Benign biliary tract diseases
Introduction
Apart from those disorders related to choledocholithiasis, benign diseases of the biliary tree are relatively uncommon (Box 11.1). The most challenging issues are in patients who present with symptoms associated with biliary strictures, which arise more commonly following iatrogenic injury during cholecystectomy. Congenital abnormalities such as choledochal cysts and biliary atresia are usually in the domain of the paediatric surgeon, although later presentation of cysts may occur after missed diagnosis or when revisional surgery is required. Most of the published literature regarding benign non-gallstone biliary disease is retrospective or at best prospectively gathered, non-randomised data, but clear guidelines can be followed based upon observation.
Congenital anomalies
Presentation is usually in the early neonatal period with prolongation of neonatal jaundice. Most patients are treated in specialist neonatal surgical units; however, occasionally patients may be referred to adult units for assessment for liver transplantation following previous unsuccessful treatment. Management in the neonate is by porto-enterostomy (Kasai’s operation), which involves anastomosis of a Roux limb of jejunum to the tissue of the hilum. Restoration of bile flow has been reported in 86% of infants treated before 8 weeks of age, but only 36% in older children.1 Four-year survival is dependent on the timing of surgery. Of 349 North American children with biliary atresia, 210 (60%) required later liver transplantation, with a 4-year transplantation survival of 82%.2 Recent evidence has suggested better outcomes following maternal liver-related liver transplantation, potentially due to tolerance to non-inherited maternal antigens.3
Choledochal cysts
The earliest description of a choledochal cyst was by Douglas in 1952,4 who described a 17-year-old girl with jaundice, fever and a painful mass in the right hypochondrium. However, presentation is usually in childhood and around 25% are diagnosed in the first year, although prenatal diagnosis is now possible with improvements in antenatal ultrasonography. Adult centres treat a small proportion of those presenting with delayed diagnosis as well as those with complications from previous cyst surgery.
The incidence of choledochal cysts in Western countries is around 1 in 200 000 live births but it is much higher in Asia. There is frequent association with other hepatobiliary disease such as hepatic fibrosis, as well as an aberrant pancreatico-biliary duct junction.5
Magnetic resonance cholangiopancreatography (MRCP) now allows images that are superior to traditional cholangiography (Fig. 11.1), and it should be recommended due to its non-invasive nature.6
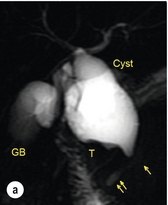
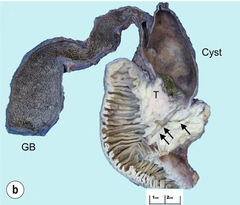
Figure 11.1 MRCP (a) and macroscopic photograph (b) demonstrating a type I choledochal cyst with a distal cholangiocarcinoma in a 42-year-old Caucasian woman requiring a pancreaticoduodenectomy. Gallbladder (GB), tumour (T), pancreatic duct (single arrow) and aberrant common channel (double arrow) are shown. Courtesy of Professor Prithi S. Bhathal, Pathology Department, University of Melbourne, Australia.
Classification
The modified Todani classification is employed to describe the various forms of choledochal cyst7 (Fig. 11.2). Type I, the most common, represents a solitary cyst characterised by fusiform dilatation of the common bile duct. Type II comprises a diverticulum of the common bile duct, whilst type III cysts are choledochocoeles. Type IV is the second most common, with extension of cysts into the intrahepatic ducts. Lastly, type V involves intrahepatic cystic disease with no choledochal cyst, which merges into the syndrome of Caroli’s disease.
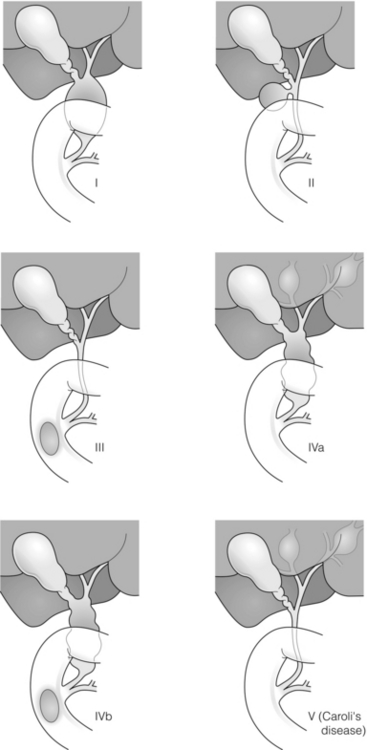
Figure 11.2 Modified Todani classification for choledochal cysts.7 Reproduced from Todani T, Watanabe Y, Narusue M et al. Congenital bile duct cysts: classification, operative procedures, and review of thirty-seven cases including cancer arising from choledochal cyst. Am J Surg 1977; 134:263–9. With permission from Elsevier.
Risk of malignancy
In the Western literature, the incidence of cholangiocarcinoma is reported to be approximately 12% (Fig. 11.1),8 compared to Todani et al.’s Japanese experience of 16% in 1353 patients.9 The incidence of malignancy is reported to be 2% at 20 years, increasing to 43% for those in their sixties.10 Cyst drainage without cyst excision does not prevent later malignant change, and there is continuing debate regarding the precise ongoing risk following cyst resection. Takeshita et al. reported 180 patients who underwent primary surgery for a choledochal cyst. Synchronous malignancy was found in 36 patients (20%), with only one of the remaining 144 patients developing malignancy during follow-up.11
Special operative techniques
During operative exposure, intraoperative ultrasound is very useful to identify the biliary confluence, the intrahepatic extension of the cyst, and the relationship to the right hepatic artery above and to the pancreatic duct below (Fig. 11.3). Small aberrant hepatic ducts may enter the cyst below the biliary confluence and these are missed frequently on preoperative imaging.12 Such aberrant ducts are usually identified once the cyst has been opened. The cyst is normally best excised in its entirety and this is facilitated by opening it along its anterior length. This aids identification of the vessels from which the cyst is freed. Early identification of the biliary confluence aids the surgeon in planning the incorporation of any segmental duct into the eventual hepatico-jejunal Roux-en-Y anastomosis. Dissection into the head of the pancreas is made easier by use of bipolar scissors and the CUSA™ (ultrasonic surgical aspiration system, ValleyLab, Boulder, CO) if the plane of dissection is obscured by fibrosis or inflammation. It may be necessary to leave a small oversewn lower common bile duct stump to avoid compromise to the pancreatic duct lumen; however, recurrent pancreatitis and possible malignant transformation remain possible complications.
Iatrogenic biliary injury
The true incidence of biliary injury following laparoscopic cholecystectomy remains obscure. It has been suggested that there was a slight increase in the incidence of injuries following initial introduction of the laparoscopic technique,13 with a reported incidence of 0.3–0.7%.14–16 Open cholecystectomy is said to have a lower incidence of biliary injury, with a rate of 0.13%.17 Recent variations in technique such as single-incision laparoscopic surgery (SILS) cholecystectomy are not immune to biliary injury. Han et al. recently reported two (1.5%) bile duct injuries in 150 patients having single-port laparoscopic surgery.18
Aetiology
Previous reports of injury during laparoscopic cholecystectomy suggested that injury was more likely to occur when performed for pancreatitis, cholangitis or acute cholecystitis.19 However, in a prospective analysis of patients referred following biliary injury, 71% occurred in patients in whom the indication for cholecystectomy was biliary colic alone,20 and thus surgeons should always be vigilant regardless of the indication.
Techniques to avoid injury
Many techniques have been described to decrease the risk of injury to the common bile duct during cholecystectomy. The main risk factors are thought to be inexperience, aberrant anatomy and inflammation.19,21 However, in an analysis of 252 laparoscopic bile duct injuries, the authors suggested that the primary cause of error was a visual perceptual illusion in 97% of cases, whilst faults in technical skill were thought to have been present in only 3% of injuries.22
Correct identification of the biliary anatomy is essential in avoiding injury to the extrahepatic bile duct. Dissection of Hartmann’s pouch should start at the junction of the gallbladder and cystic duct and continue lateral to the cystic lymph node, thus staying as close as possible to the gallbladder. The biliary tree and hepatic arterial anatomy is highly variable and therefore great care must be taken in identifying all structures within Calot’s triangle before ligation. In Couinaud’s published study of biliary anatomy, 25% had drainage of a right sectoral duct directly into the common hepatic duct.23 Sometimes this structure may follow a prolonged extrahepatic course, where it can be at greater risk from cholecystectomy. The right hepatic artery may also course through this area. All structures should be traced into the gallbladder to minimise the risk of injury (Fig. 11.4 and 11.5).
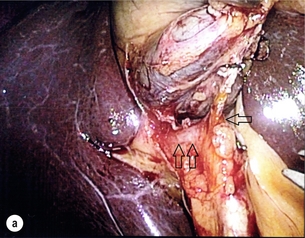
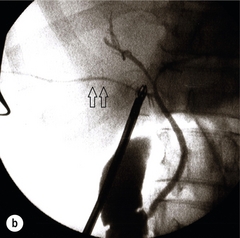
Figure 11.4 Aberrant biliary anatomy. The normal biliary anatomy is a trifurcation of the right sectoral and left hepatic ducts forming the common hepatic duct which receives the cystic duct after a variable distance. Operative photograph (a) and a cholangiogram (b) of a short cystic duct (single arrow) draining into the right posterior sectoral duct (double arrow), which has a long extrahepatic course.
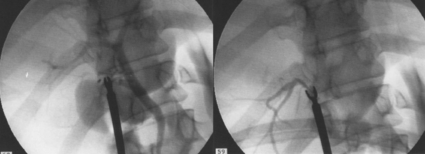
Figure 11.5 Operative cholangiography of an aberrant right sectoral duct. The injury was recognised after division of the duct following cholangiography. The cholangiogram catheter was used to obtain a cholangiogram of the aberrant duct. The surgeon obtained advice by telephone and a decision was made to ligate the duct. The patient remains asymptomatic.
Calot’s original description of gallbladder anatomy described a triangle formed by the cystic duct, common hepatic duct and superior border of the cystic artery.24 For satisfactory visualisation of the structures, dissection should also extend above the cystic artery to the liver. Extensive dissection should be avoided in Calot’s triangle as diathermy injury may occur to the lateral wall of the common hepatic duct. Furthermore, arterial bleeding in this area should not be cauterised or clipped blindly. Most bleeding can be controlled with several minutes of direct pressure with a laparoscopic forceps compressing Hartmann’s pouch on to the bleed point. During the era of open cholecystectomy many advocated complete excision of the cystic duct to its insertion into the common bile duct to avoid a cystic duct stump syndrome. However, extensive dissection around the common bile duct with or without diathermy may cause an ischaemic stricture due to damage to the intricate blood supply of the common hepatic duct.
Many authors argue that operative cholangiography is essential to avoid biliary injury.16,19 Fletcher et al. reported an overall twofold reduction in biliary injuries with the use of operative cholangiography, with an eightfold decrease in complex cases.19 Flum and colleagues analysed retrospectively the Medicare database in the USA and identified 7911 common bile duct injuries following cholecystectomy. After adjusting for patient-level factors and surgeon-level factors the relative risk was 1.49 when intraoperative cholangiography was not used.16 When the use of intraoperative cholangiography has undergone cost analysis, routine cholangiography has been found to be the most cost-effective during high-risk operations when employed by less experienced surgeons.25
Unfortunately, many operative cholangiograms are interpreted incorrectly and injuries are missed. Although this event should be less frequent with the use of modern C-arm imaging, in reported series of biliary injuries only 6–33% of operative cholangiograms are interpreted correctly.20,26 For correct anatomical interpretation of the proximal biliary tree, both right sectoral/sectional ducts and the left hepatic duct should be visualised. In the presence of an endoscopic sphincterotomy, contrast will preferentially flow into the duodenum and the patient may need to be placed in a head-down position to fill the intrahepatic ducts. If the anatomy is unclear no proximal clip should be placed on what is presumed to be the cystic duct, to avoid a crush injury to what may be the common hepatic duct.
Retrograde cholecystectomy has been described previously as a safe technique when inflammation around Calot’s triangle makes identification of the anatomy difficult. Nonetheless, care still needs to be exercised during dissection to avoid injury to the right hepatic artery and common hepatic duct, which may be adherent to an inflamed gallbladder. Eight such patients have recently been described by Strasberg and Gouma.27 If identification remains impossible then the gallbladder can be opened to facilitate identification of the cystic duct. A subtotal cholecystectomy should be considered if a safe plane of dissection cannot be established, thus avoiding injury to the common hepatic or left hepatic ducts. Originally described for open cholecystectomy, these techniques have now also been performed laparoscopically.28
Classification
Injury to the distal biliary tree is less technically demanding to repair than involvement of the biliary confluence. The success of reconstruction depends on the type of injury and the anatomical location.29 Bismuth first described a classification system for biliary strictures reflecting the relationship of the injury to the biliary confluence (Table 11.1).30 Strasberg et al. further proposed a broader classification to include a number of biliary complications, including cystic stump leaks, biliary leaks and partial injuries to the biliary tree (Fig. 11.6).17
Table 11.1
Bismuth classification of biliary strictures
| Bismuth classification | Definition |
| Bismuth 1 | Low common hepatic duct stricture – hepatic duct stump > 2 cm |
| Bismuth 2 | Proximal common hepatic duct stricture – hepatic duct stump < 2 cm |
| Bismuth 3 | Hilar stricture with no residual common hepatic duct – hepatic duct confluence intact |
| Bismuth 4 | Destruction of hepatic duct confluence – right and left hepatic ducts separated |
| Bismuth 5 | Involvement of aberrant right sectoral hepatic duct alone or with concomitant stricture of the common hepatic duct |
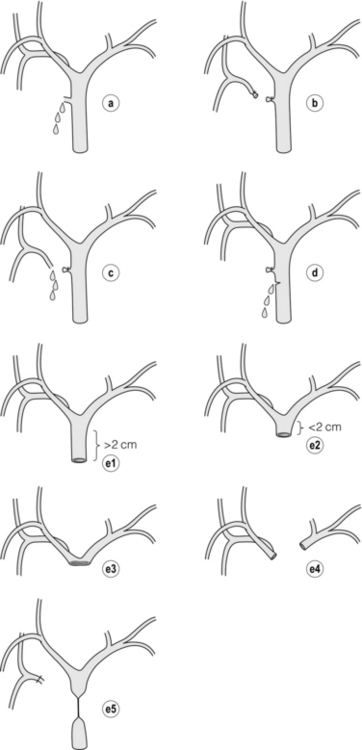
Figure 11.6 Strasberg classification. Type A injuries include leakage from the cystic duct or subvesical ducts. Type B involves occlusion of part of the biliary tree, most usually an aberrant right hepatic duct. If the former injury involves transection without ligation this is termed a type C injury. A lateral injury to the biliary tree is a type D injury. Type E injuries are those described by Bismuth and subdivided into his classification (Table 11.1). Adapted from Strasberg SM, Hertl M, Soper NJ et al. An analysis of the problem of biliary injury during laparoscopic chole cystectomy. J Am Coll Surg 1995; 180:102–25. With permission from the American College of Surgeons.
Presentation
Ligation of sectoral ducts may cause subsequent or late atrophy of the drained liver segments, which may become infected secondarily. Occasionally liver resection or transplantation may be required for fulminant hepatic failure secondary to combined biliary and vascular injuries.31,32 More commonly, injuries may present late with secondary biliary cirrhosis, which may require liver transplantation when liver failure results.31,32
In many patients there is a delay until referral, despite evidence of a biliary injury. In a report by Mirza et al., the median interval until referral was 26 days.33 This delay is not inconsequential as the opportunity for an early repair is lost and results in the liver sustaining further damage.
Management
In a review by Carroll et al., only 27% of patients underwent a successful repair by the primary surgeon responsible for the injury, whilst 79% of repairs performed following referral had a successful outcome.34 If experienced help is not at hand, no attempt should be made to remedy the situation since this may compromise subsequent successful management. A T-tube or similar drain should be placed to the biliary injury and drains left in the subhepatic space, followed by referral to a specialist centre. No attempt should be made to repair a transection or excision of the bile duct.
A partial injury to the bile duct may sometimes be managed by direct closure with placement of a T-tube through a separate choledochotomy. Primary repair with or without a T-tube for complete transection of the common bile duct is nearly always unsuccessful. This may result from unappreciated loss of common duct or an associated arterial injury, or result from local diathermy injury or devascularisation of the duct from overzealous dissection of the common bile duct35 (Fig. 11.7a,b). Succesful endoscopic treatment is possible for failed primary repair; however, as many as 32% will require subsequent hepatico-jejunostomy.36
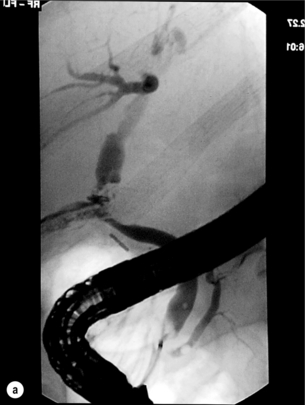
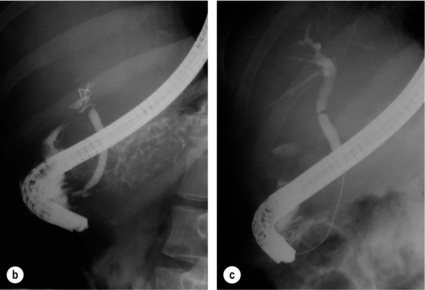
Figure 11.7 (a) Failure of primary repair with T-tube. Primary repair was performed for an injury to the common bile duct presenting with biliary peritonitis. A T-tube was inserted through the anastomosis and this was removed at 4 weeks. An anastomotic stricture developed and the patient required a hepatico-jejunostomy 2 months later. (b) Failure of primary repair for ligation of the common bile duct. A complete transection of the common bile duct identified at postoperative endoscopic retrograde cholangiopancreatography (ERCP). Immediate repair was performed with a direct duct-to-duct repair. (c) A tight anastomotic stricture is demonstrated at a later ERCP.
Postoperative recognition: biliary fistula
Further assessment depends on the clinical situation. The majority of biliary fistulas are due to leaks from the cystic duct stump or subvesicle ducts, and endoscopic retrograde cholangiopancreatography (ERCP) allows anatomical definition, endoscopic sphincterotomy or stent placement. As complete transection of the bile duct precludes ERCP, computed tomography intravenous cholangiography (CT-IVC) or MRCP can determine continuity of the biliary tree prior to endoscopy. Occasionally, persistent bile drainage is associated with choledocholithiasis requiring sphincterotomy and stone extraction. Most simple cystic duct stump leaks can be resolved by endoscopic stenting if cannulation is possible at ERCP37 and occasionally side injury to the biliary tree can be controlled with endoscopic stent placement.38
Postoperative recognition: biliary obstruction
ERCP will identify a stricture or complete transection of the bile duct; however, identification of complete transection with MRCP will avoid the risks of an unnecessary ERCP. Overzealous instillation of contrast should be avoided, and placement of an endoscopic stent should only be considered after consultation with a specialist unit since this may introduce sepsis into the biliary tree and compromise further management. Furthermore, an undrained biliary tree may allow proximal biliary dilatation, thereby facilitating later reconstruction. Although some have reported satisfactory resolution of biliary strictures with endoscopic stenting alone, the follow-up has usually been short and almost all patients require later surgery in our experience. Partial occlusion of the duct by a clip may be remedied by balloon dilatation with or without placement of a stent; however, delay in diagnosis may result in subsequent recurrent stricture formation. Nonetheless, de Reuver et al. reported 110 patients with bile duct strictures following cholecystectomy that were treated with endoscopic stenting, 48 (44%) of which had already undergone attempted surgical repair. At a mean follow-up of 7.6 years, 74% of patients had a successful outcome.37 The development of removable endoscopic expandable metal stents has recently been described, although long-term results and large series are not yet available. Furthermore stent migration can complicate treatment.
The timing of repair
Early repair: When an injury is recognised in the early postoperative period and there is minimal peritoneal contamination or sepsis, a definitive repair by an experienced surgeon can be successful (Fig. 11.8). In our series of 123 patients referred with injury to the biliary tree, 22 patients underwent primary biliary repair in the first 2 weeks following injury and three had revision of a failed biliary repair. Between 2 weeks and 6 months, a further 22 injuries were repaired selectively. Successful repair was possible in 22 of 25 early repairs compared with 20 of 22 delayed repairs.39
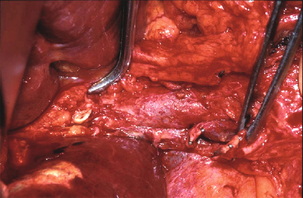
Figure 11.8 Operative picture of an early repair of an E4 injury. A right-angle forceps is placed in the opening of the left hepatic duct whilst the open right hepatic duct is visible below. The portal vein is skeletonised with ligation and excision of both the extrahepatic biliary tree and right hepatic artery (held by forceps).
Delayed repair: Many injuries continue to be unrecognised or referral delayed. In a prospective audit of major bile duct injuries from Australia, the median delay before referral was 9 days (2–28 days), and this included five patients with generalised peritonitis.20
Associated vascular injury: Abdominal CT is required to ensure resolution of intra-abdominal collections and before repair to exclude the presence of liver atrophy. Atrophy can occur from prolonged obstruction to the segmental, sectional or hepatic ducts, but is generally associated with the presence of a vascular injury, most usually of the right hepatic artery. Liver resection may occasionally be needed at the time of definitive repair to remove a source of ongoing sepsis, or if satisfactory reconstruction to the left or right duct is not possible.
Buell et al. identified associated vascular injury as an independent predictor of mortality, with 38% of patients dying compared to 3% (P < 0.001) where no arterial injury was present.40 Some authors advocate arteriography before repair to identify such associated vascular injury as a repair is less likely to be successful,41 or for consideration of hepatic arterial reconstruction at the time of hepatico-jejunostomy.41,42 However, a recent paper described 55 patients with postcholecystectomy strictures who underwent surgical reconstruction with a left duct approach and preoperative coeliac axis and superior mesenteric artery angiography.43 Twenty-six patients (47%) had an associated vascular injury, of which 20 (36%) were of the right hepatic artery. In this series only one patient in each group (vascular injury vs. no injury) developed a recurrent stricture after repair.43 A proximal anastomosis may offer a better blood supply, minimising the risk of anastomotic stricturing (Fig. 11.9). In support of this theory, Mercado et al. demonstrated that an anastomosis fashioned below the biliary confluence was more likely to require revisional surgery (16%) compared to an anastomosis performed at the biliary confluence (0%; P < 0.05).44 Recent improvements in magnetic resonance imaging (MRI) and spiral CT are producing impressive arterial and venous anatomical reconstructions, which may negate the need for invasive arteriography.
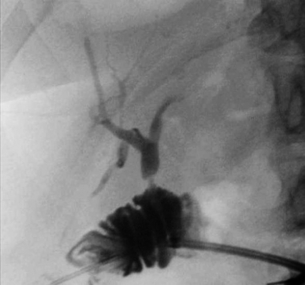
Figure 11.9 Anastomotic stricture following repair of biliary injury. Percutaneous transjejunal cholangiogram (PTJC) of a Bismuth 1 injury repaired by hepatico-jejunostomy at the level of the transection of the common bile duct (not to the left hepatic duct). Three months later the patient required reconstruction of the anastomotic stricture.
Injury to the hepatic arterial supply (usually the right hepatic artery) may present with haemobilia or intra-abdominal haemorrhage from a false aneurysm, usually associated with ongoing subhepatic sepsis. If suspected, urgent angiography is required (Fig. 11.10). Haemorrhage may be controlled by embolisation of the feeding vessel, although re-bleeding can occur and necessitate further embolisation. However, in our experience, further bleeding in the presence of ongoing sepsis usually requires laparotomy for control of bleeding and drainage of any subhepatic collection.
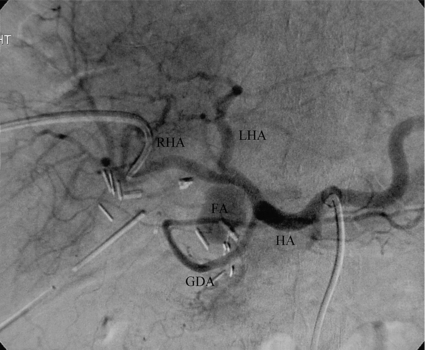
Figure 11.10 Digital subtraction angiogram demonstrating a false aneurysm of the common hepatic artery. Embolisation was required for control. The patient has undergone a primary repair for a complete transection of the common bile duct. FA, false aneurysm; GDA, gastroduodenal artery; HA, common hepatic artery; LHA, left hepatic artery; RHA, right hepatic artery.
Rarely, combined injury to the hepatic artery and portal vein can occur with resultant infarction of the affected hepatic parenchyma, usually the right liver. Such injuries may require urgent hepatic resection or transplantation.31,32,45
Further imaging: For patients with injury to the biliary confluence (E3 and E4), preoperative imaging will help in the planning of future repair. In the presence of a biliary stricture, invasive cholangiography by ERCP or percutaneous transhepatic cholangiogram (PTC) risks introducing sepsis. However, if PTC is required for external biliary drainage, an adequate cholangiogram may be obtained at this time. The quality of MRCP continues to improve, and detailed biliary anatomical reconstructions can be produced, thereby negating the need for more invasive imaging.
Operative techniques
Since the blood supply to the bile duct is often damaged at the time of injury, the common hepatic duct should be opened as proximally as possible, although frequently there has been retraction of the fibrotic remnant superiorly. Extension of the incision into the left hepatic duct allows a wide anastomosis to be fashioned with adequate views of the left- and right-sided ducts. Care should be taken since there may be a small superficial arterial branch crossing the left duct anteriorly and running above to segment 4. For injuries with separation of the confluence, the right and left hepatic ducts can be anastomosed together before formation of a hepatico-jejunostomy, allowing a single biliary anastomosis. If possible, injuries to an isolated right sectoral duct are best repaired or drained into a Roux limb of bowel (Fig. 11.5). Simple ligation will lead to atrophy of the drained segments, which may become a nidus for sepsis. However, enteric drainage of a small sectoral duct may also lead to sepsis if an anastomotic stricture occurs.
Repair should be effected by a hepatico-jejunostomy with a 70-cm Roux limb of jejunum, thereby minimising the risk of enteric reflux and chronic damage to the biliary tree. Moraca et al. advocate hepatico-duodenostomy for biliary injury on the basis that it is more physiological, quicker to perform, and allows later ERCP for imaging and intervention.46 They found no difference in outcome following hepatico-duodenostomy when compared with hepatico-jejunostomy, although median follow-up was only 54 months. Hepatico-duodenostomy has largely been abandoned in the treatment of other benign biliary disease due to ongoing enteric reflux. There have been anecdotal reports of the late development of cholangiocarcinoma,47 as well as the need to undertake liver transplantation in patients so managed when secondary biliary cirrhosis due to enteric reflux has resulted. Our own view is that hepatico-duodenostomy has no role in the management of bile duct injury.
Fine absorbable interrupted sutures of 4/0 or 5/0 polydioxanone sulphate (PDS II) should be used to fashion an end-to-side hepatico-jejunostomy, with care being taken to produce good mucosal apposition. Some authors advocate the use of an access limb, particularly for E3 and E4 injuries, to allow subsequent radiological intervention for dilatation of recurrent strictures.48 However, others believe that advances in percutaneous transhepatic techniques have made this unnecessary and have achieved satisfactory results without using this surgical approach.
Rarely, there may be no recognisable bile ducts visible in the porta hepatis. In such cases a variation of porto-enterostomy (Kasai procedure) can be considered with the Roux limb sutured to the fibrous structure of the hilar plate (S.W. Banting, personal communication).49
Management of complications related to repair
Revisional surgery: Many patients with biliary injury continue to suffer from complications despite reconstruction. Factors such as the experience of the initial surgeon, the level of injury, the associated sepsis and liver atrophy all increase the chance of an unsuccessful repair. Following primary repair of a ductal tear or laceration, further stricture formation may result if there has been extensive dissection around the common hepatic duct. In such instances, surgical revision with the formation of a Roux-en-Y hepatico-jejunostomy is indicated.
The majority of patients requiring revisional surgery will have undergone a previous biliary enteric drainage procedure. Anastomotic stricturing will require revision of the anastomosis, with extension of the choledochotomy into the left hepatic duct (Fig. 11.9).
Liver resection and transplantation: In the acute setting of bile duct injury, long-term damage to the hepatic parenchyma is difficult to predict. Major vascular injury or unrecognised segmental biliary obstruction may lead to atrophy of the liver, chronic intrahepatic infection, abscess formation or secondary biliary cirrhosis. In such patients, careful operative assessment is required; CT should be performed to identify areas of associated liver atrophy and to exclude portal vein thrombosis.
In our experience, the majority of patients requiring liver resection are those with ongoing sepsis in an obstructed segment or those where drainage of the extrahepatic biliary tree is not possible due to sectoral duct damage or fibrosis.31 A recent review identified 99 patients (5.6%) requiring hepatectomy among 1756 postcholecystectomy bile duct injury patients,50 with combined arterial and Strasberg E4 and E5 injuries more likely to require hepatic resection.50 Occasionally, early hepatic resection is required for combined arterial, portal venous and biliary injury, although results are poor.45 Very rarely, resection may be needed to gain access to the biliary tree, especially when the injury involves the biliary confluence (E4), although some authors routinely advocate resection of segments IVb and V for access to the right hepatic ducts.51 The right lobe is most commonly affected by sepsis and atrophy as the right-sided sectoral ducts and arterial supply are more likely to be damaged during cholecystectomy, although both left- and right-sided hepatic resections have been reported in patients with severe biliary injury. Resection of the right liver can be performed, for example at the time of delayed reconstruction if there is any doubt regarding the integrity of the anastomosis to the right sectoral or hepatic duct and when a satisfactory anastomosis can be achieved to the long extrahepatic left duct.
Failed reconstruction and persistent cholangitis may lead to end-stage liver failure within a few years and this may require liver transplantation31 (Fig. 11.11). A long interval between injury and referral is known to be associated with end-stage liver disease.52 Rarely, liver transplantation may be needed when the combined biliary and vascular injury is so severe as to preclude attempted reconstruction, although the results are universally poor.31
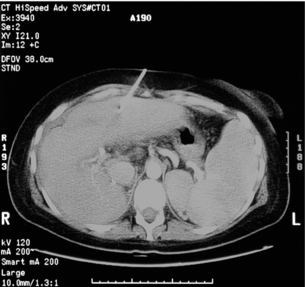
Figure 11.11 Contrast-enhanced CT image of the liver after unsuccessful revisional hepatico-jejunostomy. The surgeon who performed the laparoscopic cholecystectomy performed the hepatico-jejunostomy for an E4 injury. A revisional hepatico-jejunostomy was performed before referral, which was complicated by an anastomotic stricture and portal vein thrombosis. The CT scan shows evidence of right lobe atrophy and splenomegaly as well as a percutaneous biliary drain.
Prognosis
Success of repair: Successful repair has been well described and can be achieved in 90% of patients in a specialised unit.20,26 As for laparoscopic cholecystectomy, a learning curve for biliary repair has also been described for tertiary centres managing bile duct injury. Over a 20-year period, Mercado et al. reported an improvement with experience and a reduction of post-repair strictures from 13% to 5%.53 As well as anastomotic strictures, liver atrophy and cirrhosis may also occur many years following repair. Predictors of a poor outcome include involvement of the biliary confluence,29,52 repair by the injuring surgeon,34,54,55 three or more previous attempted repairs29 and recent active inflammation.55
Survival: Mortality following injury to the biliary tree is significant. Death may follow the acute injury itself, following the biliary repair, or occur later as a result of biliary sepsis or cirrhosis. In a recent report of a nationwide analysis of survival following biliary injury after cholecystectomy, Flum et al. identified 7911 (0.5%) injuries from 1 570 361 cholecystectomies.54 Within the first year after cholecystectomy the mortality rate was 6.6% in the uninjured group and 26.1% in those with injury to the common bile duct. The adjusted hazard ratio for death during follow-up was higher for those with an injury (2.79; 95% confidence interval 2.71–2.88). The risk of death increased significantly with advancing age and comorbidities. If the initial repair was performed by the injuring surgeon then the adjusted hazard of death increased by 11%.
Quality of life: Boerma et al. first undertook an assessment of quality of life in patients who had sustained biliary injury or leak that required additional intervention.56 Five years after injury, quality of life in the physical and mental domains was significantly worse than controls, despite a successful outcome in 84% of treated patients and regardless of the type of treatment or severity of injury. However, the length of treatment was an independent predictor of a poor mental quality of life. Melton et al. report that quality of life in 89 patients who had undergone biliary repair following laparoscopic cholecystectomy showed no difference in the physical or social domains when compared to controls.57 However, in the psychological domain, patients were significantly worse, particularly in the 31% of patients who sought legal recourse for their injury.
Associated malignancy: A small number of reports exist about the development of cholangiocarcinoma at the site of anastomosis 20–30 years following repair.47 It is possible that enteric reflux into the biliary tree with sepsis and the production of mutagenic secondary bile salts may be responsible. Furthermore, hepatocellular carcinoma may develop due to secondary biliary cirrhosis (Fig. 11.12).
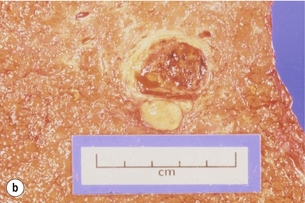
Figure 11.12 Hepatocellular carcinoma as a consequence of biliary injury. This patient required a liver transplant for secondary biliary cirrhosis, which developed following hepato-duodenostomy for a biliary injury (a). At pathological examination a hepatocellular carcinoma was detected in the explanted liver (b).
Benign biliary strictures
Mirizzi first described the syndrome of extrahepatic biliary stricture in association with cholelithiasis in 1948,58 a condition that occurs in fewer than 0.5% of cholecystectomies.59 Obstruction of the common hepatic duct may occur for two reasons: (i) a stone impacted in the cystic duct may cause direct pressure or oedema (Mirizzi type I; Fig. 11.13), or (ii) occasionally the stone may erode through the wall of the gallbladder or cystic duct and into the common hepatic duct (Mirizzi type II).
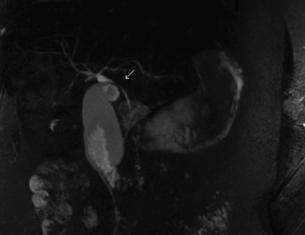
Figure 11.13 Mirizzi syndrome (type I). Magnetic resonance cholangiopancreatography (MRCP) demonstrating compression of the common bile duct (single arrow) secondary to acute cholecystitis from a stone impacted in Hartmann’s pouch.
Management
When Mirizzi’s syndrome is difficult to distinguish from a malignant stricture, CT may aid in the diagnosis. Contrast-enhanced ultrasound may allow better delineation of a benign or malignant cause; however, availability of contrast is limited at present. Occasionally laparoscopic ultrasound may be necessary to further delineate the stricture and exclude tumour dissemination. Although the two conditions may appear similar, vascular invasion may be seen and targeted biopsy may confirm a malignant diagnosis.60
Successful completion of cholecystectomy by laparoscopic means has been reported for apparent type I Mirizzi’s syndrome,61 although this would be inappropriate where there was a clear fistulous communication between gallbladder and common hepatic duct. One of these reported patients has subsequently developed a biliary stricture (O.J. Garden, personal communication). The conventional approach for type I strictures is to perform an open cholecystectomy or to convert from a laparoscopic procedure to allow adequate assessment of the associated biliary stricture. Operative cholangiography should be performed, and in those with persistent strictures a hepatico-jejunostomy should be performed. For type II, where a defect results from the removal of the gallbladder and stent, the common bile duct should be explored. The majority of patients will require a hepatico-jejunostomy, although apparently successful reconstructions using grafts of Hartmann’s pouch have been described.62 Long-term results of this innovative approach are awaited.
In a recent report, Schafer et al. identified Mirizzi’s syndrome in 39 of 13 023 patients (0.3%) undergoing a laparoscopic cholecystectomy.63 Thirty-four (87%) patients had a type I Mirizzi syndrome. Of these, 23 patients underwent cholecystectomy alone, 10 patients required bile duct exploration and T-tube insertion, and one patient had a Roux-en-Y reconstruction. Twenty-four of the 34 patients (74%) required open conversion. Of the remaining five patients with a type II Mirizzi syndrome, three underwent a hepatico-jejunostomy and two had simple closure with T-tube drainage. All had required open conversion and, interestingly, four patients (10%) were found to have a gallbladder carcinoma on histopathology.
Hepatolithiasis
Recently there has been a decline in incidence, possibly related to improved economic conditions and changes in diet.64 The cause is unknown, although Clonorchis sinensis, ascariasis and nutritional insufficiency have been suggested as associated factors in its causation.
Pathologically there is gross irregular dilatation and intrahepatic stricture formation of the biliary tree, which frequently contains stones, debris and pus. Bile duct proliferation, portal and periportal inflammation and fibrosis are seen, and occasionally there is liver abscess formation. Stone formation is frequently associated with bacterial superinfection and the bile is infected in 96% of patients with hepatolithiasis, most usually with Escherichia coli.65 There is a strong association between hepatolithiasis and cholangiocarcinoma, particularly in the presence of liver atrophy.66 Diagnosis is based on history and demographic features, and investigation includes liver biochemistry and abdominal ultrasonography. Ultrasound is usually diagnostic, although abdominal CT may add further information regarding associated liver atrophy or abscess formation. ERCP will provide important anatomical detail and will allow endoscopic stenting if required. If stricture formation or stones prevent filling of the intrahepatic ducts, MRCP should provide further information, avoiding the risk of PTC.
Management
Following resolution of an acute attack definitive surgery is required. A multidisciplinary approach is required involving radiologists, surgeons and gastroenterologists. A full spectrum of interventions from simple exploration and stone removal, hepatico-jejunostomy or liver resection through to liver transplantation may be required. Of 97 Japanese patients treated for hepatolithiasis, 49% undergoing hepatico-jejunostomy, 25% drained with T-tube and 10% treated with percutaneous transhepatic cholangioscopic lithotripsy were found to have residual stones. No patients treated with hepatic resection had residual stone disease. Furthermore, recurrent stones were found in 14% of hepatectomy patients compared to 25% or more for the other treatment options.67
Parasitic infestation causing jaundice
Echinococcus
Biliary obstruction can occur due to local compression of the common hepatic duct by the expanding cyst, or when daughter cysts pass down the common hepatic duct following rupture of the cyst into intrahepatic radicles. Secondary sclerosing cholangitis has been described following inappropriate injection of scolicidal agents into the hepatic cyst when there is communication with the biliary tree.68
Primary sclerosing cholangitis
Primary sclerosing cholangitis is a rare condition, and although the precise cause has yet to be determined there is increasing evidence of an immunological basis as well as an overall increase in the incidence.69 Around 70% of patients will have ulcerative colitis, or more rarely Crohn’s disease. There are other reports of association with Riedel’s thyroiditis, retroperitoneal fibrosis, lymphoplasmacytic sclerosing pancreatitis70 as well as a strong association with a number of human leucocyte antigens.71
Management
The prognosis of primary sclerosing cholangitis is poor, with a median survival of only 9.6 years from diagnosis to death or liver transplantation.72 Survival may improve with earlier diagnosis and liver transplantation; however, subsequent development of cholangiocarcinoma and colorectal cancer has now become the leading cause of death.73 The use of ursodeoxycholic acid in the treatment of pruritus has been associated with improvements in biochemical function and histological appearance.74 Episodes of cholangitis can be treated with antibiotics covering biliary pathogens. There is no evidence that colectomy for inflammatory bowel disease alters disease progression.
Endoscopic or transhepatic dilatation of short dominant strictures with or without endoscopic stenting has been described as effective, safe and well tolerated, although no randomised trials have been performed. Dilatation achieves palliation at 1 and 3 years in 80% and 60% of patients, respectively.75 In those patients without cirrhosis but with jaundice secondary to a dominant stricture, surgical drainage with an access limb has been described.
Liver transplantation is necessary to treat end-stage liver disease. However, it is now more usual for patients to be considered if there is persistent jaundice, intractable pruritus, recurrent cholangitis, malnutrition or fatigue. Many patients are now transplanted before liver failure, with survival rates of greater than 80% at 5 years.76
Exclusion of associated malignant stricture
Cholangiocarcinoma and gallbladder cancer complicate 10–36% of patients with primary sclerosing cholangitis,72 and need to be excluded before liver transplantation.
In the majority of patients, concern regarding occult cholangiocarcinoma is small, and liver transplantation is undertaken in the absence of a dominant stricture. Serum carbohydrate antigen (CA) 19-9 has been used in an attempt to identify cases with an occult biliary malignancy. Patients with a sudden rapid deterioration in their clinical state or with a dominant stricture must be considered to have a cholangiocarcinoma and be investigated extensively. Brush cytology at ERCP may provide the diagnosis if a malignant smear is obtained. CT or MRI may demonstrate a mass lesion in association with the biliary tree, although the usual appearance is of a stricture indistinguishable from a benign disease. Positron emission tomography (PET) scanning is superior to conventional radiological investigations to differentiate primary sclerosing cholangitis and cholangiocarcinoma.77
Laparoscopy identifies the majority of patients with unresectable biliary tract cancer,78 and may be of use in assessing those considered for transplantation in whom a cholangiocarcinoma is suspected since tumour dissemination often occurs early. Laparoscopic ultrasound may further aid assessment, and occasionally laparotomy may be required if there is diagnostic doubt regarding cholangiocarcinoma.
Biliary strictures imitating malignancy
Up to 14% of patients undergoing surgery for presumed malignant hilar obstruction are found to have a benign fibrotic stricture of the bile duct.79 It is usually impossible to differentiate them from malignancy preoperatively and thus resection is often attempted and nearly always feasible. Successful treatment has been described with the use of steroids.
Lymphoplasmacytic sclerosing pancreatitis
Lymphoplasmacytic sclerosing pancreatitis is also known as autoimmune pancreatitis, sclerosing pancreatitis or primary inflammatory pancreatitis. The disease is associated with other autoimmune diseases such as Sjögren’s syndrome, Riedel’s thyroiditis, retroperitoneal fibrosis, ulcerative colitis and primary sclerosing cholangitis.80 Although only accounting for about 2.4% of pancreatic resections, the condition is important since a proportion of patients will develop either biliary anastomotic strictures or intrahepatic strictures following resection. In a series of 31 patients, eight (28%) went on to develop recurrent jaundice after resection.80
Increased levels of IgG4 have been described in association with the disease, and successful treatment with steroids has been described, although disease recurrence is reported.81 As yet the value of steroid treatment in the prevention and treatment of recurrent strictures is not well described.
Functional biliary disorders
Most patients who present for investigation of sphincter of Oddi dysfunction have already undergone cholecystectomy for presumed gallbladder pain. However, up to 39–90% of patients with idiopathic recurrent pancreatitis may also have sphincter of Oddi dysfunction.82 In those patients with postcholecystectomy pain, the presentation and investigation identifies three types:83
• Type 1 – Abdominal pain, obstructive liver function tests, biliary dilatation and delayed emptying of contrast at ERCP.
• Type 2 – Pain with only one or two of the above-mentioned criteria.
Between 65% and 95% of group 1 patients will be found on biliary manometry to have sphincter of Oddi dysfunction compared to only 12–28% of type 3.82 Diagnosis is usually by exclusion of other causes of abdominal pain such as peptic ulcer disease and irritable bowel syndrome. Liver function tests, abdominal ultrasonography, CT, endoscopy and MRCP have often been already performed. Morphine–neostigmine and secretin provocation MRCP studies may also be of diagnostic value.
Medical therapy with calcium channel blockers, nitrates and botulinum toxin is available but long-term results are unknown. Avoidance of opiate analgesia, particularly over-the-counter preparations containing codeine, may prevent the onset of pain in the majority. Endoscopic sphincterotomy is a potential treatment; however, 5–16% of patients will develop postprocedural pancreatitis84 and good or excellent responses are only reported in 69% of patients at long-term follow-up.85 Surgical sphincterotomy is now indicated rarely due to the lower cost and lower morbidity of endoscopic sphincterotomy but it may be required if the endoscopic approach has been unsuccessful.
References
1. Mieli-Vergani, G., Howard, E.R., Portmann, B., et al, Late referral for biliary atresia: missed opportunities for effective surgery. Lancet 1989; i:421–423. 2563796
2. Schreiber, R.A., Barker, C.C., Roberts, E.A., Canadian Pediatric Hepatology Research Group, Biliary atresia: the Canadian experience. J Pediatr 2007; 151:659–665. 18035148
3. Nijagal, A., Fleck, S., Hills, N.K., et al, Decreased risk of graft failure with maternal liver transplantation in patients with biliary atresia. Am J Transplant. 2012;12(2):409–419. 22221561
4. Douglas, A.H. Case of dilatation of the common bile duct. Monthly J M Sci. 1952; 14:97–101.
5. Suda, K., Miyano, T., Suzuki, F., et al, Clinicopathologic and experimental studies on cases of abnormal pan-creatico-choledocho-ductal junction. Acta Pathol Jpn 1987; 37:1549–1562. 3434280
6. Kim, S.H., Lim, J.H., Yoon, H.K., et al, Choledochal cyst: comparison of MR and conventional cholangiography. Clin Radiol 2000; 55:378–383. 10816405
7. Todani, T., Watanabe, Y., Narusue, M., et al, Congenital bile duct cysts: classification, operative procedures, and review of thirty-seven cases including cancer arising from choledochal cyst. Am J Surg 1977; 134:263–269. 889044
8. Lenriot, J.P., Gigot, J.F., Segol, P., et al, Bile duct cysts in adults: a multi-institutional retrospective study. French Associations for Surgical Research. Ann Surg 1998; 228:159–166. 9712559
9. Todani, T., Watanabe, Y., Toki, A., et al, Carcinoma related choledochal cysts with internal drainage operations. Surg Gynecol Obstet 1987; 164:61–64. 3026058
10. Voyles, C.R., Smadja, C., Shands, W.C., et al, Carcinoma in choledochal cysts. Age-related incidence. Arch Surg 1983; 118:986–988. 6870530
11. Takeshita, N., Ota, T., Yamamoto, M., Forty-year experience with flow-diversion surgery for patients with congenital choledochal cysts with pancreaticobiliary maljunction at a single institution. Ann Surg. 2011;254(6):1050–1053. 21659852
12. Narasimhan, K.L., Chowdhary, S.K., Rao, K.L., Management of accessory hepatic ducts in choledochal cysts. J Pediatr Surg 2001; 36:1092–1093. 11431790
13. Morgenstern, L., McGrath, M.F., Carroll, B.J., et al, Continuing hazards of the learning curve in laparoscopic cholecystectomy. Am Surg 1995; 61:914–918. 7668468
14. Waage, A., Nilsson, M., Iatrogenic bile duct injury: a population-based study of 152 776 cholecystectomies in the Swedish Inpatient Registry. Arch Surg 2006; 141:1207–1213. 17178963 Population-based study in Sweden of 152 776 cholecystectomies with 613 (0.40%) requiring biliary reconstruction.
15. Richardson, M.C., Bell, G., Fullarton, G.M., The West of Scotland Laparoscopic Cholecystectomy Audit Group, Incidence and nature of bile duct injuries following laparoscopic cholecystectomy: an audit of 5913 cases. Br J Surg 1996; 83:1356–1360. 8944450 A prospective audit of biliary injury following cholecystectomy in Scotland. One of the first studies to record the rate of biliary injury and the more severe nature of the injuries at laparoscopic surgery.
16. Flum, D.R., Dellinger, E.P., Cheadle, A., et al, Intraoperative cholangiography and risk of common bile duct injury during cholecystectomy. JAMA 2003; 289:1691–1692. 12672738 A retrospective analysis of more than 1.5 million cholecystectomies detailing the risk of injury and the decreased risk if operative cholangiography is used.
17. Strasberg, S.M., Hertl, M., Soper, N.J., An analysis of the problem of biliary injury during laparoscopic cholecystectomy. J Am Coll Surg 1995; 180:101–125. 8000648 This paper describes a very useful classification system for biliary injury that includes the Bismuth classification as well as other less major injuries.
18. Han, H.J., Choi, S.B., Park, M.S., et al, Learning curve of single port laparoscopic cholecystectomy determined using the non-linear ordinary least squares method based on a non-linear regression model: an analysis of 150 consecutive patients. J Hepatobiliary Pancreat Sci. 2011;18(4):510–515. 21487757
19. Fletcher, D.R., Hobbs, M.S., Tan, P., et al, Complications of cholecystectomy: risks of the laparoscopic approach and protective effects of operative cholangiography: a population-based study. Ann Surg 1999; 229:449–457. 10203075 A retrospective audit of biliary injury in Western Australia that identified the increased risk of biliary injury after laparoscopic cholecystectomy compared to open cholecystectomy. This study also identified a significantly reduced risk of injury if operative cholangiography was performed.
20. Thomson, B.N.J., Cullinan, M.J., Banting, S.W., et al, Recognition and management of biliary complications after laparoscopic cholecystectomy. Aust N Z J Surg 2003; 73:183–188. 12662223
21. Strasberg, S.M., Avoidance of biliary injury during laparoscopic cholecystectomy. J Hepatobiliary Pancreat Surg 2002; 9:543–547. 12541037
22. Way, L.W., Stewart, L., Gantert, W., et al, Causes and prevention of laparoscopic bile duct injuries: analysis of 252 cases from a human factors and cognitive psychology perspective. Ann Surg 2003; 237:460–469. 12677139 Analysis of 252 bile duct injuries according to the principles of the cognitive science of visual perception judgment and human error showing that the majority of errors result from misperception, not errors of skill, knowledge or judgment.
23. Couinaud, C. Le foi. Etudes anatomiques et chirurgicales. Paris: Masson; 1957.
24. Calot, F., De la cholecystectomie, 1891. [Doctoral thesis, Paris].
25. Flum, D.R., Flowers, C., Veenstra, D.L., A cost-effectiveness analysis of intra-operative cholangiography in the prevention of bile duct injury during laparoscopic cholecystectomy. J Am Coll Surg 2003; 196:385–393. 12648690
26. Slater, K., Strong, R.W., Wall, D.R., et al, Iatrogenic bile duct injury: the scourge of laparoscopic cholecystectomy. Aust N Z J Surg 2002; 72:83–88. 12074081
27. Strasberg, S.M., Gouma, D.J., ‘Extreme’ vasculobiliary injuries: association with fundus-down cholecystectomy in severely inflamed gallbladders. HPB (Oxford). 2012;14(1):1–8. 22151444
28. Philips, J.A., Lawes, D.A., Cook, A.J., et al, The use of laparoscopic subtotal cholecystectomy for complicated cholelithiasis. Surg Endosc. 2008;22(7):1697–1700. 18071804
29. Chapman, W.C., Halevy, A., Blumgart, L.H., et al, Postcholecystectomy bile duct strictures. Arch Surg 1995; 130:597–604. 7763167
30. Bismuth, H. Postoperative strictures of the bile duct. In: Blumgart L.H., ed. The biliary tract. Clinical surgery international. Edinburgh: Churchill Livingstone; 1982:209–218.
31. Thomson, B.N., Parks, R.W., Madhavan, K.K., et al, Liver resection and transplantation in the management of iatrogenic biliary injury. World J Surg 2007; 31:2363–2369. 17917775
32. Ardiles, V., McCormack, L., Quiñonez, E., et al, Experience using liver transplantation for the treatment of severe bile duct injuries over 20 years in Argentina: results from a National Survey. HPB (Oxford). 2011;13(8):544–550. 21762297
33. Mirza, D.F., Narsimhan, K.L., Ferras Neto, B.H., et al, Bile duct injury following laparoscopic cholecystectomy: referral pattern and management. Br J Surg 1997; 84:786–790. 9189087
34. Carroll, B.J., Birth, M., Phillips, E.H., Common bile duct injuries during laparoscopic cholecystectomy that result in litigation. Surg Endosc 1998; 12:310–313. 9543519
35. Stewart, L., Way, L.W., Bile duct injuries during laparoscopic cholecystectomy: factors that influence the results of treatment. Arch Surg 1995; 130:1123–1129. 7575127
36. de Reuver, P.R., Busch, O.R., Rauws, E.A., et al, Long-term results of a primary end-to-end anastomosis in peroperative detected bile duct injury. J Gastrointest Surg 2007; 11:296–302. 17458601
37. de Reuver, P.R., Rauws, E.A., Vermeulen, M., et al, Endoscopic treatment of post-surgical bile duct injuries: long term outcome and predictors of success. Gut 2007; 56:1599–1605. 17595232
38. Sandha, G.S., Bourke, M.J., Haber, G.B., et al, Endoscopic therapy for bile leak based on a new classification: results in 207 patients. Gastrointest Endosc 2004; 60:567–574. 15472680
39. Thomson, B.N., Parks, R.W., Madhavan, K.K., et al, Early specialist repair of biliary injury. Br J Surg 2006; 93:216–220. 16329079
40. Buell, J.F., Cronin, D.C., Funakii, B., Devastating and fatal complications associated with combined vascular and bile duct injuries during cholecystectomy. Arch Surg 2002; 137:703–708. 12049542
41. Schmidt, S.C., Settmacher, U., Langrehr, J.M., et al, Management and outcome of patients with combined bile duct and hepatic arterial injuries after laparoscopic cholecystectomy. Surgery 2004; 135:613–618. 15179367
42. Li, J., Frilling, A., Nadalin, S., et al, Management of concomitant hepatic artery injury in patients with iatrogenic major bile duct injury after laparoscopic cholecystectomy. Br J Surg 2008; 95:460–465. 18161898
43. Alves, A., Farges, O., Nicolet, J., et al, Incidence and consequence of an hepatic artery injury in patients with postcholecystectomy bile duct strictures. Ann Surg 2003; 238:93–96. 12832970
44. Mercado, M.A., Chan, C., Orozco, H., et al, Acute bile duct injury. The need for a high repair. Surg Endosc 2003; 17:1351–1355. 12811664
45. Strasberg, S.M., Helton, W.S., An analytical review of vasculobiliary injury in laparoscopic and open cholecystectomy. HPB (Oxford). 2011;13(1):1–14. 22047762
46. Moraca, R.J., Lee, F.T., Ryan, J.A., et al, Long-term biliary function after reconstruction of major bile duct injuries with hepaticoduodenostomy or hepatico-jejunostomy. Arch Surg 2002; 137:889–893. 12146986
47. Bettschart, V., Clayton, R.A., Parks, R.W., et al, Cholangiocarcinoma arising after biliary–enteric drainage procedures for benign disease. Gut 2002; 51:128–129. 12077105
48. Al-Ghnaniem, R., Benjamin, I.S., Long-term outcome of hepaticojejunostomy with routine access loop formation following iatrogenic bile duct injury. Br J Surg 2002; 89:1118–1124. 12190676
49. Gao, J.B., Bai, L.S., Hu, Z.J., et al, Role of Kasai procedure in surgery of hilar bile duct strictures. World J Gastroenterol. 2011;17(37):4231–4234. 22072856
50. Truant, S., Boleslawski, E., Lebuffe, G., et al, Hepatic resection for post-cholecystectomy bile duct injuries: a literature review. HPB (Oxford). 2010;12(5):334–341. 20590909
51. Mercado, M.A., Chan, C., Orozco, H., et al, Long-term evaluation of biliary reconstruction after partial resection of segments IV and V in iatrogenic injuries. J Gastrointest Surg. 2006;10(1):77–82. 16368494
52. Nordin, A., Halme, L., Makisalo, H., et al, Management and outcome of major bile duct injuries after laparoscopic cholecystectomy: from therapeutic endoscopy to liver transplantation. Liver Transpl 2002; 8:1036–1043. 12424717
53. Mercado, M.A., Franssen, B., Dominguez, I., et al, Transition from a low- to a high-volume centre for bile duct repair: changes in technique and improved outcome. HPB (Oxford). 2011;13(11):767–773. 21999589
54. Flum, D.R., Cheadle, A., Prela, C., et al, Bile duct injury during cholecystectomy and survival in medicare beneficiaries. JAMA 2003; 290:2168–2174. 14570952 A retrospective analysis of survival following bile duct injury among Medicare beneficiaries in the USA. This study demonstrates the increased hazard ratio of death following injury in comparison to a control group of routine cholecystectomy patients.
55. Huang, C.S., Lein, H.H., Tai, F.C., et al, Long-term results of major bile duct injury associated with laparoscopic cholecystectomy. Surg Endosc 2003; 17:1362–1367. 12802669
56. Boerma, D., Rauws, E.A., Keulemans, Y.C., et al, Impaired quality of life 5 years after bile duct injury during laparoscopic cholecystectomy: a propsective analysis. Ann Surg 2001; 234:750–757. 11729381 A prospective analysis of quality of life that demonstrated a poor outcome at 5 years, despite successful repair.
57. Melton, G.B., Lillemoe, K.D., Cameron, J.L., et al, Major bile duct injuries associated with laparoscopic cholecystectomy: effect of surgical repair on quality of life. Ann Surg 2002; 235:888–895. 12035047 A study of the impact of biliary injury on quality of life that demonstrated a significantly worse psychological domain, especially in those pursuing legal action.
58. Mirizzi, P.L. Sindrome del conducto hepatico. J Int Chir. 1948; 8:731–777.
59. Johnson, L.W., Sehon, J.K., Lee, W.C., et al, Mirizzi’s syndrome: experience from a multi-institutional review. Am Surg 2001; 67:11–14. 11206888
60. Garden, O.J., Paterson-Brown, S. The gallbladder and bile ducts. In: Garden O.J., ed. Intraoperative and laparoscopic ultrasonography. Oxford: Blackwell Science; 1995:17–43.
61. Binnie, N.R., Nixon, S.J., Palmer, K.R., Mirizzi syndrome managed by endoscopic stenting and laparoscopic cholecystectomy. Br J Surg 1992; 79:647. 1643475
62. Shah, O.J., Dar, M.A., Wani, M.A., et al, Management of Mirizzi syndrome: a new surgical approach. Aust N Z J Surg 2001; 71:423–427. 11450919
63. Schafer, M., Schneiter, R., Krahenbuhl, L., Incidence and management of Mirizzi syndrome during laparoscopic cholecystectomy. Surg Endosc 2003; 17:1186–1190. 12739118
64. Lo, C.M., Fan, S.T., Wong, J., The changing epidemiology of recurrent pyogenic cholangitis. Hong Kong Med J 1997; 3:302–304. 11847376
65. Tabata, M., Nakayama, F., Bacteriology of hepatolithiasis. Prog Clin Biol Res 1984; 152:163–174. 6382275
66. Suzuki, Y., Mori, T., Abe, N., et al, Predictive factors for cholangiocarcinoma associated with hepatolithiasis determined on the basis of Japanese Multicenter study. Hepatol Res. 2012;42(2):166–170. 22151748
67. Uchiyama, K., Kawai, M., Ueno, M., et al, Reducing residual and recurrent stones by hepatectomy for hepatolithiasis. J Gastrointest Surg 2007; 11:626–630. 17468921
68. Belghiti, J., Benhamou, J.P., Houry, S., et al, Caustic sclerosing cholangitis. A complication of the surgical treatment of hydatid disease of the liver. Arch Surg 1986; 121:1162–1165. 3767649
69. Molodecky, N.A., Kareemi, H., Parab, R., et al, Incidence of primary sclerosing cholangitis: a systematic review and meta-analysis. Hepatology. 2011;53(5):1590–1599. 21351115
70. Montefusco, P.P., Geiss, A.C., Bronzo, R.L., et al, Sclerosing cholangitis, chronic pancreatitis, and Sjögren’s syndrome: a syndrome complex. Am J Surg 1984; 147:822–826. 6731702
71. Chapman, R.W., Kelly, P.M., Heryet, A., et al, Expression of HLA-DR antigens on bile duct epithelium in primary sclerosing cholangitis. Gut 1988; 29:422–427. 3286382
72. Tischendorf, J.J., Hecker, H., Krüger, M., et al, Characterization, outcome, and prognosis in 273 patients with primary sclerosing cholangitis: a single center study. Am J Gastroenterol 2007; 102:107–114. 17037993
73. Fevery, J., Henckaerts, L., Van Oirbeek, R., et al, Malignancies and mortality in 200 patients with primary sclerosering cholangitis: a long-term single-centre study. Liver Int. 2012;32(2):214–222. 21745316
74. Beuers, U., Spengler, U., Kruis, W., et al, Ursodeoxycholic acid for treatment of primary sclerosing cholangitis: a placebo-controlled trial. Hepatology 1992; 16:707–714. 1505913 A prospective randomised double-blind trial that demonstrated the efficacy of ursodeoxycholic acid in the treatment of primary sclerosing cholangitis.
75. Aljiffry, M., Renfrew, P.D., Walsh, M.J., et al, Analytical review of diagnosis and treatment strategies for dominant bile duct strictures in patients with primary sclerosing cholangitis. HPB (Oxford). 2011;13(2):79–90. 21241424
76. Roberts, M.S., Angus, D.C., Bryce, C.L., et al, Survival after liver transplantation in the United States: a disease-specific analysis of the UNOS database. Liver Transpl 2004; 10:886–897. 15237373
77. Prytz, H., Keiding, S., Björnsson, E., et al, Dynamic FDG-PET is useful for detection of cholangiocarcinoma in patients with PSC listed for liver transplantation. Hepatology 2006; 44:1572–1580. 17133469
78. Weber, S.M., DeMatteo, R.P., Fong, Y., et al, Staging laparoscopy in patients with extrahepatic biliary carcinoma. Analysis of 100 patients. Ann Surg 2002; 235:392–399. 11882761
79. Uhlmann, D., Wiedmann, M., Schmidt, F., et al, Management and outcome in patients with Klatskin-mimicking lesions of the biliary tree. J Gastrointest Surg 2006; 10:1144–1150. 16966034
80. Weber, S.M., Cubukcu-Dimopulo, O., Palesty, J.A., et al, Lymphoplasmacytic sclerosing pancreatitis: inflammatory mimic of pancreatic carcinoma. J Gastrointest Surg 2003; 7:129–137. 12559194
81. Church, N.I., Pereira, S.P., Deheragoda, M.G., et al, Autoimmune pancreatitis: clinical and radiological features and objective response to steroid therapy in a UK series. Am J Gastroenterol 2007; 102:2417–2425. 17894845
82. Corazziari, E., Sphincter of Oddi dysfunction. Dig Liver Dis 2003; 35:S26–S29. 12974506
83. Geenen, J.E., Hogan, W.J., Dodds, W.J., et al, The efficacy of endoscopic sphincterotomy after cholecystectomy in patients with sphincter of Oddi dysfunction. N Engl J Med 1989; 320:82–87. 2643038
84. Lehman, G.Y., Sherman, S., Sphincter of Oddi dysfunction. Int J Pancreatol 1996; 20:11–25. 8872520
85. Freeman, M.L., Gill, M., Overby, C., et al, Predictors of outcomes after biliary and pancreatic sphincterotomy for sphincter of Oddi dysfunction. J Clin Gastroenterol 2007; 41:94–102. 17198071