Chapter 44 Benign Biliary Strictures and Leaks
Introduction
Accidental injuries of the bile ducts leading to biliary leaks and strictures may occur during any surgical procedure involving the biliary tract. However, the main cause of injury of the bile ducts at the present time is laparoscopic cholecystectomy (LC). Although LC has proved to be superior to open cholecystectomy in terms of shorter hospitalization, lower overall morbidity, faster recovery, and better cosmetic outcome, the risk of bile duct injury during LC is two to six times greater compared with open cholecystectomy.1,2 Bergman and colleagues3 described four types of postoperative bile duct injuries, as follows:
Epidemiology
LC was first performed by Mouret in France in 1987. The technique was standardized by two other French surgeons, Dubois in Paris and Perissat in Bordeaux.4,5 This new technique spread very rapidly around the world; in the United States, the percentage of cholecystectomies done laparoscopically grew from zero in 1987 to almost 80% in 1992.6 The advent of LC also induced an estimated increase of at least 25% in the overall number of cholecystectomies performed,7,8 so that the likely number of cholecystectomies performed at the present time in the United States is 800,000 per year.9 The number of iatrogenic injuries to the bile duct has increased accordingly.10
Many reasons may explain the increased incidence of biliary complications at the beginning of the laparoscopic era, most related to the new technical skills required to perform laparoscopically what had been done by open surgery: bidimensional vision, loss of tactile sensations, different visual approach of the hepatic pedicle, difficult hemostatic maneuvers, abuse of electrocoagulation, and lack of confidence with the new instrumentation.11 The rate of injuries seemed to be related to the surgeon’s learning curve and his or her personal experience. An inversely proportional relationship between the number of cholecystectomies performed and the rate of injuries was suggested by earlier reported series.1,12 In a review of 77,604 LCs performed in the United States, the incidence of biliary injuries decreased from 0.6% to 0.4% (P < .001) for surgical teams having an experience of more than 100 LCs.1 A Belgian survey11 suggested the number of 50 LCs as the threshold of a completed learning curve; however, the same authors emphasized that one-third of the biliary injuries in their country had occurred with surgeons with an experience of more than 100 LCs. When reviewing several multicenter series published before 1995, totaling 198,267 LCs, the incidence of biliary injuries was 0.55% in 13 European series and 0.49% in 17 series outside Europe.13
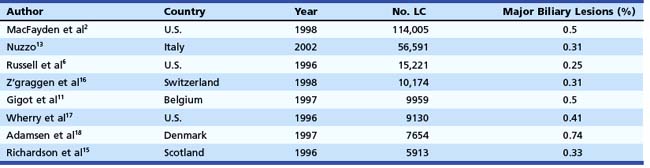
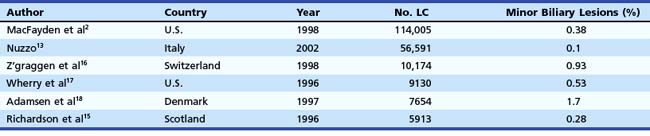
In the mid-1990s, the incidence of biliary injury seemed to be three times higher for LC than for open cholecystectomy. However, these figures most likely underestimated reality because there was a tendency not to declare all the lesions, as revealed by the low rate of reply to most surveys and by the increasing number of reported lesions in direct proportion with the collected replies.14 At the present time, the incidence of biliary injuries has not substantially changed, even if a trend toward reduction has been reported by some authors.6,15 The estimated overall incidence is 0.25% to 0.74% for major biliary lesions (Table 44.1) and 0.1% to 1.7% for minor biliary lesions (Table 44.2).16–18 These figures are only partially explained by the still increasing number of LCs performed around the world and by the activity of young surgeons at the beginning of their learning curve. However, at least one-third of biliary injuries may be ascribed to technical mistakes during surgery.19 The learning curve is not the only risk factor of LC.
Pathogenesis
Unrecognized CBD stones are one of the major risk factors of cystic duct leakage after LC. The mechanism and the cause of a biliary injury remain unexplainable in at least one-third of cases.13 In more than 50% of cases, the injury occurs during the dissection of the cystic duct or during separation of the gallbladder neck from the CBD. Misinterpretation of the cystic duct and the CBD is the most common cause of injury.12 Excessive traction on the gallbladder neck, especially if the tissues are not inflamed, may facilitate the injury of the CBD. Conversely, when the area is acutely or chronically inflamed or when a stone is trapped into the gallbladder infundibulum, the risk of CBD injury is higher during the dissection of the gallbladder neck from the hepatic pedicle. Other recurrent reasons for bile duct injury are related to incorrect hemostatic maneuvers in case of bleeding from the cystic artery; inappropriate use of electrocautery; and other specific maneuvers, such as intraoperative cholangiography, cystic duct dilation, and transcystic CBD instrumental exploration.
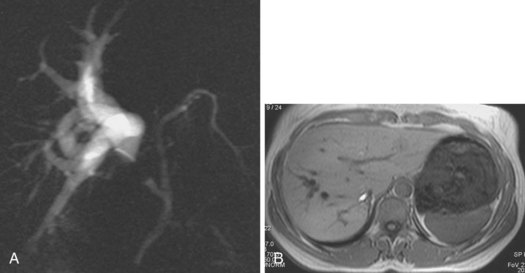
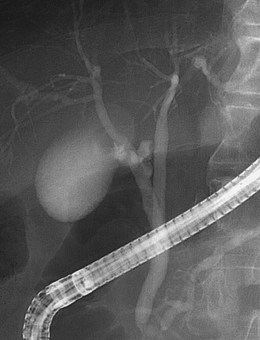
An anatomic anomaly is often reported by the surgeon as having caused a biliary injury. Variations of the biliary anatomy, especially at the level of the main hepatic confluence, are present in 50% of patients (see the section on interpretation of intrahepatic cholangiography). Surgeons must be aware of such variations and must keep in mind the danger of injuring aberrant ducts originating in the right liver during dissection of the gallbladder pedicle. Aberrant ducts must not be interpreted as accessory ducts because the biliary distribution within the liver parenchyma is of a terminal type; this implies that there are no intrahepatic anastomoses between the ducts and that every injury of an aberrant duct would determine functional exclusion of the corresponding liver area. Injury to a small aberrant duct may still be considered a minor lesion; however, it would cause a bile leak into the peritoneal space with all the related consequences. Another cause of injury is clipping or ligation of an aberrant duct. This injury does not involve a bile leak but entails the functional exclusion of the corresponding liver area leading to its progressive atrophy and hypertrophy of the remaining liver parenchyma. This possible event may be clinically totally asymptomatic and noted only by an increase in biochemical parameters of cholestasis and cytolysis (Fig. 44.1). Although there is no indication of treatment in asymptomatic cases, if the obstructed ducts become infected, recurrent cholangitis is the typical clinical manifestation often requiring operative reestablishment of an adequate bile flow.
Treatment
Endoscopic Retrograde Cholangiopancreatography and Biliary Leak
The presence of a bile leak invariably indicates a break in the continuity of the biliary system. However, the severity of the injury (ranging from simple leakage of the cystic stump to complete transection of the bile duct) and the complexity of its repair are extremely variable. The magnitude of the bile output does not usually help in presuming the origin and the size of the leak. A direct cholangiogram is of utmost importance for accurate anatomic depiction and to classify the type of injury to plan therapy. The usefulness of magnetic resonance cholangiopancreatography (MRCP) in the delineation of postoperative biliary leaks20 and of iatrogenic strictures21 has been reported. However, in contrast to ERCP, MRCP has no therapeutic capability. MRCP may be recommended in anatomic situations in which the endoscopic approach is presumably difficult or occasionally impossible, such as in patients with a Billroth II anatomy and with Roux-en-Y hepaticojejunostomy.
In case of external biliary fistula through an abdominal drain, it is not advisable to start the procedure by injecting contrast medium through the drain (fistulography). In most instances, especially in minor lesions, the contrast medium freely flows into the peritoneal space without depicting the biliary tree. The presence of contrast medium overlapping the area involved by the lesion may hinder the correct interpretation of subsequent cholangiography and occasionally disguise the picture entirely. In contrast, fistulography is indicated whenever endoscopic cholangiography shows an incomplete filling of the biliary system resulting from a complete transection of the main bile duct or lack of visualization of a sectorial or segmental intrahepatic branch. As an alternative to contrast medium, which occasionally might not fill the missing branch, air may be used to obtain a pneumocholangiogram.22
The technique of ERCP in the setting of a suspected biliary injury does not substantially differ from the routine examination. Special attention should be paid to the injection of contrast medium, however, which should be slow and careful to allow precise delineation of the lesions. Massive injection of the biliary tree should be avoided. Minimal injection and early filling x-ray films are also important in detecting small residual CBD stones, which are present in 20% of patients with biliary leakage originating from the cystic duct stump. If the suspected lesion is located in an intrahepatic biliary branch, it is of paramount importance to obtain a complete intrahepatic cholangiogram; to achieve an adequate pressure of injection, especially if a sphincterotomy has been previously performed, the use of an occlusion balloon catheter is advisable. Intrahepatic biliary anatomy is better shown by multiple x-ray films taken in different projections. Percutaneous transhepatic cholangiography and MRCP should be reserved for patients in whom ERCP fails technically or fails to show the intrahepatic biliary anatomy because of proximal ductal disruption.23
Interpretation of Intrahepatic Cholangiography
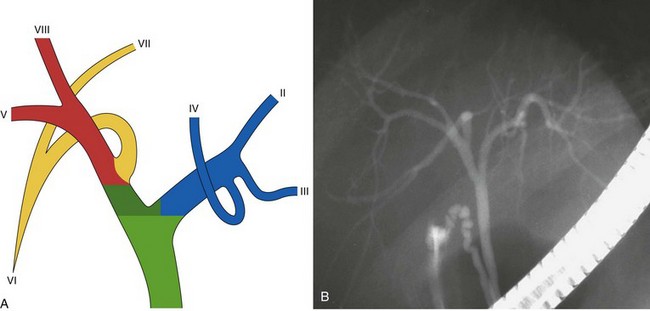
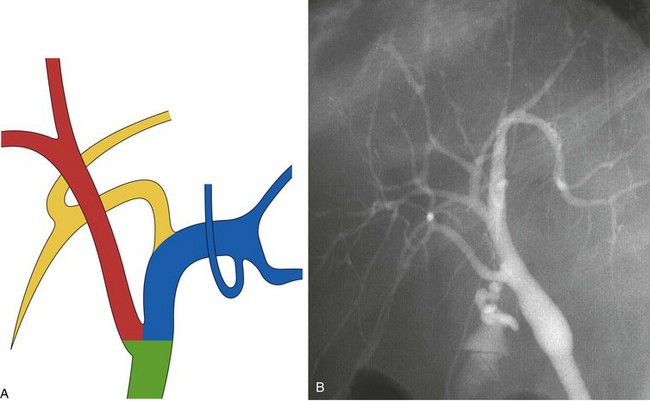
The main biliary confluence is formed by the union of the right and left hepatic ducts that drain the bile originating in the right hemiliver and the left hemiliver. The main confluence is often incorrectly called bifurcation in the English literature; actually, although the portal vein and the hepatic artery carrying the blood to the liver have bifurcations, the fusion of ducts collecting the bile from the liver with a flow directed toward the CBD generates a confluence. According to the segmental liver anatomy described by Couinaud,24 the left hepatic duct collects the bile originating from segments II and III (left anatomic liver lobe or left lateral sector) and from segment IV (quadrate lobe). One or more small ducts originating from segment I (caudate lobe) also join the left hepatic duct close to the main confluence. The anatomic variations occurring in the left hepatic system are rare and are irrelevant in this perspective. The right hepatic duct is shorter than the left hepatic duct and follows the same axis of the common hepatic duct. The right hepatic duct originates from the confluence of the right anteromedial sectorial duct (segments V and VIII) and of the right posterolateral sectorial duct (segments VI and VII). The right anteromedial duct is recognizable thanks to its orientation, which follows the same axis of the right hepatic duct, whereas the right posterolateral duct joins the right anteromedial duct on its medial aspect with a typical umbrella handle–like shape.
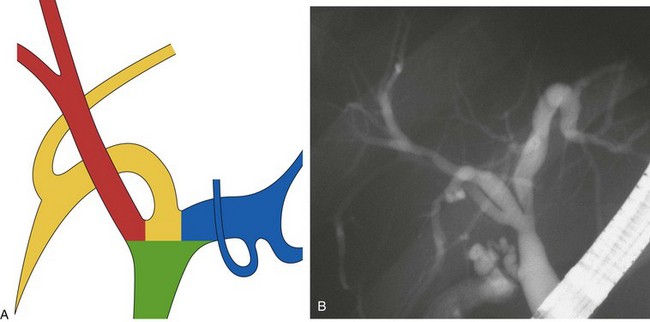
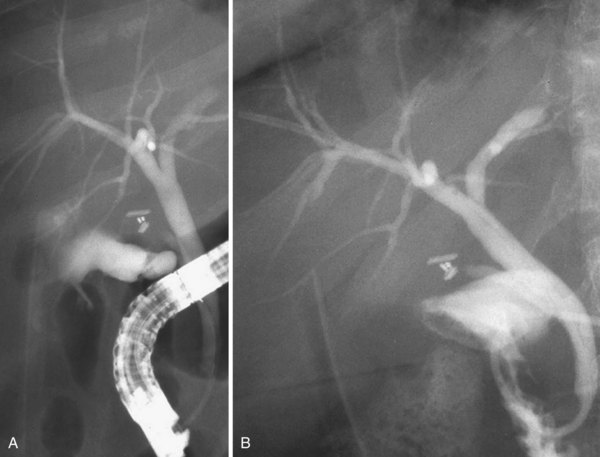
This normal anatomy (called “modal” by Couinaud) is present in approximately 60% of the population (Fig. 44.2). The main hepatic confluence is usually located high in the hilar region. A lower position at the level of the hepatic pedicle may also occur, causing a much closer proximity to the insertion of the cystic duct into the hepatic duct (Fig. 44.3). The main variations of the main hepatic confluence are the absence of the right hepatic duct with the anteromedial and posterolateral right ducts joining independently the left duct to form the confluence (Fig. 44.4) or with one of the two right sectorial ducts joining the CBD at a more distal level closer to the insertion of the cystic duct (Fig. 44.5). More rarely, an isolated segmental or subsegmental duct may join the CBD away from the main confluence, usually on the lateral aspect of the CBD close to the insertion of the cystic duct. Most aberrant ducts arise from the right liver and drain into the common hepatic duct or cystic duct within 30 mm of the hepatocystic angle.25
These are the most dangerous anatomic variations for the surgeon during the dissection of the gallbladder pedicle. Apart from complete transection of the CBD (type D of the Bergman classification) (Fig. 44.6), which is typically an indication for open surgical repair, an attempt at endoscopic treatment may be envisaged in all other circumstances of biliary injury with concomitant bile leak. The basic principle of endoscopic treatment is abolition of the transpapillary pressure gradient, equalizing the bile duct and duodenal pressures and allowing flow of bile into the duodenum.26
Endoscopic Treatment of Minor Lesions
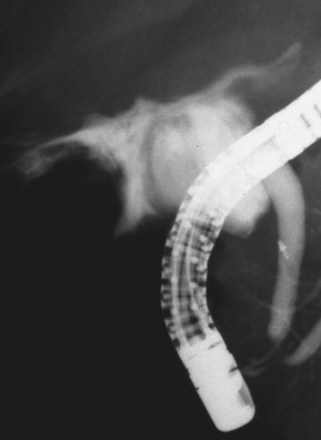
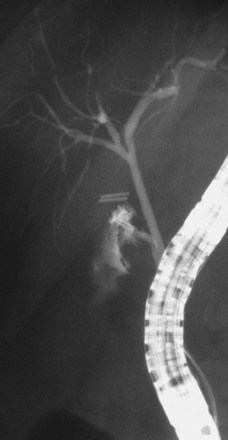
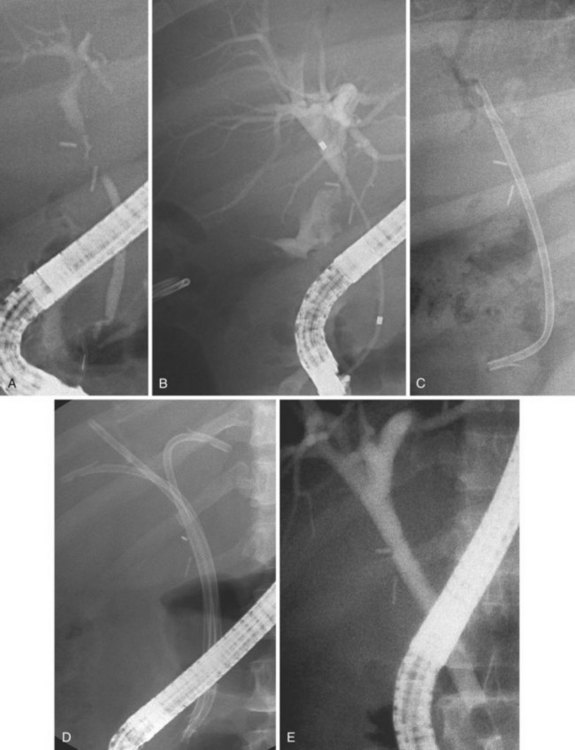
The largest part of biliary leaks from minor lesions originates from the cystic duct stump (Fig. 44.7). Bile leakage from the cystic duct stump may be due to stump dehiscence resulting from defective technique in clips positioning, inadvertent injury to the cystic wall below the closure, or partial disruption of the cystic duct implantation into the bile duct resulting from excessive traction. Biliary hypertension resulting from temporary impaction of a residual bile duct stone into the sphincter of Oddi in the early postoperative period is most likely the cause of cystic duct stump dehiscence in almost one-fifth of patients. Similarly, a simple spasm of the sphincter of Oddi theoretically may create enough pressure to induce dislodgment of clips placed on the cystic duct stump. Bile leaks can also originate from severed ducts of Luschka (small peripheral ducts connecting the intrahepatic system with the gallbladder lumen) (Fig. 44.8), small subsegmental ducts running in the gallbladder bed, and segmental or subsegmental aberrant branches joining the CBD in the proximity of the cystic duct.
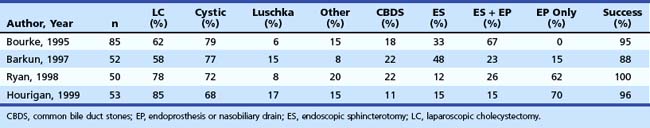
Treatment of these leaks does not differ from treatment employed when the leak arises from the cystic duct stump (Table 44.3). The transpapillary pressure gradient can be equalized by endoscopic sphincterotomy (ES) alone,27,28 ES and stent or nasobiliary drain (NBD) placement,29 and stent30,31 or NBD placement alone31 without preliminary ES (Fig. 44.9). All methods seem to be equally effective in facilitating the closure of the biliary leak usually within 1 week of treatment.3,26,30–33 The endoscopic approach of choice is controversial. However, if stones are present in the CBD, ES and stone extraction with or without stent or NBD placement seems the most logical approach. However, each option has specific limitations. ES is associated with inherent immediate and potential long-term complications; stent placement requires a second procedure to remove the stent, which can also become clogged or can migrate; and NBD requires a prolonged hospital stay, is uncomfortable for the patient, and may be accidentally displaced. Advantages and disadvantages of the different options are summarized in Table 44.4.
Table 44.4 Endoscopic Options to Treat Postoperative Bile Leaks (Type A according to Bergman)
| Procedure | Advantages | Disadvantages |
|---|---|---|
| ES | Treatment of associated CBD stones | Complications |
| Nasobiliary drain (days) | Avoids ES | Uncomfortable |
| Allows check cholangiography | Prolongs hospitalization | |
| Stent placement (wks) | Avoids ES | Repeat ERCP required |
| Clogging, dislocation |
CBD, common bile duct; ERCP, endoscopic retrograde cholangiopancreatography; ES, endoscopic sphincterotomy.
To select the optimal endoscopic therapy, Sandha and colleagues34 proposed classifying the bile leaks by ERCP into two categories: low-grade (identified only after intrahepatic opacification) and high-grade (observed before intrahepatic opacification). Of 104 low-grade leaks, 75 were treated by ES alone with a success rate of 91%. Of 100 high-grade leaks, 97 were treated by stent insertion with a final success rate (4 patients had to undergo retreatment) of 100%. The investigators concluded that this simple, practical endoscopic classification system might be clinically relevant in the choice of endoscopic therapy. Endoscopic local injection of botulinum toxin (Botox) to decrease the transpapillary bilioduodenal pressure gradient has also been reported.35 Injection of 100 IU of botulinum toxin into the sphincter of Oddi was shown to lower CBD pressure significantly within 24 hours. This effect lasted for 2 weeks on average in the animal model.
Endoscopic Treatment of Major Lesions
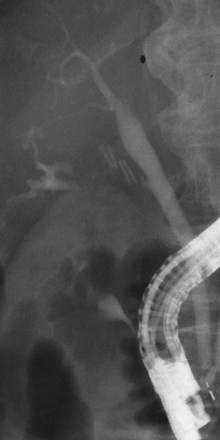
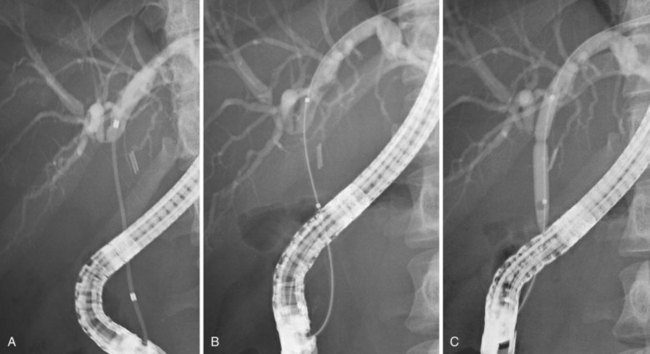
Bile leakage in major lesions originates from a tear on the CBD or on one of the biliary branches that form the main hepatic confluence (type B). In both instances, ES alone may be inadequate to seal the leak. It is preferable to insert at least one large-bore plastic stent (10-Fr to 11.5-Fr) long enough to bypass the site of injury. The secondary intent of stent placement is to prevent the development of stricture at the site of the injured bile duct wall.23,26 For this purpose, the stent should be left in place for several months to allow the healing process to stabilize. In case of secondary stricture formation at the site of injury, the presence of a stent already in place facilitates successive endoscopic maneuvers to dilate the stricture. Therapeutic success may be obtained in 71% to 79% of cases (Fig. 44.10).3,36,37
Biliary stents have also been successfully used to reestablish the continuity of disrupted segmental branches at the level of the main hepatic confluence22 and for leaks from aberrant bile ducts.23,38 In major biliary injury with bile leakage, the primary therapeutic objective is to seal the leak to convert an acute problem into a stabilized condition. The high efficacy of the endoscopic approach in this setting justifies its use as a first-line treatment whenever possible. Treatment of postoperative bile duct strictures in the prelaparoscopic era was traditionally surgical. The role of ERCP was limited to the diagnostic phase and particularly to the definition of the level and extent of the lesion.39
Along with the increasing use of ERCP in the evaluation and treatment of acute complications of LC, therapeutic ERCP has been increasingly employed to manage postoperative strictures occurring both early and late postoperatively. The first nonoperative alternative in the management of bile duct strictures has been the percutaneous transhepatic approach. After establishment of percutaneous access to the intrahepatic bile ducts, the stricture is crossed with a guidewire, and pneumatic balloon dilation is performed. Although instantly very effective, this approach has a very limited value in the long-term because of the high rate of stricture recurrence.40 Approximately one-third of patients undergoing this treatment modality experience complications, and stricture recurrence develops in at least 25% of cases during follow-up.41,42 In another series published by the group at The Johns Hopkins University, the success rate of these procedures was only 55%, with 20% of patients having significant hemobilia.43 The high recurrence rate after percutaneous pneumatic dilation is most likely due to the forceful disruption of the scar, which can add further traumatic damage to the tissue and consequential development of new local fibrogenic reaction.
Description of Technique
Getting over the Stricture
The morphologic requirement that allows getting over the stricture is the continuity of the CBD. In case of complete transection or obstruction of the bile duct, the endoscopic option alone is applicable in only a very few cases. A combined percutaneous and endoscopic approach with an aim at reconstructing the missing segment of the bile duct has been described.44 A similar combined approach has also been described in the case of complete obstruction of the distal biliary stump; percutaneous puncture of the distal stump was performed under radiologic guidance with a device designed for nonbiliary use (a set marketed for placing transjugular intrahepatic portosystemic shunts).45 However, because of the lack of standardization, these approaches cannot be recommended on a routine basis.
Dilation of the Stricture
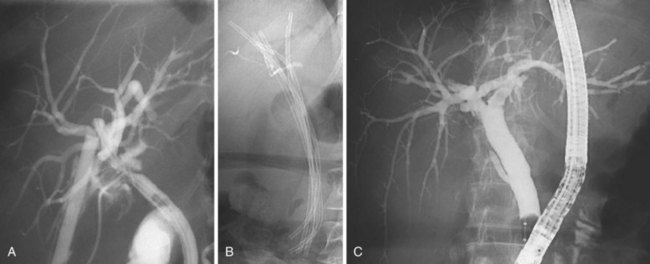
Dilation of the stricture has two objectives: (1) to reopen the bile duct to achieve a regular bile flow and (2) to secure the dilation to avoid restricture in the long-term. In the beginning of endoscopic treatment, only the first objective was pursued; in the percutaneous approach, the mainstay of treatment was pneumatic dilation alone.46 However, it soon became clear that even if immediately very effective, pneumatic dilation alone was ineffective in granting good results in a long-term follow-up. At the present time, pneumatic dilation is mainly used as a preliminary step before placement of one or more plastic stents (Fig. 44.11). The role of stent placement is to keep the stricture open for a long time (months to years according to different treatment strategies), while allowing scar modeling and its consolidation.47
Typically, two 10-Fr stents are placed, exchanged every 3 months to avoid cholangitis resulting from stent occlusion, and left in place for 1 year. In a retrospective study from the Amsterdam group reporting on the multidisciplinary experience obtained during a decade (1981–1990), the long-term results of endoscopic treatment were compared with the long-term results of surgery.48 Surgery (Roux-en-Y hepaticojejunostomy) was performed in 35 patients, and endoscopic treatment was performed in 66 patients. Patient characteristics, type of initial injury, and level of obstruction were not significantly different in the two groups. At a mean follow-up of 50 months and 42 months for the surgical and the endoscopic groups, 83% of patients in both groups had an excellent (asymptomatic patient with normal or stable laboratory parameters) or good (single episode of cholangitis) result. Immediate complication rate was in favor of endoscopic treatment (8% vs. 26% for surgical treatment), whereas 21% of the patients had at least one episode of cholangitis resulting from stent malfunction during the stent period (two 10-Fr stents for 1 year with stent exchange every 3 months).
When analyzing the long-term results, it becomes immediately evident how the time interval between the end of treatment and the symptomatic recurrence of the stricture is much shorter in the group with endoscopic treatment compared with the surgical group (on average 3 ± 11 months vs. 40 ± 11 months), indicating possible undertreatment in the endoscopy group. However, this important study showed that endoscopic treatment may be considered at least as effective as surgical treatment in terms of long-term results, having the big advantage of not hindering any further surgery if necessary. Several other experiences of endoscopic treatment with plastic stents of postoperative biliary strictures have been published in recent years.3,47–56 From the analysis of the available data, however, this treatment modality still seems far from standardized; the published experiences differ in terms of number of stents placed, their caliber, exchange intervals, and definition of treatment objectives and of outcomes. Examples of two different methodologic approaches follow:
 The treatment protocol used by the Amsterdam group (74 patients) is the classic one, entailing placement of two 10-Fr stents, exchanged every 3 months for 1 year (the period of stent placement).53 Preliminary pneumatic dilation had been performed in approximately one-fourth of the patients before stent insertion. A combined percutaneous-endoscopic approach to bypass the stricture with a guidewire was required in only three cases. Stents were removed after 1 year.
The treatment protocol used by the Amsterdam group (74 patients) is the classic one, entailing placement of two 10-Fr stents, exchanged every 3 months for 1 year (the period of stent placement).53 Preliminary pneumatic dilation had been performed in approximately one-fourth of the patients before stent insertion. A combined percutaneous-endoscopic approach to bypass the stricture with a guidewire was required in only three cases. Stents were removed after 1 year. The protocol described by our group (55 patients)52 involved the placement of the maximum possible number of stents (ideally 10-Fr) in relation to the tightness of the stricture and diameter of the CBD at every treatment session with a trimonthly interval. Treatment was continued until complete morphologic disappearance of the stricture at cholangiography (Fig. 44.12).
The protocol described by our group (55 patients)52 involved the placement of the maximum possible number of stents (ideally 10-Fr) in relation to the tightness of the stricture and diameter of the CBD at every treatment session with a trimonthly interval. Treatment was continued until complete morphologic disappearance of the stricture at cholangiography (Fig. 44.12). In the Amsterdam series, the technical success of stenting was 80%; however, only 44 patients (59% of the initial cohort and 75% of patients in whom an initial technical success had been obtained) concluded the 12-month stent period for different reasons. At a median follow-up of 9 years, 9 of 44 patients (20%) developed recurrent strictures. In eight of nine cases, recurrent strictures developed within the first 6 months of follow-up (median 2.6 months). On an intention-to-treat basis, this protocol was able to resolve definitively the bile duct stricture in 47% of the initial cohort. The results of this study suggest that endoscopic stent placement is not the best treatment option for patients with low compliance to repeated treatment sessions. Similar results, with 81% of patients symptom-free at a mean follow-up of 9.5 years, were reported in an abstract form by the Toronto group by using the same treatment protocol.51
In the Amsterdam series, the technical success of stenting was 80%; however, only 44 patients (59% of the initial cohort and 75% of patients in whom an initial technical success had been obtained) concluded the 12-month stent period for different reasons. At a median follow-up of 9 years, 9 of 44 patients (20%) developed recurrent strictures. In eight of nine cases, recurrent strictures developed within the first 6 months of follow-up (median 2.6 months). On an intention-to-treat basis, this protocol was able to resolve definitively the bile duct stricture in 47% of the initial cohort. The results of this study suggest that endoscopic stent placement is not the best treatment option for patients with low compliance to repeated treatment sessions. Similar results, with 81% of patients symptom-free at a mean follow-up of 9.5 years, were reported in an abstract form by the Toronto group by using the same treatment protocol.51 In our study, 42 of 55 patients initially considered were evaluable at a mean follow-up of 49 months after the end of treatment. Ten patients were excluded from the protocol because of complete CBD section (n = 5) or use of self-expandable metallic stents (n = 5). Another three patients were not evaluable for different reasons. Two patients died of unrelated causes during follow-up. Among the remaining 40 patients, there was no recurrence of symptoms caused by relapsing biliary stricture. One patient sustained two episodes of cholangitis but without stricture recurrence. By an intention-to-treat analysis, the success rate was 89% (40 of 45). Although the follow-up period in our series is shorter compared with the Amsterdam series, it is longer than the typical period during which all the recurrences after endoscopic treatment have been described (2 years). This more aggressive approach to endoscopic treatment with stents seems to improve long-term results for patients with postoperative biliary strictures. We reported more recently on the very long-term results of this aggressive approach in the same cohort of patients57; of 35 evaluable patients after a mean follow-up of 13.7 years, 7 (20%) had recurrent cholangitis owing to relapse of the stricture in 4 patients (all successfully retreated with stents) and newly formed bile duct stones in 3 patients. The remaining 28 patients remained completely asymptomatic with normal liver function tests and abdominal ultrasound.
In our study, 42 of 55 patients initially considered were evaluable at a mean follow-up of 49 months after the end of treatment. Ten patients were excluded from the protocol because of complete CBD section (n = 5) or use of self-expandable metallic stents (n = 5). Another three patients were not evaluable for different reasons. Two patients died of unrelated causes during follow-up. Among the remaining 40 patients, there was no recurrence of symptoms caused by relapsing biliary stricture. One patient sustained two episodes of cholangitis but without stricture recurrence. By an intention-to-treat analysis, the success rate was 89% (40 of 45). Although the follow-up period in our series is shorter compared with the Amsterdam series, it is longer than the typical period during which all the recurrences after endoscopic treatment have been described (2 years). This more aggressive approach to endoscopic treatment with stents seems to improve long-term results for patients with postoperative biliary strictures. We reported more recently on the very long-term results of this aggressive approach in the same cohort of patients57; of 35 evaluable patients after a mean follow-up of 13.7 years, 7 (20%) had recurrent cholangitis owing to relapse of the stricture in 4 patients (all successfully retreated with stents) and newly formed bile duct stones in 3 patients. The remaining 28 patients remained completely asymptomatic with normal liver function tests and abdominal ultrasound.Despite some controversies,58 at the present time, multiple stent placement is a well-accepted strategy adopted worldwide for postoperative bile duct strictures.59,60 According to the published data, endoscopic treatment with stents of major bile duct injuries and strictures is at least as effective as surgical treatment. The advantages of endoscopic treatment are its simplicity, reversibility, and minimal invasiveness. Endoscopic treatment should always be considered, whenever available, in the therapeutic algorithm of most patients with major bile duct injuries. For most patients, it may be the only treatment required. Endoscopy and surgery should be considered as complementary treatments. This complex and difficult pathology is best managed in centers in which a multidisciplinary approach is available.
Future Trends
The main limitation of endoscopic treatment of postoperative bile strictures with the current method of multiple plastic stent placement is the need for repeat interventions over a long time (1 year on average). The ideal stent would allow progressive dilation of the stricture during weeks or months and would dissolve once the goal had been reached. Uncovered self-expanding metal stents (SEMS) have proved to be a bad alternative to plastic stents for several reasons.54 First, SEMS invariably induce a hyperplastic response of the inflammatory tissue at the level of the stricture. This hyperplastic reaction ultimately leads to occlusion of SEMS, on average less than 1 year after their placement. Second, SEMS are usually not removable; treatment of secondary stricture resulting from hyperplastic reaction requires repeated balloon dilations and plastic stent placement. Third, biliary SEMS have been developed to produce abrupt recanalization of a stricture resulting from neoplastic invasion; the radial force exerted by the stent is much higher than the force desirable to induce progressive dilation of a scar, such as the scar of postoperative bile duct strictures.
Partially covered SEMS have the advantage of avoiding ingrowth of hyperplastic tissue at the level of the stricture and of being often removable61; however, no clinical experience is available. The advent of fully covered, removable SEMS has raised new interest concerning their potential use in benign conditions.62 However, the experience in postoperative biliary strictures is still scanty and controversial.63 Drug-eluting, self-expandable stents have been used in the vascular system to inhibit endothelial growth; it is conceivable that this technology might become available for use in the biliary system. Local release of antiinflammatory drugs able to control the fibrogenetic process that occurs during healing of a biliary injury may be valuable in this setting.
1 Deziel DJ, Millikan KW, Economou SG, et al. Complications of laparoscopic cholecystectomy: A national survey of 4,292 hospitals and an analysis of 77,604 cases. Am J Surg. 1993;165:9-14.
2 MacFadyen BVJr, Vecchio R, Ricardo AE, et al. Bile duct injury after laparoscopic cholecystectomy: The United States experience. Surg Endosc. 1998;12:315-321.
3 Bergman JJ, van den Brink GR, Rauws EA, et al. Treatment of bile duct lesions after laparoscopic cholecystectomy. Gut. 1996;38:141-147.
4 Dubois F, Berthelot G, Levard H. [Cholecystectomy by coelioscopy]. Presse Med. 1989;18:980-982.
5 Perissat J, Collet D, Belliard R, et al. Laparoscopic cholecystectomy: The state of the art. A report on 700 consecutive cases. World J Surg. 1992;16:1074-1082.
6 Russell JC, Walsh SJ, Mattie AS, et al. Bile duct injuries, 1989–1993: A statewide experience. Connecticut Laparoscopic Cholecystectomy Registry. Arch Surg. 1996;131:382-388.
7 Legorreta AP, Silber JH, Costantino GN, et al. Increased cholecystectomy rate after the introduction of laparoscopic cholecystectomy. JAMA. 1993;270:1429-1432.
8 Shea JA, Healey MJ, Berlin JA, et al. Mortality and complications associated with laparoscopic cholecystectomy: A meta-analysis. Ann Surg. 1996;224:609-620.
9 Moody FG. Bile duct injury during laparoscopic cholecystectomy. Surg Endosc. 2000;14:605-607.
10 Strasberg SM, Hertl M, Soper NJ. An analysis of the problem of biliary injury during laparoscopic cholecystectomy. J Am Coll Surg. 1995;180:101-125.
11 Gigot J, Etienne J, Aerts R, et al. The dramatic reality of biliary tract injury during laparoscopic cholecystectomy: An anonymous multicenter Belgian survey of 65 patients. Surg Endosc. 1997;11:1171-1178.
12 Davidoff AM, Pappas TN, Murray EA, et al. Mechanisms of major biliary injury during laparoscopic cholecystectomy. Ann Surg. 1992;215:196-202.
13 Nuzzo G: Personal communication, unpublished data, 2002.
14 Fletcher DR. Biliary injury at laparoscopic cholecystectomy: Recognition and prevention. Aust N Z J Surg. 1993;63:673-677.
15 Richardson MC, Bell G, Fullarton GM. Incidence and nature of bile duct injuries following laparoscopic cholecystectomy: An audit of 5913 cases. West of Scotland Laparoscopic Cholecystectomy Audit Group. Br J Surg. 1996;83:1356-1360.
16 Z’graggen K, Wehrli H, Metzger A, et al. Complications of laparoscopic cholecystectomy in Switzerland: A prospective 3-year study of 10,174 patients. Swiss Association of Laparoscopic and Thoracoscopic Surgery. Surg Endosc. 1998;12:1303-1310.
17 Wherry DC, Marohn MR, Malanoski MP, et al. An external audit of laparoscopic cholecystectomy in the steady state performed in medical treatment facilities of the Department of Defense. Ann Surg. 1996;224:145-154.
18 Adamsen S, Hansen OH, Funch-Jensen P, et al. Bile duct injury during laparoscopic cholecystectomy: A prospective nationwide series. J Am Coll Surg. 1997;184:571-578.
19 Archer SB, Brown DW, Smith CD, et al. Bile duct injury during laparoscopic cholecystectomy: Results of a national survey. Ann Surg. 2001;234:549-558.
20 Vitellas KM, El-Dieb A, Vaswani K, et al. Detection of bile duct leaks using MR cholangiography with mangfodipir trisodium (Teslascan). J Comput Assist Tomogr. 2001;25:102-105.
21 Khalid TR, Casillas VJ, Montalvo BM, et al. Using MR cholangiopancreatography to evaluate iatrogenic bile duct injury. AJR Am J Roentgenol. 2001;177:1347-1352.
22 Mutignani M, Shah SK, Tringali A, et al. Endoscopic therapy for biliary leaks from aberrant right hepatic ducts severed during cholecystectomy. Gastrointest Endosc. 2002;55:932-936.
23 Mehta SN, Pavone E, Barkun JS, et al. A review of the management of post-cholecystectomy biliary leaks during the laparoscopic era. Am J Gastroenterol. 1997;92:1262-1267.
24 Couinaud C. Le foie. Etude anatomiques et chirurgicales. Paris: Masson Ed; 1957.
25 Moosman DA, Collier FA. Prevention of traumatic injury to the bile ducts: A study of the structures of the cystohepatic angle encountered in cholecystectomy and supraduodenal choledochostomy. Am J Surg. 1951;82:132-143.
26 Bjorkman DJ, Carr-Locke DL, Lichtenstein DR, et al. Postsurgical bile leaks: Endoscopic obliteration of the transpapillary pressure gradient is enough. Am J Gastroenterol. 1995;90:2128-2133.
27 Liguory C, Vitale GC, Lefebre JF, et al. Endoscopic treatment of postoperative biliary fistulae. Surgery. 1991;110:779-783.
28 Ponchon T, Gallez JF, Valette PJ, et al. Endoscopic treatment of biliary tract fistulas. Gastrointest Endosc. 1989;35:490-498.
29 Chow S, Bosco JJ, Heiss FW, et al. Successful treatment of post-cholecystectomy bile leaks using nasobiliary tube drainage and sphincterotomy. Am J Gastroenterol. 1997;92:1839-1843.
30 Foutch PG, Harlan JR, Hoefer M. Endoscopic therapy for patients with a post-operative biliary leak. Gastrointest Endosc. 1993;39:416-421.
31 Marks JM, Ponsky JL, Shillingstad RB, et al. Biliary stenting is more effective than sphincterotomy in the resolution of biliary leaks. Surg Endosc. 1998;12:327-330.
32 Sugiyama M, Mori T, Atomi Y. Endoscopic nasobiliary drainage for treating bile leak after laparoscopic cholecystectomy. Hepatogastroenterology. 1999;46:762-765.
33 Binmoeller KF, Katon RM, Shneidman R. Endoscopic management of postoperative biliary leaks: Review of 77 cases and report of two cases with biloma formation. Am J Gastroenterol. 1991;86:227-231.
34 Sandha GS, Bourke MJ, Haber GB, et al. Endoscopic therapy for bile leak based on a new classification: Results in 207 patients. Gastrointest Endosc. 2004;60:567-574.
35 Marks JM, Bower AL, Goormastic M, et al. A comparison of common bile duct pressures after botulinum toxin injection into the sphincter of Oddi versus biliary stenting in a canine model. Am J Surg. 2001;181:60-64.
36 Traverso LW, Kozarek RA, Ball TJ, et al. Endoscopic retrograde cholangiopancreatography after laparoscopic cholecystectomy. Am J Surg. 1993;165:581-586.
37 Woods MS, Traverso LW, Kozarek RA, et al. Characteristics of biliary tract complications during laparoscopic cholecystectomy: A multi-institutional study. Am J Surg. 1994;167:27-33.
38 Mergener K, Strobel JC, Suhocki P, et al. The role of ERCP in diagnosis and management of accessory bile duct leaks after cholecystectomy. Gastrointest Endosc. 1999;50:527-531.
39 Vallon AG, Mason RR, Laurence BH, et al. Endoscopic retrograde cholangiography in post-operative bile duct strictures. Br J Radiol. 1982;55:32-35.
40 Trambert JJ, Bron KM, Zajko AB, et al. Percutaneous transhepatic balloon dilatation of benign biliary strictures. AJR Am J Roentgenol. 1987;149:945-948.
41 Mueller PR, vanSonnenberg E, Ferrucci JTJr, et al. Biliary stricture dilatation: Multicenter review of clinical management in 73 patients. Radiology. 1986;160:17-22.
42 Williams HJJr, Bender CE, May GR. Benign postoperative biliary strictures: Dilation with fluoroscopic guidance. Radiology. 1987;163:629-634.
43 Pitt HA, Kaufman SL, Coleman J, et al. Benign postoperative biliary strictures: Operate or dilate? Ann Surg. 1989;210:417-425.
44 Bezzi M, Silecchia G, Orsi F, et al. Complications after laparoscopic cholecystectomy: Coordinated radiologic, endoscopic, and surgical treatment. Surg Endosc. 1995;9:29-36.
45 Dumonceau JM, Baize M, Deviere J. Endoscopic transhepatic repair of the common hepatic duct after excision during cholecystectomy. Gastrointest Endosc. 2000;52:540-543.
46 Geenen DJ, Geenen JE, Hogan WJ, et al. Endoscopic therapy for benign bile duct strictures. Gastrointest Endosc. 1989;35:367-371.
47 Berkelhammer C, Kortan P, Haber GB. Endoscopic biliary prostheses as treatment for benign postoperative bile duct strictures. Gastrointest Endosc. 1989;35:95-101.
48 Davids PH, Tanka AK, Rauws EA, et al. Benign biliary strictures: Surgery or endoscopy? Ann Surg. 1993;217:237-243.
49 Davids PH, Rauws EA, Coene PP, et al. Endoscopic stenting for post-operative biliary strictures. Gastrointest Endosc. 1992;38:12-18.
50 Smith MT, Sherman S, Lehman GA. Endoscopic management of benign strictures of the biliary tree. Endoscopy. 1995;27:253-266.
51 Duvall A, Haber GB, Kortan P, et al. Long term follow up of endoscopic stenting for benign postoperative biliary strictures [abstract]. Gastrointest Endosc. 1997;45:AB129.
52 Costamagna G, Pandolfi M, Mutignani M, et al. Long-term results of endoscopic management of postoperative bile duct strictures with increasing numbers of stents. Gastrointest Endosc. 2001;54:162-168.
53 Bergman JJ, Burgemeister L, Bruno MJ, et al. Long-term follow-up after biliary stent placement for postoperative bile duct stenosis. Gastrointest Endosc. 2001;54:154-161.
54 Dumonceau JM, Deviere J, Delhaye M, et al. Plastic and metal stents for postoperative benign bile duct strictures: The best and the worst. Gastrointest Endosc. 1998;47:8-17.
55 Draganov P, Hoffman B, Marsh W, et al. Long-term outcome in patients with benign biliary strictures treated endoscopically with multiple stents. Gastrointest Endosc. 2002;55:680-686.
56 Familiari L, Scaffidi M, Familiari P, et al. An endoscopic approach to the management of surgical bile duct injuries: Nine years’ experience. Dig Liver Dis. 2003;35:493-497.
57 Costamagna G, Tringali A, Mutignani M, et al. Endotherapy of postoperative biliary strictures with multiple stents: Results after more than 10 years of follow-up. Gastrointest Endosc. 2010;72:551-557.
58 de Reuver PR, Rauws EA, Vermeulen M, et al. Endoscopic treatment of post-surgical bile duct injuries: Long term outcome and predictors of success. Gut. 2007;56:1599-1605.
59 Nguyen-Tang T, Frossard JL, Dumonceau JM. [Endoscopic management of benign biliary strictures]. Rev Med Suisse. 2009;5:1714-1719.
60 Judah JR, Draganov PV. Endoscopic therapy of benign biliary strictures. World J Gastroenterol. 2007;13:3531-3539.
61 Familiari P, Bulajic M, Mutignani M, et al. Endoscopic removal of malfunctioning biliary self-expandable metallic stents. Gastrointest Endosc. 2005;62:903-910.
62 Kahaleh M, Behm B, Clarke BW, et al. Temporary placement of covered self-expandable metal stents in benign biliary strictures: A new paradigm? (with video). Gastrointest Endosc. 2008;67:446-454.
63 Costamagna G. Covered self-expanding metal stents in benign biliary strictures: Not yet a “new paradigm” but a promising alternative. Gastrointest Endosc. 2008;67:455-457.