possible PSA testing. Acupuncture and moxibustion can increase blood flow, and some studies have provided evidence that it may be considered as a viable adjunctive treatment,133–135 particularly when combined with hypnotherapy.136 Numerous studies on the effect of acupuncture on urinary frequency have been conducted; however, no studies exist on the use of acupuncture for BPH specifically, and many of these studies involve ageing women.137–141 Other studies suggest trigger point release therapy and relaxation as being beneficial for addressing ED and BPH, particularly if chronic pelvic pain is involved.142 Counselling and psychiatry may be beneficial for addressing the psychological roots or manifestations of ED and the symptoms of BPH. Pelvic floor (kegal) exercises have been shown to be beneficial in up to 35% of ED patients143,144 with some potential shown for their ability to improve urinary function.145
ERECTILE DYSFUNCTION
Overview
Conventional treatments
Conventional treatments focus on providing symptomatic relief through pharmacological substances (phosphodiesterase type-5 (PDE-5) inhibitors such as sildenafil or tadalafil) as first-line therapy, removing causative agents (the side effects of beta-adrenergic blockers, antidepressants or spironolactone), and counselling.13,121
CAM treatments
Arginine
Three small, pilot, randomised, controlled studies provided relative support for the clinical effectiveness of L-arginine in doses ranging from 1500 mg—5 g/day used over a 2–6-week period for ED.122–124 While the study123 using 1500 mg/day for 2 weeks did not demonstrate a significant benefit over placebo, the other two studies demonstrated a 20–40% self-reported improvement in symptoms of ED. It is unclear whether foods rich in L-arginine such as legumes, whole grains and nuts consumed in moderate-to-large amounts would have a similar effect. Other studies using L-arginine in combination with other natural substances such as pycnogenol125 or yohimbine126 generally supported the notion that L-arginine can have some positive effect on the symptoms of erectile dysfunction; however, further research is necessary.
Herbs
Panax ginseng has the most promising evidence for safe and effective treatment of ED, with 900 mg/day for 8 weeks showing clinical and physiological effectiveness in up to 60% of men with ED.8,127 Yohimbine (5.4 mg t.i.d. for 8 weeks) also shows promising results, although significant side effects associated with its therapeutic action as an alpha-2-adrenergic receptor make it a less desirable treatment option.128 Based on vasodilatory actions, Ginkgo biloba may benefit some men where penile blood flow is impaired (however, the clinical evidence is mixed).129,130 Tribulus terrestris may be a viable option to treat ED, via increasing the release of nitric oxide from the endothelium and nitrergic nerve endings in penile tissue (clinical studies have not yet, however, confirmed its efficacy).131,132
Example treatment
Expected outcomes and follow-up protocols
If PSA levels are used as one means of monitoring prostate health, this test should be done once every 6 months (especially if there is a significantly enlarged prostate on digital rectal exam). Reassessment of symptoms of BPH should occur every 6 weeks if moderate, and at least every 4 weeks if severe. Patients should keep a diary of daily trips
to the toilet, fluid intake and attempts to collect urine output, as a means of assessing the effectiveness of treatment as well as whether adjunctive or emergency care is warranted.
KEY POINTS
Edwards J.L. Diagnosis and management of benign prostatic hyperplasia. Am Fam Physician. 2008;77(10):1403-1410.
Mantzoros C.S., et al. Insulin-like growth factor 1 in relation to prostate cancer and benign prostatic hyperplasia. Br J Canc. 1997;76(9):1115-1118.
Moyad M.A., et al. Prevention and treatment of erectile dysfunction using lifestyle changes and dietary supplements: what works and what is worthless, parts 1 and 2. Uro Clin N Am. 2004;31:249-273.
Rosenberg M.T. Diagnosis and management of erectile dysfunction in the primary care setting. Int J Clin Prac. 2007;61(7):1198-1208.
Zakaria L., et al. Common conditions of the aging male: erectile dysfunction, benign prostatic hyperplasia, cardiovascular disease and depression. Int Urol Nephrol. 2001;33(2):283-292.
Zhu Y.S., Imperato-McGinley J.L. 5 alpha-reductase isozymes and androgen actions in the prostate. Ann N Y Acad Sci. 2009;1155:43-56.
1. Edwards J.L. Diagnosis and management of benign prostatic hyperplasia. Am Fam Physician. 2008;77(10):1403-1410.
2. Arrighi H.M., et al. Natural history of benign prostatic hyperplasia and risk of prostatectomy. The Baltimore Longitudinal Study of Aging. Urology. 1991;38(1 Suppl):4-8.
3. Dull P., et al. Managing benign prostatic hyperplasia. Am Fam Physician. 2002;66(1):77-84.
4. Feldman H.A., et al. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol. 1994;151(1):54-61.
5. Goldstein I. Male sexual circuitry. Working Group for the Study of Central Mechanisms in Erectile Dysfunction. Sci Am. 2000;283(2):70-75.
6. Shankar P.R., Subish P. Disease mongering. Singapore Med J. 2007;48(4):275-280.
7. Nehra A., Kulaksizoglu H. Global perspectives and controversies in the epidemiology of male erectile dysfunction. Curr Opin Urol. 2002;12(6):493-496.
8. McKay D. Nutrients and botanicals for erectile dysfunction: examining the evidence. Altern Med Rev. 2004;9(1):4-16.
9. Thomas J.A. Diet, micronutrients, and the prostate gland. Nutr Rev. 1999;57(4):95-103.
10. Comhaire F., Mahmoud A. Preventing diseases of the prostate in the elderly using hormones and nutriceuticals. Aging Male. 2004;7(2):155-169.
11. Zakaria L., et al. Common conditions of the aging male: erectile dysfunction, benign prostatic hyperplasia, cardiovascular disease and depression. Int Urol Nephrol. 2001;33(2):283-292.
12. Sullivan M.E., et al. Nitric oxide and penile erection: is erectile dysfunction another manifestation of vascular disease? Cardiovasc Res. 1999;43(3):658-665.
13. Rosenberg M.T. Diagnosis and management of erectile dysfunction in the primary care setting. Int J Clin Pract. 2007;61(7):1198-1208.
14. Jacobsen S.J. Risk factors for benign prostatic hyperplasia. Curr Urol Rep. 2007;8(4):281-288.
15. Moyad M.A. Lifestyle changes to prevent BPH: heart healthy = prostate healthy. Urol Nurs. 2003;23(6):439-441.
16. Engl T., et al. Uropharmacology: current and future strategies in the treatment of erectile dysfunction and benign prostate hyperplasia. Int J Clin Pharmacol Ther. 2004;42(10):527-533.
17. Soygur T., et al. Effect of obesity on prostatic hyperplasia: its relation to sex steroid levels. Int Urol Nephrol. 1996;28(1):55-59.
18. Suzuki S., et al. Intakes of energy and macronutrients and the risk of benign prostatic hyperplasia. Am J Clin Nutr. 2002;75(4):689-697.
19. Platz E.A., et al. Physical activity and benign prostatic hyperplasia. Arch Intern Med. 1998;158(21):2349-2356.
20. Platz E.A., et al. Interrelation of energy intake, body size, and physical activity with prostate cancer in a large prospective cohort study. Cancer Res. 2003;63(23):8542-8548.
21. Wisard M. [Erectile dysfunction: physical exercise, losing weight, stop smoking, reducing alcohol, relaxing, it could also work!]. Rev Med Suisse. 2007;3(136):2773-2774. 2776, 2778
22. Morrison A.S. Epidemiology and environmental factors in urologic cancer. Cancer. 1987;60(3 Suppl):632-634.
23. Lagiou P., et al. Diet and benign prostatic hyperplasia: a study in Greece. Urology. 1999;54(2):284-290.
24. Gass R. Benign prostatic hyperplasia: the opposite effects of alcohol and coffee intake. BJU Int. 2002;90(7):649-654.
25. Demark-Wahnefried W., et al. Pilot study to explore effects of low-fat, flaxseed-supplemented diet on proliferation of benign prostatic epithelium and prostate-specific antigen. Urology. 2004;63(5):900-904.
26. Kappas A., et al. Nutrition-endocrine interactions: induction of reciprocal changes in the delta 4-5 alpha-reduction of testosterone and the cytochrome P-450-dependent oxidation of estradiol by dietary macronutrients in man. Proc Natl Acad Sci U S A. 1983;80(24):7646-7649.
27. Zhang S.X., et al. [Comparison of incidence of BPH and related factors between urban and rural inhabitants in district of Wannan]. Zhonghua Nan Ke Xue. 2003;9(1):45-47.
28. Fahim M.S., et al. Zinc arginine, a 5 alpha-reductase inhibitor, reduces rat ventral prostate weight and DNA without affecting testicular function. Andrologia. 1993;25(6):369-375.
29. Leake A. The effect of zinc on the 5 alpha-reduction of testosterone by the hyperplastic human prostate gland. J Steroid Biochem. 1984;20(2):651-655.
30. Leake A. Subcellular distribution of zinc in the benign and malignant human prostate: evidence for a direct zinc androgen interaction. Acta Endocrinol (Copenh). 1984;105(2):281-288.
31. Chyou P.H., et al. A prospective study of alcohol, diet, and other lifestyle factors in relation to obstructive uropathy. Prostate. 1993;22(3):253-264.
32. Zaichick V., et al. Zinc in the human prostate gland: normal, hyperplastic and cancerous. Int Urol Nephrol. 1997;29(5):565-574.
33. Leake A., et al. The effect of zinc on the 5 alpha-reduction of testosterone by the hyperplastic human prostate gland. J Steroid Biochem. 1984;20(2):651-655.
34. Leake A., et al. Subcellular distribution of zinc in the benign and malignant human prostate: evidence for a direct zinc androgen interaction. Acta Endocrinol (Copenh). 1984;105(2):281-288.
35. Wallace A.M., Grant J.K. Effect of zinc on androgen metabolism in the human hyperplastic prostate. Biochem Soc Trans. 1975;3(4):540-542.
36. Johnson A.R., et al. High dose zinc increases hospital admissions due to genitourinary complications. J Urol. 2007;177(2):639-643.
37. Silk R., LeFante C. Safety of zinc gluconate glycine (Cold-Eeze) in a geriatric population: a randomized, placebo-controlled, double-blind trial. Am J Ther. 2005;12(6):612-617.
38. Meyer F., et al. Antioxidant vitamin and mineral supplementation and prostate cancer prevention in the SU.VI.MAX trial. Int J Cancer. 2005;116(2):182-186.
39. Neuhouser M.L., et al. Dietary supplement use in the Prostate Cancer Prevention Trial: implications for prevention trials. Nutr Cancer. 2001;39(1):12-18.
40. Patterson R.E., et al. Vitamin supplements and cancer risk: the epidemiologic evidence. Cancer Causes Control. 1997;8(5):786-802.
41. Judd A.M., et al. Zinc acutely, selectively and reversibly inhibits pituitary prolactin secretion. Brain Res. 1984;294(1):190-192.
42. Login I.S., et al. Zinc may have a physiological role in regulating pituitary prolactin secretion. Neuroendocrinology. 1983;37(5):317-320.
43. Farnsworth W.E., et al. Interaction of prolactin and testosterone in the human prostate. Urol Res. 1981;9(2):79-88.
44. De Rosa G., et al. Prolactin secretion after beer. Lancet. 1981;2(8252):934.
45. Corenblum B., Whitaker M. Inhibition of stress-induced hyperprolactinaemia. Br Med J. 1977;2(6098):1328.
46. Farrar D.J., Osborne J.L. The use of bromocriptine in the treatment of the unstable bladder. Br J Urol. 1976;48(4):235-238.
47. Boyd A. Bromocriptine and psychosis: a literature review. Psychiatr Q. 1995;66(1):87-95.
48. Miettinen T.A., et al. Serum plant sterols and cholesterol precursors reflect cholesterol absorption and synthesis in volunteers of a randomly selected male population. Am J Epidemiol. 1990;131(1):20-31.
49. Tilvis R.S., Miettinen T.A. Serum plant sterols and their relation to cholesterol absorption. Am J Clin Nutr. 1986;43(1):92-97.
50. Berges R.R., et al. Randomised, placebo-controlled, double-blind clinical trial of beta-sitosterol in patients with benign prostatic hyperplasia. Beta-sitosterol Study Group. Lancet. 1995;345(8964):1529-1532.
51. Denis L., et al. Diet and its preventive role in prostatic disease. Eur Urol. 1999;35(5–6):377-387.
52. Engelhardt P.F., Riedl C.R. Effects of one-year treatment with isoflavone extract from red clover on prostate, liver function, sexual function, and quality of life in men with elevated PSA levels and negative prostate biopsy findings. Urology. 2008;71(2):185-190. discussion 190
53. Messina M.J. Emerging evidence on the role of soy in reducing prostate cancer risk. Nutr Rev. 2003;61(4):117-131.
54. Jarred R.A., et al. Anti-androgenic action by red clover-derived dietary isoflavones reduces non-malignant prostate enlargement in aromatase knockout (ArKo) mice. Prostate. 2003;56(1):54-64.
55. Drewes S.E., et al. Hypoxis hemerocallidea—not merely a cure for benign prostate hyperplasia. J Ethnopharmacol. 2008;119(3):593-598.
56. Katz A.E. Flavonoid and botanical approaches to prostate health. J Altern Complement Med. 2002;8(6):813-821.
57. Wilt T., et al. Beta-sitosterols for benign prostatic hyperplasia. Cochrane Database Syst Rev. (2):2000. CD001043
58. Wilt T.J., et al. Beta-sitosterol for the treatment of benign prostatic hyperplasia: a systematic review. BJU Int. 1999;83(9):976-983.
59. Gerber G.S. Phytotherapy for benign prostatic hyperplasia. Curr Urol Rep. 2002;3(4):285-291.
60. Yamashita A., et al. Influence of diethylstilbestrol, Leuprolelin (a luteinizing hormone-releasing hormone analog), Finasteride (a 5 alpha-reductase inhibitor), and castration on the lobar subdivisions of the rat prostate. Prostate. 1996;29(1):1-14.
61. Lahtonen R. Zinc and cadmium concentrations in whole tissue and in separated epithelium and stroma from human benign prostatic hypertrophic glands. Prostate. 1985;6(2):177-183.
62. Sinquin G., et al. Testosterone metabolism by homogenates of human prostates with benign hyperplasia: effects of zinc, cadmium and other bivalent cations. J Steroid Biochem. 1984;20(3):773-780.
63. Sivkov A.V., et al.[Morphological changes in prostatic tissue of patients with benign prostatic hyperplasia treated with permixon]. Urologiia. 2004(5):10-16.
64. Buck A.C. Phytotherapy for the prostate. Br J Urol. 1996;78(3):325-336.
65. Dedhia R.C., et al. Impact of phytotherapy on utility scores for 5 benign prostatic hyperplasia/lower urinary tract symptoms health states. J Urol. 2008;179(1):220-225.
66. Fleshner N., et al. Evidence for contamination of herbal erectile dysfunction products with phosphodiesterase type 5 inhibitors. J Urol. 2005;174(2):636-641. discussion 641; quiz 801
67. Gryniewicz C.M., et al. Detection of undeclared erectile dysfunction drugs and analogues in dietary supplements by ion mobility spectrometry. J Pharm Biomed Anal. 2009;49(3):601-606.
68. Buck A.C. Is there a scientific basis for the therapeutic effects of serenoa repens in benign prostatic hyperplasia? Mechanisms of action. J Urol. 2004;172(5 Pt 1):1792-1799.
69. Magri V., et al. Activity of Serenoa repens, lycopene and selenium on prostatic disease: evidences and hypotheses. Arch Ital Urol Androl. 2008;80(2):65-78.
70. Vela Navarrete R., et al. BPH and inflammation: pharmacological effects of Permixon on histological and molecular inflammatory markers. Results of a double blind pilot clinical assay. Eur Urol. 2003;44(5):549-555.
71. Wilt T., et al. Serenoa repens for benign prostatic hyperplasia. Cochrane Database Syst Rev. (3):2002. CD001423
72. Wilt T.J., et al. Phytotherapy for benign prostatic hyperplasia. Public Health Nutr. 2000;3(4A):459-472.
73. Tacklind J., et al. Serenoa repens for benign prostatic hyperplasia. Cochrane Database Syst Rev. (2):2009. CD001423
74. Gordon A.E., Shaughnessy A.F. Saw palmetto for prostate disorders. Am Fam Physician. 2003;67(6):1281-1283.
75. Asakawa K., et al. [Effects of cernitin pollen-extract (Cernilton) on inflammatory cytokines in sex-hormone-induced nonbacterial prostatitis rats]. Hinyokika Kiyo. 2001;47(7):459-465.
76. Yasumoto R., et al. Clinical evaluation of long-term treatment using Cerniton pollen extract in patients with benign prostatic hyperplasia. Clin Ther. 1995;17(1):82-87.
77. Buck A.C., et al. Treatment of outflow tract obstruction due to benign prostatic hyperplasia with the pollen extract, cernilton. A double-blind, placebo-controlled study. Br J Urol. 1990;66(4):398-404.
78. Dutkiewicz S. Usefulness of Cernilton in the treatment of benign prostatic hyperplasia. Int Urol Nephrol. 1996;28(1):49-53.
79. Hayashi J., et al. [Clinical evaluation of Cernilton in benign prostatic hypertrophy]. Hinyokika Kiyo. 1986;32(1):135-141.
80. Horii A., et al. [Clinical evaluation of Cernilton in the treatment of the benign prostatic hypertrophy]. Hinyokika Kiyo. 1985;31(4):739-746.
81. MacDonald R., et al. A systematic review of Cernilton for the treatment of benign prostatic hyperplasia. BJU Int. 2000;85(7):836-841.
82. Maekawa M., et al. [Clinical evaluation of Cernilton on benign prostatic hypertrophy–a multiple center double-blind study with Paraprost]. Hinyokika Kiyo. 1990;36(4):495-516.
83. Ueda K., et al. [Clinical evaluation of Cernilton on benign prostatic hyperplasia]. Hinyokika Kiyo. 1985;31(1):187-191.
84. Habib F.K., et al. Identification of a prostate inhibitory substance in a pollen extract. Prostate. 1995;26(3):133-139.
85. Yablonsky F., et al. Antiproliferative effect of Pygeum africanum extract on rat prostatic fibroblasts. J Urol. 1997;157(6):2381-2387.
86. Boulbes D., et al. Pygeum africanum extract inhibits proliferation of human cultured prostatic fibroblasts and myofibroblasts. BJU Int. 2006;98(5):1106-1113.
87. Edgar A.D., et al. A critical review of the pharmacology of the plant extract of Pygeum africanum in the treatment of LUTS. Neurourol Urodyn. 2007;26(4):458-463. discussion 464
88. Kamatenesi-Mugisha M., Oryem-Origa H. Traditional herbal remedies used in the management of sexual impotence and erectile dysfunction in western Uganda. Afr Health Sci. 2005;5(1):40-49.
89. Zenico T., et al. Subjective effects of Lepidium meyenii (maca) extract on well-being and sexual performances in patients with mild erectile dysfunction: a randomised, double-blind clinical trial. Andrologia. 2009;41(2):95-99.
90. Gonzales G.F., et al. Effect of Lepidium meyenii (maca), a root with aphrodisiac and fertility-enhancing properties, on serum reproductive hormone levels in adult healthy men. J Endocrinol. 2003;176(1):163-168.
91. Gonzales G.F., et al. Effect of Lepidium meyenii (maca) on sexual desire and its absent relationship with serum testosterone levels in adult healthy men. Andrologia. 2002;34(6):367-372.
92. Lopatkin N., et al. Efficacy and safety of a combination of sabal and urtica extract in lower urinary tract symptoms–long-term follow-up of a placebo-controlled, double-blind, multicenter trial. Int Urol Nephrol. 2007;39(4):1137-1146.
93. Safarinejad M.R. Urtica dioica for treatment of benign prostatic hyperplasia: a prospective, randomized, double-blind, placebo-controlled, crossover study. J Herb Pharmacother. 2005;5(4):1-11.
94. Sokeland J. Combined sabal and urtica extract compared with finasteride in men with benign prostatic hyperplasia: analysis of prostate volume and therapeutic outcome. BJU Int. 2000;86(4):439-442.
95. Wagner H., et al. [Biologically active compounds from the aqueous extract of Urtica dioica]. Planta Med. 1989;55(5):452-454.
96. Schottner M., et al. Lignans from the roots of Urtica dioica and their metabolites bind to human sex hormone binding globulin (SHBG). Planta Med. 1997;63(6):529-532.
97. Ducrey B., et al. Inhibition of 5 alpha-reductase and aromatase by the ellagitannins oenothein A and oenothein B from Epilobium species. Planta Med. 1997;63(2):111-114.
98. Hevesi Toth B., Kery A. [Epilobium parviflorum—in vitro study of biological action]. Acta Pharm Hung. 2009;79(1):3-9.
99. Hevesi B.T., et al. Antioxidant and antiinflammatory effect of Epilobium parviflorum Schreb. Phytother Res. 2009;23(5):719-724.
100. Steenkamp V., et al. Studies on antibacterial, anti-inflammatory and antioxidant activity of herbal remedies used in the treatment of benign prostatic hyperplasia and prostatitis. J Ethnopharmacol. 2006;103(1):71-75.
101. Vlahos C.J., et al. Platelet-derived growth factor induces proliferation of hyperplastic human prostatic stromal cells. J Cell Biochem. 1993;52(4):404-413.
102. Gleason P.E., et al. Platelet derived growth factor (PDGF), androgens and inflammation: possible etiologic factors in the development of prostatic hyperplasia. J Urol. 1993;149(6):1586-1592.
103. Bravi F., et al. Macronutrients, fatty acids, cholesterol, and risk of benign prostatic hyperplasia. Urology. 2006;67(6):1205-1211.
104. Boyd E., Berry N. Prostatic hypertrophy as part of generalized metabolic disease. J Urol. 1939;41:406-411.
105. Chaudry A.A., et al. Arachidonic acid metabolism in benign and malignant prostatic tissue in vitro: effects of fatty acids and cyclooxygenase inhibitors. Int J Cancer. 1994;57(2):176-180.
106. Narayan P., Dahiya R. Alterations in sphingomyelin and fatty acids in human benign prostatic hyperplasia and prostatic cancer. Biomed Biochim Acta. 1991;50(9):1099-1108.
107. Kristal A.R., et al. Dietary patterns, supplement use, and the risk of symptomatic benign prostatic hyperplasia: results from the prostate cancer prevention trial. Am J Epidemiol. 2008;167(8):925-934.
108. Edinger M.S., Koff W.J. Effect of the consumption of tomato paste on plasma prostate-specific antigen levels in patients with benign prostate hyperplasia. Braz J Med Biol Res. 2006;39(8):1115-1119.
109. Schwarz S., et al. Lycopene inhibits disease progression in patients with benign prostate hyperplasia. J Nutr. 2008;138(1):49-53.
110. Bullock T.L., Andriole Jnr G.L. Emerging drug therapies for benign prostatic hyperplasia. Expert Opin Emerg Drugs. 2006;11(1):111-123.
111. Damrau F. Benign prostatic hypertrophy: amino acid therapy for symptomatic relief. J Am Geriatr Soc. 1962;10:426-430.
112. Feinblatt H.M., Gant J.C. Palliative treatment of benign prostatic hypertrophy; value of glycine-alanine-glutamic acid combination. J Maine Med Assoc. 1958;49(3):99-101. passim
113. Thor P.J., et al. [The autonomic nervous system function in benign prostatic hyperplasia]. Folia Med Cracov. 2006;47(1–4):79-86.
114. Delmulle L., et al. Anti-proliferative properties of prenylated flavonoids from hops (Humulus lupulus L.) in human prostate cancer cell lines. Phytomedicine. 2006;13(9–10):732-734.
115. Kozlowski R., et al. Chronic ischemia alters prostate structure and reactivity in rabbits. J Urol. 2001;165(3):1019-1026.
116. Hinman F., editor. Benign prostatic hypertrophy. New York: Springer-Verlag, 1983.
117. Baldwin D.S. Sexual dysfunction associated with antidepressant drugs. Expert Opin Drug Saf. 2004;3(5):457-470.
118. Sachs B.D. The false organic-psychogenic distinction and related problems in the classification of erectile dysfunction. Int J Impot Res. 2003;15(1):72-78.
119. Price D., Hackett G. Management of erectile dysfunction in diabetes: an update for 2008. Curr Diab Rep. 2008;8(6):437-443.
120. Mikhail N. Management of erectile dysfunction by the primary care physician. Cleve Clin J Med. 2005;72(4):293-294. 296–297, 301–305 passim
121. Guay A.T., et al. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the evaluation and treatment of male sexual dysfunction: a couple’s problem—2003 update. Endocr Pract. 2003;9(1):77-95.
122. Chen J., et al. Effect of oral administration of high-dose nitric oxide donor L-arginine in men with organic erectile dysfunction: results of a double-blind, randomized, placebo-controlled study. BJU Int. 1999;83(3):269-273.
123. Klotz T., et al. Effectiveness of oral L-arginine in first-line treatment of erectile dysfunction in a controlled crossover study. Urol Int. 1999;63(4):220-223.
124. Zorgniotti A.W., Lizza E.F. Effect of large doses of the nitric oxide precursor, L-arginine, on erectile dysfunction. Int J Impot Res. 1994;6(1):33-35. discussion 36
125. Stanislavov R., Nikolova V. Treatment of erectile dysfunction with pycnogenol and L-arginine. J Sex Marital Ther. 2003;29(3):207-213.
126. Kernohan A.F., et al. An oral yohimbine/L-arginine combination (NMI 861) for the treatment of male erectile dysfunction: a pharmacokinetic, pharmacodynamic and interaction study with intravenous nitroglycerine in healthy male subjects. Br J Clin Pharmacol. 2005;59(1):85-93.
127. Rowland D.L., Tai W. A review of plant-derived and herbal approaches to the treatment of sexual dysfunctions. J Sex Marital Ther. 2003;29(3):185-205.
128. Ernst E., Pittler M.H. Yohimbine for erectile dysfunction: a systematic review and meta-analysis of randomized clinical trials. J Urol. 1998;159(2):433-436.
129. Wheatley D. Triple-blind placebo-controlled trial of Ginkgo biloba in sexual dysfunction due to antidepressant drugs. Hum Psychopharmacol. 2004;19(8):545-548.
130. Cohen A.J., Bartlik B. Ginkgo biloba for antidepressant-induced sexual dysfunction. J Sex Marital Ther. 1998;24(2):139-143.
131. Adimoelja A. Phytochemicals and the breakthrough of traditional herbs in the management of sexual dysfunctions. Int J Androl. 2000;23(Suppl 2):82-84.
132. Adaikan P.G., et al. Proerectile pharmacological effects of Tribulus terrestris extract on the rabbit corpus cavernosum. Ann Acad Med Singapore. 2000;29(1):22-26.
133. Lee M.S., et al. Acupuncture for treating erectile dysfunction: a systematic review. BJU Int. 2009;104(3):366-370.
134. Yang Y.K., et al. [Effects of moxibustion on erectile function and NO-cGMP pathway in diabetic rats with erectile dysfunction]. Zhongguo Zhen Jiu. 2007;27(5):353-356.
134. Zhao L. Clinical observation on the therapeutic effects of heavy moxibustion plus point-injection in treatment of impotence. J Tradit Chin Med. 2004;24(2):126-127.
136. Rowland D.L., Slob A.K. Understanding and diagnosing sexual dysfunction: recent progress through psychophysiological and psychophysical methods. Neurosci Biobehav Rev. 1995;19(2):201-209.
137. Kim J.H., et al. Randomized control trial of hand acupuncture for female stress urinary incontinence. Acupunct Electrother Res. 2008;33(3–4):179-192.
138. Tian F.S., et al. [Study on acupuncture treatment of diabetic neurogenic bladder]. Zhongguo Zhen Jiu. 2007;27(7):485-487.
139. Emmons S.L., Otto L. Acupuncture for overactive bladder: a randomized controlled trial. Obstet Gynecol. 2005;106(1):138-143.
140. Katz A.R. Urinary tract infections and acupuncture. Am J Public Health. 2003;93(5):702. author reply 702–703
141. Alraek T., et al. Acupuncture treatment in the prevention of uncomplicated recurrent lower urinary tract infections in adult women. Am J Public Health. 2002;92(10):1609-1611.
142. Anderson R.U., et al. Sexual dysfunction in men with chronic prostatitis/chronic pelvic pain syndrome: improvement after trigger point release and paradoxical relaxation training. J Urol. 2006;176(4 Pt 1):1534-1538. discussion 1538–1539
143. Dorey G., et al. Pelvic floor exercises for treating post-micturition dribble in men with erectile dysfunction: a randomized controlled trial. Urol Nurs. 2004;24(6):490-497. 512
144. Dorey G., et al. Pelvic floor exercises for erectile dysfunction. BJU Int. 2005;96(4):595-597.
145. Vasconcelos M., et al. Voiding dysfunction in children. Pelvic-floor exercises or biofeedback therapy: a randomized study. Pediatr Nephrol. 2006;21(12):1858-1864.
146. Rohrmann S. Meat and dairy consumption and subsequent risk of prostate cancer in a US cohort study. Cancer Causes Control. 2007;18(1):41-50.
147. Rohrmann S., et al. Association of cigarette smoking, alcohol consumption and physical activity with lower urinary tract symptoms in older American men: findings from the third National Health And Nutrition Examination Survey. BJU Int. 2005;96(1):77-82.
148. Shiels M.S., et al. Association of cigarette smoking, alcohol consumption, and physical activity with sex steroid hormone levels in US men. Cancer Causes Control. 2009;20(6):877-886.