Benign liver lesions
Classification
Although a variety of benign liver tumours have been described, many are rare and a detailed description of these various lesions is beyond the scope of the current text (Box 4.1). The majority of benign hepatic lesions encountered in clinical practice include haemangioma, liver cell adenoma, focal nodular hyperplasia, bile duct hamartoma and hepatic cysts. For completeness, a brief résumé is provided of less common and miscellaneous lesions that may give rise to diagnostic and management dilemmas.
Haemangiomas
Haemangiomas are the most common benign hepatic tumours of mesenchymal origin. Small capillary haemangiomas are more common than the larger cavernous haemangiomas and are often multiple. Small lesions are asymptomatic and an incidental finding; however, they may give rise to diagnostic difficulty in patients undergoing investigation. Once accurate diagnosis has been made no further therapy is needed. Haemangiomas are probably of congenital origin and do not undergo malignant transformation. The incidence of cavernous haemangioma in autopsy series varies considerably but has been reported to be as high as 8%. These lesions are the second most common hepatic tumour in the USA, exceeded only by hepatic metastases.1 With the more widespread use of sensitive imaging studies of the upper abdomen, the identification of such lesions as an incidental finding will undoubtedly be more common. Cavernous haemangiomas may reach an enormous size, and lesions weighing up to 6 kg are well documented. There is poor agreement in the literature as to the exact definition of what constitutes a giant haemangioma. Some are defined as greater than 4 cm in diameter, others greater than 6 cm. Such haemangiomas are usually solitary, but multiple lesions have been described in about 10% of cases.1 They may be associated with similar lesions in the skin and other organs. Lesions are usually evenly distributed throughout the liver and its substance but large lesions situated peripherally may form a pedicle.
Pathology
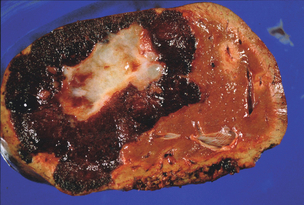
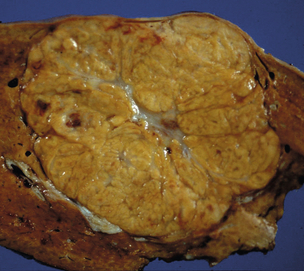
Cavernous haemangiomas are seen most frequently in patients in the third to fifth decades of life. They are more common and more likely to become clinically manifest at a younger age in women, are more common with increasing parity and may enlarge during pregnancy.2–4 This indicates a possible role of female sex hormones in their development, although an association with the oral contraceptive pill has not been proven. The aetiology of liver haemangiomas is still unclear but they may represent benign congenital hamartomas. These lesions appear to grow by progressive ectasia rather than hyperplasia or hypertrophy. At operation, they appear as well-circumscribed, reddish-purple, hypervascular lesions, which may be multilobulated or have a smooth surface. When sectioned, the lesion will partially collapse due to the escape of blood, and it has a honeycombed cut surface. There may be gross evidence of thrombosis, fibrosis or calcification (Fig. 4.1). Microscopically, haemangiomas are composed of cystically dilated vascular spaces, lined by endothelial cells and separated by fibrous septa of varying thickness. There is usually a clear plane between haemangioma and normal liver tissue as these lesions are usually encapsulated by a rim of fibrous tissue.
Clinical presentation
Most haemangiomas are asymptomatic but subcapsular lesions and larger lesions causing compression of adjacent organs may produce clinical features. Symptoms may include vague abdominal pain or fullness, early satiety, nausea, vomiting or fever. Rare presentations include obstructive jaundice, gastric outlet obstruction and spontaneous rupture. Although abdominal pain or discomfort is the most frequent indication for removing a liver haemangioma, it must be remembered that associated pathology may coexist and be the cause of the symptoms. Farges et al.5 reported that 42% of the patients in their series had other pathology, such as gallbladder disease, liver cysts, gastroduodenal ulcers or hiatus hernia. The difficulty of attributing symptoms to the haemangioma is evidenced by the occasional persistence of symptoms after resection.6
Pain related to an uncomplicated haemangioma is likely due to stretching or inflammation of Glisson’s capsule. Occasionally, large lesions located in the left lobe of the liver may cause pressure effects on adjacent structures and infarction or necrosis may account for the sudden onset of pain. Intra-abdominal haemorrhage due to spontaneous or traumatic rupture of haemangioma is a very rare complication.4 A past review of the literature included only 28 reports of spontaneous, life-threatening haemorrhage due to liver haemangiomas, a minimal figure considering the prevalence of the tumour.7 Thrombocytopenia and hypofibrinogenaemia have also been associated with cavernous haemangiomas of the liver (Kasabach–Merritt syndrome), and this effect may be related to consumption of coagulation factors.1
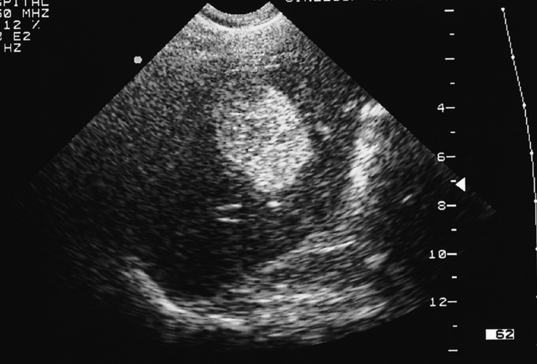
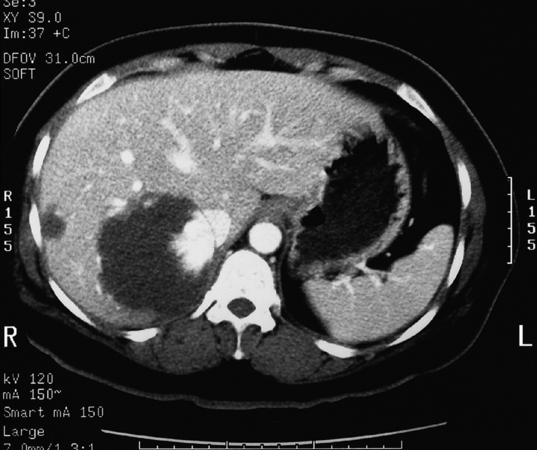
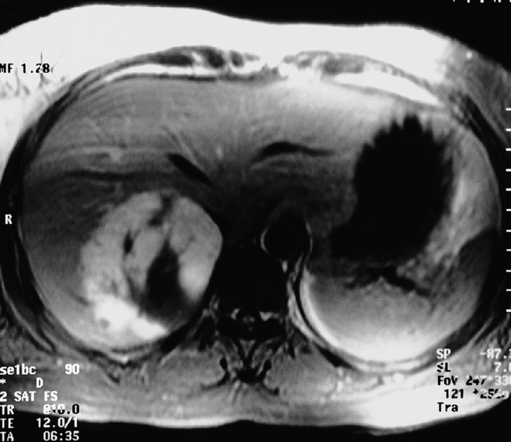
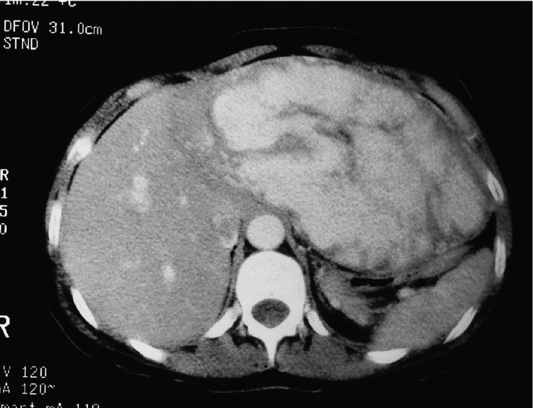
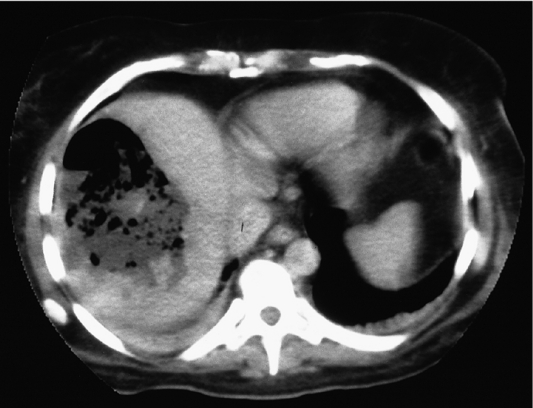
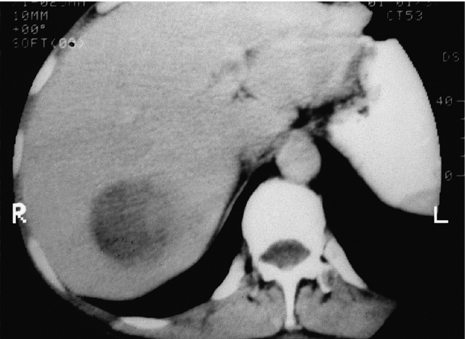
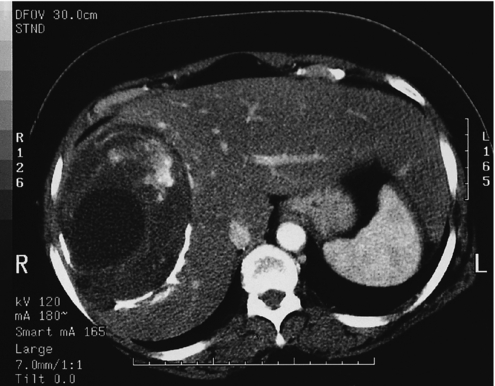
Such lesions are generally hyperechoic on ultrasound examination (Fig. 4.2). Farges et al.5 found the diagnosis to be established by US alone in 80% of patients with haemangiomas smaller than 6 cm. However, this investigation alone cannot differentiate a haemangioma from hepatocellular carcinoma, liver cell adenoma, focal nodular hyperplasia or a solitary metastasis. CT has proven useful in the diagnosis of haemangiomas.8 Prior to intravenous contrast infusion, CT shows the haemangioma to consist of a well-demarcated hypodense mass. After the intravenous injection of contrast medium, serial scans will reveal a zone of progressive enhancement peripherally that varies in thickness and often demonstrates an irregular margin (Fig. 4.3). The centre of the haemangioma remains hypodense and the overall lesion size does not change. Over the past decade MRI has emerged as a highly accurate technique for diagnosing and characterising liver haemangioma, with a reported 90% sensitivity, 95% specificity and 93% accuracy9,10 (Fig. 4.4). Haemangiomas are typically very bright (light bulb sign) on T2-weighted images and show peripheral nodular enhancement on dynamic gadolinium-enhanced T1-weighted images.11 Single-photon emission CT (SPECT) using technetium-99m-labelled red blood cells has been shown to increase the spatial resolution of planar scintigraphy and has been shown to have a sensitivity and accuracy close to that of MRI.12 Fluorodeoxyglucose (FDG)-PET has been reported as useful to differentiate giant hepatic cavernous haemangiomas from malignant hepatic tumours.13 In practice, a combination of these diagnostic modalities is preferred. Superficial lesions may be identified incidentally during abdominal laparoscopic procedures and should be recognised by their gross appearance and characteristic compressibility when gently palpated with a laparoscopic instrument. Needle biopsy of vascular liver lesions should not be performed. Diagnostic uncertainty is seldom a problem with cavernous haemangiomas, except in lesions not large enough to show cavernous characteristics.
Management
A wide range of management strategies from observation to resection has been advocated for such lesions. Simple reassurance should be given to patients in whom small lesions (i.e. < 6 cm) have been detected as an incidental finding. For larger cavernous haemangiomas, consideration should be given to weighing the risk of operation against the natural history of untreated lesions. Trastek et al.4 followed up 34 untreated patients over a maximum period of 15 years. No patient had a lesion that bled, none reported abdominal symptoms and no patient had compromise of quality of life. A further report from the same group, when the observation period had been extended to 21 years, reported two patients with large symptomatic lesions of questionable resectability at initial presentation who remained symptomatic but with little documented growth of the haemangioma. The remainder were asymptomatic and there was no instance of rupture.14 Two more recent longitudinal studies have supported the accepted view that asymptomatic giant haemangiomas of the liver can be managed safely by observation.15,16
Nichols et al.14 reported no operative deaths and the single postoperative complication was a wound infection in 41 patients undergoing resection of such lesions. In a similar series of 69 patients, Weimann et al.17 reported no postoperative deaths and a morbidity rate of 19%. Also in this series were 104 patients with haemangioma and 53 patients with focal nodular hyperplasia who were observed for a median of 32 months (range 7–132 months). There was no evidence of malignant transformation or tumour rupture. Therefore, safe resection is possible but there is no evidence that asymptomatic patients should undergo resection since the risk of rupture is minimal.5,18
The choice of excision requires consideration of the size and anatomical location of the lesion. Haemangiomas can often be enucleated19 to avoid loss of functional liver parenchyma, diminish blood loss and minimise postoperative bile leakage, although in some cases it may be wiser and safer to perform a formal anatomical liver resection. At enucleation, a plane between the lesion and the liver is easily found and this can be developed by blunt dissection. This can be facilitated by the use of the Cavitron ultrasonic surgical aspiration system (CUSA™) with concomitant control of the inflow vessels. Laparoscopic resection of liver haemangioma is increasingly reported,20 and orthotopic liver transplantation has been used successfully to treat symptomatic patients with technically unresectable complicated giant haemangioma.21
Liver cell adenoma
Although liver cell adenoma requires differentiation from any solid hepatic lesion, it is often considered alongside focal nodular hyperplasia.22
Hepatic adenomas arise in otherwise normal liver and present as a focal abnormality or mass. The true prevalence of the disease is difficult to assess but 90% develop in women in the third to fifth decades of life.23 These tumours were rarely reported before 1960, but their apparent increase in incidence since then corresponded with the introduction of oral contraceptives at that time. The causal relationship between liver cell adenoma and oral contraceptives was first suggested by Baum et al.24 in 1973. Ninety per cent of patients with liver cell adenomas have used oral contraceptives and the annual incidence among oral contraceptive users has been reported to be 3–4 per 100 000 if the contraceptives are taken for more than 2 years. The risk of developing a liver cell adenoma increases with the dose and duration of use of the contraceptive preparation.23 Furthermore, pregnancy has been associated with increased symptoms and an increased risk of complications in patients with liver cell adenomas.23,25 The introduction of low-oestrogen-containing contraceptive preparations may result in a reduction in incidence, although adenomas are also associated with non-contraceptive oestrogen use, androgenic steroid use, diabetes, glycogen storage disease, galactosaemia and iron overload. This association implicates altered carbohydrate metabolism in the formation of liver cell adenomas.26
Pathology
Liver cell adenomas are usually solitary, round and occasionally encapsulated. Lesions are soft and smooth surfaced, but occasionally may be pedunculated. The cut surface has a pale yellow fleshy appearance unless haemorrhage and necrosis produce discoloration (Fig. 4.5). They are sharply demarcated from normal liver but without a fibrous capsule. Approximately 12–30% of these tumours are multiple, and if more than 10 adenomas are present, the condition is regarded as liver adenomatosis.27 This may be a separate pathological entity from isolated liver cell adenoma as both sexes are equally affected and oral contraceptive usage is unusual. Microscopically, there are uniform masses of benign-appearing hepatocytes without ducts or portal triads. The hepatocytes appear paler than normal because of increased glycogen or fat content. Venous lakes (peliosis hepatis) are often seen.
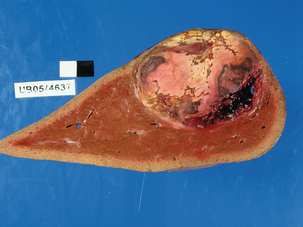
Figure 4.5 Large liver cell adenoma showing the pale yellow fleshy appearance of its cut surface. There are areas of discoloration from haemorrhage.
Historically, liver cell adenomas have been considered precancerous. Rooks et al.23 reported the finding of hepatocellular carcinoma 5 years after resection of a liver cell adenoma, and other authors have recognised unequivocal areas of hepatocellular carcinoma adjacent to or within liver cell adenomas.23,28,29 Also reported is the development of hepatocellular carcinoma several years after diagnosis of biopsy-proven benign liver cell adenoma.26,30,31 More recent studies have shown that there are different subtypes of liver cell adenoma and that the risk of malignant transformation varies. Liver cell adenoma occurring in men and large tumours are at highest risk, but telangiectatic or unclassified liver cell adenomas have an increased risk whereas steatotic liver cell adenomas have a lower risk.32 Recent innovations in molecular biology and immunohistochemistry have identified β-catenine mutation as a significant risk factor for malignant transformation.33,34
Clinical presentation
These lesions present frequently with abdominal pain from haemorrhage into the tumour or adjacent liver. Some patients develop severe acute abdominal pain due to intraperitoneal rupture and haemoperitoneum, which may present as hypovolaemic shock. The risk of bleeding is reported as 21–50% and is not related to tumour size.32 Up to one-third of patients sense the presence of an abdominal mass. The remainder of adenomas are discovered incidentally at autopsy, laparotomy or during radiological assessment for another problem.
Although the clinical presentation may be suggestive of liver cell adenoma, definitive preoperative diagnosis may be difficult. Liver function tests are generally normal unless tumour necrosis or haemorrhage is present. Anaemia may therefore occur. US can detect small adenomas, which characteristically display a lesion of mixed echogeneity and heterogeneous texture. CT may show evidence of recent haemorrhage or necrosis. Lesions are generally hypodense prior to infusion of contrast medium and demonstrate a wide range of densities after intravenous contrast administration. They often appear as well-demarcated, fat-containing or haemorrhagic lesions on MRI. Conventional radiological imaging may not be able to differentiate between liver cell adenoma and hepatocellular carcinoma; however, promising results have been reported with the use of FDG-PET to differentiate benign from malignant lesions.35
Management
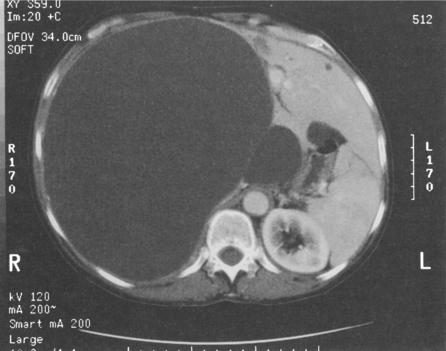
In the symptomatic patient, surgical intervention will be required. A minority of patients will present with intraperitoneal bleeding, the cause of which might only be identified at laparotomy. Most deaths from liver cell adenomas are secondary to haemorrhage, with intraperitoneal bleeding carrying a 20% mortality rate in one series.22 Hepatic arterial embolisation36 or packing might be considered to facilitate transfer of the patient to a specialist centre. Definitive control of bleeding is best achieved by formal hepatic resection. In some patients, haemorrhage may be contained within the liver or subcapsularly. If the patient remains haemodynamically stable, it may be prudent to defer elective surgical intervention to enable resolution of the haematoma, thereby enabling a more limited hepatic resection (Fig. 4.6). Orthotopic liver transplantation has been described for unresectable benign liver tumours with severe symptoms and for patients with multiple adenomas.17,37
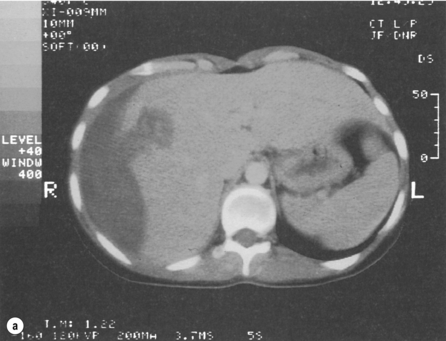
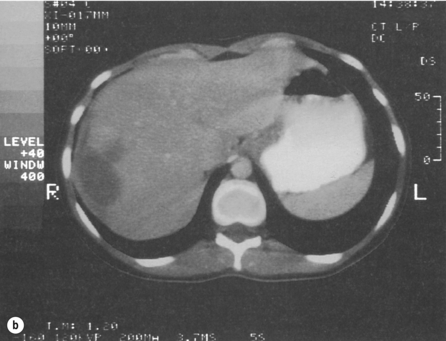
Figure 4.6 (a) CT scan showing extensive subcapsular haematoma resulting from spontaneous haemorrhage into the liver. (b) CT scan taken 2 months later showing a reduction in the size of the haematoma. Contrast is now present within a small adenoma lying adjacent to the haematoma.
For the asymptomatic patient, surgical intervention should be considered; however, with new insights and understanding of the clinical–pathological and radiological features and recent innovations in molecular biology and immunohistochemistry, selected patients can now be managed conservatively.32,38 Steatotic liver cell adenomas have a minimal risk of bleeding or malignant transformation. Furthermore, several case reports document regression of liver cell tumours following cessation of oral contraceptives,39,40 although this is not a consistent finding, and development of hepatocellular carcinoma in the site of adenoma regression has been reported.32 Non-operative discrimination between liver cell adenoma and hepatocellular carcinoma remains challenging.
Focal nodular hyperplasia
Pathology
FNH consists of a firm lobulated localised lesion in an otherwise normal liver. These nodules are generally several centimetres in size and occasionally can grow much larger. Lesions are well circumscribed but have no capsule. On sectioning, there is generally a central scar with fibrous radiations which account for the nodular and sometimes umbilicated appearance. Lesions are usually similar or slightly lighter in colour than adjacent normal hepatic parenchyma (Fig. 4.7). FNH is multifocal in up to 20% of cases and may coexist with haemangiomas in 5–10% of patients.1
Clinical features
CT and MRI are important imaging modalities to characterise FNH. Classical CT appearance of FNH is of hyperattenuation during the arterial phase that becomes isoattenuating during the portal and delayed phase (Fig. 4.8). In approximately 40–60% of patients, the central scar will inially be hypoattenuating but becomes hyperintense in the delayed phase due to delayed washout of contrast. Typical MRI features of FNH are iso- or hypointensity on T1-weighted images, slight hyper- or isointensity on T2-weighted images and the presence of a central scar that appears hyperintense on T2-weighted imaging. After administration of gadolinium chelates, the appearance is similar to that seen on contrast-enhanced CT, i.e. dramatic enhancement in the arterial phase followed by isointensity during the portal venous phase with a high-intensity signal in the scar during the delayed phase. Cherqui et al.41 reported a 70% sensitivity and 98% specificity for MRI in detecting FNH.
Management
Treatment of a patient with FNH depends essentially on the certainty of the diagnosis. In asymptomatic patients with the typical features of FNH unequivocally demonstrated by one or more radiological investigation, no further treatment is required. However, a malignant tumour will be found in up to 6% of patients with an undetermined, presumed benign lesion.42 FNH on occasions may be difficult to differentiate from liver cell adenoma. If this is the case, it is advisable to proceed to biopsy of these lesions before committing to hepatic resection.
Data on the natural history of FNH have been gathered by Kerlin et al.43 Of 41 patients studied, 11 had lesions found incidentally at autopsy. Sixteen patients had open surgical biopsies of clinically apparent lesions, with the majority of the lesions left in situ. These patients were observed for up to 15 years, during which time none of the lesions bled or increased in size. The vast majority of patients with FNH can be managed conservatively, with surgical excision (enucleation or resection) only rarely considered for large symptomatic or complicated lesions.
Bile duct adenoma (bile duct hamartoma)
Surgeons should be aware of bile duct adenomas since they are common and may be mistaken at operation as liver metastases. They do not manifest clinically but are incidental findings at laparotomy or autopsy.44 They rarely exceed 1 cm in diameter and appear as raised greyish-white areas on the liver capsule. Histologically, they are composed of a mass of mature bile ducts surrounded by fibrous stroma, which blends indistinctly into the adjacent liver. They require to be distinguished from the nests of hyperplastic bile ducts that occur in focal nodular hyperplasia and also in undifferentiated adenocarcinoma of the biliary tract type.
Hepatic pseudotumours
Hepatic pseudotumours may be considerable in size and can occur in any age group. These lesions are essentially overgrowths of chronic inflammatory tissue but may be mistaken for other neoplastic lesions of the liver.45 The aetiology is not known but they may be secondary to thrombosis and infarction of a major vessel, represent a form of immune reaction, or result from resolution of an abscess. They may be either hyperechoic or hypoechoic on US and appear as a hypodense lesion on CT. Such pseudotumours may require resection to prevent reactivation of infection. The clinical history and presentation are likely to point towards a diagnosis of pseudotumour.
Miscellaneous benign tumours
Mesenchymal hamartomas are exceptional and probably of congenital origin. They are most commonly described in infants under 12 months; however, a few have been documented in adults.46 Although they are entirely benign, hamartomas can compromise the liver and the individual by progressive enlargement, and therefore these lesions should be resected. Microscopically, the tumour is characterised by a myxoid background of highly cellular embryonal mesenchyme, throughout which are found random groups of hepatic cells, bile ducts and multiple cysts, which may produce a honeycomb appearance. Recurrence following excision has not been reported.
Primary myxoma in the adult is exceptional. Primary lipomas are rarely described in life but have been identified incidentally at post-mortem.1 Other solid tumours include leiomyoma, mesothelioma and fibroma. Benign teratoma of the liver has been reported but this generally occurs in children.
Liver abscess
The incidence of pyogenic liver abscess has remained relatively constant over the past century despite earlier diagnosis and treatment of underlying causes and more aggressive antibiotic therapies. In recent years, the decrease in cases resulting from haematogenous spread from infected foci has been mirrored by an increase in cases secondary to hepatobiliary pathology. In almost half the patients reviewed over a 5-year period, biliary sepsis was the major predisposing factor.47 In 20% of patients, the presumed source of infection was from the portal route, but few cases were thought to have arisen from systemic infection. Hepatic abscesses secondary to ascending cholangitis are often multiple due to the distribution of the infecting organism along the biliary ductal system.48 Early reports implicated choledocholithiasis as the main causative factor; however, more recent series document malignant biliary obstruction as a more common aetiological factor.47,49
Clinical presentation
Patients present with a spectrum of symptoms and signs, the most consistent being fever associated with malaise, anorexia, weight loss and upper abdominal pain. Jaundice is a feature in approximately 50% of cases. Laboratory studies typically reflect a systemic bacterial infection. Commonly reported findings are of leucocytosis, anaemia, hyperbilirubinaemia, hypoalbuminaemia and raised levels of acute-phase proteins. US is invariably diagnostic and will often demonstrate a fluid-filled cavity. There may be a hyperechoic wall, the presence of which is dependent on the chronicity of the abscess. CT may be useful to exclude the presence of other abscesses and to identify a primary source within the abdomen (Fig. 4.9). Magnetic resonance cholangiography should be undertaken in patients with biliary symptoms, obstructive liver function tests or a dilated common bile duct, and can be combined with cross-sectional MRI to identify any hepatic parenchymal abnormality. Barium enema or colonoscopy may be indicated to exclude a colonic source of portal pyaemia.
Management
The key to successful management is drainage of the purulent collection combined with appropriate antibiotic therapy, which is determined by the results of culture of blood and aspirated pus. Although virtually all pathogenic organisms have been identified, enteric organisms predominate. Polymicrobial infection is seen frequently when hepatic abscess is secondary to infection arising from the portal venous system. Although antibiotic therapy as the sole treatment for hepatic abscess is rarely successful, prolonged systemic antibiotic administration may be the only option for patients with diffuse multiple microabscesses. In general, macroscopic hepatic collections require drainage of the purulent material. Over the past two decades, the introduction and refinement of percutaneous drainage techniques have dramatically altered the management of patients with pyogenic hepatic abscesses. Percutaneous drainage has become the first-line therapeutic option in most centres for patients with single or multiple liver abscesses.48,50,51 Abscess communication with the intrahepatic biliary tree does not prevent pyogenic collections being successfully treated by percutaneous techniques, although the period of drainage may be prolonged. The use of percutaneous aspiration combined with systemic antibiotics without drainage has been advocated by some groups;52 however, in the only randomised trial comparing the two techniques, aspiration was successful in only 60% of patients whereas percutaneous catheter drainage was successful in 100% of patients.53
Regular irrigation of drainage catheters reduces the risk of catheter blockage due to necrotic debris. Surgical drainage is rarely employed but is usually reserved for patients who have failed percutaneous drainage and those who require surgical management of the underlying problem. Liver resection is occasionally required for patients with liver abscess.54 The indication is usually failed non-operative management, hepatolithiasis, intrahepatic biliary stricture or gross parenchymal destruction.
Amoebic abscess
This form of abscess is sufficiently common that it should be considered in the differential diagnosis of hepatic lesions. About 10% of the world’s population is chronically infected with Entamoeba histolytica, although less than 10% of individuals are symptomatic. Liver abscess is the most common extraintestinal manifestation of amoebiasis and is reported in 3–10% of affected patients. Males are more commonly affected than females, and the highest incidence is in the 20- to 50-year-old age group.55
The diagnosis is likely to be straightforward in areas where amoebiasis is endemic but the liver abscess may present many years after previous intestinal infection. Some 75–90% of abscesses are in the right lobe, and involvement of the left lobe usually indicates more advanced disease. Rupture occurs in 2–17% of cases and usually occurs into the peritoneal cavity and rarely into the pleural cavity, the bronchial tree or pericardium. Signs and symptoms of amoebic infection are the same as for pyogenic abscess. On US and CT, the boundaries of the abscess are generally poorly defined (Fig. 4.10). Patients with amoebic liver abscess virtually always have serum antiamoebic antibodies, which can be detected by an indirect haemagglutination test or an enzyme-linked immunosorbent assay (ELISA) technique. Percutaneous aspiration produces a sterile and odourless fluid, which is described as having the appearance of ‘anchovy paste’. Routine percutaneous aspiration is now regarded as superfluous in the management of amoebic liver abscess unless serology is inconclusive, a therapeutic trial with antiamoebic drugs is deemed inappropriate (as in pregnancy), or rupture is suspected to be imminent. A preliminary diagnosis can be made on the basis of a dramatic clinical response to metronidazole, which should be commenced empirically in endemic areas.55 If clinical symptoms do not resolve within 48–72 hours of treatment, an incorrect diagnosis or secondary bacterial infection should be suspected. Percutaneous aspiration may be beneficial for patients when medical treatment has failed. Percutaneous catheter drainage is indicated rarely as the abscess contents are viscous and bacterial superinfection may occur. Open surgical drainage is indicated in complicated cases and in those who fail to respond to conservative therapy. In a meta-analysis of 3081 patients with amoebic liver abscess the mortality rate was 4%, compared with a mortality rate of 46% in patients with pyogenic liver abscess.56
Hydatid cyst
Clinical presentation
Echinococcal disease may occasionally mimic a primary liver tumour or metastatic disease. Serology may be helpful in establishing a diagnosis. Plain abdominal radiographs may reveal a calcified cyst wall. US and CT may demonstrate septa, ‘hydatid sand’ or daughter cysts within the main cyst cavity, which are important signs for differentiating hydatid from other benign liver cysts (Fig. 4.11). Percutaneous aspiration and drainage should be avoided because of the risk of dissemination or anaphylaxis.
Management
The main principle of surgical treatment is to eradicate the parasite, prevent intraoperative spillage of cyst contents and obliterate the residual cavity.57 At open operation, the operating field is generally packed off with swabs. After decompression, the cyst and contents are shelled out by peeling the endocyst off the host ectocyst layer. The fibrous host wall of the residual cavity should be carefully examined for any bile leakage from biliary–cyst communications, which are then sutured. The residual cyst cavity can be marsupialised, packed with omentum or plicated.58 Pericystectomy is advocated by some but should preserve those portions of the cyst wall that come into contact with major blood vessels. For smaller, peripheral lesions, formal hepatic resection may be considered, particularly if a diagnostic dilemma remains. The mortality for surgery of hydatid disease should be low and confined to complicated disease. In a series of 505 patients, Milicevic reported a mortality rate of 1.5% and a morbidity rate of 30%.58
Simple cysts of the liver
Non-parasitic cystic disease of the liver can result from a congenital malformation of the intrahepatic bile ducts. These cysts may be single, multiple or diffuse (polycystic liver disease). They contain serous fluid and do not communicate with the intrahepatic biliary tree. Small cysts are surrounded by normal liver tissue, although as these enlarge there is displacement and atrophy of adjacent hepatic tissue. A large cyst may occupy an entire lobe of the liver and result in compensatory hypertrophy of the residual liver. Such cysts have no vascularised septa and are unilocular. Microscopically, they are lined by a single layer of cuboidal or columnar epithelial cells, which resemble those of biliary epithelium. Simple cysts have a prevalence of about 3.6%. The female to male ratio is 4:1 in asymptomatic cases, but rises to 10:1 in symptomatic or complicated simple cysts.59
Clinical presentation
Diagnosis can be made on the basis of abdominal US, which demonstrates a circular anechoic area that has a well-defined boundary with the liver. No wall is evident and there is posterior acoustic enhancement. Intracystic haemorrhage may cause internal acoustic shadowing; however, the presence of cyst wall nodules or solid intracystic components must be considered neoplastic. US examination of the kidneys is useful in patients with multiple liver cysts to exclude the presence of polycystic disease. Further diagnostic investigation is rarely required, although where intervention is contemplated, CT or MRI will provide more accurate anatomical localisation and exclude the presence of other cysts. Cysts appear as well-rounded, water-dense lesions without septa on CT (Fig. 4.12). Intravenous contrast enhancement will confirm the avascularity of these lesions. Where complications such as haemorrhage occur, the simple cyst may appear relatively thick-walled and may contain cystic debris. In such instances, serological tests should be undertaken to exclude parasitic infection. It should be borne in mind that calcification is rarely present in simple cysts but may be present with hydatid cysts.
Management
Asymptomatic simple cysts require no treatment; however, symptomatic or complicated simple cysts may require intervention. Percutaneous aspiration risks introducing infection and does not provide definitive therapy; however, this technique may be useful as a diagnostic test for patients with questionable symptoms.60 Aspiration followed by percutaneous instillation of sclerosant agents has shown promising results in reducing symptomatic and radiological cyst recurrence.61 Open deroofing of simple liver cysts has, in the past, been the established conventional treatment. Total cystectomy is not required and may be hazardous since there is no plane of dissection between the cyst and the liver. In recent years, laparoscopic deroofing of such solitary cysts has been advocated. This technique was first described in 1991,62 and is associated with higher patient acceptability and shorter postoperative stay compared with open surgical techniques. In a recent comprehensive review of 21 papers on the laparoscopic management of hepatic cysts, Klingler et al.63 reported 61 laparoscopic deroofing procedures with an overall morbidity rate of 10%.
Polycystic liver disease (PCLD)
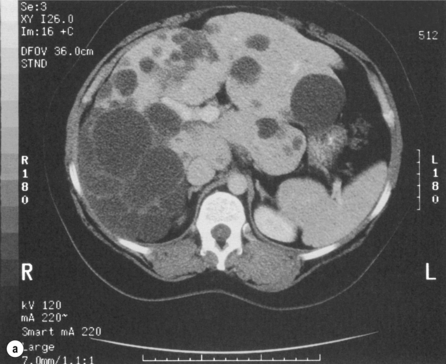
Adult polycystic kidney disease is frequently associated with multiple liver cysts, which are macroscopically and microscopically similar to simple cysts of the liver. However, in this condition the liver cysts are multiple when present and may extensively replace both lobes of the liver (Fig. 4.13). In addition to the macroscopic cysts, there are usually numerous microscopic cysts and clusters of multiple bile ductules, designated as von Meyenburg complexes. The condition is an autosomal dominant disorder and carries a much more sinister prognosis because of the risk of chronic renal failure. There is an increased prevalence associated with increasing age and the female sex.64
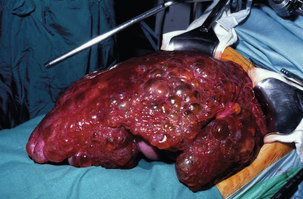
Figure 4.13 Massive polycystic liver delivered from abdomen and pelvis before resection and deroofing.
Clinical presentation
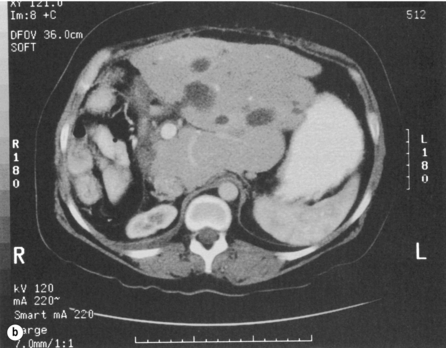
In most patients with adult polycystic kidney disease, the polycystic hepatomegaly is clinically silent. The commonest symptoms are related to increase in liver size, and include abdominal and pelvic discomfort and respiratory compromise. An abdominal mass will be present in three-quarters of patients. There are rarely signs of cholestasis, liver failure or portal hypertension, and liver function tests are usually normal. Both US and CT will demonstrate multiple fluid-filled cysts with well-defined margins in the liver and the kidneys (Fig. 4.14). Liver cysts increase in size slowly and complications are uncommon. Rupture and bacterial infection are reported to be more common with immunosuppression following kidney transplantation.65
Management
Asymptomatic patients require no treatment. Percutaneous aspiration of cysts and instillation of sclerosant rarely produce satisfactory long-term relief of symptoms. Surgical deroofing or fenestration according to the technique described by Lin et al.66 is the most widely used treatment modality for symptomatic patients but must be extensive and radical to achieve satisfactory results. Some have suggested that laparoscopic deroofing may provide good relief of symptoms.67 However, in our own series this technique was associated with a high recurrence rate.59 Recent evidence suggests that a more aggressive open surgical approach involving resection of the liver may provide longer-lasting relief of symptoms,68,69 but it should be appreciated that hepatic resection is difficult in such patients and is associated with significant morbidity. Nonetheless, extensive resection and cyst deroofing may allow the abdomen to better accommodate the enlarged residual liver. Surgical intervention is often associated with transient but massive ascites in the postoperative period.70 Liver transplantation may be indicated in selected patients with hepatic failure.71
Cystadenoma
Cystadenoma of the liver is rare, but it has a strong tendency to recur and has a malignant potential. It is usually solitary and mainly affects women over 40 years of age. Cystadenomas are often multiloculated and may measure up to 20 cm in diameter. Histologically, the locules are mostly lined by a single layer of cuboidal or columnar cells; however, in areas the epithelium may form papillary projections. The presenting features are similar to other mass-forming hepatic pathologies, namely abdominal discomfort, anorexia, nausea and abdominal swelling. A large hepatic mass may be palpable. Liver function tests are usually normal. Diagnosis is based on US, MRI or CT (Fig. 4.15). US characteristics are of a large, anechoic, fluid-filled area with irregular margins. Internal echoes may be seen due to septa or papillary projections from the cyst wall. CT provides more accurate localisation, but may be less sensitive than US for demonstrating the thin septations. Cystadenomas grow very slowly and complications include biliary obstruction, intracystic haemorrhage, bacterial infection, rupture, recurrence after partial excision and transformation into cystadenocarcinoma. This may be suspected radiologically by the presence of large projections into the cyst lobules and septal calcification.72 Cystadenoma of the liver, even if asymptomatic, must be treated by complete excision.
References
1. Ishak, K.G., Rabin, L., Benign tumors of the liver. Med Clin North Am 1975; 59:995–1013. 167242
2. Schwartz, S.I., Husser, W.C., Cavernous hemangioma of the liver: a single institution report of 16 resections. Ann Surg 1987; 205:456–465. 3555360
3. Sewell, J.H., Weiss, K., Spontaneous rupture of hemangioma of the liver. Arch Surg 1961; 83:729–733. 13911012
4. Trastek, V.F., van Heerden, J.A., Sheedy, P.F., et al, Cavernous hemangiomas of the liver: resect or observe? Am J Surg 1983; 145:49–53. 6849494
5. Farges, O., Daradkeh, S., Bismuth, H., Cavernous hemangiomas of the liver: are there any indications for resection? World J Surg 1995; 19:19–24. 7740805
6. Bornman, P.C., Terblanche, J., Blumgart, R.L., et al, Giant hemangiomas: diagnostic and therapeutic dilemmas. Surgery 1987; 101:445–449. 3563891
7. Yamamoto, T., Kawarada, Y., Yano, T., et al, Spontaneous rupture of haemangioma of the liver: treatment with transcatheter hepatic arterial embolisation. Am J Gastroenterol 1991; 86:1645–1649. 1951244
8. Johnson, C.M., Sheedy, P.F., Stanson, A.W., et al, Computed tomography and angiography of cavernous hemangiomas of the liver. Radiology 1985; 138:115–121. 3975582
9. Birnbaum, B.A., Weinreb, J.C., Mengibow, A.J., et al, Definitive diagnosis of hepatic hemangiomas: MR imaging versus Tc-99m labelled red blood cell SPECT. Radiology 1990; 176:95–102. 2191377
10. Choi, B.I., Shin, Y.M., Chung, J.W., et al, MR findings of hepatic cavernous hemangioma after intraarterial infusion of iodized oil. Abdom Imaging. 1994;19(6):507–511. 7820021
11. Mahfouz, A.E., Hamm, B., Taupitz, M., et al, Hypervascular liver lesions: differentiation of focal nodular hyperplasia from malignant tumours with dynamic gadolinium-enhanced MR imaging. Radiology 1993; 186:133–142. 8416554
12. Krause, T., Hauenstein, K., Studier-Fischer, B., et al, Improved evaluation of technetium-99m-red blood cell SPECT in haemangioma of the liver. J Nucl Med 1993; 34:375–380. 8441026
13. Shimada, K., Nakamoto, Y., Isoda, H., et al, FDG PET for giant cavernous haemangioma: important clue to differentiate from a malignant vascular tumour in the liver. Clin Nucl Med 2010; 35:924–926. 21206221
14. Nichols, F.C., van Heerden, J.A., Weiland, L.H., Benign liver tumors. Surg Clin North Am 1989; 69:297–314. 2538935
15. Pietrabissa, A., Giulianotti, P., Campatelli, A., et al, Management and follow-up of 78 giant haemangiomas of the liver. Br J Surg 1996; 83:915–918. 8813773
16. Terkivatan, T., Vrijland, W.W., Den Hoed, P.T., et al, Size of lesion is not a criterion for resection during management of giant liver haemangioma. Br J Surg 2002; 89:1240–1244. 12296890
17. Weimann, A., Ringe, B., Klempnauer, J., et al, Benign liver tumours: differential diagnosis and indications for surgery. World J Surg 1997; 21:983–991. 9361515
18. Foster, J.H., Adson, M.A., Schwartz, S.I., et al. Symposium: benign liver tumours. Contemp Surg. 1982; 21:67–102.
19. Baer, H.U., Dennison, A.R., Mouton, W., et al, Enucleation of giant hemangiomas of the liver. Ann Surg 1992; 216:673–676. 1466621
20. Descottes, B., Glineur, D., Lachachi, F., et al, Laparoscopic liver resection of benign liver tumors. Surg Endosc 2003; 17:23–30. 12364994
21. Longeville, J.H., de-la-Hall, P., Dolan, P., et al, Treatment of a giant haemangioma of the liver with Kasabach–Merritt syndrome by orthotopic liver transplant, a case report. HPB Surg 1997; 10:159–162. 9174860
22. Nagorney, D.M., Benign hepatic tumors: focal nodular hyperplasia and hepatocellular adenoma. World J Surg 1995; 19:13–18. 7740799
23. Rooks, J.B., Ory, H.W., Ishak, K.G., et al, Epidemiology of hepatocellular adenoma: the role of oral contraceptive use. JAMA 1979; 242:644–648. 221698
24. Baum, J.K., Bookstein, J.J., Holtz, F., et al, Possible association between benign hepatomas and oral contraceptives. Lancet 1973; 2:926–929. 4126557
25. Kent, D.R., Nissen, E.D., Nissen, S.E., et al, Effect of pregnancy on liver tumour associated with oral contraceptives. Obstet Gynecol 1978; 51:148–151. 622225
26. Leese, T., Farges, O., Bismuth, H., Liver cell adenomas: a 12 year surgical experience in a specialist hepatobiliary unit. Ann Surg 1988; 208:558–564. 3190282
27. Chiche, L., Dao, T., Salame, E., et al, Liver adenomatosis: reappraisal, diagnosis, and surgical management: eight new cases and review of the literature. Ann Surg 2000; 231:74–81. 10636105
28. Ferrell, L.D., Hepatocellular carcinoma arising in a focus of multilobular adenoma. Am J Surg Pathol 1993; 17:525–529. 8385884
29. Scott, F.R., El-Rafaie, A., More, L., et al, Hepatocellular carcinoma arising in an adenoma: value of Qbend 10 immunostaining in diagnosis of liver cell carcinoma. Histopathology 1996; 28:472–474. 8735726
30. Gordon, S.C., Reddy, K.R., Livingstone, A.S., et al, Resolution of a contraceptive steroid-induced hepatic adenoma with subsequent evolution into hepatocellular carcinoma. Ann Intern Med 1986; 105:547–549. 3019201
31. Gyorffy, E.J., Bredfeldt, J.E., Black, W.C., Transformation of hepatic cell adenoma to hepatocellular carcinoma due to oral contraceptive use. Ann Intern Med 1989; 110:489–490. 2537593
32. Dardenne, S., Hubert, C., Sempoux, C., et al, Conservative and operative management of benign solid hepatic tumours: a successful stratified algorithm. Eur J Gastroenterol Hepatol 2010; 22:1337–1344. 20683192
33. Zucman-Rossi, J., Jeannot, E., Nhieu, J.T., et al, Genotype–phenotype correlation in hepatocellular adenoma: new classification and relationship with HCC. Hepatology 2006; 43:515–524. 16496320
34. Bioulac-Sage, P., Balabaud, C., Bedossa, P., et al, Pathological diagnosis of liver cell adenoma and focal nodular hyperplasia: Bordeaux update. J Hepatol 2007; 46:521–527. 17239484
35. Delbeke, D., Martin, W.H., Sandler, M.P., et al, Evaluation of benign vs malignant hepatic lesions with positron emission tomography. Arch Surg 1998; 133:510–516. 9605913
36. Stoot, J.H.M.B., van der Linden, E., Terpstra, O.T., et al, Life-saving therapy for haemorrhaging liver adenomas using selective arterial embolisation. Br J Surg 2007; 94:1249–1253. 17696216
37. Tepetes, K., Selby, R., Webb, M., et al, Orthoptic liver transplantation for benign hepatic neoplasms. Arch Surg 1995; 130:153–156. 7848084
38. Laumonier, H., Biolac-Sage, P., Laurent, C., et al, Hepatocellular adenomas: magnetic resonance features as a function of molecular pathological calssification. Hepatology 2008; 48:808–818. 18688875
39. Buhler, H., Pirovino, M., Akobiantz, A., et al, Regression of liver cell adenoma. A follow-up study of three consecutive cases after discontinuation of oral contraceptive use. Gastroenterology 1982; 82:775–782. 6277724
40. Aseni, P., Sansalone, C.V., Sammartino, C., et al, Rapid disappearance of hepatic adenoma after contraceptive withdrawal. J Clin Gastroenterol 2001; 33:234–236. 11500616
41. Cherqui, D., Rahmouni, A., Charlotte, F., et al, Management of focal nodular hyperplasia and hepatocellular adenoma in young women: a series of 41 patients with clinical, radiological, pathological correlations. Hepatology 1995; 22:1674–1681. 7489973
42. Belghiti, J., Pateron, D., Panis, Y., et al, Resection of presumed benign liver tumours. Br J Surg 1993; 80:380–383. 8472159
43. Kerlin, P., Davis, G.L., McGill, D.B., et al, Hepatic adenoma and focal nodular hyperplasia: clinical, pathologic and radiologic features. Gastroenterology 1983; 84:994–1002. 6299876
44. Allaire, G.S., Rabin, L., Ishak, K.G., Bile duct adenoma: a study of 152 cases. Am J Surg Pathol 1988; 12:708–715. 3046396
45. Shek, T.W., Ng, I.O., Chan, K.W., Inflammatory pseudotumor of the liver: report of four cases and review of the literature. Am J Surg Pathol 1993; 17:231–238. 8382011
46. Grases, P.J., Matos-Villalobos, M., Arcia-Romero, F., et al, Mesenchymal hamartoma of the liver. Gastroenterology 1979; 76:1466–1469. 437445
47. Rintoul, R., O’Riordain, M.G., Laurenson, I.F., et al. The changing management of pyogenic liver abscess. Br J Surg. 1996; 83:215–218.
48. Chou, F.F., Sheen-Chen, S.M., Chen, Y.S., et al, Single and multiple pyogenic liver abscesses: clinical course, etiology and results of treatment. World J Surg 1997; 21:384–389. 9143569
49. Huang, C.J., Pitt, H.A., Lipsett, P.A., et al, Pyogenic hepatic abscess: changing trends over 42 years. Ann Surg 1996; 223:600–609. 8651751
50. Chu, K.M., Fan, S.T., Lai, E.C., et al, Pyogenic liver abscess: an audit of experience over the past decade. Arch Surg 1996; 131:148–152. 8611070
51. Pearce, N.W., Knight, R., Irving, H., et al, Non-operative management of pyogenic liver abscess. HPB (Oxford) 2003; 5:91–95. 18332963
52. Giorgio, A., Tarantino, L., Mariniello, N., et al, Pyogenic liver abscesses: 13 years of experience in percutaneous needle aspiration with US guidance. Radiology 1995; 195:122–124. 7892451
53. Rajak, C.L., Gupta, S., Jain, S., et al, Percutaneous treatment of liver abscess: needle aspiration versus catheter drainage. AJR Am J Roentgenol 1998; 170:1035–1039. 9530055
54. Strong, R.W., Fawcett, J., Lynch, S.V., et al, Hepatectomy for pyogenic liver abscess. HPB (Oxford) 2003; 5:86–90. 18332962
55. Akgun, Y., Tacyildiz, I.H., Celik, Y., Amebic liver abscess: changing trends over 20 years. World J Surg 1999; 23:102–106. 9841772
56. Pitt, H.A., Surgical management of hepatic abscesses. World J Surg 1990; 14:498–504. 2200212
57. Agaoglu, N., Turkyilmaz, S., Arslan, M.K., Surgical treatment of hydatid cysts of the liver. Br J Surg 2003; 90:1536–1541. 14648733
58. Milicevic, M. Hydatid disease. In: Blumgart L.H., ed. Surgery of the liver and biliary tract. 3rd ed. London: WB Saunders; 2000:1167–1204.
59. Martin, I.J., McKinley, A.J., Currie, E.J., et al, Tailoring the management of nonparasitic liver cysts. Ann Surg 1998; 228:167–172. 9712560
60. Gigot, J.F., Legrand, M., Hubens, G., et al, Laparoscopic treatment of nonparasitic liver cysts: adequate selection of patients and surgical technique. World J Surg 1996; 20:556–561. 8661625
61. Montorsi, M., Torzilli, G., Fumagalli, U., et al, Percutaneous alcohol sclerotherapy of simple hepatic cysts. Results from a multicentre survey in Italy. HPB Surg 1994; 8:89–94. 7880778
62. Paterson-Brown, S., Garden, O.J., Laser assisted laparoscopic excision of liver cyst. Br J Surg 1991; 78:1047. 1834297
63. Klingler, P.J., Gadenstatter, M., Schmid, T., et al, Treatment of hepatic cysts in the laparoscopic era. Br J Surg 1997; 84:438–444. 9112889
64. Milutinovic, J., Failkow, P.J., Rudd, T.G., et al, Liver cysts in patients with autosomal dominant polycystic kidney disease. Am J Med 1980; 68:741–744. 7377224
65. Bourgeois, N., Kinnaert, P., Vereerstraeten, P., et al, Infection of hepatic cysts following kidney transplantation in polycystic disease. World J Surg 1983; 7:629–631. 6356635
66. Lin, T.Y., Chen, C.C., Wang, S.M., Treatment of nonparasitic disease of the liver: a new approach to therapy of the polycystic liver. Ann Surg 1968; 168:921–927. 5684196
67. Morino, M., De Giuli, M., Festa, V., et al, Laparoscopic management of symptomatic non-parasitic cysts of the liver: indications and results. Ann Surg 1994; 219:157–164. 8129486
68. Que, F., Nagorney, D.M., Gross, J.B., Jr., et al, Liver resection and cyst fenestration in the treatment of severe polycystic liver disease. Gastroenterology 1995; 108:487–494. 7835591
69. Gigot, J.F., Jadoul, P., Que, F., et al, Adult polycystic liver disease: is fenestration the most adequate opearation for long-term management ? Ann Surg 1997; 225:286–294. 9060585
70. Farges, O., Bismuth, H., Fenestration in the management of polycystic liver disease. World J Surg 1995; 19:25–30. 7740806
71. Starzl, T.E., Reyes, J., Tzakis, A., et al, Liver transplantation for polycystic liver disease. Arch Surg 1990; 125:575–577. 2331212
72. Korobkin, M., Stephens, D.H., Lee, J.K.T., et al, Biliary cystadenoma and cystadenocarcinoma: CT and sonographic findings. AJR Am J Roentgenol 1989; 153:507–511. 2669463