CHAPTER 87 Benign Neoplasms of the Salivary Glands
Better understanding of the histogenesis of neoplasms of the salivary glands, however, has allowed a more consistent and rational classification of these tumors. Recent advances in molecular biology and tumor genomics have shed some light on the genetic basis of certain types of tumors of the salivary glands.1 The role of fine-needle aspiration biopsy and high-resolution imaging in the management of patients with salivary neoplasms continues to evolve. This chapter discusses some of the advances made in understanding the etiology and histogenesis of salivary tumors and describes a contemporary approach to the diagnosis and management of patients with benign neoplasms of the salivary glands.
Embryology
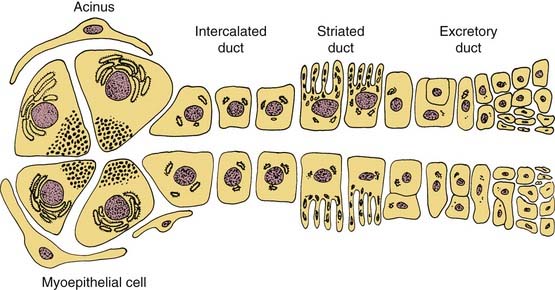
Understanding the origin of benign salivary gland tumors requires knowledge of the embryology and ultrastructure of the normal salivary gland. The major salivary glands originate from ectoderm and begin their development during the sixth week of gestation as solid, ridgelike ingrowths of the oral epithelium.2 These ingrowths continue to develop into tubules that later become the ductal system of the salivary glands. In the major salivary glands both serous and mucous cells are arranged into acini that are drained by a series of ducts. An intercalated duct drains into a striated duct, which empties into an excretory duct. Contractile myoepithelial cells surround the acini and intercalated ducts and help in draining saliva through the ductal system (Fig. 87-1). The parotid gland consists of predominately serous acini. The submandibular gland is composed of a mixture of serous and mucinous acini, whereas sublingual gland and minor salivary glands scattered throughout the upper aerodigestive tract contain predominantly mucinous acini.
Histogenesis of Salivary Neoplasms
At least two theories of tumorigenesis have been proposed for salivary gland neoplasms.3 In the multicellular theory, each type of neoplasm is thought to originate from a distinctive cell type within the salivary gland unit. According to this theory, Warthin’s and oncocytic tumors are thought to arise from striated ductal cells, acinic cell tumors from acinar cells, and mixed tumors from intercalated duct and myoepithelial cells.4 This theory is supported by the observation that all differentiated salivary cell types retain the ability to undergo mitosis and regenerate.5,6 An alternative theory, the bicellular reserve cell theory, assumes that the origin of the various types of salivary neoplasms can be traced to the basal cells of either the excretory or the intercalated duct. According to this theory, either of these two cells can act as a reserve cell with the potential for differentiation into a variety of epithelial cells.7 Hence despite the seeming heterogeneity of salivary tumors, all of them are thought to arise from one of two pluripotential cell populations. In this model, adenomatoid tumors including pleomorphic adenoma and oncocytic tumors are derived from the reserve cell of the intercalated duct, whereas epidermoid tumors, such as squamous cell carcinoma and mucoepidermoid carcinomas, are derived from the reserve cell of the excretory duct.8 Some reports provide molecular evidence to support the reserve cell theory of salivary gland tumorigenesis.9
Recent efforts to identify biomarkers of salivary neoplasia have focused on methylation patterns. Williams and colleagues10 investigated methylations status and protein expression of four tumor suppressor genes (DAPK, MGMT, RARB2, and RASSF1) in the largest cohort study of benign and malignant salivary neoplasms to date. The authors found benign and malignant salivary tumor differed in the frequency and pattern of gene methylation. They postulate methylation may aid in diagnosis and targeted therapy for salivary neoplasms.10 Maruya and colleagues analyzed the gene expression profile of 18 primary tumors representing the major benign subtypes using a membrane-based DNA microarray platform. Of the 5000 genes arrayed, S-100 beta, galectin-9 isoform, vimentin, and tyrosine kinase receptor were more highly expressed in adenomas than in carcinomas.11
Etiology
Radiation
There is increasing evidence to suggest that exposure to ionizing radiation may increase the risk of developing tumors of the salivary glands. In 1996 the Radiation Epidemiology Branch of the National Cancer Institute published a study on the risk of developing salivary gland tumors among atomic bomb survivors.12 The study indicated a higher radiation-related risk of developing both benign and malignant salivary gland tumors, as compared with the general population. Dose-response analyses found statistically significant increases in risk with increasing A-bomb dose for both cancer and benign tumors. The risk was higher for malignant tumors, especially mucoepidermoid carcinoma. Among those with benign neoplasms, Warthin’s tumor showed the highest dose-response–related risk.
Radiation therapy to the head and neck, especially if it encompassed the salivary glands, may also be a risk factor in the development of salivary gland tumors.13 A recent study found a 4.5-fold increase in the incidence of salivary gland cancer and a 2.6-fold increase of benign tumors among persons exposed to scalp irradiation compared with matched controls.14 The mean length of latency period until tumor development was 11 years for malignant tumors and 21.5 years for benign. The study also demonstrated a clear dose response effect for cancer and benign tumors, suggesting that ionizing radiation may play an important role in salivary gland tumorigenesis.
Viral
Although Epstein-Barr virus (EBV) has been consistently associated with lymphoepithelial carcinoma of the salivary gland in the Asian population, there is no evidence of a causal role of EBV in other primary salivary gland neoplasms.15 Pollock and colleagues16 failed to demonstrate any positive signal by in situ hybridization for EBV RNA in 42 benign salivary gland neoplasms. Other viruses including human papillomavirus, human herpesvirus-8, and cytomegalovirus do not appear to have any etiologic role in salivary gland neoplasms.17
Other Factors
Although tobacco use was not associated with a higher incidence of malignant salivary neoplasms, Warthin’s tumor is strongly associated with cigarette smoking.18 Occupational exposure to silica dust was linked to a 2.5-fold increased risk of cancer of the salivary gland.19 The risk was also elevated among rubber workers exposed to nitrosamines.20 Dietary analyses revealed a possible protective effect for a diet high in polyunsaturated fatty acids.19,21 Similar to breast cancer, women with history of early menarche and nulliparity had an increased risk of cancer of the salivary gland, which may be due to a hormonal effect.22 Whether these occupational, dietary, or hormonal factors have any association with the risk of developing benign tumors of the salivary glands remains unknown.
Genetic Factors
Recent developments in the field of molecular biology and human genomics have shed light on the genetic basis of developing several types of neoplasms including tumors of salivary gland origin. A study of several types of salivary gland tumors using comparative genomic hybridization found specific genetic alterations in different types of salivary gland tumors and involvement of several chromosomes in the alterations.23 Genetic aberrations associated with the salivary gland neoplasia include allelic loss and point mutation, structural rearrangement of chromosomal units (most commonly translocations), the absence of one chromosome (monosomy), and the presence of an extra chromosome (polysomy). Any of these genetic aberrations may be the only karyotypic anomaly indicating an early event in tumor initiation, or several genetic alterations may coexist, which are more likely to be associated with tumor progression and more aggressive biologic behavior.9,24
Incidence
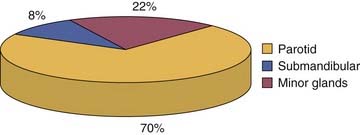
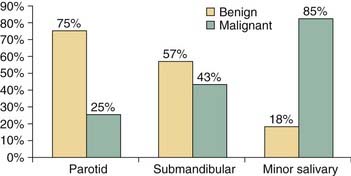
Salivary gland tumors are relatively rare and constitute 3% to 4% of all head and neck neoplasms. The majority (70%) of salivary gland tumors arise in the parotid gland (Fig. 87-2). Although the majority of minor salivary gland tumors are malignant, three fourths of parotid tumors are benign (Fig. 87-3). Spiro25 reviewed the Memorial Sloan-Kettering experience with salivary neoplasms over a 35-year period. The distribution of these 2807 patients is shown in Table 87-1. Benign neoplasms constituted 54% (1529 patients) of all tumors. Pleomorphic adenoma (1280 patients) constituted 84% of benign tumors and 45% of all salivary gland neoplasms. Warthin’s tumor was the second most benign tumor and comprised 12% (183 patients) of all benign tumors.
| Histology | Number of Patients | Percent |
|---|---|---|
| Pleomorphic adenoma | 1274 | 45.4 |
| Warthin’s tumor | 183 | 6.5 |
| Benign cyst | 29 | 1.0 |
| Lymphoepithelial lesion | 17 | 0.6 |
| Oncocytoma | 20 | 0.7 |
| Monomorphic adenoma | 6 | 0.2 |
| Mucoepidermoid carcinoma | 439 | 15.7 |
| Adenoid cystic carcinoma | 281 | 10.0 |
| Adenocarcinoma | 225 | 8.0 |
| Malignant mixed tumor | 161 | 5.7 |
| Acinic cell carcinoma | 84 | 3.0 |
| Epidermoid carcinoma | 53 | 1.9 |
| Other (anaplastic) | 35 | 1.3 |
| Total | 2807 | 100 |
From Spiro RH. Salivary neoplasms: overview of a 35-year experience with 2,807 patients. Head Neck Surg. 1986;8:177-84. © 1986 John Wiley & Sons.
Patient Evaluation
Clinical Features
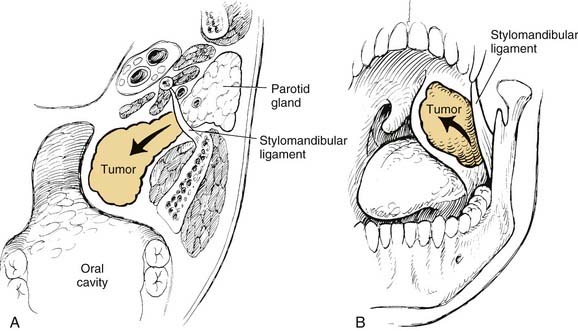
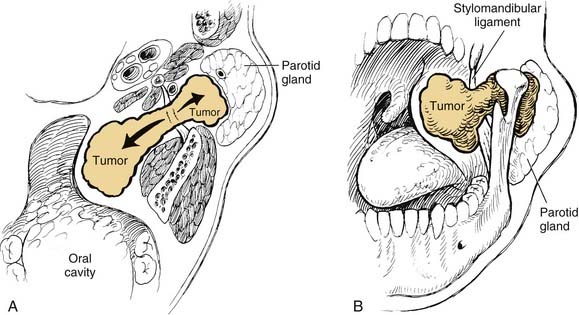
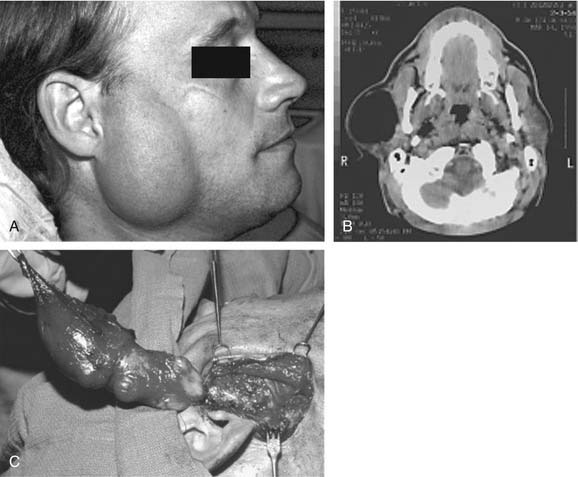
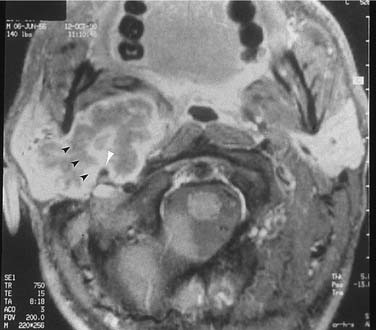
On examination, benign tumors of the parotid gland are usually well defined, nontender, and freely mobile. They are commonly located in the “tail” of the parotid gland but may be present anywhere in the superficial or deep lobe. Tumors may originate entirely from the deep lobe (Fig. 87-4) or extend from the superficial to the deep lobe through the relatively narrow stylomandibular tunnel, giving the appearance of a dumbbell tumor (Fig. 87-5). In either case, the tumor may extend to the parapharyngeal space and displace the oropharyngeal wall medially (Fig. 87-6). The presence of facial nerve paresis or paralysis, pain, fixation of the mass to the overlying skin or underlying structures, and associated cervical adenopathy usually indicate the presence of malignancy. It should be noted, however, that these findings usually indicate local or regional extension of the tumor, and the diagnosis of parotid malignancy should not await the development of these signs and symptoms. The possibility of malignancy should be ruled out in patients presenting with any mass in the parotid gland. This will usually require cytologic or histologic evaluation with a fine-needle biopsy, or parotidectomy, respectively.
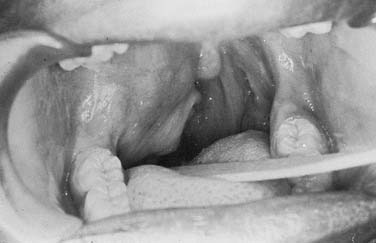
Figure 87-6. Pleomorphic adenoma of the deep lobe of a parotid gland, causing medial displacement of the palate and tonsil.
Benign tumors of the minor salivary glands are rare. Most tumors arising from the minor salivary glands are malignant (see Fig. 87-3). However, similar to the major salivary glands, pleomorphic adenoma is the most frequent benign tumor of the minor salivary glands. The clinical presentation depends on the site of origin, and the most common sites include the palate, parapharyngeal space, and the lacrimal gland.
Fine-Needle Aspiration Biopsy
Fine-needle aspiration biopsy (FNAB) has been widely recognized and well established as an accurate technique in the diagnosis of salivary gland neoplasms. Numerous studies have reported an exceptionally high degree of sensitivity, specificity, and predictive value for FNAB. The overall sensitivity ranges from 85.5% to 99%, and the overall specificity ranges from 96.3% to 100%.26–28 In general, diagnostic accuracy is higher for benign than for malignant salivary gland tumors.29,30 The accuracy of FNAB, however, depends greatly on the experience of the cytopathologist, as well as the overall volume of patients with salivary neoplasms evaluated in any given institution. The most common source of diagnostic error of FNAB is inadequate sampling.31 The use of ultrasound-guided FNAB may be of help in cases in which it is difficult to obtain a representative sample, and it enhances the overall diagnostic accuracy of the technique.32,33 Despite its overall high accuracy, several significant but uncommon areas can lead to diagnostic difficulties, with the potential for clinically important diagnostic errors.29 The most frequent problems involve variations in the expected cytology of pleomorphic adenoma. Also, there are several benign-malignant “look-alike” pairs of lesions. The first of these is related to small-cell epithelial neoplasms of low nuclear grade; the most frequent problem is between basal cell adenomas and adenoid cystic carcinoma, particularly the solid (anaplastic) type. The next area contrasts mucoepidermoid carcinoma with its cytologic mimic, benign salivary gland duct obstruction. The final difficulty in salivary gland aspiration contrasts large-cell epithelial lesions of low nuclear grade: oncocytic proliferations and acinic cell carcinoma.34
In addition to being accurate, FNAB is safe, simple to perform, and relatively inexpensive. However, one essential question is worth asking. Is FNAB really necessary in the evaluation of salivary gland masses? Would it change the course of management on the basis of clinical assessment? In an attempt to answer this question, Heller and colleagues35 performed a study to determine the impact of FNAB on patient management. One hundred patients underwent FNAB of major salivary gland masses. The physician’s initial clinical impression was compared with the FNAB diagnosis and the final diagnosis in each case. Overall, FNAB resulted in a change in the clinical approach to 35% of the patients. Examples of such changes in the planned management included avoiding surgical resection for lymphomas and inflammatory masses and adopting a more conservative approach with benign tumors in elderly and high-surgical-risk patients. FNAB also allows better preoperative counseling of patients regarding the nature of the tumor, the likely extent of resection, management of the facial nerve, and the likelihood of a neck dissection. Such information is not only important in treatment planning but also helps alleviate an already high level of anxiety among patients and their families.
The possibility of tumor seeding is a commonly cited argument against the routine use of FNAB in patients with salivary gland tumors. To address these issues, Mukunyadzi and colleagues36 recently reviewed 94 resected salivary gland masses for infarction, hemorrhage, and needle track tumor seeding, as well as fibrosis after FNAB. They concluded FNAB with a 25-gauge needle is safe and does not significantly alter the histologic diagnosis because tissue effects did not preclude accurate diagnostic interpretation in any case.36
Imaging
The routine use of imaging in patients with small, well-defined masses of the superficial lobe of the parotid gland is probably not warranted because the results of imaging are not likely to change the treatment plan. However, tumors that present with clinical findings suggestive of malignancy, tumors arising from the deep lobe of the parotid gland or the parapharyngeal space, and, tumors of the submandibular and minor salivary gland should be evaluated with high-resolution imaging. In such cases, imaging provides accurate delineation of the location and extent of tumor, its relation to major neurovascular structures, perineural spread, skull base invasion, and intracranial extension.37
Computed Tomography and Magnetic Resonance Imaging
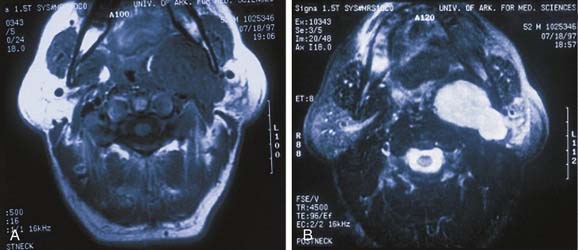
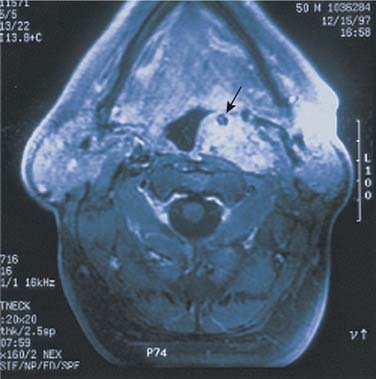
In evaluating patients with tumors of the salivary glands, both computed tomography (CT) and magnetic resonance imaging (MRI) provide information that is superior to that provided by other imaging techniques or by physical examination.38 The normal parotid gland has a high fat content and is easily visualized on both CT and MRI. Therefore both techniques can demonstrate whether a mass in that region is intraglandular or extraglandular. Generally, neither CT nor MRI provides information regarding the specific histologic diagnosis, except rarely.39 An example of such a rare scenario is with lipoma of the parotid gland (Fig. 87-7). The MRI signal characteristics of parotid masses, however, may be suggestive of certain diagnosis.40 For example, if a parotid gland mass is bilateral, it is more likely to be Warthin’s tumor, especially if it does not enhance. Less likely, it could be lymphoepithelial cyst or necrotic lymph node. A unilateral, nonenhancing mass with a high T2 signal is more likely to be a Warthin’s tumor and less likely a necrotic lymph node or first branchial cleft cyst. If the mass is unilateral, shows postcontrast enhancement, has a high T2 signal, and does not invade surrounding tissue planes, it is more likely to be a pleomorphic adenoma (Fig. 87-8). An intermediate to low T2 signal mass, with or without invasion of surrounding tissue planes, is more likely to be a malignant mass such as adenoid cystic or mucoepidermoid carcinoma.41
MRI is also superior to CT in demonstrating the internal architecture of salivary gland tumors in a multiplanar fashion and in delineating the interface between tumor and normal salivary gland.42 In contrast to benign tumors, which invariably have well-defined margins (see Fig. 87-8), malignant tumors may exhibit irregular margins (Fig. 87-9). Extension of the tumor beyond the fascial confines of the gland can be adequately seen on both CT and MRI and should raise the suspicion of malignancy.43 Bone destruction of the mandible or skull base is best visualized on CT, whereas bone marrow involvement is better demonstrated on MRI. Both studies can adequately evaluate the neck for metastatic adenopathy. CT has the advantage of being less expensive and more available than MRI.
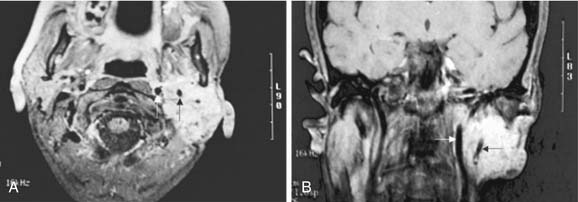
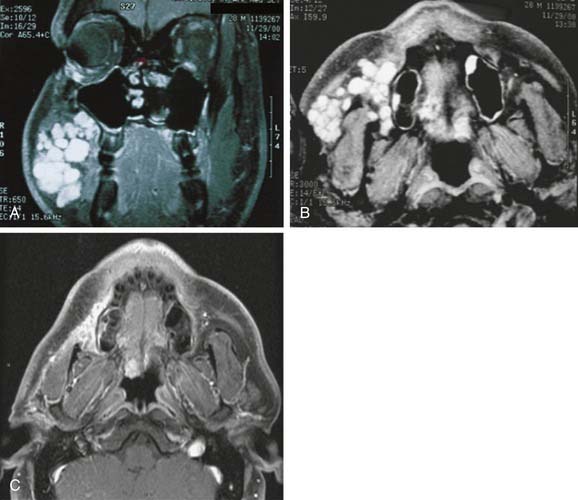
Although parapharyngeal space masses are well visualized by both techniques, they are better delineated with MRI than CT. This is because of the different signal intensity of tumor, fat, and muscle on MRI. The differential diagnosis of parapharyngeal masses includes deep lobe parotid tumors, minor salivary gland tumors, and neurogenic and vascular tumors. Most salivary tumors have low-to-intermediate T1 signal intensities and intermediate-to-high T2 signal intensities. Deep lobe parotid tumors and minor salivary gland tumors of the parapharyngeal space lie in the prestyloid compartment, anterior to the carotid artery, and displace the parapharyngeal fat medially (Figs. 87-10 and 87-11). Deep lobe tumors are connected to the parotid gland at least in one imaging section, whereas minor salivary gland tumors are completely surrounded by fat (see Fig. 87-11). By contrast, neurogenic tumors and glomus tumors lie in the poststyloid compartment, posterior to the carotid artery, which is displaced anteriorly (Fig. 87-12). Neurogenic tumors usually enhance intensely with gadolinium, whereas glomus tumors have characteristic serpiginous flow voids (salt and pepper appearance) on MRI.
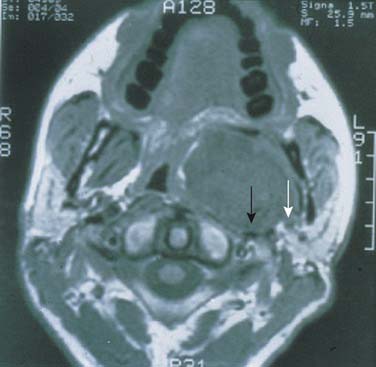
Figure 87-11. In contrast to the dumbbell tumor depicted in Figure 87-10, this pleomorphic adenoma originates entirely from the deep lobe of the parotid gland (black arrow) and lies entirely medial to the stylomandibular tunnel. Pleomorphic adenomas originating from the minor salivary glands of the parapharyngeal space have a similar appearance on high-resolution imaging but are not connected to the parotid gland. Regardless of their origin, salivary gland tumors of the parapharyngeal space will occupy the prestyloid compartment and displace the carotid artery posteriorly (white arrow).
Recently, new MR technologies such as dynamic contrast-enhanced MRI, diffusion-weighted MRI, and proton MR spectroscopy have shown promising results in the differentiation between benign and malignant salivary gland tumors.44 Further research with larger-scale studies on these particular MR methods is necessary.
Ultrasonography
Ultrasonography has the advantage of being inexpensive, noninvasive, simple to perform, and is virtually free of complications. It can be used to differentiate solid from cystic masses in the salivary glands, and most salivary gland diseases can be diagnosed sonographically.32 Ultrasound guidance may also enhance the accuracy of FNAB in nonpalpable tumors and in masses with a highly heterogeneous architecture.45
Color Doppler Sonography
Color Doppler sonography has been recently used to evaluate the vascular anatomy of the salivary glands. It can distinguish between the physiologic changes that occur during salivary stimulation in normal subjects and the flow alterations that occur in diseased glands.46
Positron Emission Tomography
PET scans have not gained wide application in imaging of tumors of the salivary gland for several reasons. First, tumors of the salivary glands have a variable and inconsistent uptake of F-18 fluorodeoxyglucose (FDG), which is the most commonly used radiotracer with PET scans, and therefore FDG PET is unreliable both in tumor detection and in distinguishing benign from malignant salivary tumors.47 Second, competing techniques such as CT, MRI, and even clinical examination already have a high degree of accuracy. Third, high-resolution studies such as CT and MRI provide anatomic detail required for treatment planning that is unavailable from FDG PET images. Finally, the high cost of FDG PET prohibits its widespread use. Research is ongoing with PET scans. Uchida and colleagues48 recently proposed combining FDG PET with Tc-99m pertechnetate salivary gland scintigraphy for differentiating various parotid gland tumors in patients with nondiagnostic needle aspiration.
Pleomorphic Adenomas
In the parotid gland, approximately 90% of pleomorphic adenomas originate superficial to the facial nerve. Occasionally, tumors of the superficial lobe extend to the deep lobe medial to the facial nerve. If they extend to the parapharyngeal space through the stylomandibular “tunnel” bounded by the stylomandibular ligament, they may have a narrow isthmus connecting the superficial and deep components, giving the tumor a dumbbell appearance (see Figs. 87-5 and 87-10). Approximately 10% of pleomorphic adenomas originate entirely from the deep lobe and are usually located deep to the stylomandibular ligament. Therefore they have a more rounded appearance on high-resolution imaging (see Figs. 87-4 and 87-11). Pleomorphic adenomas may also arise from minor salivary gland tissue that naturally occurs in the parapharyngeal space. Regardless of their origin, pleomorphic adenomas of the parapharyngeal space occupy the prestyloid compartment (see Figs. 87-10 and 87-11) and, like other parapharyngeal tumors, may displace the oropharynx medially (see Fig. 87-6). Pleomorphic adenomas of the minor salivary glands most commonly occur in the palate (Fig. 87-13).49 The second most common site is the upper lip. Despite the abundance of minor salivary glands in the lower lip, pleomorphic adenomas are exceedingly rare in this location. Pleomorphic adenomas are the most common benign tumors of the lacrimal gland (Fig. 87-14).50–52
Clinically, pleomorphic adenomas are characterized as painless, slow-growing masses. Multiple primary pleomorphic adenomas are extremely rare, although reported.53 Pathologically, pleomorphic adenomas are solitary, firm, round tumors. The cut surface is characteristically solid and may be hard, rubbery, or soft in consistency with a whitish gray to pale yellow color. Pleomorphic adenomas of the major salivary glands have a capsule, although varying in thickness and completeness, whereas pleomorphic adenomas of the minor salivary glands are usually nonencapsulated.54 On histologic examination, pleomorphic adenomas consist of epithelial components that may take the form of ducts, nests, cords, or solid sheets of cells and myoepithelial cells that appear plasmacytoid or spindled in a fibrocollagenous, myxochondroid, or chondroid background. Myoepithelium in pleomorphic adenoma is considered to be responsible for the development of the characteristic myxoid or chondroid stroma. Variations in the histologic appearance of this tumor type are common and may include squamous metaplasia, calcification, cartilage-appearing tissue, oxyphilic cells, and a palisading appearance of the underlying stroma. Lipomatous55 and osseous56 changes have been occasionally reported in pleomorphic adenoma.
Histopathologic studies have demonstrated that almost all pleomorphic adenomas have focally thin capsules, and one fourth of pleomorphic adenomas demonstrate satellite nodules or pseudopodia.57 Therefore the recommended treatment is surgical excision with a surrounding cuff of normal tissue to prevent recurrence from failure to address these pseudopod-like extensions of tumor.58 In patients with tumors of the submandibular gland, the gland is generally removed with careful preservation of the marginal mandibular nerve. Tumors of the parotid gland usually involve removal of the tumor with an adequate margin and facial nerve preservation. Because most of the tumors arise from the superficial lobe, a superficial parotidectomy is often required.
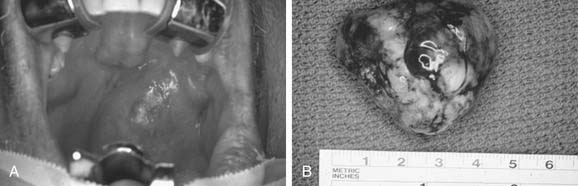
Recurrent pleomorphic adenomas are an uncommon but challenging problem. Frequently, multiple foci of recurrence continue to manifest over several years.59 This multicentric pattern is characteristic of recurrent pleomorphic adenomas (Fig. 87-15). Currently, no reliable criteria have been established to predict those tumors with a greater tendency to recur. Surgical excision, however, remains the mainstay of treatment for recurrent pleomorphic adenomas. Patients who have undergone previous surgical excision, especially those with previous parotidectomy, are at increased risk for facial nerve injury, as well as recurrence with revision surgery.60,61 Neutron radiotherapy offers excellent local control rates and survival rates in patients with multiple recurrences who are not surgical candidates even in the presence of gross residual disease.62
Malignant transformation of pleomorphic adenoma is rare and occurs most frequently in patients with long-standing tumors. The risk of malignant transformation in pleomorphic adenoma is 1.5% within the first 5 years of diagnosis but increases to 10% if observed for more than 15 years.63 Cases of benign pleomorphic adenoma metastasizing to cervical lymph nodes have been reported.64–66
Basal Cell Adenomas
Most basal cell adenomas occur in the parotid gland and minor salivary glands of the upper lip. As with other benign neoplasms, they present as a slow-growing, asymptomatic mass. Gross pathologic examination reveals a well-circumscribed, solid tumor with a gray-white to pink-brown cut surface. Parotid basal cell adenomas are characteristically encapsulated, whereas those of minor salivary gland origin may lack a capsule.67 Histologically, an intact basement membrane helps differentiate basal cell adenomas from pleomorphic adenomas. Four histologic growth patterns have been recognized: solid, trabecular, tubular, and membranous.68 Basal cell adenomas may be difficult to distinguish from the solid variety of adenoid cystic carcinomas. Cytologic features that help differentiate basal cell adenomas from adenoid cystic carcinomas include lack of true invasion of surrounding tissues, lack of perineural invasion, a vascular stroma, and a peripheral palisading of the outer layer of basaloid cells. The cell-stroma interface is also useful in distinguishing basal cell adenomas from adenoid cystic carcinomas. In basal cell adenomas the collagenous stroma interdigitates with adjacent cells, whereas in those with adenoid cystic carcinomas the two are separated by a sharp, smooth border. Further, the stroma of basal cell adenomas may contain rare spindle cells or capillaries, but the cylinders of adenoid cystic carcinoma are acellular. The treatment of these benign tumors is surgical excision with a cuff of normal surrounding tissue.
Canalicular Adenomas
Canalicular adenomas are benign neoplasms of predominately oral minor salivary glands, the upper lip being the most common site. They have been reported to account for less than 1% of salivary gland tumors and are rarely reported to be multifocal.69 The patient presents with complaint of an asymptomatic, slowly enlarging mass. These tumors may be clinically similar to a mucocele or varix and may be multifocal. Histologically, they are characterized by cells arranged in parallel rows forming ductlike structures—hence the term “canalicular.” Canalicular adenomas may be distinguished from adenoid cystic carcinomas by their well-circumscribed appearance and lack of perineural or soft tissue invasion. Surgical excision is the treatment of choice for canalicular adenomas.
Papillary Cystadenoma Lymphomatosum—Warthin’s Tumors
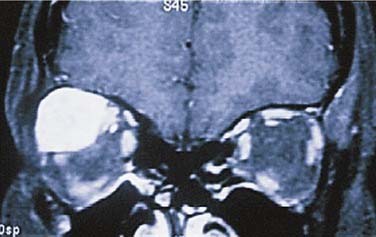
First described by Aldred Warthin in 1929, Warthin’s tumor is the second most common benign salivary gland neoplasm behind pleomorphic adenoma. Warthin’s tumor is also known as papillary cystadenoma lymphomatosum or adenolymphoma. The term “adenolymphoma” is often confused with malignant lymphoma and therefore the use of this term should be discouraged. Papillary cystadenoma lymphomatosum is an accurate descriptive term but is somewhat cumbersome compared with the most favored term of Warthin’s tumor. Warthin’s tumor accounts for approximately 10% of all parotid tumors and is found almost exclusively in the parotid gland. Warthin’s tumor is associated with a higher male preponderance and is more prevalent in whites than in other racial groups. Bilateral Warthin’s tumors occur in 10% of cases and may be simultaneous. As previously discussed, Warthin’s tumor is associated with cigarette smoking, which may be due to irritation of ductal epithelium by tobacco smoke, which initiates tumorigenesis.18
Oncocytoma
On gross pathologic examination, oncocytomas of the major salivary glands are well circumscribed and encapsulated. Those originating from minor salivary glands have less well-defined borders and are not encapsulated. The surface is pink to rust in color. Microscopically, the tumor has a granular appearance as a result of the abundant, hyperplastic mitochondria. The concentration of mitochondria differentiates oncocytomas from other tumors. A true oncocytoma contains no lymphoid tissue. The extensive lymphoid component that is typical of a Warthin’s tumor is never encountered. Histologically, oncocyte-predominant benign lesions may have a degree of epithelial atypia, squamous metaplasia, or necrosis that may mimic some types of malignant salivary tumors with oncocytic components such as adenoid cystic carcinoma, mucoepidermoid carcinoma, and adenocarcinoma.70 Metastatic thyroid carcinoma or renal cell carcinoma with a large number of oncocytes should also be differentiated from oncocytoma.
Oncocytomas originating from minor salivary glands tend to grow in an irregular and locally invasive pattern. They are less predictable than those originating in the parotid glands. Those originating from minor salivary glands in the respiratory tract may invade surrounding cartilage or bone. Although histologically benign, they have a destructive potential. Rarely do they demonstrate features indicative of malignancy such as increased mitoses and perineural or vascular invasion. Detection of cellular proliferation using immunohistochemical staining may be helpful in distinguishing a benign oncocytoma from a malignant oncocytoma.71 Treatment of benign oncocytomas is surgical excision.
Myoepithelioma
Myoepithelioma originates from contractile myoepithelial cells that lie beneath the ductal and acinar epithelium in the salivary glands. These cells also have been identified in sweat glands and mammary glands. They have the ability to contract, and their pumping action helps expel (or discharge) secretions from the salivary glands. Diagnosing myoepitheliomas by fine-needle aspiration is difficult, and immunohistochemistry with CD 10, SMA, and S-100 markers may aid in diagnosis.72 On gross pathology, myoepitheliomas are well demarcated and the external surface is smooth. The cut surface is homogeneous and white. Microscopically, these tumors have a thin capsule, except when they occur in the palate, wherein they lack encapsulation. The cells are benign appearing with rare mitosis and often assume spindle, polygonal, or plasmacytoid features. Clinically, these tumors are difficult to distinguish from pleomorphic adenomas due to their painless, slow-growing nature. There is no gender predilection, and they occur in the major and minor salivary glands. Histologically, they should be differentiated from plasmacytomas and any tumors with spindle cells including neurilemoma, fibroma, meningioma, and leiomyoma. The majority of these tumors behave in a benign manner, although there have been reports of local aggressiveness. The treatment is surgical excision.
Congenital Lesions
Hemangiomas
Congenital vascular lesions are divided into two groups: hemangiomas and vascular malformations. Juvenile hemangiomas are the most common tumors of infancy, occurring in up to 10% of all births.73 They may be present at birth but commonly appear several days to weeks later. A rapid growth phase occurs around the age of 1 to 6 months, followed by gradual involution over 1 to 12 years. During the growth phase, endothelial cells proliferate rapidly and become flat during involution. Facial infantile hemangiomas occur in two distinct patterns of tissue involvement: a focal type with a tumor-like appearance and a less common diffuse type with a plaquelike appearance. The diffuse lesions are more likely to be complicated by ulceration or airway obstruction and show a strikingly segmental distribution pattern. Focal hemangiomas, in contrast, show a predilection for regions of embryologic fusion.74 Hemangiomas are more common in the parotid than in the submandibular gland. Hemangiomas of the parotid area are more often seen in female patients and are usually asymptomatic, unilateral, and compressible masses. The overlying skin may be normal or may be involved. It is not uncommon to see other skin lesions present. On gross pathologic examination, this tumor is dark red, lobulated, and nonencapsulated. Microscopically, these tumors are composed of capillaries lined by proliferative endothelial cells. Blood vessels are uniform in size. Mitoses occur frequently but are not indicators of behavior.75
Hemangiomas may become extremely large with complications such as bleeding, heart failure, and even death. Treatment initially should consist of steroids administered 2 to 4 mg/kg per day. If the hemangioma is responsive to steroids, the result is usually immediate and often dramatic. Unfortunately, the response rate to steroids is only 40% to 60%. In such patients, interferon may be useful, but it should be reserved for life-threatening situations because of its toxicity and the necessity to continue the drug for prolonged periods. Surgical excision or various types of laser treatments may be performed for select circumstances.76 Hemangiomas usually involute completely, but they may take years and may result in medical or psychological problems for children.77
Vascular and Lymphatic Malformations
Venous Malformations
Venous malformations are usually noted at the time of birth and continue to grow as the patient gets older. They may become extremely large in size by the time the child is fully grown. Unlike hemangiomas, which grow by cellular proliferation, enlargement of venous malformations is primarily by dilation of the existing vessels and does not represent cellular division.76 Treatment is usually by surgical excision; however, large venous malformations involving the parotid gland may be difficult to remove. An MRI scan is the best imaging study to determine the extent of disease. Arteriograms are not helpful.
Management of Benign Salivary Gland Neoplasms
Management of benign salivary gland tumors has not changed dramatically for years. Management of tumors of the salivary glands requires a detailed understanding of the anatomy and pathologic processes affecting these glands. Benign salivary gland tumors should be excised completely with an adequate margin to avoid local recurrences.57 Generally tumors in the parotid gland are removed with an adequate cuff of surrounding normal tissue, and the facial nerve is dissected and carefully preserved. The extent of dissection of the facial nerve and the amount of parotid tissue resection depend on the size, location, and histology of the tumor.78
Larger tumors of the superficial lobe, however, usually require a complete superficial parotidectomy. Deep lobe tumors require a total parotidectomy, with facial nerve preservation. Parapharyngeal tumors are most commonly excised through a cervical-parotid approach and only occasionally in conjunction with a mandibulotomy.79
A tumor of the submandibular gland requires submandibular gland resection. If the tumor originates from a minor salivary gland, the tumor and a cuff of normal tissue should be excised. Simple enucleation should be discouraged because of the increased risk of recurrence.58 Pediatric hemangiomas will generally involute but may be resected in special cases. Vascular malformations involving the parotid or submandibular glands may require surgical excision.
Parotidectomy (see Key Indicator Video online)
The patient is placed in the supine position, and the head turned to the opposite side. The standard incision is a modified Blair’s incision (Fig. 87-16A). The skin incision is placed in a preauricular crease and extends superiorly to the level of the root of the helix. The incision extends inferiorly around the lobule of ear over the mastoid tip. It then gently curves down along the sternocleidomastoid muscle and then slightly forward in a natural skin crease in the upper neck (see Fig. 87-16A). Alternatively, a “face-lift” incision may be done in selected patients with benign tumors of the lower or mid regions of the parotid gland. The preauricular portion of the incision passes on or just inside the tragal margin. The incision is then carried below the lobule of the ear, turns posteriorly over the mastoid, and then is extended within the hairline (Fig. 87-17A). The face-lift incision is less conspicuous and therefore offers better cosmesis (see Fig. 87-17B).
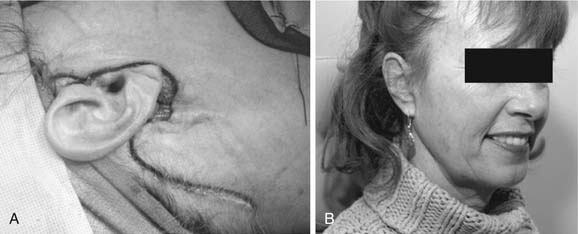
Figure 87-17. Modified “face-lift” incision for parotidectomy. A, Unlike the modified Blair’s incision, which has a cervical extension (see Fig. 87-16A), the face-lift incision extends posteriorly and inferiorly along the hairline. B, The hairline portion of the incision is inconspicuous, making this approach more cosmetically pleasing.
To expose the parotid gland, skin flaps are elevated in the plane superficial to the parotid fascia in the preauricular region and in the subplatysmal plane in the cervical portion of the incision (see Fig. 87-16B). The greater auricular nerve and the external jugular vein are identified over the sternocleidomastoid muscle and are divided to free the tail of the parotid gland (see Fig. 87-16B). In some cases the greater auricular nerve can be preserved, but whether this preservation has a significant impact on the patients’ quality of life is debatable (as will be discussed under Complications).80,81 The posterior belly of the digastric muscle is exposed proximal to its attachment to the mastoid bone. The fascia between the parotid gland and the cartilaginous external auditory canal is dissected, and the parotid gland is retracted anteriorly. This exposes the tragal pointer. The most common method of identifying the main trunk of the facial nerve is in its course in the region located between the tragal pointer and the attachment of the posterior belly of the digastric muscle to the mastoid bone (see Fig. 87-16C). Unless displaced by tumor, the nerve is usually located approximately 1 to 1.5 cm deep and inferior to the tragal pointer. Another reliable, and perhaps a more constant, landmark to identify the facial nerve is the tympano-mastoid suture line, which could be followed medially to the main trunk of the nerve. The nerve is usually 6 to 8 mm deep to the tympano-mastoid suture line. In certain cases where the tumor is directly overlying the region of the main trunk of the facial nerve, one or more of the peripheral branches of the nerve are identified distally and traced proximally toward the main trunk.82 The marginal mandibular branch can be identified below the lower border of the mandible, as it crosses superficial to the facial vessels, in the plane immediately underneath the deep cervical fascia. Alternatively, the buccal branch can be identified underneath the parotido-masseteric fascia, coursing parallel to the parotid duct.
Once the facial nerve is identified, the overlying parotid tissue is meticulously elevated from the nerve with a fine clamp and the bridge of tissue between the blades of the clamp carefully divided. If the main trunk of the nerve is identified first, then the overlying parotid tissue is progressively dissected free of the nerve to the first branching of the nerve, usually into an upper and a lower division (see Fig. 87-16C). Subsequent branching of the nerve (pes anserinus) is sequentially dissected in a similar fashion, until the entire superficial lobe of the parotid gland lying lateral to the facial nerve is delivered (see Fig. 87-16D). This completes a superficial or lateral parotidectomy.
Submandibular Gland Excision
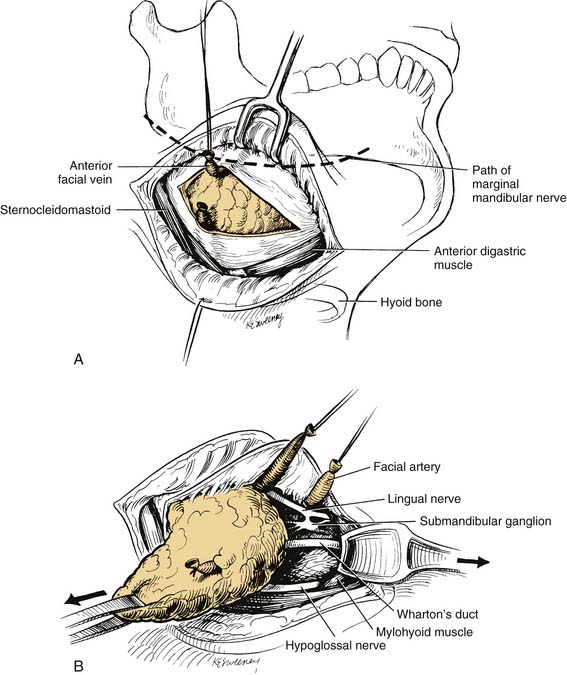
A curvilinear incision is placed, preferably in a natural skin crease, 3 to 4 cm below the lower border of the mandible, overlying the submandibular gland. The incision is carried down through the subcutaneous fat, and the platysma muscle. Great care should be taken to avoid injury of the marginal mandibular nerve. The nerve lies immediately beneath the deep cervical fascia, and can be identified crossing the anterior facial vein. The vein is doubly ligated and transected well below the nerve, and upward retraction of the superior ligature displaces the nerve superiorly and protects it from injury during further dissection (Fig. 87-18A). Superior dissection proceeds by double ligation and transection of the facial artery, which frees the superior attachment of the gland. Anteriorly the vessels to the mylohyoid muscle are divided, and the gland is mobilized posteriorly, exposing the free edge of the mylohyoid muscle. The free (posterior) edge of the mylohyoid muscle is retracted anteriorly, while gentle posterior traction on the gland is maintained. This exposes the deep portion of the gland and its duct, the submandibular ganglion, and the lingual and hypoglossal nerves (see Fig. 87-18B). These structures lie superficial to the hyoglossus muscle. The contribution of the lingual nerve to the submandibular ganglion is transected, and the Warthin’s duct is doubly ligated and divided. This delivers the deep portion of the gland. Care should be taken to avoid injury to the lingual and hypoglossal nerves. Finally the facial artery is divided a second time, and the gland is removed.
Excision of Parapharyngeal Salivary Gland Tumors
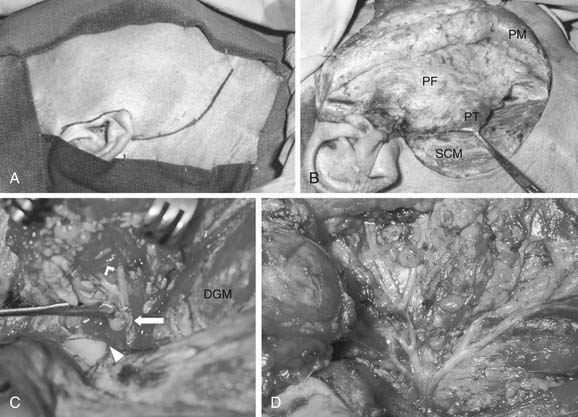
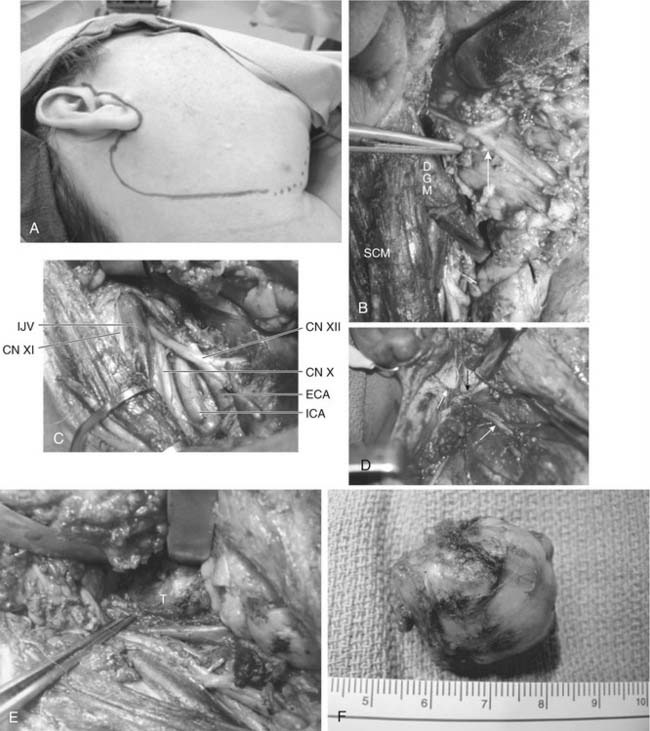
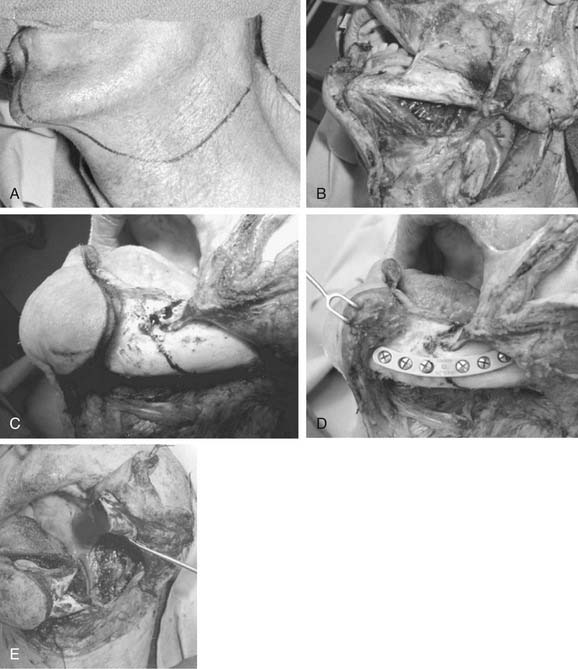
Surgical excision of salivary gland tumors within the parapharyngeal space is best done via an external approach.37,79 The incision is that of a parotidectomy with a cervical extension (Fig. 87-19A). If the tumor involves the deep lobe of the parotid gland, a superficial parotidectomy is done first. If the tumor arises from the minor salivary glands of the parapharyngeal space, the inferior division of the facial nerve is identified and carefully preserved (see Fig. 87-19B). Next, the sternocleidomastoid is retracted laterally and the upper neck is dissected to expose the internal jugular vein, external and internal carotid arteries, and the last four (IX, X, XI, and XII) cranial nerves (see Fig. 87-19C). The posterior belly of the digastric and the stylohyoid muscle are identified, divided near their mastoid and styloid attachments, respectively, and retracted medially. This allows further superior exposure of the internal carotid artery, internal jugular vein, and adjacent nerves, as well as visualization of the stylomandibular ligament and the styloid process. The stylomandibular ligament is divided (see Fig. 87-19D), which allows further anterior retraction of the mandible and provides a wide opening into the parapharyngeal space (see Fig. 87-19E). The styloid process may be resected for further exposure and to assist delivery of larger tumors. Through this exposure, the tumor can be easily visualized and safely excised (see Fig. 87-19E and F).
In patients with tumors located in the superior aspect of the parapharyngeal space approaching the eustachian tube and skull base, the addition of a mandibulotomy may be necessary.37,83 The enhanced exposure offered by the mandibulotomy allows the visualization necessary for resection of the tumor with adequate margins. First, a cervical-parotid approach is performed as described in the previous section. If a mandibulotomy is indicated, a preliminary tracheostomy is performed. Next, the cervical portion of the incision is extended through the chin to split the lower lip in the midline (Fig. 87-20A). After dissection of the upper carotid sheath, the mandible is exposed carefully, identifying and preserving the mental nerve (see Fig. 87-20B). A stair-step or “Chevron” osteotomy is outlined either in the midline (median mandibulotomy) or just anterior to the mental foramen (paramedian mandibulotomy) (see Fig. 87-20C). These types of osteotomies are more mechanically stable than a straight-line osteotomy. In order to obtain normal dental occlusion, a metal plate is preregistered and fixed to the mandible before performing the planned osteotomy (see Fig. 87-20D). The osteotomy is performed using a micro-reciprocating saw in order to minimize bony loss at the osteotomy site. A paralingual incision is then made along the floor of the mouth. The mylohyoid muscle is divided while carefully preserving the hypoglossal and lingual nerves. The tongue is retracted medially, and the parapharyngeal and retropharyngeal spaces are opened widely (see Fig. 87-20E). The tumor can now be excised safely and adequately while protecting the critical neurovascular structures of the parapharyngeal space. Closure involves a water-tight intraoral suture line and rigid fixation of the mandibulotomy. A nasogastric tube is inserted for feeding. Within 7 to 10 days most patients will have their tracheostomy decannulated and be able to resume oral feeding.
Complications
Facial Nerve Paresis or Paralysis
Facial nerve dysfunction may result from traction injury to the facial nerve during dissection. Facial nerve monitoring has helped decrease the morbidity of a parotidectomy.84 As long as the anatomic integrity of the nerve is preserved, this type of injury usually results in neuropraxia and, therefore, complete recovery is anticipated. The degree of weakness or paralysis may range from minimal partial weakness of one or more branches of the facial nerve to complete paralysis of all branches of the nerve. Recovery of facial nerve function may be prompt and complete within days or may be delayed for several months. In a study of 256 consecutive patients who underwent parotid surgery at the Cleveland Clinic over a period of 15 years, immediate postoperative facial nerve dysfunction was frequently encountered (46%), but permanent dysfunction was uncommon (4%).85 The incidence of long-term dysfunction was higher in revision cases and when an extended (total or subtotal) parotidectomy was performed. In order to minimize facial nerve injury, the parotid surgeon should adhere to meticulous dissection and gentle handling of the facial nerve. Excessive traction on the nerve must be avoided, as should overzealous use of the nerve stimulator.
If the facial nerve is sacrificed, nerve repair may be done by using either direct neurorrhaphy of the cut edges or a cable graft, depending on the length of the resected segment (see Fig. 87-18). Immediate rehabilitation of the paralyzed face requires diligent eye care to prevent exposure keratitis. This involves liberal use of artificial tears, lubricating ointment, and protection with an appropriate eye dressing and eyewear. A gold weight implant should be used routinely in patients with complete facial paralysis. A temporary tarsorrhaphy may be necessary for patients with lower eyelid ectropion. If the facial nerve is not repaired or grafted, then one or more of the various surgical procedures for static or dynamic rehabilitation of the paralyzed face may be indicated.
Sensory Abnormalities
The greater auricular nerve (GAN) is frequently divided during the operation of parotidectomy. This usually results in sensory deficits in the dermatomal distribution of the GAN, which comprises the lower third of the pinna including the earlobe, as well as the adjacent preauricular and postauricular skin. Although many patients experienced sensory deficits, the overall quality of life was not significantly affected after GAN sacrifice during parotidectomy.80
Gustatory Sweating—”Frey’s Syndrome”
Patients with Frey’s syndrome experience flushing and sweating of the ipsilateral facial skin during mastication (gustatory sweating). The symptoms may vary in severity from barely noticeable to severe and bothersome. The syndrome is a sequela of parotidectomy and may follow other surgical, traumatic, and inflammatory injuries of the parotid and submandibular glands and the cervical and upper thoracic portions of the sympathetic trunk.86 The true incidence of postoperative Frey’s syndrome is unknown, but it is estimated to be between 35% and 60%. The incidence would probably be higher if the syndrome were searched for by directed, symptom-specific questions.87 The presumed pathophysiology of Frey’s syndrome involves aberrant cross-reinnervation between the postganglionic secretomotor parasympathetic fibers to the parotid gland and the postganglionic sympathetic fibers supplying the sweat glands of the skin.
The diagnosis of Frey’s syndrome depends largely on the patient’s symptoms. An objective method of confirming the diagnosis is the Minor’s starch/iodine test. This involves painting the ipsilateral side of the face and neck with iodine solution, which is allowed to dry. Starch powder is then dusted over the painted area. The patient then chews on a sialagogue (e.g., lemon wedge) for several minutes. The appearance of dark blue spots along the face confirms gustatory sweating. This discoloration is the result of the reaction of the dissolved starch with iodine. If the Minor’s starch iodine test is used for diagnosis, up to 96% of patients who did not complain of Frey’s syndrome after surgery actually had a subclinical manifestation of this complication.86 This is usually detected within the first year after surgery, but the onset may occasionally be delayed for many years.
If the symptoms are bothersome enough, treatment involves simply applying an antiperspirant over the involved skin. Glycopyrrolate (1%) roll-on lotion is also effective in controlling the symptoms. In cases not responding to these simple measures, surgical interruption of the secretory fibers may be attempted by performing a tympanic neurectomy. Recently, intracutaneous injection of botulinum toxin A has been described as an effective treatment in severe cases of Frey’s syndrome.87,88
Thick skin flap and partial superficial parotidectomy are the most important techniques to minimize the risk of developing symptomatic Frey’s syndrome.89 The incidence was not altered by the use of sternocleidomastoid muscle flap or other implantation material.90
Carlson GW. The salivary glands. Embryology, anatomy, and surgical applications. Surg Clin North Am. 2000;80:261-273.
Cheuk W, Chan JKC. Advances in salivary gland pathology. Histopathology. 2007;51:1-20.
Dardick I, Burford-Mason AP. Current status of histogenetic and morphogenetic concepts of salivary gland tumorigenesis. Crit Rev Oral Biol Med. 1993;4:639-677.
De Bree R, Van der Waal I, Leemans R. Management of Frey syndrome. Head Neck. 2007;29:773-778.
El-Naggar AK, Klijanienko J. Advances in clinical investigations of salivary gland tumorigenesis. Ann Pathol. 1999;19:19-22.
Maruya S, Kim HW, Weber RS, et al. Gene expression screening of salivary gland neoplasms. J Mol Diagn. 2004;6:180-190.
Mehle ME, Kraus DH, Wood BG, et al. Facial nerve morbidity following parotid surgery for benign disease: the Cleveland Clinic Foundation experience. Laryngoscope. 1993;103:386-388.
O’Brien CJ. Current management of benign parotid tumors—the role of limited superficial parotidectomy. Head Neck. 2003;25:946-952.
Patel N, Har-El G, Rosenfeld R. Quality of life after great auricular nerve sacrifice during parotidectomy. Arch Otolaryngol Head Neck Surg. 2001;127:884-888.
Scianna JM, Petruzzelli GJ. Contemporary management of tumors of the salivary glands. Curr Opin Rep. 2007;9:134-138.
Spiro RH. Salivary neoplasms: overview of a 35-year experience with 2,807 patients. Head Neck Surg. 1986;8:177-184.
Thoeny HC. Imaging of salivary gland tumours. Cancer Imaging. 2007;7:52-62.
Uchida Y, Minoshima S, Kawata T, et al. Diagnostic value of FDG PET and salivary gland scintigraphy for parotid tumors. Clin Nucl Med. 2005;30:170-176.
Witt RL. The significance of the margin in parotid surgery for pleomorphic adenoma. Laryngoscope. 2002;112:2141-2154.
Zheng W, Shu XO, Ji BT, et al. Diet and other risk factors for cancer of the salivary glands: a population-based case-control study. Int J Cancer. 1996;67:194-198.
1. Cheuk W, Chan JKC. Advances in salivary gland pathology. Histopathology. 2007;51:1-20.
2. Carlson GW. The salivary glands: embryology, anatomy, and surgical applications. Surg Clin North Am. 2000;80:261-273.
3. Levin RJ, Bradley MK. Neuroectodermal antigens persist in benign and malignant salivary gland tumor cultures. Arch Otolaryngol Head Neck Surg. 1996;122:551-557.
4. Dardick I. Mounting evidence against current histogenetic concepts for salivary gland tumorigenesis. Eur J Morphol. 1998;36:257-261.
5. Dardick I, Byard RW, Carnegie JA. A review of the proliferative capacity of major salivary glands and the relationship to current concepts of neoplasia in salivary glands. Oral Surg Oral Med Oral Pathol. 1990;69:53-67.
6. Dardick I, Burford-Mason AP. Current status of histogenetic and morphogenetic concepts of salivary gland tumorigenesis. Crit Rev Oral Biol Med. 1993;4:639-677.
7. Batsakis JG, Luna MA, el-Naggar AK. Basaloid monomorphic adenomas. Ann Otol Rhinol Laryngol. 1991;100:687-690.
8. Batsakis JG, Regezi JA, Luna MA, et al. Histogenesis of salivary gland neoplasms: a postulate with prognostic implications. J Laryngol Otol. 1989;103:939-944.
9. El-Naggar AK, Callender D, Coombes MM, et al. Molecular genetic alterations in carcinoma ex-pleomorphic adenoma: a putative progression model? Genes Chromosomes Cancer. 2000;27:162-168.
10. Willams MD, Chakravarti N, Kies MS, et al. Implications of methylation patterns of cancer genes in salivary gland tumors. Clin Cancer Res. 2006;12:7353-7358.
11. Maruya S, Kim HW, Weber RS, et al. Gene expression screening of salivary gland neoplasms. J Mol Diagn. 2004;6:180-190.
12. Land CE, Saku T, Hayashi Y, et al. Incidence of salivary gland tumors among atomic bomb survivors, 1950-1987: evaluation of radiation-related risk. Radiat Res. 1996;146:28-36.
13. Spitz MR, Batsakis JG. Major salivary gland carcinoma: descriptive epidemiology and survival of 498 patients. Arch Otolaryngol. 1984;110:45-49.
14. Modan B, Chetrit A, Alfandary E, et al. Increased risk of salivary gland tumors after low-dose irradiation. Laryngoscope. 1998;108:1095-1097.
15. Tsai CC, Chen CL, Hsu HC. Expression of Epstein-Barr virus in carcinomas of major salivary glands: a strong association with lymphoepithelioma-like carcinoma. Hum Pathol. 1996;27:258-262.
16. Pollock AM, Toner M, McMenamin M, et al. Absence of Epstein-Barr virus encoded RNA and latent membrane protein (LMP1) in salivary gland neoplasms. J Laryngol Otol. 1999;113:906-908.
17. Atula T, Grenman R, Klemi P, et al. Human papillomavirus, Epstein-Barr virus, human herpesvirus 8 and human cytomegalovirus involvement in salivary gland tumours. Oral Oncol. 1998;34:391-395.
18. Pinkston JA, Cole P. Cigarette smoking and Warthin’s tumor. Am J Epidemiol. 1996;144:183-187.
19. Zheng W, Shu XO, Ji BT, et al. Diet and other risk factors for cancer of the salivary glands: a population-based case-control study. Int J Cancer. 1996;67:194-198.
20. Straif K, Weiland SK, Bungers M, et al. Exposure to nitrosamines and mortality from salivary gland cancer among rubber workers. Epidemiology. 1999;10:786-787.
21. Actis AB, Eynard AR. Influence of environmental and nutritional factors on salivary gland tumorigenesis with a special reference to dietary lipids. Eur J Clin Nutr. 2000;54:805-810.
22. Horn-Ross PL, Morrow M, Ljung BM. Menstrual and reproductive factors for salivary gland cancer risk in women. Epidemiology. 1999;10:528-530.
23. Toida M, Balazs M, Mori T, et al. Analysis of genetic alterations in salivary gland tumors by comparative genomic hybridization. Cancer Genet Cytogenet. 2001;127:34-37.
24. Morio T, Morimitsu Y, Hisaoka M, et al. DNA copy number changes in carcinoma in pleomorphic adenoma of the salivary gland: a comparative genomic hybridization study. Pathol Int. 2002;52:501-507.
25. Spiro RH. Salivary neoplasms: overview of a 35-year experience with 2,807 patients. Head Neck Surg. 1986;8:177-184.
26. Michael CW, Hunter B. Interpretation of fine-needle aspirates processed by the ThinPrep technique: cytologic artifacts and diagnostic pitfalls. Diagn Cytopathol. 2000;23:6-13.
27. Stewart CJ, MacKenzie K, McGarry GW, et al. Fine-needle aspiration cytology of salivary gland: a review of 341 cases. Diagn Cytopathol. 2000;22:139-146.
28. Al-Khafaji BM, Afify AM. Salivary gland fine needle aspiration using the ThinPrep technique: diagnostic accuracy, cytologic artifacts and pitfalls. Acta Cytol. 2001;45:567-574.
29. Oka K, Chikamatsu K, Eura M, et al. Clinical significance of fine-needle aspiration biopsy in major salivary gland tumors. Nippon Jibiinkoka Gakkai Kaiho [J Oto-Rhino-Laryngol Soc Japan]. 2002;105:1109-1115.
30. Verma K, Kapila K. Role of fine needle aspiration cytology in diagnosis of pleomorphic adenomas. Cytopathology. 2002;13:121-127.
31. MacLeod CB, Frable WJ. Fine-needle aspiration biopsy of the salivary gland: problem cases. Diagn Cytopathol. 1993;9:216-224.
32. Gritzmann N, Hollerweger A, Macheiner P, et al. Sonography of soft tissue masses of the neck. J Clin Ultrasound. 2002;30:356-373.
33. Ivanova S, Slobodnikova J, Janska E, et al. Fine needle aspiration biopsy in a diagnostic workup algorithm of salivary gland tumors. Neoplasma. 2003;50:144-147.
34. Stanley MW. Selected problems in fine needle aspiration of head and neck masses. Mod Pathol. 2002;15:342-350.
35. Heller KS, Dubner S, Chess Q, et al. Value of fine needle aspiration biopsy of salivary gland masses in clinical decision-making. Am J Surg. 1992;164:667-670.
36. Mukunyadzi P, Bardales RH, Palmer HE, et al. Tissue effects of salivary gland fine-needle aspiration: does this procedure preclude accurate histologic diagnosis? Am J Clin Pathol. 2000;114:741-745.
37. Hanna EY, Suen JY. Malignant tumors of the salivary glands. In: Myers EN, Suen JY, Myers JN, et al, editors. Cancer of the Head and Neck. Philadelphia: Saunders; 2003:475-510.
38. Rabinov JD. Imaging of salivary gland pathology. Radiol Clin North Am. 2000;38:1047-1057.
39. Kurabayashi T, Ida M, Tetsumura A, et al. MR imaging of benign and malignant lesions in the buccal space. Dentomaxillofacial Radiol. 2002;31:344-349.
40. Yabuuchi H, Fukuya T, Tajima T, et al. Salivary gland tumors: diagnostic value of gadolinium-enhanced dynamic MR imaging with histopathologic correlation. [erratum appears in Radiology. 2003 Jun;227(3):909]. Radiology. 2003;226:345-354.
41. Shah GV. MR imaging of salivary glands. Magn Reson Imaging Clin N Am. 2002;10:631-662.
42. Takashima S, Wang J, Takayama F, et al. Parotid masses: prediction of malignancy using magnetization transfer and MR imaging findings. AJR Am J Roentgenol. 2001;176:1577-1584.
43. Kim KH, Sung MW, Yun JB, et al. The significance of CT scan or MRI in the evaluation of salivary gland tumors. Auris Nasus Larynx. 1998;25:397-402.
44. Thoeny HC. Imaging of salivary gland tumours. Cancer Imaging. 2007;7:52-62.
45. Feld R, Nazarian LN, Needleman L, et al. Clinical impact of sonographically guided biopsy of salivary gland masses and surrounding lymph nodes. Ear Nose Throat J. 1999;78:905.
46. Martinoli C, Derchi LE, Solbiati L, et al. Color Doppler sonography of salivary glands. AJR. Am J Roentgenol. 1994;163:933-941.
47. Keyes JWJr, Harkness BA, Greven KM, et al. Salivary gland tumors: pretherapy evaluation with PET. Radiology. 1994;192:99-102.
48. Uchida Y, Minoshima S, Kawata T, et al. Diagnostic value of FDG PET and salivary gland scintigraphy for parotid tumors. Clin Nucl Med. 2005;30:170-176.
49. Jansisyanont P, Blanchaert RHJr, Ord RA. Intraoral minor salivary gland neoplasm: a single institution experience of 80 cases. Int J Oral Maxillofac Surg. 2002;31:257-261.
50. Becelli R, Carboni A, Cassoni A, et al. Pleomorphic adenoma of the lachrymal gland: presentation of a clinical case of relapse. J Craniofac Surg. 2002;13:49-52.
51. Cates CA, Manners RM, Rose GE. Pleomorphic adenoma of the lacrimal gland in a 10 year old girl. Br J Ophthalmol. 2002;86:249-250.
52. Chandrasekhar J, Farr DR, Whear NM. Pleomorphic adenoma of the lacrimal gland: case report. Br J Oral Maxillofac Surg. 2001;39:390-393.
53. Tanimoto H, Kumoi K, Otsuki N, et al. Multiple primary pleomorphic adenomas in a single parotid gland: report of a new case. Ear Nose Throat J. 2002;81:341-345.
54. Webb AJ, Eveson JW. Pleomorphic adenomas of the major salivary glands: a study of the capsular form in relation to surgical management. Clin Otolaryngol Allied Sci. 2001;26:134-142.
55. Korkmaz H, Adsay NV, Jacobs JR, et al. Lipomatous pleomorphic adenoma of parotid gland. Histopathology. 2002;40:487-488.
56. Shigeishi H, Hayashi K, Takata T, et al. Pleomorphic adenoma of the parotid gland with extensive bone formation. Pathol Int. 2001;51:883-886.
57. Stennert E, Guntinas-Lichius O, Klussmann JP, et al. Histopathology of pleomorphic adenoma in the parotid gland: a prospective unselected series of 100 cases. Laryngoscope. 2001;111:2195-2200.
58. Witt RL. The significance of the margin in parotid surgery for pleomorphic adenoma. Laryngoscope. 2002;112:2141-2154.
59. Koral K, Sayre J, Bhuta S, et al. Recurrent pleomorphic adenoma of the parotid gland in pediatric and adult patients: value of multiple lesions as a diagnostic indicator. AJR Am J Roentgenol. 2003;180:1171-1174.
60. Carew JF, Spiro RH, Singh B, et al. Treatment of recurrent pleomorphic adenomas of the parotid gland. Otolaryngol Head Neck Surg. 1999;121:539-542.
61. Glas AS, Hollema H, Nap RE, et al. Expression of estrogen receptor, progesterone receptor, and insulin-like growth factor receptor-1 and of MIB-1 in patients with recurrent pleomorphic adenoma of the parotid gland. Cancer. 2002;94:2211-2216.
62. Douglas JG, Einck J, Austin-Seymour M, et al. Neutron radiotherapy for recurrent pleomorphic adenomas of major salivary glands. Head Neck. 2001;23:1037-1042.
63. Seifert G. Histopathology of malignant salivary gland tumours. Eur J Cancer Part B Oral Oncol. 1992;28B:49-56.
64. Chen I, Tu H. Pleomorphic adenoma of the parotid gland metastasizing to the cervical lymph node. Otolaryngol Head Neck Surg. 2000;122:455-457.
65. Hay MA, Witterick IJ, Mock D. Recurrent pleomorphic adenoma of the parotid gland with cervical metastasis. J Otolaryngol. 2001;30:361-365.
66. Raja V, China C, Masaki KH, et al. Unusual presentations of uncommon tumors: case 1. Benign metastasizing pleomorphic adenoma. J Clin Oncol. 2002;20:2400-2403.
67. Batsakis JG, Regezi JA. The pathology of head and neck tumors: salivary glands, part 1. Head Neck Surg. 1978;1:59-68.
68. Dardick I, Lytwyn A, Bourne AJ, et al. Trabecular and solid-cribriform types of basal cell adenoma: a morphologic study of two cases of an unusual variant of monomorphic adenoma. Oral Surg Oral Med Oral Pathol. 1992;73:75-83.
69. Liess BD, Lane RV, Fraizer S, et al. Bilateral canalicular adenoma of the parotid gland. Arch Otolarnygol. Head Neck Surg. 2006;132:339-341.
70. Verma K, Kapila K. Salivary gland tumors with a prominent oncocytic component: cytologic findings and differential diagnosis of oncocytomas and Warthin’s tumor on fine needle aspirates. Acta Cytol. 2003;47:221-226.
71. Ito K, Tsukuda M, Kawabe R, et al. Benign and malignant oncocytoma of the salivary glands with an immunohistochemical evaluation of Ki-67. ORL J Otorhinolaryngol Rel Spec. 2000;62:338-341.
72. Ramdall RB, Cai G, Levine PH, et al. Fine-needle aspiration biopsy findings in epithelioid myoepithelioid myoepithelioma of the parotid gland: a case report. Diagn Cytopathol. 2006;34:776-779.
73. Walter JW, North PE, Waner M, et al. Somatic mutation of vascular endothelial growth factor receptors in juvenile hemangioma. Genes Chromosomes Cancer. 2002;33:295-303.
74. Waner M, North PE, Scherer KA, et al. The nonrandom distribution of facial hemangiomas. Arch Dermatol. 2003;139:869-875.
75. Childers EL, Furlong MA, Fanburg-Smith JC. Hemangioma of the salivary gland: a study of ten cases of a rarely biopsied/excised lesion. Ann Diagn Pathol. 2002;6:339-344.
76. Raveh E, Waner M, Kornreich L, et al. The current approach to hemangiomas and vascular malformations of the head and neck. Harefuah. 2002;141:783-788.
77. Waner M, Suen JY. Management of congenital vascular lesions of the head and neck. Oncology (Huntington). 1995;9:989-994.
78. O’Brien CJ. Current management of benign parotid tumors—the role of limited superficial parotidectomy. Head Neck. 2003;25:946-952.
79. Malone JP, Agrawal A, Schuller DE. Safety and efficacy of transcervical resection of parapharyngeal space neoplasms. Ann Otol Rhinol Laryngol. 2001;110:1093-1098.
80. Patel N, Har-El G, Rosenfeld R. Quality of life after great auricular nerve sacrifice during parotidectomy. Arch Otolaryngol Head Neck Surg. 2001;127:884-888.
81. Porter MJ, Wood SJ. Preservation of the great auricular nerve during parotidectomy. Clin Otolaryngol. 1997;22:251-253.
82. Yu GY. Superficial parotidectomy through retrograde facial nerve dissection. J Royal Coll Surg Edinburgh. 2001;46:104-107.
83. Olsen KD, Lewis JE. Carcinoma ex pleomorphic adenoma: a clinicopathologic review. Head Neck. 2001;23:705-712.
84. Scianna JM, Petruzzelli GJ. Contemoporary management of tumors of the salivary glands. Curr Opin Rep. 2007;9:134-138.
85. Mehle ME, Kraus DH, Wood BG, et al. Facial nerve morbidity following parotid surgery for benign disease: the Cleveland Clinic Foundation experience. Laryngoscope. 1993;103:386-388.
86. Malatskey S, Rabinovich I, Fradis M, et al. Frey syndrome—delayed clinical onset: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:338-340.
87. Kuttner C, Berens A, Troger M, et al. [Frey syndrome after lateral parotidectomy. Follow-up and therapeutic outlook]. Mund Kiefer Gesichtschir. 2001;5:144-149.
88. von Lindern JJ, Niederhagen B, Berge S, et al. Treatment of Frey’s syndrome with type A botulinum toxin: case report. J Oral Maxillofac Surg. 2000;58:1411-1414.
89. De Bree R, Van der Waal I, Leemans R. Management of Frey syndrome. Head Neck. 2007;29:773-778.
90. Guntinas-Lichius O, Gabriel B, Klussmann JP. Risk of facial palsy and severe Frey’s syndrome after conservative parotidectomy for benign disease: analysis of 610 operations. Acta Otolaryngol. 2006;126:1104-1109.