Chapter 42A Benign biliary strictures
Overview
Benign biliary strictures may affect the intrahepatic or extrahepatic bile ducts or both and may be solitary or multiple. Several causes of benign biliary strictures have been described (Table 42A.1). Iatrogenic injuries after cholecystectomy are the most commonly reported and best characterized causes, and they are emphasized in this chapter to illustrate the principles of diagnosis and management. Strictures and other injuries also may occur after common bile duct (CBD) exploration or biliary reconstruction. Other procedures in the upper abdomen may endanger the biliary tract, especially procedures involving the liver, pancreas, and stomach/duodenum. The true incidence of biliary injury after these procedures is difficult to assess, but it seems to be substantially lower than after cholecystectomy.
Bile Duct Injury at Cholecystectomy
Incidence
Because of the great frequency with which the operation is performed, cholecystectomy remains the greatest source of postoperative biliary injuries. Open cholecystectomy has long been associated with a modest incidence of biliary injuries. In a review of more than 42,000 open cholecystectomies performed in the United States in 1989, Roslyn and others (1993) documented a 0.2% incidence of biliary injuries. Strasberg and colleagues (1995) reported a 0.3% incidence of injuries in a literature review of more than 25,000 open cholecystectomies since 1980. The advent of laparoscopic cholecystectomy (see Chapter 34) has refocused attention on this issue, however, because of the significant increase in the number of injuries.
Early studies worldwide documented a marked increase in the frequency of bile duct injuries associated with the laparoscopic approach, ranging from 0.4% to 1.3% (Deziel et al, 1993; Richardson et al, 1996; Wherry et al, 1996; Adamsen et al, 1997; MacFadyen et al, 1998; Fletcher et al, 1999). In a review of nearly 125,000 laparoscopic cholecystectomies reported in the literature in the years 1991 through 1993, Strasberg and colleagues (1995) reported an overall incidence of biliary injuries of 0.85% and incidence of major injuries of 0.52%. Recent studies evaluating biliary injury rates more than a decade since the widespread introduction of the laparoscopic technique confirm a stable yet high indicence of iatrogenic injury, with the incidence ranging between 0.3% and 0.6% in larger studies (Nuzzo et al, 2005; Kaman et al, 2006; Waage & Nilsson, 2006; Tantia et al, 2008). Given the inherent difficulties in collecting data on postcholecystectomy biliary injuries, the true incidence at any one time may never be known precisely; however, the figures reported for laparoscopic cholecystectomy represent a substantial increase over historical controls reported for open cholecystectomy.
The bile duct is at increased risk of injury during laparoscopic cholecystectomy compared with open cholecystectomy for several reasons that will be discussed later. It has long been argued that surgeon inexperience is a major factor, and that with increased familiarity with the procedure, the number of injuries would decrease—the so-called learning curve effect (Southern Surgeons Club, 1991). Considerable evidence supports this view. Several authors have shown an inverse relationship between the incidence of bile duct injuries and the number of cases performed (Deziel et al, 1993; Gigot et al, 1997; Woods et al, 1994) and a decreased bile duct injury rate when performed by specialized hepatobiliary surgeons (Boddy et al, 2007). Also, large population-based reviews have documented a decline in injuries over time (Richardson et al, 1996; Russell et al, 1996). However, these trends have not been observed by all investigators. A recent report spanning more than 12 years showed that an initial decline in injuries was not sustained at the end of the study (Waage & Nilsson, 2006). Several other reports have suggested no change in the incidence of bile duct injuries over time (Adamsen et al, 1997; Fletcher et al, 1999; Wherry et al, 1996), and the number and complexity of cases referred for repair have remained static at some specialist units (Walsh et al, 1998). Reports of major bile duct injuries inflicted by surgeons with considerable experience continue to appear (Carroll et al, 1996; Gigot et al, 1997). Moreover, the role of hospital volume does not seem to affect the incidence of injuries (Waage & Nilsson, 2006; Csikesz et al, 2009). The data suggest that laparoscopic postcholecystectomy bile duct injuries will remain a significant problem for the foreseeable future.
Pathogenesis
Several factors are associated with an increased risk of bile duct injury at cholecystectomy, some of which are general and some unique to the laparoscopic approach. Ultimately, the final common pathway of most injuries is either a technical error or misinterpretation of the anatomy (Table 42A.2; Strasberg et al, 1995; Olsen, 1997). Many authors classify biliary injuries as major, such as transection of the CBD, or minor, such as biliary leak; but the line separating the two is often blurred. In general, major injuries are more serious and usually require reoperation to repair. Minor injuries are not always trivial, however, and they may require operative intervention. The classification of biliary injuries is discussed later.
Table 42A.2 Classification of Causes of Laparoscopic Biliary Injuries
Modified from Strasberg SM, et al, 1995: An analysis of the problem of biliary injury during laparoscopic cholecystectomy. J Am Coll Surg 180:101–125.
Anatomic Variations
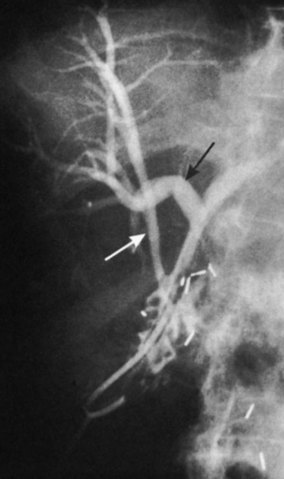
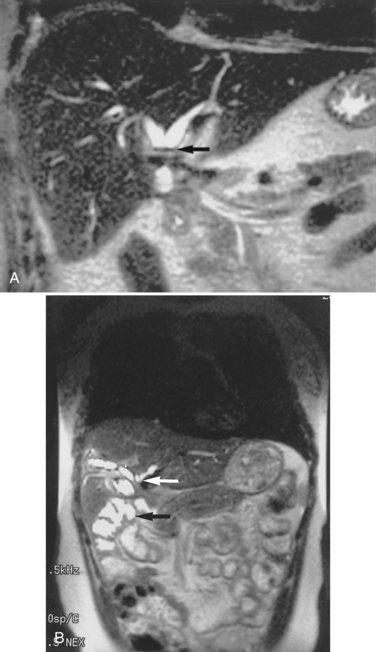
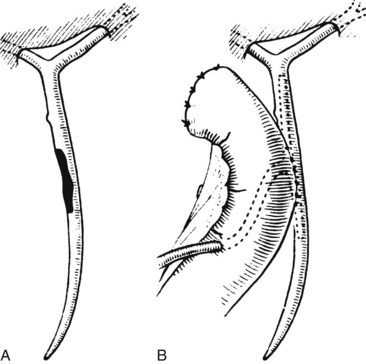
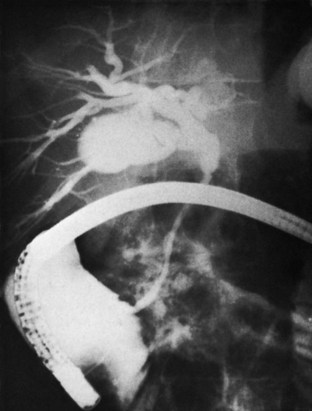
Any surgeon operating on the biliary tree must be familiar with the wide range of anatomic variations that may be encountered (see Chapter 1B). Anomalies of the cystic duct insertion into the common hepatic duct are most commonly seen (Fig. 42A.1). The cystic duct may join the common hepatic duct quite high, almost at the biliary confluence, or it may run parallel to the common hepatic duct for a long distance before joining it very low, occasionally at the level of the ampulla. In many patients, perhaps 25%, the right hepatic duct per se is absent, and the right anterior and posterior sectoral hepatic ducts join the left hepatic duct independently. In some cases, one of the right sectoral hepatic ducts, usually the anterior, may follow a prolonged extrahepatic course and enter the common hepatic duct quite low, and it may receive drainage from the cystic duct (see Fig. 42A.1D). Such unrecognized, low-entry right sectoral hepatic ducts (Bismuth type 5) are at particular risk of injury during laparoscopic cholecystectomy (Fig. 42A.2; Wherry et al, 1996).
In the future, routine use of preoperative imaging may help minimize bile duct injuries by identifying aberrant biliary anatomy. Already, some centers are examining the utility of preoperative CT cholangiography and MRI in preventing this complication (Hirano et al, 2006). Widespread adoption of this strategy will clearly be costly, but it could be justified: some imaging studies document right ductal aberrancy in the general population ranging from 4.8% to 8.4% (Kullman et al, 1996; Dusunceli et al, 2004). In some cases, the cystic duct is quite short, and misinterpretation of the anatomy can occur easily. Misinterpretation is especially likely if, on intraoperative cholangiography, the catheter has been advanced well into the CBD, and the proximal ducts are not visualized. The surgeon may mistake the common duct for the cystic duct, ligate it, and remove it with the attached gallbladder (Fig. 42A.3). This type of injury seems to be much more common after laparoscopic rather than open cholecystectomy and is well described (Davidoff et al, 1992). A related situation can occur in cases of long-standing cholelithiasis and chronic cholecystitis, resulting in obliteration of the cystic duct, which we will discuss later.
Vascular anomalies also are common, occurring in 20% of patients (see Chapter 1B). In the most common of these, the right hepatic arterial supply arises in part or in whole from the superior mesenteric artery. Such an aberrant vessel usually courses to the right of the portal vein, lateral and posterior to the CBD. During cholecystectomy, this vessel is prone to injury at the junction of the cystic and common hepatic ducts. Vascular injuries are not limited to patients with anatomic anomalies. The classic biliary injury during laparoscopic cholecystectomy, in which the surgeon mistakes the CBD for the cystic duct, is frequently associated with injury to the right hepatic artery, which will be discussed (Davidoff et al, 1992). This problem has been documented to occur in as many as 0.6% of patients after laparoscopic cholecystectomy and may not be apparent at the initial operation but may come to attention later as a hepatic artery pseudoaneurysm (Balsara et al, 1998; Nicholson et al, 1999). Rarely, concomitant vascular injury may involve both the right hepatic artery and right portal vein, which can be life threatening (Fig. 42A.4).
Ischemia
In the absence of overt injury, delayed biliary stricture formation may result from extensive periductal dissection and consequent interruption of the major ductal arterial supply (see Chapter 1B). Most of the arterial supply to the extrahepatic bile duct is provided by two small arteries that travel along the lateral duct borders in the 3 o’clock and 9 o’clock positions. Sixty percent of the blood supply runs upward from the major inferior vessels (retroduodenal, gastroduodenal), and approximately 38% runs downward from the right hepatic artery (Northover & Terblanche, 1979). Extensive circumferential dissection of the duct may disrupt this axial blood flow and has been proposed as a major mechanism of biliary stricture formation. Although there is no direct evidence to support this, it would seem reasonable not to pursue extensive dissection of the common duct during cholecystectomy. Also, damage to these vessels is likely to be a contributing, if not a central factor, in the formation of strictures attributed to electrocautery use.
Pathologic Factors
Patients with acute cholecystitis may have severe inflammation in the porta hepatis and triangle of Calot, which greatly distorts the anatomy. In addition, the gallbladder is often friable and difficult to grasp, and persistent oozing of blood often obscures the field. Some or all of these factors may conspire to preclude safe laparoscopic cholecystectomy. During the early era of laparoscopic cholecystectomy, acute cholecystitis was considered an absolute contraindication. However, as experience with laparoscopy grew, surgeons became more comfortable extending the indication to include acute cholecystitis. Several reports later documented that the laparoscopic approach not only is possible but also is safe in selected cases of acute cholecystitis (Kum et al, 1996; Wherry et al, 1996; Adamsen et al, 1997; Fletcher et al, 1999; Neri et al, 2003). As a result, laparoscopic cholecystectomy for acute cholecystitis is now widely practiced; in fact, the issue regarding timing of operation (early vs. delayed) has been settled (see Chapter 31; Casillas et al, 2008; Gurusamy et al, 2009). When laparoscopic cholecystectomy is performed for acute cholecystitis, studies have also revealed a greater potential for biliary injury (Kholdebarin et al, 2008). Fletcher and colleagues (1999) reported that complex cases that include patients with acute cholecystitis, cholangitis, and gallstone pancreatitis are associated with a dramatically increased incidence of bile duct injuries (1.7%), although acute cholecystitis alone is not an independent risk factor. Other authors have shown that acute cholecystitis increases the incidence of bile duct injuries twofold to threefold above baseline (Adamsen et al, 1997; Russell et al, 1996; Soderlund et al, 2005). In patients seen initially with acute cholecystitis, we recommend early operation by laparoscopic approach, even in patients with a history of prior open abdominal surgery. Given the increased risk of biliary injury in this setting, however, the threshold for conversion to an open procedure should always be low.
On occasion, the inflammatory reaction is so severe that even conversion to an open procedure does not clarify the anatomy. Fibrosis and inflammation within the triangle of Calot make dissection of the cystic duct particularly hazardous. In severe cases, the gallbladder neck and cystic duct may be fused to the common duct within a sheath of scar tissue. This finding should lead the surgeon away from an extensive dissection to define the cystic duct, which risks a major biliary injury. Placement of a cholecystostomy tube may be a safer approach. An alternative option is partial (subtotal) cholecystectomy with amputation of the gallbladder, well away from the cystic duct/common hepatic duct junction, and placement of a drain. This procedure is generally safe and effective, and in a review of 1100 patients, only 11.2% required operative reintervention (Soleimani et al, 2007). Moreover, centers have reported a significant decrease in the incidence of bile duct injuries since the introduction of laparoscopic subtotal cholecystectomy (Nakajima et al, 2009). In such heavily fibrosed cases, the cystic duct is nearly always obliterated, and postoperative biliary leak is infrequent.
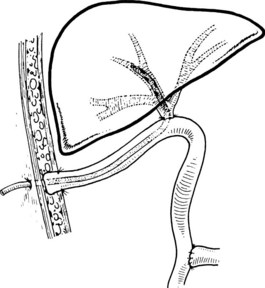
A potentially more difficult problem is the small, contracted, fibrotic gallbladder with extensive surrounding inflammation, sometimes partially embedded into the liver and obliterating the triangle of Calot such that the gallbladder neck and cystic duct are adherent to the common hepatic duct (Fig. 42A.5B). In such cases, the cystic duct is obliterated, and safe dissection in Calot’s triangle is impossible. With long-standing disease, particularly associated with large impacted gallstones in the gallbladder neck, a cholecystocholedochal fistula is possible. A common hepatic duct stricture may be present, resulting from an impacted stone and the subsequent inflammatory response (Mirizzi, 1948; see Chapter 42B). A cholecystocholedochal fistula should be suspected in any patient with gallstones, jaundice, cholangitis, and imaging studies or operative findings that suggest an inflamed and contracted gallbladder. In these cases, an attempt to perform a total cholecystectomy should be abandoned, because this would lead the surgeon directly into the common hepatic duct (Sharma et al, 1998). Rather, partial cholecystectomy with extraction of the stones allows inspection of the depths of the gallbladder and is preferable. A rush of bile into the operative field at this point is strong evidence for a cholecystocholedochal fistula, because the cystic duct is nearly always obliterated. Because a biliary stricture often lies distal to the fistula, direct closure of the defect should be avoided, and it should instead be reconstructed as a cholecystocholedochoduodenostomy (see Chapter 42B and Fig. 42A.5B).
Technical Factors
General
Early reports of laparoscopic biliary injury cited bleeding and subsequent attempts to achieve hemostasis as major contributing factors to bile duct injury (Davidoff et al, 1992; Rossi & Tsao, 1994). At laparoscopy, even a small amount of blood obscures the field and hinders dissection. If necessary, an additional port should be placed for a high-powered suctioner/irrigator to precisely define the site of bleeding. Hemorrhage must be controlled, regardless of the approach, and blind placement of clips or indiscriminate use of electrocautery must be avoided. Early demonstration of the cystic artery is desirable, and it should be divided close to the gallbladder to avoid injury to the right hepatic or common hepatic artery.
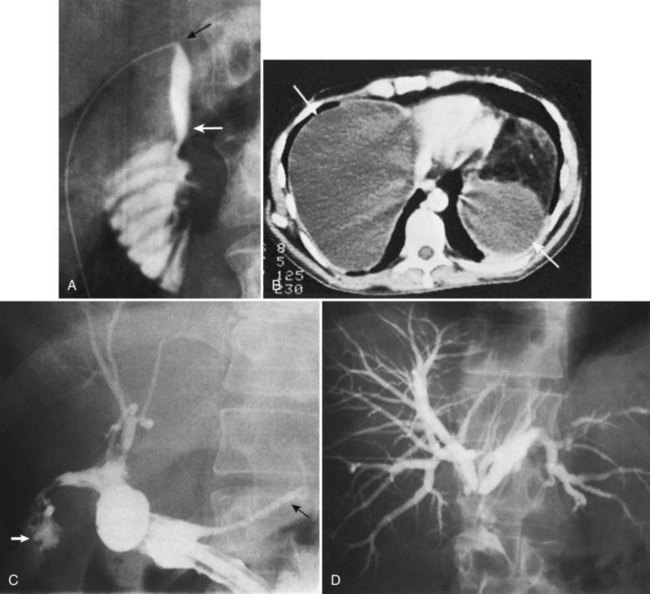
The observation of bile draining from the gallbladder fossa always suggests the possibility of a biliary injury, perhaps to an aberrant right sectoral hepatic duct. It is tempting to ascribe such a finding to drainage from a subvesical duct of Lushka, a slender, 1- to 2-mm in diameter duct that passes from the right lobe in the gallbladder fossa to join the right hepatic or common hepatic duct (McMahon et al, 1995). In this situation, a major bile duct injury must be considered the leading diagnosis until disproved, and the surgeon is compelled to convert to an open procedure or to place a drain and investigate the cause postoperatively. Regarding the duct of Lushka, although some surgeons question its existence, several detailed anatomic and radiologic imaging studies have reliably identified its structure and course (Kocabiyik et al, 2009). Most patients with bile leaks attributable to a subvesical duct experience a delayed onset of symptoms and are usually seen in the first or second week following the procedure (Fig. 42A.6). In a review of nearly 10,000 patients, the incidence of bile leakage was 0.7%, of which 52% were leaks from subvesical ducts (Spanos & Syrakos, 2006).
The utility of routine cystic duct cholangiography is controversial (see Chapter 21), particularly with respect to reducing the incidence of biliary injury at laparoscopic cholecystectomy, which will be discussed later. Occasionally, operative cholangiography reveals a very small CBD with a filling defect, suggesting a small stone. In this situation, simple cholecystectomy without exploration of the common duct is probably safer than operative pursuit of a tiny stone in a small duct. Postoperative endoscopic cholangiography with stone extraction is nearly always possible if subsequently indicated. In general, exploration of a small caliber, otherwise normal-appearing common duct is frequently unrewarding, potentially dangerous, and more likely to result in postoperative symptoms than if such a stone were left in situ. Biliary strictures are more than just a theoretical concern after exploration of a small bile duct (Fig. 42A.7). Rarely, bile duct stones retained after cholecystectomy that are not removable by endoscopic means require reoperation (Fig. 42A.8; see Chapters 35 and 36). Under these circumstances, laparoscopic removal is usually possible via a transcholedochotomy approach (see Chapter 34). If this approach fails, an open exploration can often be avoided by placing a T-tube into the CBD for subsequent operative retrieval by intervential radiology techniques, which are successful in more than 95% of cases (Burhenne, 1980). When indicated at cholecystectomy or after failed attempts by other methods, open exploration of the CBD should be performed through a supraduodenal choledochotomy with careful exposure of a sufficient length of duct but without excessive dissection (see Chapters 29 and 35). Exploration must be gentle and performed only with soft bougies, Fogarty-type balloon catheters, or a choledochoscope (rigid or flexible). Metal bougies such as Bake’s dilators or stone forceps should be used with caution and should never be forced through the papilla of Vater into the duodenum; otherwise, secondary postinflammatory papillary stenosis may occur, or a false passage into the duodenum or pancreas may be created. After bile duct exploration, the choledochotomy should be closed with care, because this is also a potential source of biliary stricture (see Chapters 29 and 35).
Transduodenal sphincteroplasty probably is best reserved for patients with stones impacted in the distal bile duct (see Chapters 29 and 35). Stenosis or stricture of the distal CBD may occur after surgical or endoscopic sphincteroplasty or sphincterotomy. Side-to-side choledochoduodenostomy is an option that should be considered in patients with multiple stones, primary stasis stones, or distal obstruction by stricture or papillary stenosis. The results are generally good, provided that the CBD is dilated to at least 15 mm in diameter, and a stoma of at least 2 cm can be created (Schein & Gliedman, 1981). Choledochoduodenostomy performed to a decompressed duct is more likely to result in stricture formation and cholangitis (see Chapter 29; Johnson & Stevens, 1969).
Laparoscopic-Specific Factors
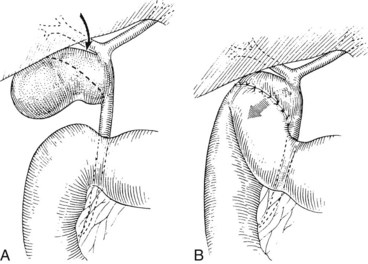
Several technical factors specific to laparoscopic cholecystectomy predispose to biliary injury (see Chapter 34). Mistaking the common duct for the cystic duct is a frequent technical error (Davidoff et al, 1992; Gigot et al, 1997; Olsen, 1997). In the most common sequence of events, a long length of the common duct is excised up to the proximal common hepatic duct, which is either occluded or left to drain bile into the peritoneal cavity. This devastating injury—referred to as the “classic” laparoscopic injury, rarely seen at open cholecystectomy—also may be associated with damage to the right hepatic artery (Davidoff et al, 1992; Strasberg et al, 1995). Less severe injuries also may occur, such as lacerations of the duct wall or near-complete transections. Proper exposure is essential to avoid injuries arising from such anatomic misinterpretations. Maximum cephalad traction on the gallbladder fundus is necessary to expose the cystic duct and triangle of Calot. Concomitant lateral traction on the infundibulum is equally important, however, to open the angle between the cystic and common hepatic ducts. If lateral traction is inadequate, Calot’s triangle is not opened sufficiently, and the course of the common duct is placed directly in line with that of the cystic duct (Fig. 42A.9; Rossi & Tsao, 1994; McMahon et al, 1995). The common duct may be damaged during dissection of the cystic duct because of its proximity, or because it is misidentified. Before any structures are divided, Calot’s triangle must be cleared of all fatty and areolar tissue. The peritoneal reflection along the posterolateral aspect of the gallbladder should be opened to fully expose the junction of the gallbladder neck and cystic duct. At the completion of the dissection, only the cystic artery and cystic duct should be seen entering the gallbladder, and the bottom of the gallbladder fossa should be visible. The use of a 30-degree laparoscope has been advocated to improve visualization of this area (Hunter, 1991), and the resulting anatomically satisfying final view has been described by Strasberg (1995) as the “critical view of safety.” The dissection should be maintained close to the gallbladder wall, and the cystic duct should be divided as close as possible to its junction with the gallbladder.
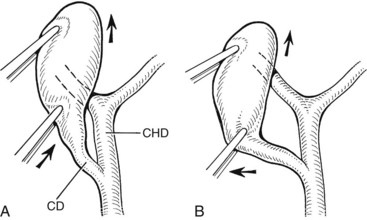
FIGURE 42A.9 Area of the triangle of Calot as it appears during laparoscopic cholecystectomy. A, Upward traction on the gallbladder, with insufficient lateral traction on the infundibulum, fails to open Calot’s triangle adequately. This causes the cystic duct (CD) and common hepatic duct (CHD) to become aligned within the same plane. Injury to the CHD can occur during dissection of the CD because of its proximity or as a result of mistaking the CHD for the CD. B, Proper lateral traction on the infundibulum opens Calot’s triangle, allowing safer dissection of the CD. In some cases, obliteration of Calot’s triangle by dense fibrosis prevents adequate exposure of this area, even with adequate traction (see Fig. 42A.5).
Routine intraoperative cholangiography has been advocated by many to minimize the incidence of laparoscopic biliary injuries (Way, 1992; Moossa et al, 1992; Asbun et al, 1993), but it remains controversial. In a meta-analysis encompassing more than 327,000 laparoscopic cholecystectomies, Ludwig and colleagues (2002) noted contribution of intraoperative cholangiography to incidence and outcome of CBD injuries during laparoscopic cholecystectomy and found injury rates to be halved in a routine cholangiogram group (0.21%) compared with a selective group (0.43%). A valid concern regarding the conclusions from these studies is that surgeons who use selective cholangiography do so appropriately in situations of inflammation or unclear anatomy inherently associated with increased risk of bile duct injuries. Thus, the injury rate under these circumstances can be artificially elevated, because the denominator—that is, the number cholecystectomies done without cholangiogram—is not typically included in the overall analysis. In a large review of cholecystectomies from Western Australia, Fletcher and colleagues (1999) reported that intraoperative cholangiography reduced the incidence of biliary injury twofold overall and eightfold in complex cases (acute cholecystitis, cholangitis, pancreatitis). In addition, some authors noted a strong inverse correlation between biliary injury and nonperformance of cholangiography (Torkington et al, 1999). Cholangiography is clearly not preventive if the injury occurs after a normal study, or if the study serves only to identify an injury that already has been inflicted (Andren-Sandberg et al, 1985).
Other groups, however, have touted the lack of statistical benefit with the routine use of intraoperative cholangiography. A survey analysis of more than 184 Italian hospitals accounting for more than 56,000 operations demonstrated no significant difference between bile duct injury rate when cholangiogram was used routinely or selectively (Nuzzo et al, 2005). In contrast, several other reports have suggested that routine cholangiography may not reduce the incidence of biliary injury but may minimize injury severity and allow immediate recognition and definitive repair (Gigot et al, 1997; Woods et al, 1994; Carroll et al, 1996; Russell et al, 1996); this depends on proper interpretation of the study. In a review of 177 biliary injuries, Olsen (1997) found that only two of 32 cholangiographies were correctly interpreted, despite evidence of impending bile duct transection in most. The most common error was accepting an incomplete study as normal, without opacification of the right and left hepatic ducts. In a similar study, Stewart and Way (2003) showed that in their series of 252 bile duct injuries, cholangiograms were obtained in 43 patients, but only nine were correctly interpreted intraoperatively. They concluded that these injuries stemmed from a misperception that was often so compelling that adjunctive cholangiographic data was likewise misperceived. Failure to opacify the proximal bile ducts in their entirety should prompt the surgeon to strongly consider open conversion. Laparoscopic ultrasound has been described as an alternative to cholangiography for delineation of the cystic duct and identification of common duct calculi (Tomonaga et al, 1999). Recent studies demonstrate the efficacy of the technique as an alternative to cholangiography (Machi et al, 2009; Hublet et al, 2009), but it is not widely used.
Bile leakage occurs with greater frequency after laparoscopic cholecystectomy than after open cholecystectomy, which may be related to the use of clips rather than ties or suture ligatures (McMahon et al, 1995). Clips are less secure than sutures and may become dislodged if improperly placed or if manipulated after placement (Nelson et al, 1992; Fig. 42A.10). Reports of clip migration leading to complications such as choledocholithiasis and pancreatitis have also been reported (Raoul et al, 1992; Cetta et al, 1997; Dolay et al, 2007). Also, incomplete occlusion may occur if clips are placed across a thickened, rigid cystic duct or a cystic duct containing stones or if other tissues are included. When placing clips, it is important that the tips meet to ensure adequate occlusion. If both tips cannot be visualized as the clip is being applied, damage to adjacent structures may occur. If the clip does not fit across the entire width of the cystic duct, the surgeon must consider the possibility that the structure about to be divided is not the cystic duct but the common duct. If the anatomy is clear, and the cystic duct is simply thickened, placement of a ligature may be more appropriate (Michalowski et al, 1998). Also, using electrocautery in areas adjacent to clips should be avoided, because this may result in conduction of thermal energy to the adjacent common duct and lead to delayed biliary structure.
Location and Classification
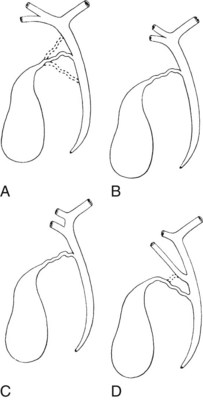
The ease of management, operative risk, and outcome of biliary injuries vary considerably and depend on the type of injury and its location (Chapman et al, 1995). It has long been recognized that strictures involving the CBD or distal common hepatic duct are easier to repair than are more proximal injuries. The anatomic classification of Bismuth has been widely adopted in recognition of this fact (Fig. 42A.11; Bismuth & Lazorthes, 1981). In this classification scheme, five stricture types are recognized, reflecting the location with respect to the hepatic duct confluence (types 1 through 4) or the involvement of an aberrant right sectoral hepatic duct with or without a concomitant hepatic duct stricture (type 5; Table 42A.3).
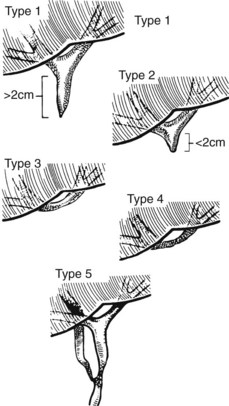
FIGURE 42A.11 Classification of bile duct strictures based on location with respect to the hepatic duct confluence (see Table 42A.3).
(From Bismuth H, 1982: Postoperative strictures of the bile duct. In Blumgart LH [ed]: The Biliary Tract: Clinical Surgery International. Edinburgh, Churchill Livingstone, pp 209-218.)
The Bismuth classification is useful for localization and for prognosis after repair, but it does not encompass the entire spectrum of injuries that are possible. No provision is made for biliary leaks or major ductal injuries without stricture. Even within the group of patients with biliary leaks, there are varying degrees of severity. In an effort to fill this gap, Strasberg and colleagues (1995) proposed a comprehensive classification system that incorporates Bismuth’s scheme but is much broader in scope (Fig. 42A.12). Injuries are classified as type A to type E, with the latter representing biliary strictures and further subdivided as E1 to E5 according to the Bismuth classification. Type A injuries are bile leaks from minor ducts still in continuity with the CBD. This encompasses the most common causes of biliary leaks seen after laparoscopic cholecystectomy—leakage from the cystic duct and from a subvesical duct of Luschka (see Figs 42A.6 and 42A.10). Type B injuries involve occlusion of part of the biliary tree, which for practical purposes almost always is an aberrant right sectoral hepatic duct. When this duct is transected without ligation, the injury is termed type C, reflecting the differences in presentation and management between the two. A lateral injury to an extrahepatic bile duct is termed type D, which is similar to type A injuries in that the extrahepatic biliary tree remains in continuity but is classified separately to underscore the greater severity and potential need for major reconstruction (Fig. 42A.13).
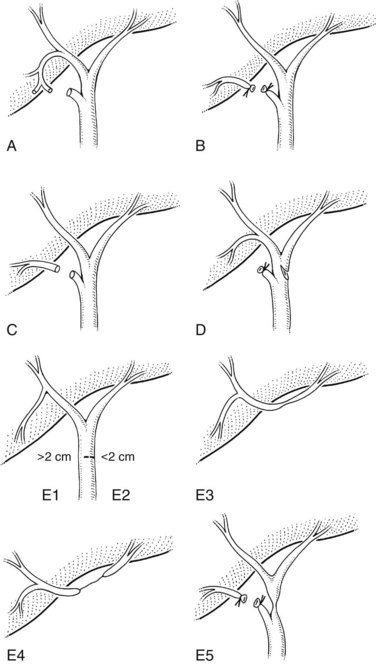
FIGURE 42A.12 Classification of laparoscopic biliary injuries according to Strasberg and colleagues (1995). Injuries are stratified from type A to type E. Type E injuries are subdivided further into E1 to E5 according to the Bismuth classification system.
The incidence of each type of biliary injury is difficult to ascertain with certainty. Most literature reports are derived from tertiary referral centers and are biased toward more severe injuries. Most surgical series focus on management of type E biliary strictures, whereas endoscopic and radiologic reports are concerned mainly with the treatment of biliary leaks. Type A injuries may be the most common, but many are managed successfully without referral and are underrepresented in reports from major centers (Strasberg et al, 1995).
Clinical Presentation
Most patients with postcholecystectomy biliary injuries, if not diagnosed at operation, come to medical attention early in the postoperative period. After open cholecystectomy, only about 10% of injuries are suspected after the first week, but nearly 70% are diagnosed within the first 6 months after operation (Pitt et al, 1982). By contrast, injuries after laparoscopic cholecystectomy seem to be recognized earlier (Davidoff et al, 1992; Lillemoe et al, 1997). This probably reflects differences in the pattern of injuries between the two approaches combined with a heightened awareness of the potential for injury at laparoscopy (Strasberg et al, 1995).
The clinical presentation depends on the type of injury; conversely, the type of injury may be inferred based on the clinical picture at presentation. Patients with significant bile leaks—types A, C, and D—generally present within the first week after operation, but some leaks may not become apparent for several weeks, and few are diagnosed intraoperatively (Strasberg et al, 1995). Most patients have abdominal pain coupled with fever or other signs of sepsis, which is most common, or bile leakage from an incision. A few patients have none these signs and symptoms but rather have nonspecific complaints of weakness, fatigue, or anorexia. Elevated alkaline phosphatase levels are characteristic, as is mild hyperbilirubinemia, but markedly elevated serum bilirubin levels (>3 mg/dL) are uncommon (vanSonnenberg et al, 1993; Brooks et al, 1993).
Major injuries to the common duct (type E injuries) are more likely to be discovered intraoperatively, although most remain unrecognized until after operation. Similar to bile leaks, these injuries are diagnosed more often within the first few postoperative weeks, although patients with a slowly evolving stricture may not come to attention for several months (Strasberg et al, 1995), which is distinctly uncommon for patients with bile leaks. Most patients with these injuries present with jaundice often coupled with pain and occasionally sepsis. Jaundice is not always present early in the course of the illness. In some patients, the stricture may evolve slowly or cause only partial obstruction. Such patients may have nonspecific complaints, pruritus, or derangements in liver function tests (LFTs), any or all of which should prompt an investigation. In addition, patients with an isolated right sectoral hepatic duct injury (type B) or an internal biliary fistula may be seen initially with a history of unexplained fevers, pain, or general debilitation.
Pathologic Consequences
Fibrosis
Biliary obstruction is associated with the formation of high local concentrations of bile salts at the canalicular membrane, and these initiate pathologic changes in the liver (Schaffner et al, 1971). Bile thrombi form within dilated centrilobular bile canaliculi, and secondary changes develop in adjacent hepatocytes. A complex cascade of molecular and cellular events ensues, collectively termed fibrogenesis (Friedman, 2008), which ultimately leads to the deposition of collagen and other extracellular matrix proteins and eventually to fibrosis and scarring around bile ducts and ductules (Maher & McGuire, 1990; Jarnagin et al, 1994; Friedman, 1997; see Chapter 6). As this process progresses, mechanical interference with bile flow develops in these intrahepatic biliary radicles and perpetuates cholestasis.
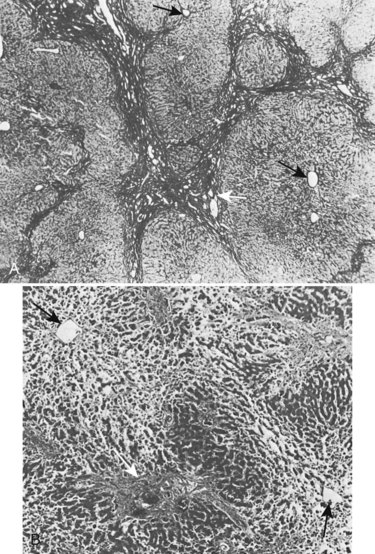
Fibrosis is accompanied by liver cell hyperplasia (Weinbren et al, 1985); this is not true in secondary biliary cirrhosis, because the lobular structure of the liver is usually well preserved (Fig. 42A.14) and the marked fibrosis that occurs in advanced cases only rarely progresses to true cirrhosis. This knowledge is important in planning therapy because many of these pathologic changes are reversible (Duffield et al, 2005). A histologic return of normal liver parenchyma is seen after relief of obstruction in both animal and human models (Sikora et al, 2008; Kirkland et al, 2009), which correlates to the return to near-normal liver function after relief of biliary obstruction (Blumgart, 1978). Fibrosis also may develop in the extrahepatic ducts proximal to the stricture, which is especially likely after biliary intubation. Upward retraction of the ducts is accompanied by a sequence of mucosal atrophy, squamous metaplasia, inflammatory infiltration, and further fibrosis in the subepithelial layers of the ducts.
It usually takes several years for liver fibrosis and portal hypertension (see Chapter 70A, Chapter 70B ) to become evident, although it may manifest 2 years after the development of the stricture. Major stigmata of hepatocellular dysfunction, such as spider angiomata and encephalopathy, are uncommon in benign biliary obstruction and should raise the possibility of preexisting parenchymal disease (e.g., chronic hepatitis, alcoholic liver disease). If liver biopsy reveals significant primary hepatocellular disease in association with a benign stricture, the prognosis is especially poor.
Atrophy
The distribution of liver mass is regulated by a poorly understood balance of bile flow, portal venous flow, and hepatic venous flow. Segmental or lobar atrophy may result from portal venous obstruction or bile duct occlusion in the affected area (see Chapter 5). Unilobar atrophy is associated with hypertrophy of the contralateral lobe and may present diagnostic and operative difficulties. Liver lobe atrophy and compensatory hypertrophy is frequently found in benign strictures and may be associated with asymmetric involvement of lobar or sectoral hepatic ducts, interference with portal venous blood supply, or decreased portal perfusion owing to secondary fibrosis. In benign strictures, the dilated ducts within the atrophic segments often are filled with infected bile and debris, and even though drainage of an atrophic segment would not be effective in relieving jaundice, cholangitis may continue unabated unless satisfactory drainage of the atrophic and hypertrophic segments is achieved.
The presence of significant atrophy and compensatory hypertrophy greatly influences the approach to repair. The most common situation is gross hypertrophy of the left lobe accompanied by right lobe atrophy (Czerniak et al, 1986). Anastomosis in the region of the hilum is made difficult by the rotational deformity and anatomic distortion imposed by this condition (see Chapters 5 and 29). A thoracoabdominal approach to such strictures provides more direct exposure and access for repair by allowing rotation of the liver to the left (Bismuth & Lazorthes, 1981). Recent reports verify that the presence of atrophy and contralateral hypertrophy are associated with significantly longer reconstructive operations, higher intraoperative blood loss, and greater blood transfusion requirements (Pottakkat et al, 2009). Similar problems may occur in bile duct strictures after right hepatic resection.
Portal Hypertension (See Chapter 70A, Chapter 70B )
It is estimated that approximately 15% to 20% of patients with benign biliary stricture have concomitant portal hypertension (Blumgart & Kelley, 1984; Chapman et al, 1995). Patients with biliary strictures may develop portal hypertension as a result of secondary hepatic fibrosis or direct damage to the portal vein. Alternatively, portal hypertension may be due to preexisting liver disease. It is important that these patients undergo further workup, including a liver biopsy, to exclude underlying chronic parenchymal disease. Iatrogenic biliary injuries are often the subject of medicolegal proceedings, and precise documentation of all injuries is essential to provide an accurate assessment of the cause of symptoms and prognosis. The outcome of patients with biliary strictures and portal hypertension is much worse than for patients without portal hypertension, with an in-hospital mortality rate of 25% to 40% (Blumgart & Kelley, 1984; Chapman et al, 1995). It has been suggested, however, that adequate biliary drainage may be followed by some resolution of fibrosis and perhaps a reduction in portal pressure (Blumgart, 1978).
Management
Radiologic Investigations (See Chapter 13, Chapter 15, Chapter 16, Chapter 17, Chapter 17 )
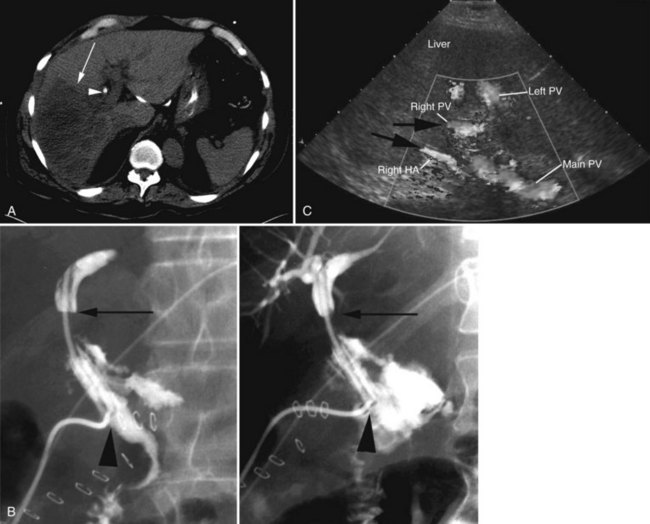
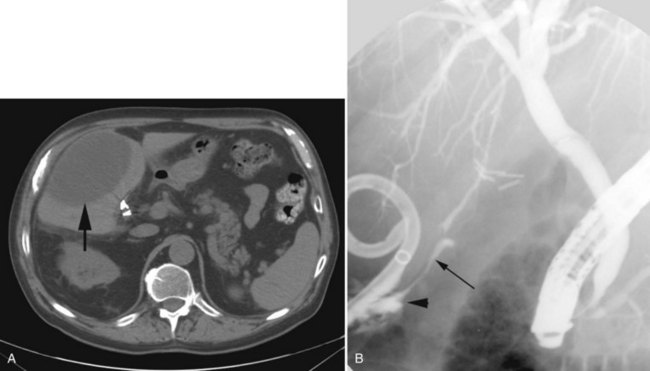
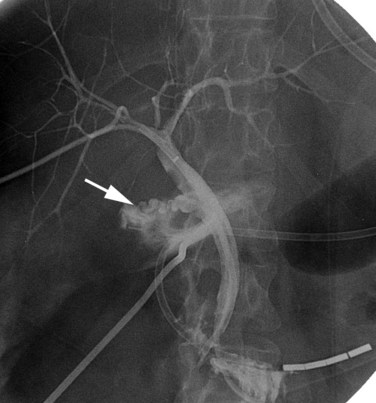
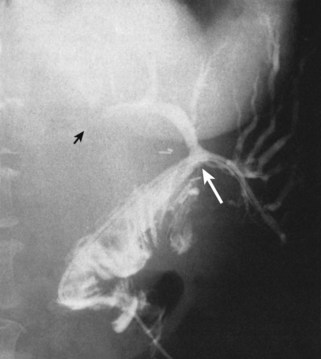
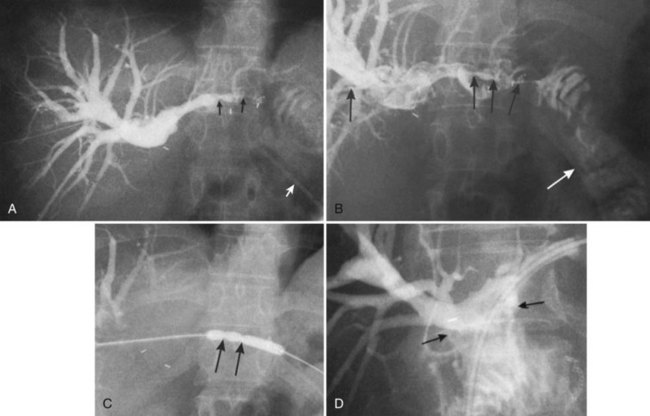
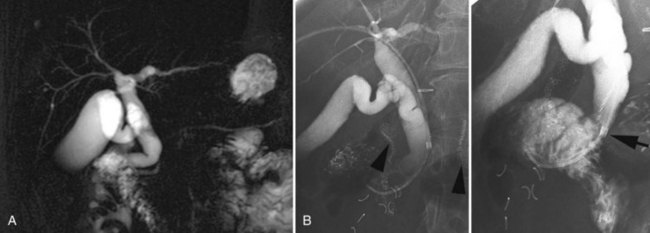
In patients with biliary strictures, complete delineation of the level and extent of injury is necessary. All branches of the right and left intrahepatic biliary tree must be outlined, particularly in cases of high bile duct stricture and recurrent stricture after previous reconstruction. Displaying the hepatic duct confluence (if intact) and the left ductal system and its branches is especially important in selecting the appropriate reconstruction. Percutaneous transhepatic cholangiography (PTC) is much more likely than endoscopic retrograde cholangiopancreatography (ERCP) to provide this information, and PTC remains the standard investigation in this setting (Fig. 42A.15). Drainage catheters should be left in place following PTC if a complex injury is identified on cholangiogram, because palpation of the catheter intraoperatively often helps guide identification of ductal structures later during definitive repair. The risk of cholangitis can be reduced with prophylactic antibiotics.
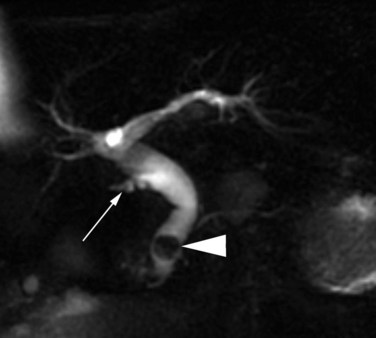
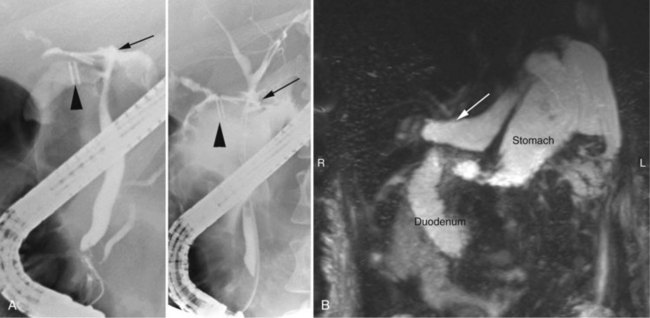
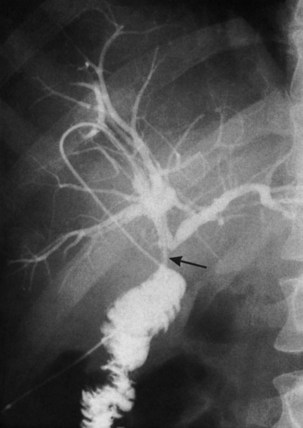
Magnetic resonance cholangiopancreatography (MRCP) has emerged as a valuable tool in evaluating proximal bile duct injuries (Coakley et al, 1998). This noninvasive modality provides striking images of the biliary tree and yields anatomic information in a single study that was previously obtainable only with CT and PTC (Fig. 42A.16). Multiple studies have now confirmed the high accuracy and detailed information provided by MRCP (Bujanda et al, 2003; Jarnagin et al, 1994; Ragozzino et al, 2004). However, in some cases involving severely distorted anatomy as a result of atrophy, hypertrophy, or dense scarring, preoperative PTC and catheter placement should still be strongly considered, not only for additional cholangiographic information or intraoperative guidance but also because catheters can be easily exchanged for soft tubing to stent across worrisome small caliber anastomoses.
ERCP is seldom of value in the precise diagnosis of complete proximal bile duct strictures because there is often discontinuity of the CBD preventing visualization of the intrahepatic ductal system. ERCP may be more helpful for incomplete strictures (stenoses) and is appropriate for patients with a history of sphincteric damage at previous common duct exploration, or if there is suspicion of papillary stenosis or other periampullary pathology. ERCP also has a role in the diagnosis and treatment of patients with bile leakage from the cystic duct stump or from a laceration of the common duct (Brooks et al, 1993). These patients have evidence of an intraabdominal fluid collection on CT. After percutaneous drainage, biliary scintigraphy (e.g., hepatobiliary iminodiacetic acid [HIDA] scan) can be used to establish the presence of a persistent bile leak. ERCP may then be applied to identify the location of the leak, and placement of a stent may reduce or eliminate bile leakage; however, many bile leaks resolve with percutaneous drainage alone, and ERCP is probably unnecessary in the absence of radiographic or clinical evidence of ongoing bile drainage (Brooks et al, 1993).
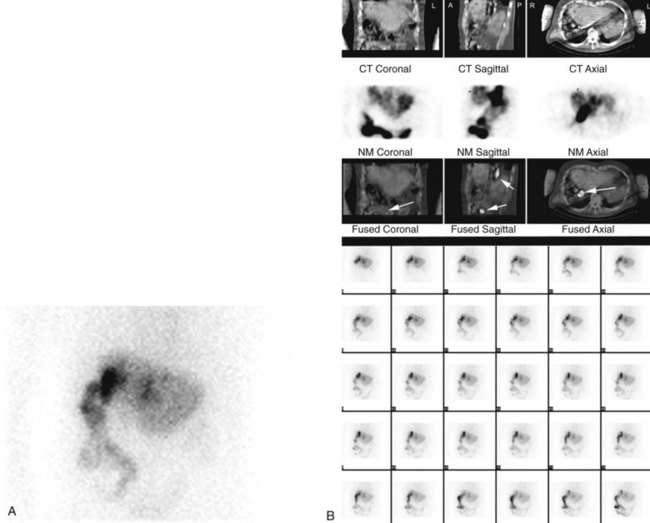
Isotopic scanning techniques may be valuable in assessing bile duct strictures, particularly the functional assessment of incomplete strictures, previous biliary reconstructions, and isolated sectoral hepatic duct strictures (see Chapter 15). HIDA scanning offers a dynamic and quantitative assessment of liver function and of the clearance of bile across anastomoses and stenoses (Fig. 42A.17; McPherson et al, 1982). In patients with hepatocellular disease, HIDA scanning may be valuable in distinguishing the contribution of the biliary obstruction from that of the intrinsic liver disease to the overall biochemical and symptomatic picture. In such cases, the bilirubin level may be normal, but the alkaline phosphatase level is increased. HIDA scanning also is valuable during follow-up of patients after surgical repair. Because it can be repeated and is noninvasive, it is of particular value in showing anastomotic patency and function when no tube has been left across the anastomosis at the time of repair (see Fig. 42A.17). An isolated sectoral hepatic duct stricture is suggested by delayed clearance of isotope from a portion of the liver.
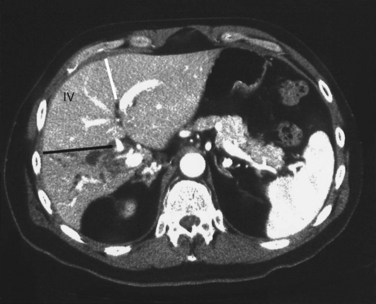
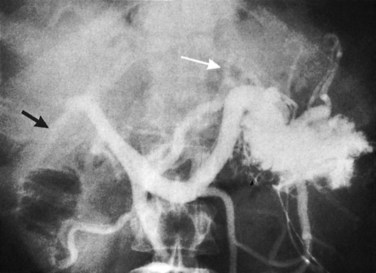
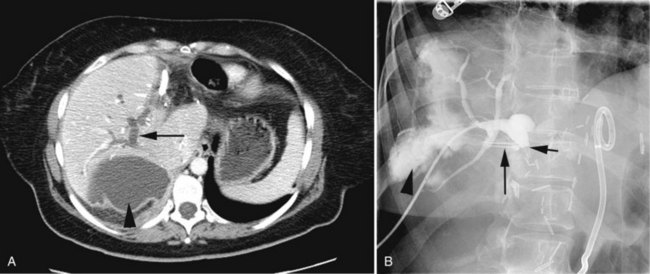
Arteriography and delayed-phase portography (see Chapter 19) can be obtained to confirm vascular injury on the initial studies, a suspicion of portal hypertension from the history and physical examination, or a history of excessive bleeding at the time of cholecystectomy (Li et al, 2008). A biliary injury in the setting of portal hypertension dramatically increases the morbidity and mortality of operative intervention, and its presence should redirect the management approach (Chapman et al, 1995). The combination of biliary and vascular injuries often leads to segmental or lobar atrophy, but it may also be seen with long-standing biliary obstruction alone. An atrophic lobe may be evident on the initial ultrasound or CT scan and appears as a small, often hypoperfused area with dilated, irregular, and crowded bile ducts (Fig. 42A.18). Isotopic scanning may show what appears to be a filling defect in the affected area. It is important to recognize lobar atrophy on the cross-sectional imaging studies, not only because it is an indicator of concomitant vascular injury needing further evaluation, but also because it would change the operative approach during repair (see Chapter 29). In addition, patients with combined bile duct and hepatic artery injuries seem to be at increased risk for severe complications, such as hepatic necrosis and abscess, after reconstructive surgery. A patient undergoing biliary reconstruction in the setting of hepatic artery occlusion may also be at greater risk of late stricture recurrence (Gupta et al, 1998; Schmidt et al, 2005).
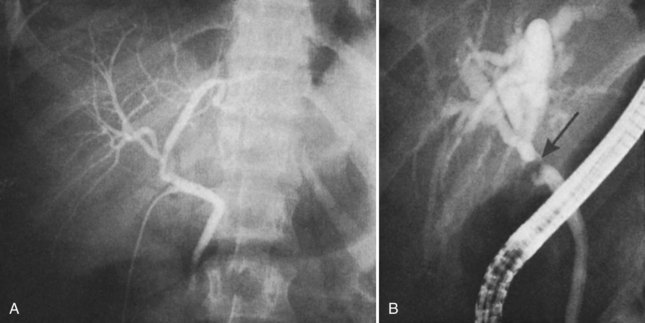
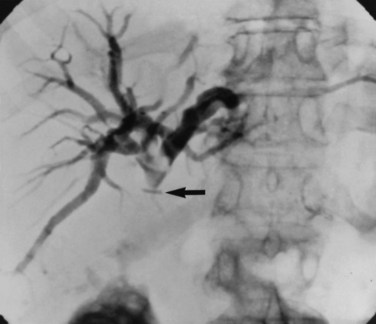
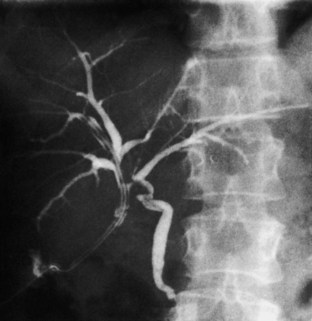
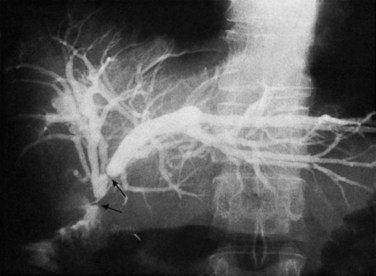
Occasionally, injection of contrast material into an established biliary fistula or percutaneous drain outlines the ductal system (Fig. 42A.19). Such studies may complement information provided by formal PTC, particularly if the fistula or tube tract drains an excluded sectoral duct (see Chapter 42B). Because bacterial colonization or biliary infection is inevitable in such cases, it is wise to administer prophylactic antibiotics before and after these studies.
Preoperative Preparation
In general, operative repair of bile duct injuries need not be rushed, the exceptions being treatment of bile duct injuries recognized at the time of initial cholecystectomy or, rarely, emergency treatment of suppurative cholangitis or peritonitis. For most patients, there is ample time to treat coexisting conditions and to perform a full investigation, both of which increase the likelihood of a successful outcome (Stewart & Way, 1995; Chapman et al, 1995).
Cholangitis is a frequent occurrence in patients with bile duct strictures, especially after ductal intubation (see Chapter 43). Administration of intravenous antibiotics is important prior to surgical treatment, and the results from bile cultures obtained at PTC should be used to direct therapy. Patients with severe cholangitis and sepsis are unlikely to respond to antibiotics alone and should be submitted to percutaneous drainage before surgery. Preoperative antibiotics are usually adequate to manage milder attacks and should be used in patients with no clinical evidence of cholangitis, given the high incidence of bacterial contamination. Antibiotic regimens should take into account the frequent presence of anaerobic organisms and enterococci in patients with biliary obstruction (Thompson et al, 1990; Hochwald et al, 1999). We begin antibiotics immediately before operation and continue appropriate treatment for 5 to 7 days postoperatively if cholangitis is a preoperative feature. Although no firm data exist to guide the duration of treatment, the markedly increased incidence of postoperative infectious complications in patients with endoscopic or percutaneous stents suggests that a prolonged course of treatment is justified (Hochwald et al, 1999). These data further suggest that jaundice without evidence of cholangitis is not an indication for biliary intubation.
Anemia should be corrected, if necessary, by blood transfusion; coagulation defects, which typically manifest as a prolongation of the prothrombin time, should be treated with vitamin K or fresh frozen plasma. Patients with prolonged illness may be seen with malnutrition (see Chapter 24). Enteral feedings through a fine-bore nasal catheter may be successful in some cases but may not be tolerated in sufficient amounts, and parenteral nutrition may be necessary. Despite these measures, however, weight gain is sometimes difficult to achieve, and to date no prospective randomized trials have shown a benefit from short-term preoperative nutritional support. A significant external bile fistula predisposes to excessive fluid and electrolyte loss and may lead to hyponatremia and acidosis (McPherson et al, 1982). It is imperative to correct fluid deficits and electrolyte imbalances before operation (see Chapter 24).
Surgical Treatment
Injury Recognized at Initial Operation
If injury to the extrahepatic biliary tree is recognized at the time of initial cholecystectomy, the surgeon should consider his or her experience and ability to repair it immediately. The advice of a more experienced surgeon should be sought if possible. The situation is not immediately desperate, and there is always time to wait a short while for another opinion and for additional assistance. Substantial evidence suggests that immediate open conversion and repair by an experienced surgeon is associated with reduced morbidity, shorter duration of illness, and lower cost (Stewart & Way, 1995; Savader et al, 1997). A recent multivariate analysis of bile duct injuries by Stewart and Way (2009) identified timing of biliary reconstruction as being insignificant when patient preoperative condition, complete cholangiography, surgical technique, and surgeon experience are optimized.
Despite the apparent ability to perform biliary reconstruction electively, several studies have cited the benefits of early referral to a tertiary center, as such institutions have specialized hepatobiliary surgeons and radiologists to diagnose and treat biliary complications in a timely fashion (Sicklick et al, 2005; Fischer et al, 2009). Each failed repair is associated with some loss of bile duct length (Tocchi et al, 1996) and greatly exacerbates an already difficult situation. This is particularly true of injuries involving the biliary confluence, in which failure of the initial repair and loss of bile duct length may result in isolation of the right and left hepatic ducts; repair becomes more difficult, and the likelihood of a successful outcome is reduced (Chapman et al, 1995). If the surgeon cannot effect a reasonable repair, and competent help is unavailable, drains should be placed to control any biliary leak, and the patient should be referred to a specialist center.
Two options are available for repairing a complete duct transection. The first is end-to-end repair over a T-tube. This repair is feasible only if the transected ends can be apposed without tension, which usually requires full mobilization of the duodenum and head of the pancreas. The anastomosis is created using a single layer of interrupted absorbable sutures with the T-tube brought out of the bile duct away from the anastomotic line. Silk sutures should be avoided for all biliary reconstructions, because they can promote an inflammatory reaction and act as a nidus for stone formation. End-to-end anastomoses are associated with a high incidence of late stricture formation, up to 60% (Csendes et al, 1989; Pellegrini et al, 1984; Rossi & Tsao, 1994; Stewart & Way, 1995). Other reports support the use of end-to-end repair, because more than two thirds of the associated complications, such as stricture or leak, can be managed nonoperatively (de Reuver et al, 2007). In general, we recommend Roux-en-Y hepaticojejunostomy, which will be discussed later; this approach is more likely to give better long-term results.
An injury to the lateral duct wall may be amenable to direct suture repair, and relatively small and simple lacerations may be repaired with interrupted 4-0 or 5-0 absorbable suture. The area of injury should be adequately exposed, but extensive dissection risks further injury and late stricture formation. Some authors recommend T-tubes for all such repairs, but they are probably unnecessary for small lacerations, and their placement into a decompressed bile duct may exacerbate the injury (Rossi & Tsao, 1994). An analogous situation is primary choledochorrhaphy after CBD exploration, and several authors have reported excellent results without using T-tubes (Sheen-Chen & Chou, 1990; Sorensen et al, 1994; Tu et al, 1999). Long, lateral injuries that are not circumferential may be impossible to repair transversely without compromising the lumen, and direct repair over a T-tube is likely to result in future stenosis. Some authors have suggested that vein patches may be used to cover such defects (Ellis & Hoile, 1980), whereas others have described using the cystic duct stump or pedicled flaps of jejunum (Okamura et al, 1985).
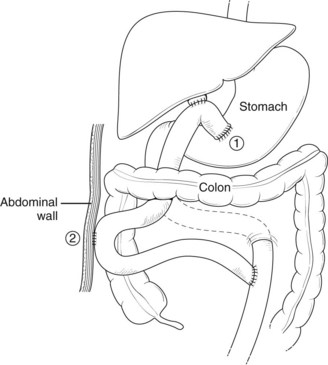
Although these approaches may have merit in some situations, we prefer instead to use a Roux-en-Y loop of jejunum as a serosal patch (Fig. 42A.20). A T-tube is placed across the defect, and its long limb is led out through the Roux loop and exteriorized through the abdominal wall. This approach has several advantages: First, bile duct length is maintained. Second, the jejunal serosa is used to cover the defect, secured in place with fine, interrupted absorbable sutures to the bile duct wall without attempting direct approaches to the ragged edge of the damaged duct. Finally, the T-tube provides biliary decompression across the jejunum so that when it is removed, any leaking bile drains into the bowel lumen rather than the abdominal cavity. This method may be particularly useful to an inexperienced surgeon in a difficult situation.
Injury Recognized in the Immediate Postoperative Period
Biliary injuries not appreciated intraoperatively may appear in the first few days after operation. The mode of presentation may be bile drainage through the wound, bile peritonitis, or progressive jaundice, depending on the injury type. In the setting of an external biliary fistula (see Chapter 42B), the essential consideration in management is to avoid early reoperation. It is wiser to take full stock of the situation, to carry out appropriate investigations as described earlier, and to keep the patient well nourished and free of infection. If fistulography or other studies reveal continuity between the biliary system and the gastrointestinal tract, a prolonged period of drainage is warranted and may result in spontaneous closure, provided that there is no distal obstruction to bile flow. This is particularly true of bile leakage from the cystic duct or a subvesical duct of Luschka (type A) or from a noncircumferential laceration (type D).
More severe lacerations or complete transections of the common duct or an aberrant right sectoral hepatic duct with ongoing bile leakage require careful consideration. Because the biliary tree is decompressed, the proximal ducts are small in caliber. Immediate surgical treatment of these injuries is difficult. Adequate repair requires exposing healthy bile duct mucosa within a sufficiently dilated proximal duct to allow precise anastomosis. In the setting of a decompressed biliary tree and significant inflammation, this exposure can be quite demanding or even impossible. A delayed approach remains the most appropriate course of action, because such fistulas also may close without operative intervention, and this may be associated with eventual proximal duct dilation and easier subsequent repair (see Fig. 42A.3). If fluid losses from the biliary fistula remain high over a prolonged period, a useful but rarely used technique is creation of a temporary internal fistulojejunostomy, with definitive repair deferred for a later date (Smith et al, 1982). Alternatively, placement of an endoscopic or percutaneous stent across the defect may reduce output from the fistula, hasten closure, and make management easier. Long-term results of such interventions have shown excellent results, and oftentimes more than 90% of patients with peripheral leaks or strictures can be treated endoscopically (Weber et al, 2009). Some authors advocate early endoscopic sphincterotomy to decrease the relative resistance of transpapillary bile drainage to promote closure (Liguory et al, 1991; Abdel et al, 1996; Inui et al, 1998; Fujii et al, 1998). Although these approaches are occasionally useful, no evidence is available to show that they provide a significant advantage, and a waiting period of 2 to 3 weeks is recommended, because most fistulas close or decrease substantially.
Patients with bile peritonitis are often desperately ill, especially if the bile is infected, although some patients with sterile bile may accumulate large volumes without overt signs of sepsis (see Fig. 42A.3B). Drainage of the bile collection and control of ongoing bile leak is the primary objective and often requires percutaneous abscess drains in combination with percutaneous biliary catheters. Definitive repair is seldom possible initially, with the bile ducts collapsed, deeply bile stained, and friable; repair is best delayed, until the biliary leak has been controlled completely, and the patient has been fully resuscitated.
Injury Presenting at an Interval After Initial Operation
It may be necessary to consider a staged approach to stricture repair in the presence of intraabdominal infection, portal hypertension, or poor general condition of the patient. Repair attempts in the face of uncontrolled local infection, acute complications of portal hypertension, or general debilitation are doomed to fail. The initial approach in such cases should be limited to establishing external bile drainage, controlling sepsis, and treating other coexisting conditions that are life threatening. In this manner, the clinical condition of the patient may be improved before attempts at definitive repair (Cattell & Braasch, 1959). In a patient with portal hypertension, initial interventional radiologic management by percutaneously placed biliary drainage catheters is probably safer than operation, given the danger of intraoperative hemorrhage (Pellegrini et al, 1984).
Technical Approaches to Biliary Repair (See Chapter 29)
End-to-End Duct Repair
Excision of the stricture with end-to-end anastomosis was one of the earliest techniques used for reconstruction (Cattell & Braasch, 1959). This repair reestablishes normal anatomic continuity and drainage via an intact sphincter of Oddi. Such an approach has been tried even for high strictures after extensive mobilization of the duodenum and common duct. End-to-end repair is associated with a 50% to 60% incidence of long-term failure (Csendes et al, 1989; Pellegrini et al, 1984; Stewart & Way, 1995). These figures reflect the failure rates in the most favorable of cases, because patients with more extensive injuries usually were submitted to biliary-enteric anastomosis (Rossi & Tsao, 1994). These data suggest that end-to-end repair has a limited role in the surgical treatment of benign biliary strictures.
Biliary-Enteric Repair
For most cases of bile duct transection or stricture, biliary-enteric anastomosis is the procedure of choice. For strictures of the retropancreatic or immediate supraduodenal portion of the CBD, choledochoduodenostomy is an ideal procedure. The anastomosis may be performed either side-to-side or end-to-side (see Chapter 29). This technique is appropriate only in the setting of a dilated bile duct and almost certainly would result in recurrent stricture if created to a decompressed duct. Most postcholecystectomy strictures are not amenable to choledochoduodenostomy, because such low injuries are unusual in contrast to postgastrectomy strictures, which often involve the distal bile duct. Strictures involving the common hepatic duct are more difficult to manage, especially strictures involving the biliary confluence, and they almost always require Roux-en-Y hepaticojejunostomy for reconstruction. The technical aspects of biliary-enteric anastomosis have been described (Voyles & Blumgart, 1982; Blumgart & Kelley, 1984) and are outlined in Chapter 29.
There are a variety of approaches to the proximal hepatic ducts. When the stricture is below the confluence (Bismuth type 1 or 2), a direct anastomosis to the hepatic duct stump is usually straightforward. By contrast, when the stricture encroaches on the confluence of the right and left hepatic ducts (type 3) or extends proximally so as to isolate the ducts (type 4), the problem becomes more complex, and good results are more difficult to achieve. The choice of surgical approach should be tailored to the height and extent of the lesion. The technical descriptions that follow should be read in conjunction with Chapter 29.
Strictures at or above the level of the biliary confluence are much more challenging. Adequate exposure of the bile ducts is usually achievable by dissecting the left hepatic duct system. This approach is based on the anatomic studies of Couinaud (1953, 1955; Hepp & Couinaud, 1956) and is well described (Bismuth et al, 1978; Blumgart & Kelley, 1984). For type 2 and type 3 strictures, biliary-enteric anastomosis to the left hepatic duct provides complete drainage of the left and right ductal systems. For type 4 strictures, however, the confluence is obliterated, and it is necessary to provide drainage of the right lobe as well, often by dissection across the stricture and creation of a second anastomosis to the right ductal system. Occasionally, mobilization or even partial excision of the quadrate lobe may be necessary in some type 4 strictures.
Although most high strictures may be approached and managed as described previously, it is occasionally difficult to expose the left hepatic duct because of adhesions or fibrosis. Excessive bleeding may be encountered, or a large, overhanging quadrate lobe may prevent access to the left duct. Occasionally, the extrahepatic length of the left duct, normally much greater than the right, is short, and access to it is difficult. In such instances, repair can be accomplished by dissection of the left duct within the umbilical fissure, known as the ligamentum teres or round ligament approach (Fig. 42A.21; Soupault & Couinaud, 1957). This approach rarely is indicated for benign strictures and should not be used unless the biliary confluence is intact, so that a left-sided anastomosis would drain the entire liver.
The mucosal graft procedure of Smith (1964, 1969) is a procedure of historical interest only. This method was introduced for treating high strictures in which hilar dissection was thought to be impossible, and the proximal ducts could not be delivered for mucosa-to-mucosa anastomosis. The object of the procedure is to use a transhepatic tube to draw the jejunal mucosa high up into the hepatic ducts and allow apposition. The procedure was claimed to be easier and quicker, because no sutures were inserted. The transhepatic tube was left in place for 2 to 6 months, occasionally longer for difficult cases. Initially hailed as an important advance in technique (Kune & Sali, 1981), it is now clear that the dome of the jejunal mucosa drawn into the hepatic ducts may block significant secondary intrahepatic ducts, occluding and isolating segments of liver tissue (Blumgart, 1978; Blumgart et al, 1984). In addition, the mucosa may slip away postoperatively, and the jejunal loop may become detached (Fig. 42A.22). Recurrent stricture at the site of previous mucosal grafting is common (Blumgart & Kelley, 1984). This technique fell out of favor after several series of complex biliary strictures documented good to excellent long-term results using biliary-enteric anastomotic techniques without using the mucosal graft approach (Schol et al, 1995; McDonald et al, 1995; Chapman et al, 1995; Stewart & Way, 1995; Nealon & Urrutia, 1996; Tocchi et al, 1996; Lillemoe et al, 1997).
Liver Split and Liver Resection
To expose the bile ducts for repair, it is sometimes necessary to open the liver tissue as a hepatotomy (Blumgart, 1980). The most frequent situations involve opening the umbilical fissure to obtain access to the segment III duct or extending the subhepatic approach to expose the origin of the right hepatic duct. This latter approach involves opening the liver in the line of the gallbladder fossa. Upward mobilization of the entire quadrate lobe by this maneuver combined with opening the umbilical fissure facilitates access for selected type 4 strictures, especially when approaching the right hepatic ducts is difficult. Fiddian-Green and colleagues (1988) described a similar approach. Resection of the lower parts of segments IV and V to obtain exposure to the hilar plate has been described for Strasberg E3 injuries; in this setting, the surgeon must be diligent to stay away from the hepatoduodenal ligament (Sirichindakul et al, 2009). Division of liver tissue in this setting must be accomplished patiently, because it may be accompanied by significant hemorrhage. These techniques are unnecessary if approaches to the left duct are possible. Actual liver resection is rarely necessary for exposure of the bile ducts in benign postcholecystectomy strictures. Most often, excision of the anterior portion of the quadrate lobe is done if exposure of the umbilical fissure and incision in the gallbladder bed are insufficient to expose the ducts in difficult type 4 strictures.
More rarely, benign strictures may be treated by means of an intrahepatic hepaticojejunostomy as described by Longmire and Sandford (1948, 1949), which involves resection of a portion of the left lateral segments (II and III) and anastomosis to ducts exposed on the cut surface of the liver. In general, this procedure is difficult and potentially dangerous, because the liver is often fibrous, and resection is met by a fair degree of hemorrhage. The bleeding vessels are generally in close proximity to the ducts needed for anastomosis. The use of this procedure is limited to cases in which there is left-sided hypertrophy (Czerniak et al, 1986), and it is rarely the only option available to secure access to the bile ducts.
Isolated Sectoral Hepatic Duct Injuries
Injuries to aberrant or “low entry” right sectoral hepatic ducts can be particularly difficult to diagnose and manage. Patients with a stricture but no bile leak (type B) may remain asymptomatic for months or years after the injury and only then present with pain or evidence of cholangitis. Some patients may remain essentially asymptomatic and come to attention because of abnormal LFTs. These injuries are not associated with jaundice, unless concomitant stricture of the common hepatic duct (type E5) is present. Long-standing obstruction may result in atrophy of the corresponding hepatic sector drained by the occluded duct (right anterior or right posterior). Some of these patients will have had ERCP in the course of their evaluation that was interpreted as normal, but in retrospect, it is found to actually show partial nonfilling of the right biliary tree. Biliary drainage to a Roux-en-Y loop of jejunum should be done in symptomatic patients. In patients with symptoms, especially recurrent cholangitis, and evidence of liver atrophy, drainage alone may be insufficient, and resection of the atrophic sector may be required. Asymptomatic patients may not require intervention, especially if the injury was remote, and significant atrophy is already evident; however, patients with a relatively recent injury and no atrophy probably are best served with operative drainage to prevent future problems (Strasberg et al, 1995).
Patients with sectoral hepatic duct injuries associated with biliary leak (type C) are a particularly difficult group to manage. After appropriate initial investigation, control of biliary leak, and resuscitation, reconstruction to a Roux-en-Y jejunal loop should be performed. Because the transected duct is small and decompressed, achieving good results is difficult even in experienced hands, and restenosis is common. It has been suggested that percutaneous transhepatic drainage of the affected duct before operation (Lillemoe et al, 2000; Strasberg et al, 1995) and prolonged postoperative anastomotic stenting are crucial for successful management (Lillemoe et al, 2000). We believe that neither of these maneuvers is necessary as a matter of routine, but they may be helpful in selected cases.
Combined Modality Approaches
Under such adverse circumstances, when the risk of recurrent stricture or stone formation is believed to be high, hepaticojejunostomy may be performed over a transjejunal tube brought to the exterior across the blind end of the Roux limb. The defunctionalized Roux limb is deliberately left long, and the end is secured subcutaneously or subperitoneally (Fig. 42A.23); this allows easy subsequent access for cholangiography, cholangioscopy, dilation, or stone removal. The blind end of the Roux limb may be reaccessed by percutaneous puncture under fluoroscopic guidance or via a small incision made under local anesthetic for late diagnostic or therapeutic procedures long after the transjejunal tube has been removed. In our experience, this approach is rarely necessary but can provide an improved chance of excellent outcome in difficult cases and may spare the patient the need for repeated major surgical intervention months or years later (Schweizer et al, 1991; McPherson et al, 1998). It is generally unnecessary to add the morbidity of a formal stoma, although such an approach has been reported (Barker & Winkler, 1984).
Hepatic Resection
Patients who have undergone prior unsuccessful repairs or have concomitant vascular injury or long-standing cholangitis often develop sectional duct strictures or interruptions between the right-sided and left-sided biliary tree, effectively precluding biliary-enteric revision. Such patients may require formal liver resection to eliminate atrophied liver or the biliary duct confluence. The surgical aim in such instances is to create a more functional biliary-enteric anastomosis using the remnant liver. Although mostly described as isolated cases (Uenishi et al, 1999; Heinrich et al, 2003), Laurent and colleagues (2008) reported their experience with 18 cases of formal resections (14 right hepatectomies, 3 left hepatectomies, and 1 left trisectionectomy) for benign biliary stricture (see Chapter 90B). At a median follow-up of 8 years, 94% of patients had good or excellent results. Another report described eight patients who underwent either right hepatectomy or left lateral sectionectomy, and it demonstrated similar, excellent results (Thomson et al, 2007). Hepatic resection remains an important option in the treatment of refractory benign bilary stricture, especially in patients suffering from recurrent bouts of cholangitis or sepsis.
Liver Transplantation
Transplantation for iatrogenic biliary injury can be performed acutely but is usually done because of a devastating combined vascular and biliary injury (Felekouras et al, 2007; Zaydfudim et al, 2009). Only rarely does secondary biliary fibrosis resulting from longstanding biliary obstruction progress to true cirrhosis (see Chapter 97A). In such cases, orthotopic liver transplantation should be considered as an alternative to biliary reconstruction. Thomson and colleagues (2007) reported their series of 14 patients who either underwent hepatic resection or transplantation for secondary biliary cirrhosis. Of the five patients who ultimately required transplant for survival, two were deemed unsuitable for the procedure, one died awaiting an organ, and two underwent the procedure. Even in experienced transplantation centers, surgical reconstruction is preferred for most patients with benign strictures.
Portal Hypertension and Biliary Stricture
Patients with coexisting portal hypertension and biliary stricture are an especially difficult group to manage (see Chapter 70A, Chapter 70B ). This combination has been reported in 10% to 20% of patients at the time of referral (Blumgart & Kelley, 1984; Chapman et al, 1995). Portal hypertension may result from secondary biliary fibrosis or direct injury to the portal vein, or coincident hepatocellular disease may be evident from alcohol abuse, chronic hepatitis, or other causes. The presence of splenomegaly or a history of gastrointestinal bleeding in a patient with biliary stricture should prompt further investigation with esophagogastroduodenoscopy and angiography with delayed-phase splenoportography. Bleeding esophageal varices, particularly if accompanied by hypersplenism or ascites, render the overall prognosis far worse (Chapman et al, 1995; Way & Dunphy, 1972). Collateral venous channels in the subhepatic region and within adhesions make dissection difficult and bloody. Patients with portal hypertension often have a proximal stricture, and some have had multiple previous attempts at repair, further reducing the likelihood of a successful outcome.
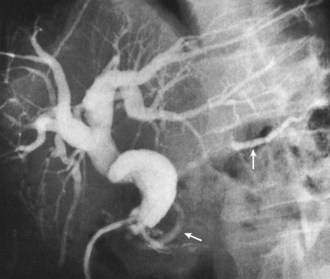
In seriously ill patients with jaundice and portal hypertension, it is preferable to attempt nonoperative stenting or balloon dilation than to proceed to immediate definitive repair (Fig. 42A.24; Toufanian et al, 1978; Molnar & Stockum, 1978; Teplick et al, 1980; Schwarz et al, 1981; Pellegrini et al, 1984). In the face of severe gastrointestinal bleeding, initial measures must be directed at stopping the hemorrhage and resuscitating the patient (see Chapters 75A and 75B). If bleeding continues, consideration should be given to immediate splenorenal or transjugular intrahepatic portosystemic shunting (TIPS; see Chapter 76A, Chapter 76B, Chapter 76C, Chapter 76D, Chapter 76E ) to achieve control, with bile duct reconstruction deferred until a later date. Occasionally, successful biliary-enteric reconstruction can be done under these circumstances with no need to create an intraoperative vascular shunt (Perakath et al, 2003; Agarwal et al, 2008). The best and most convenient form of operative shunt is the splenorenal shunt (see Chapter 76C). TIPS has a limited patency, and such shunting is used most often as a bridge to transplantation. The risk of biliary injury and subsequent biliary-venous fistula is high in the setting of a dilated biliary tree and seems to be a major mechanism of early shunt failure (Jalan et al, 1996; Saxon et al, 1998). TIPS is unlikely to play a major role in the management of these patients; instead, percutaneous drainage or balloon dilation may be used along with the measures described earlier.
If hemorrhage is encountered during stricture repair, hepaticostomy drainage may be performed as an initial measure, and a splenorenal shunt may be undertaken at a later date. Bile duct stricture repair done at the time of the shunting procedure can be extremely difficult and in any event is unwise at a time of severely compromised liver function. Occasionally, patients present with variceal hemorrhage after an otherwise successful stricture repair (Fig. 42A.25). In these instances, portosystemic shunting may be indicated as an emergency measure or, preferably, after initial conservative management. Injection sclerotherapy may control the bleeding successfully and obviate the need for operative intervention (see Chapter 75B).
Results of Biliary Reconstruction
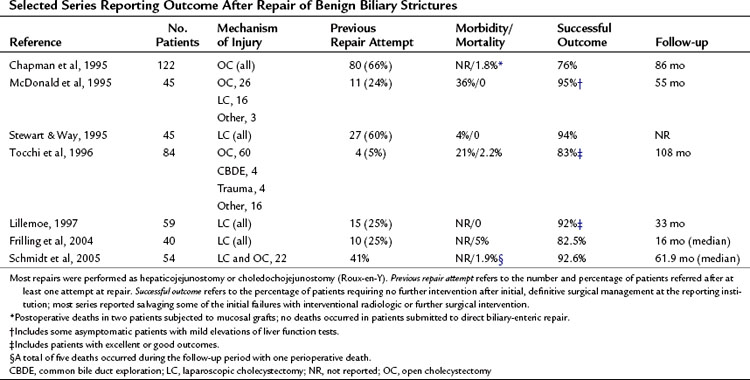
Given the wide spectrum of injuries that are possible at laparoscopic cholecystectomy, it is difficult to assess fully the results of operative repair. Most surgical series report results of repair of biliary strictures (type E) and do not address operative intervention for other injury types. In addition, many patients are subjected to immediate repair at the time of initial injury; the more difficult cases and the failures generally are referred to specialist units, but the long-term outcome of the other cases is often not reported. As a result, most literature reports—generated from tertiary referral centers—include significant numbers of patients requiring difficult reconstructions or with multiple previous repair attempts, which may underestimate the overall success rate. Relatively few reports with sufficient follow-up focus on the results of repair of laparoscopic injuries alone (Table 42A.4). It remains to be determined whether the results of repair of bile duct strictures after open cholecystectomy or other procedures can be extrapolated to repair of laparoscopic injuries. It has been suggested that laparoscopic bile duct injuries may have a less satisfactory outcome because of the more complex nature of many of these injuries and the frequent inflammation and fibrosis secondary to bile leakage (Lillemoe et al, 1997).
Operative Morbidity and Mortality
Several studies have suggested significant morbidity associated with repair of benign strictures (Blumgart et al, 1984; Kune & Sali, 1981). The most common postoperative complications seen were intraabdominal abscess, wound infection, cholangitis, sepsis, biliary fistula, postoperative hemorrhage, and pneumonia. The perioperative mortality rate in many series ranged from 5% to 8% (Cattell & Braasch, 1959; Warren et al, 1982; Way & Dunphy, 1972); however, since the 1990s, several authors have documented a considerable decline in mortality, with many citing no perioperative deaths (see Table 42A.4). These figures generally do not take into account patients who die as a result of biliary tract–related sepsis while awaiting definitive treatment. Factors frequently associated with perioperative death include advanced age, significant comorbid medical conditions, and biliary sepsis. Significant underlying liver disease is perhaps the most important predictor of adverse outcome. Chapman and colleagues (1995) reported a perioperative mortality rate of 23% in patients with biliary strictures and portal hypertension subjected to any operative procedure.
Long-Term Results and Follow-up
Several factors must be considered when analyzing the long-term results of biliary reconstruction. First, there is no consistently used algorithm for postoperative investigation. Likewise, the measures used to assess outcome vary considerably. Approaches to follow-up range from simple observation and measurement of LFTs to performance of cholangiography or HIDA scans. Consistency in analyzing long-term results is important and should include an assessment of symptoms, LFTs, and radiographic studies. Such a triad of criteria is useful for clarifying the results and is the only way to compare accurately the results from various series or interventions (Table 42A.5; Schweizer et al, 1991). In addition, long-term follow-up is crucial in analyzing the final results of any series and when comparing different treatment modalities. Pitt and colleagues (1982) reported that two thirds of recurrent strictures were apparent within 2 years, but 20% were diagnosed 5 or more years after the initial repair. Similarly, Tocchi and colleagues (1996) observed that 40% of restrictures were identified more than 5 years after the initial operation. Complete and accurate assessment of the results of surgery or any other intervention requires a minimum follow-up of 5 years, but probably longer (Bismuth, 1982).
Numerous studies assessing the impact of postcholecystectomy bile duct injuries on quality of life (QoL) in long-term follow-up have reported mixed results. Three studies showed significant impairment in several measured QoL categories (Boerma et al, 2001; Moore et al, 2004; de Reuver et al, 2008), whereas three others showed minimal (Melton et al, 2002) or no difference (Sarmiento et al, 2004; Hogan et al, 2009) compared with a matched cohort. Tornqvist and colleagues (2009) analyzed more than 374,000 laparoscopic cholecystectomies over 40 years’ time in Sweden and identified 1386 injuries requiring reconstructive surgery; of note, injured patients had a fourfold increase in the risk of death from liver disease and a signficantly decreased overall survival. However, differences in study design and in the QoL instrument used make direct comparisons between studies difficult. In addition, pending litigation, which is common in such cases, is a potential confounding variable that likely has some influence on the results. Nevertheless, QoL measurement adds another dimension to the assessment of long-term outcome.
Several specialist centers reported satisfactory outcomes in 80% to 90% of patients reconstructed with Roux-en-Y biliary-enteric anastomosis, (see Table 42A.4). Many of these studies also identified factors associated with an adverse outcome (Table 42A.6). Chapman and colleagues (1995) found that long-term failure and the need for reintervention were significantly greater in patients with injuries involving the biliary confluence and those subjected to three or more repair attempts before referral. Tocchi and colleagues (1996) observed that the best results correlated directly with the degree of biliary ductal dilation, independent of stricture location. A report from Schmidt and colleagues (2005) confirmed that a repair in the presence of uncontrolled infection, a concomitant hepatic arterial injury, and injury location (i.e., at or above the bifurcation) were independent predictors of the development of major biliary complications. Surgeon experience and the type of repair also are important determinants of outcome. In the series reported by Stewart and Way (1995), primary end-to-end repairs over a T-tube were always unsuccessful when the duct had been completely transected.
Table 42A.6 Factors Associated with Stricture Recurrence or Poor Outcome After Operative Reconstruction
Many authors have advocated the use of anastomotic stents (Smith, 1969; Crist et al, 1987; Lillemoe, 1997), but the impact of such stents on long-term patency is questionable. We and other authors rarely use stents, and we get excellent results (Bismuth, 1982; Bismuth et al, 1978; Stewart & Way, 1995; Tocchi et al, 1996). Transanastomotic stents seem to have little impact on outcome and probably should not be used routinely. Although stents may be useful in selected cases, they cannot prevent the inevitable failure of a poorly constructed anastomosis.
The results of surgical reconstruction for injuries other than those involving the main extrahepatic bile duct have not been reported in great numbers and are not known precisely. Lillemoe and colleagues (2000) reported the results of 9 cases of reconstruction for isolated sectoral hepatic duct strictures over an 18-year period. All patients in this series incurred injuries to aberrant right sectoral hepatic ducts at laparoscopic or open cholecystectomy, and all had associated bile leaks. After Roux-en-Y hepaticojejunostomy, six (66.6%) of the nine patients had a successful outcome, whereas three patients developed late strictures that required reintervention. Colovic (2009) likewise analyzed 19 patients with isolated segmental, sectoral, and right hepatic duct injuries over a 26-year period. Twelve patients underwent surgical reconstruction, and the rest were managed expectantly or with percutaneous drainage with good to excellent results.
Patients with recurrent strictures after reconstruction at a specialist unit may be salvaged with further intervention, either anastomotic revision or balloon dilation. Several authors have reported some measure of success with surgical revision in these patients (Chapman et al, 1995; Lillemoe et al, 1997; Stewart & Way, 1995; Tocchi et al, 1996; Walsh et al, 2004). The likelihood of a good outcome is less than with the initial reconstruction, however. In the series reported by Chapman and colleagues (1995), 11 of 22 operative failures were treated a second time successfully and had satisfactory outcomes, but this was substantially lower than the nearly 80% success rate after the initial repair. Other groups have reported nearly equivalent surgical outcomes for reoperated patients when compared with primary repairs at the same institution (Pottakkat et al, 2007). As discussed previously, the loss of bile duct length associated with a failed repair is a major factor that limits the success of subsequent interventions.
Duodenal ulceration has been reported in 10% of patients after Roux-en-Y biliary-enteric reconstruction and constitutes an additional cause of late morbidity (McArthur & Longmire, 1971; Sato et al, 1982). Most patients respond to antiulcer medication, but some may develop significant hemorrhage.
Nonoperative Approaches
Advances in interventional radiology have resulted in a broader application of nonoperative approaches to bile duct stricture (see Chapter 28). The largest reported experience has been with percutaneous balloon dilation. With this technique, the biliary tree is accessed percutaneously, and a guidewire is passed through the stricture. The stricture is dilated with an angioplasty-type balloon catheter, after which a transhepatic stent is left in place for follow-up cholangiography and repeat dilation. In most cases, multiple dilations and prolonged biliary intubation are required. Early results have been encouraging, with several authors reporting good results in 55% to 85% of patients (Vogel et al, 1985; Mueller et al, 1986; Williams et al, 1987; Moore et al, 1987; Pitt et al, 1989; Lillemoe, 1997).
These results must be viewed with considerable caution, however. First, the mean follow-up in most of these studies was less than 3 years, which is insufficient to make a definitive comment regarding long-term efficacy. However, Cantwell and colleagues (2008) reported their long-term results with balloon dilation of 85 patients with benign postoperative biliary stricture and reported that most patients (61.9%) were successful after the first dilation, and 75% of patients had clinical patency after multiyear (mean, 8 years) follow-up. Second, balloon dilation is limited in its application to patients in whom biliary continuity is intact or has been restored by a previous attempt at repair; it has no role for strictures at or above the confluence and cannot be used if the bile duct has been transected (Lillemoe et al, 1997). Third, complications related to balloon dilation or to the percutaneous catheter are frequent and include hemobilia, bile leak, and cholangitis in 20% of patients.
Another nonoperative approach used is endoscopic stenting. With this technique, multiple plastic or metallic stents are placed across the stricture endoscopically, necessitating removal at a later date. Kassab and colleagues (2006) reported their experience with 88 patients with benign biliary stricture after cholecystectomy who underwent endoscopic stenting during ERCP. They reported that 69.1% of patients were successfully treated, with a mean of 1.6 stents placed for an average duration of 14 months and no stricture recurrence at a mean follow-up of 28 months after stent removal. Other groups have reported higher success rates, albeit at shorter follow-up intervals (Kuzela et al, 2005; van Boeckel et al, 2009). As with balloon dilation, the results of stenting are tempered with caveats of short follow-up periods and no randomized studies.
Comparing the results of balloon dilation or endoscopic stenting with operative reconstruction is difficult because of differences in the types of injuries selected for each type of treatment, inconsistencies in defining successful outcomes, and differences in reporting complications and length of follow-up. To date, no randomized studies have compared these techniques. In two retrospective analyses, biliary reconstruction was more likely to result in a successful outcome: 89% versus 52% (Pitt et al, 1989), 92% versus 64% (Lillemoe et al, 1997), and 94% versus 58% (Misra et al, 2004). Because balloon dilation requires multiple readmissions and repeat interventions, the overall cost and morbidity seem to be similar to that of operatively treated patients (Pitt et al, 1989). The data suggest that, in most cases, biliary-enteric anastomosis is more effective and provides more durable relief of symptoms than balloon dilation, although balloon dilation is preferable in patients who otherwise would not tolerate an operation; it may be tried as an initial treatment in patients with biliary anastomotic strictures, for which the success rate seems to be greater than for primary bile duct strictures (Millis et al, 1992).
Bile Duct Injury After Other Operations
Nontraditional Cholecystectomy
The advent of novel surgical technologies has ushered in a new potential for causation of iatrogenic biliary stricture. Natural orifice translumenal endoscopic surgery (NOTES)—a technique to obtain intraabdominal access via transgastric, transvaginal, transvesical, or transcolonic routes—has been recently applied for cholecystectomy. Multiple groups have described both the feasibility and safety of the technique (Chamberlain & Sakpal, 2009) as well as the ability to achieve Strasberg’s critical view of safety (Auyang et al, 2009). However, hepatic complications, including bile leak and hepatic injury (Zornig et al, 2009; Salinas et al, 2010; Chow et al, 2009), have occurred in addition to other NOTES-specific complications such as gastric hematoma, abdominal sepsis, and esophageal perforation.
Another recent surgical trend is single-incision laparosopic surgery (SILS) to perform cholecystectomy via a single port at the umbilicus. Similar to NOTES, complications include hepatic injury, cystic artery hemorrage, and biliary leakage (Palanivelu et al, 2008; Tacchino et al, 2009). As with any new technology, initial results are tempered by the anticipated learning curve. An important limitation for both SILS and NOTES for cholecystectomy is inadequate retraction and maneuverability of the gallbladder to achieve critical views. Percutaneous large-bore–needle T-fasteners inserted into the gallbladder for retraction are fragile and may increase the likelihood of bile leakage. The effect on iatrogenic biliary injury remains to be seen with these techniques. On the other hand, the implementation of robotic technologies in theory gives the ability for more refined movement and views. Groups have already reported using robotic surgical platforms as a salvage mechanism for completion cholecystectomy, as it is believed the increased precision allows for better visualization and dissection in a hostile abdomen than that provided by traditional open or laparoscopic approaches (Kohn & Martinie, 2009). These new techniques need further careful safety evaluations before they should be widely accepted. The authors believe that the minor cosmetic benefit of these procedures does not outweigh the unknown potential harmful affects when compared with the standard laparoscopic approach.
Biliary Reconstructive Operation
Postoperative stricture or fistula can complicate procedures that require biliary-enteric anastomoses, such as reconstruction after pancreaticoduodenectomy, bile duct resection for mid–bile duct tumors, and excision of choledochal cysts. Typically, these procedures involve choledochoenteric or hepaticoenteric anastomosis. Late strictures after such procedures are most likely to occur when enteric anastomosis is performed to a normal caliber duct, or when the duct itself is diseased, as in cases of choledochal cysts. When biliary-enteric anastomosis has been performed for longstanding biliary obstruction, the duct is dilatated and thickened. In these cases, the anastomosis is usually easy to construct, and late stenosis is uncommon (Tocchi et al, 1996). In most cases, stricture of a biliary-enteric anastomosis after resection for malignancy is the result of cancer recurrence. However, in a retrospective study of 1595 patients, no difference was apparent in postoperative stricture rate (2.6%) between benign and malignant indications for resection following pancreaticoduodenectomy (House et al, 2006). Only 9% of strictures were caused by recurrent malignant disease, and preoperative biliary drainage and postoperative biliary stenting were the only risk factors for stricture.
Late stricture may also occur after side-to-side choledochoduodenostomy performed for choledocholithiasis or as a bypass procedure for chronic pancreatitis. This complication is rare if the anastomosis is performed to a sufficiently dilated duct (at least 1.5 cm), and the final diameter of the anastomosis is at least 2 to 2.5 cm (Schein & Gliedman, 1981; Escudero-Fabre et al, 1991). The so-called sump syndrome after choledochoduodenostomy—in which particulate matter, stones, and food debris accumulate and stagnate in the distal, “blind” end of the common duct—is an occasional cause of recurrent cholangitis that can result in anastomotic stricture (Matthews et al, 1993; Venerito et al, 2009). Endoscopic management, consisting of sphincterotomy with or without balloon dilation of the anastomosis or even placement of an occlusion device, has been reported for this condition (Baker et al, 1985; Ell et al, 2006). This approach may not be adequate, however, to remove the thick, infected debris that is often densely adherent to the wall of the inflamed distal CBD, and restricture of the anastomosis is common. We prefer reoperation, with end-to-side hepaticojejunostomy, Roux-en-Y, to prevent persistent regurgitation of intestinal contents and to remove the “sump” permanently (Matthews et al, 1993). When dissection in the hilus is rendered hazardous because of dense scarring, an alternative maneuver is to perform a pyloric exclusion and gastrojejunostomy, which accomplishes the same objective of preventing reflux of intestinal contents into the biliary tree.
Recurrent cholangitis after biliary-enteric anastomosis almost invariably is attributed to anastomotic stricture; however, this assumption has been questioned (Goldman et al, 1983). Some patients develop repeated episodes of cholangitis despite apparently free drainage of bile through the anastomosis in the absence of a clear stricture. In these cases, there may be an overlooked intrahepatic stricture or intrahepatic stone disease (Matthews et al, 1993) or even parasitic infection (Mercado et al, 2006).
Gastric Resection
Injury to the bile duct at gastrectomy is particularly likely when the pyloric region and duodenal bulb are severely distorted or inflamed (Florence et al, 1981). The most common situation is biliary injury during Billroth II gastrectomy. Patients may be seen with jaundice or biliary fistula in the postoperative period, which may be mistakenly attributed to a duodenal stump leak. Less commonly, bile duct injury occurs during gastrectomy (Fig. 42A.26), which can result in biliary stricture. Chronic inflammation from a duodenal ulcer or deeply placed sutures for management of hemorrhage can present with a delayed distal bile duct stricture or papillary stenosis (Fig. 42A.27).
Hepatic Resection
Biliary injury during hepatic resection is uncommon in experienced hands, but it can occur, particularly when resection is done for lesions near the hilus. In such instances, we recommend that the bile duct be freed from the tumor by careful dissection of hilum and clear identification of the right and left main ducts. Special care should be exercised when using the pedicle ligation and stapler technique to prevent inadvertent injury or complete occlusion of the left bile duct in right-sided resections (Fig. 42A.28). If there is a suspicion of iatrogenic ductal injury that cannot be readily identified, or if the biliary anatomy is unclear, intraoperative cholangiography should be performed; deliberate choledochotomy with passage of fine bougies into the right and left ducts may assist identification. If a choledochostomy has been performed, a T-tube usually is inserted.
The management of injuries that occur after partial hepatectomy can be extremely difficult (Johnson et al, 1979; Smith et al, 1982; Matthews et al, 1991). The problems are similar to those encountered with liver atrophy discussed earlier. A biliary fistula after liver resection should be treated expectantly for a long time, because many resolve spontaneously. Operative repair should be undertaken only for persistent fistula despite continued observation or for jaundice or recurrent episodes of cholangitis.
Other Procedures
Late biliary stenosis may follow any other procedure requiring dissection in or near the porta hepatis, such as portocaval shunt or lymphadenectomy (Ishizuka et al, 1998). Biliary strictures also have been described after external-beam radiation therapy (Schmets et al, 1996) and after endoscopic injection of sclerosant into a bleeding duodenal ulcer (Luman, 1994). Bile duct strictures also may complicate orthotopic liver transplantation (see Chapter 98A), although their frequency seems to have decreased (Calne, 1976; Colonna et al, 1992; Vougas et al, 1996; Macfarlane et al, 1996; Lemmer et al, 1997; Wojcicki et al, 2008). Direct end-to-end choledochostomy has been the preferred method of reconstruction, but Roux-en-Y choledochojejunostomy also is used (Lopez-Santamaria et al, 1999). The use of T-tubes after biliary reconstruction, once considered essential, has been shown to offer no significant benefit (Vougas et al, 1996), and their routine use has been discontinued in many centers (Macfarlane et al, 1996; Vougas et al, 1996; see Chapter 35). Hepatic artery thrombosis is an uncommon but well-recognized cause of biliary stricture after liver transplantation (Colonna et al, 1992; Orons et al, 1995; Sanchez-Urdazpal et al, 1993; see Chapter 100). Late stricture is seen in 10% to 15% of cases (Macfarlane et al, 1996; Vougas et al, 1996), which is much lower than the reported rate of recurrent stricture after direct anastomosis for bile duct injuries. Many of these patients require operative revision, but some may be managed successfully with endoscopic or interventional radiologic approaches (Colonna et al, 1992; Lemmer et al, 1997; Macfarlane et al, 1996). In addition, the burgeoning of living donor transplantation has exposed the potential for significant biliary injury to the donor, a problem that seems to be uncommon in experienced hands (see Chapters 98B and 100; Lo, 2003; Liu et al, 2005).
Bile Duct Injury Resulting from Blunt or Penetrating Injury
Hepatic, pancreatic, and biliary injuries from trauma are discussed more fully in Chapters 102, 103, and 104. The gallbladder or biliary tree may be damaged by blunt (Fig. 42A.29) or penetrating abdominal injuries. The CBD is susceptible to disruption from deceleration injuries, usually at the level of the pancreaticoduodenal junction, where it is relatively fixed compared with the more proximal duct. Delayed common duct stricture has been reported after blunt abdominal trauma (Yoon et al, 1998; Horiguchi et al, 1998). Late problems also may arise after hepatic trauma, in which prolonged fistulization occurs from a segment of liver isolated by the injury, particularly when bile drains from a large portion of the liver through the fistulous tract.
Management of ductal injuries depends on the extent of associated injuries. Major hemorrhage and other life-threatening injuries take priority, and in this setting, external diversion of bile flow through a tube passed proximally into the duct prevents bile leak and allows for later definitive repair. Roux-en-Y biliary-enteric reconstruction is the preferred approach for complete ductal transections (Degiannis et al, 2001). Traumatic strictures are probably also best approached in this manner, although successful dilation has been reported (Horiguchi et al, 1998). Management of persistent bile leak after liver injury is a much more difficult problem, because the fistula may persist even after prolonged observation. The possibility of an associated distal stricture, which perpetuates bile drainage, should be excluded. One approach to management is by anastomosis of a well-developed fistula tract to a loop of jejunum (fistulojejunostomy) or to the gallbladder. Fistulojejunostomy may yield a permanent cure, although late stenosis or stricture still may develop, for which secondary repair must be performed (Smith et al, 1982). Resection of an isolated segment of liver that is the source of the drainage is difficult and seldom warranted, and direct oversewing of the fistula is rarely successful.
Postinflammatory Biliary Strictures
Longstanding Cholelithiasis
Repeated attacks of cholecystitis may result in progressive fibrosis and shrinking of the gallbladder, which ultimately obliterates the triangle of Calot, as described earlier. The inflammatory process may spread to involve the common hepatic duct, causing inflammatory stenosis and resulting in jaundice and cholangitis (Mirizzi, 1948; see Chapter 42B). The presentation may be associated with a history of acute or chronic cholecystitis, and an overt stricture may be present at the time of cholecystectomy. Intraoperative cholangiography may be difficult to obtain, and inadvertent bile duct injury may ensue during attempts to remove the gallbladder. A large stone in the region of the Hartmann pouch may erode into the common hepatic duct and cause a cholecystocholedochal fistula (see Fig. 42A.5). In such cases, attempts to remove the gallbladder result in injury to the common hepatic duct or removal of a portion of the duct. Patients with long-standing gallstones and jaundice should never be submitted to operation without preliminary percutaneous or endoscopic cholangiography.
Patients with inflammatory strictures of the common hepatic duct in association with chronic cholelithiasis may be seen with radiologic features that are indistinguishable from cholangiocarcinoma (see Chapter 50B); this possibility must be considered in all patients suspected to harbor a tumor at the biliary confluence with coincident choledocholithiasis (Hadjis et al, 1985; Standfield et al, 1989; Wetter et al, 1991). Biliary cytology and percutaneous biopsy generally are not helpful, because a benign result never excludes a malignancy; the diagnosis usually is established at operation.
Recurrent Pyogenic Cholangitis
Recurrent pyogenic cholangitis is addressed in detail in Chapters 39 and 40. This condition is seen mainly in Southeast Asia and is associated with intrahepatic calcium bilirubinate stones and intrahepatic strictures (Ong, 1962; Maki et al, 1972; Choi et al, 1982; Chen et al, 1984). Recurrent episodes of cholangitis and sepsis are the major threat to life in these patients, along with a significantly increased risk of cholangiocarcinoma (Sato et al, 1995, 1998; Chijiiwa et al, 1995; Chu et al, 1997). Hepatic resection combined with Roux-en-Y biliary-enteric reconstruction is effective in relieving symptoms and clearing stones. This is particularly true if the disease is unilateral, but even patients with bilateral disease may benefit from a combination of resection and postoperative balloon dilation and stone extraction (Jeng et al, 1996). Because of the high incidence of recurrent stricture and stone formation, a team approach that includes experienced surgeons, interventional radiologists, and gastroenterogists is required to obtain the best results (Kim et al, 1998). Careful operative planning is important, and the operation can be tailored to help provide future access to the biliary tract. For example, we have sucessfully used a Roux-en-Y biliary-enteric reconstruction that not only includes the long-segment limb anchored to the abdominal wall for interventional radiology access, but a jejunogastrostomy is also constructed, using the proximal end of the Roux limb, for easy endoscopic access in patients at high risk for recurrent stones (Fig. 42A.30). Some authors have reported success using balloon dilation combined with transhepatic choledochoscopic lithotripsy, either as initial therapy or for recurrent stones after surgery (Yoshida et al, 1998; Sheen-Chen et al, 1998). Numerous patients have advanced parenchymal disease at presentation and die as a result of related complications despite aggressive intervention (Chijiiwa et al, 1995).
Chronic Pancreatitis (See Chapter 55A, Chapter 55B )
Chronic pancreatitis is a well-known cause of distal bile duct stenosis and stricture. The incidence of biliary stricture in these patients is difficult to know with certainty, but it has been reported in 30% of patients (Sarles & Sahel, 1978). The characteristic lesion is a long, narrow stricture involving the retropancreatic portion of the CBD (Fig. 42A.31), but other variants have been described (Sarles & Sahel, 1978; Barthet et al, 1994). Although more common in association with alcohol-related chronic pancreatitis, it may occur in chronic pancreatitis unrelated to alcohol use. In addition to jaundice, pain is common, which may be intermittent and similar to biliary colic; cholangitis and fever are less frequent (Stabile et al, 1987; Kalvaria et al, 1989; Barthet et al, 1994).
The presence of a bile duct stenosis or stricture in association with chronic pancreatitis is not an indication for therapy. Quite severe stenosis may be found in the presence of absolutely normal LFTs and in patients who have never had jaundice. If cholestasis or cholangitis occurs, however, biliary bypass becomes necessary. Occasionally, operation is necessary in the management of chronic pancreatitis, and if it is known that a high-grade biliary stricture is present, biliary bypass may be performed concomitantly. In a series of 240 patients with chronic pancreatitis, 39 of whom came to operation, nine had symptomatic biliary obstruction, and 13 were subjected to some form of biliary-enteric anastomosis, either alone or associated with excisional surgery for pancreatic disease. Most patients with CBD stenosis associated with chronic pancreatitis can be managed without biliary bypass (Kalvaria et al, 1989; Smits et al, 1996). Temporary endoscopic biliary stenting may be useful in some cases and may be definitive therapy in 10% to 25% (Barthet et al, 1994; Itani & Taylor, 1995; Smits et al, 1996).
For patients requiring biliary bypass, side-to-side choledochoduodenostomy may be used, but this is often not the best option, because the surrounding inflammatory reaction may render the duodenum rigid and less mobile. Also, the CBD may be involved in periductal fibrosis and may be only minimally dilated. Roux-en-Y choledochojejunostomy is the preferred reconstructive approach. Cholecystojejunostomy should not be used, and transduodenal sphincteroplasty is generally not suitable because of the great length of the stricture. Biliary-enteric anastomosis generally affords excellent long-term relief of obstruction, but relief from chronic pain associated with the pancreatitis itself is unusual (Barthet et al, 1994; Stabile et al, 1987).
Abdel WM, et al. Postcholecystectomy bile duct injuries: experience with 49 cases managed by different therapeutic modalities. Hepatogastroenterology. 1996;43:1141-1147.
Adamsen S, et al. Bile duct injury during laparoscopic cholecystectomy: a prospective nationwide series. J Am Coll Surg. 1997;184:571-578.
Agarwal AK, et al. Management of patients of postcholecystectomy benign biliary stricture complicated by portal hypertension. Am J Surg. 2008;195:421-426.
Andren-Sandberg A, Alinder G, Bengmark S. Accidental lesions of the common bile duct at cholecystectomy: pre- and perioperative factors of importance. Ann Surg. 1985;201:328-332.
Asbun HJ, et al. Bile duct injury during laparoscopic cholecystectomy: mechanism of injury, prevention, and management. World J Surg. 1993;17:547-551.
Auyang ED, et al. Natural orifice translumenal endoscopic surgery (NOTES): dissection for the critical view of safety during transcolonic cholecystectomy. Surg Endosc. 2009;23:1117-1118.
Baker AR, et al. Sump syndrome following choledochoduodenostomy and its endoscopic treatment. Br J Surg. 1985;72:433-435.
Balsara KP, Dubash C, Shah CR. Pseudoaneurysm of the hepatic artery along with common bile duct injury following laparoscopic cholecystectomy: a report of two cases. Surg Endosc. 1998;12:276-277.
Barker EM, Winkler M. Permanent-access hepaticojejunostomy. Br J Surg. 1984;71:188-191.
Barthet M, et al. Biliary stenting in benign biliary stenosis complicating chronic calcifying pancreatitis. Endoscopy. 1994;26:569-572.
Bismuth H. Postoperative strictures of the bile duct. In: Blumgart, LH, editor. The Biliary Tract: Clinical Surgery International. Edinburgh: Churchill Livingstone; 1982:209-218.
Bismuth H, et al. Long term results of Roux-en-Y hepaticojejunostomy. Surg Gynecol Obstet. 1978;146:161-167.
Bismuth H, Lazorthes F. [83rd Congress of the French Surgical Society (Paris, 21-24 September 1981): second report—operative injuries of the common biliary duct]. J Chir (Paris). 1981;118:601-609.
Blumgart LH. Biliary tract obstruction—new approaches to old problems. Am J Surg. 1978;135:19-31.
Blumgart LH. Hepatic resection. In: Taylor, S, editor. Recent Advances in Surgery. Edinburgh: Churchill Livingstone; 1980:1-26.
Blumgart LH, Kelley CJ. Hepaticojejunostomy in benign and malignant high bile duct stricture: approaches to the left hepatic ducts. Br J Surg. 1984;71:257-261.
Blumgart LH, et al. Benign bile duct stricture following cholecystectomy: critical factors in management. Br J Srug. 1984;71:836-843.
Boddy AP, et al. Who should perform laparoscopic cholecystectomy? A 10-year audit. Surg Endosc. 2007;21:1492-1497.
Boerma D, et al. Impaired quality of life 5 years after bile duct injury during laparoscopic cholecystectomy: a prospective analysis. Ann Surg. 2001;234:750-757.
Brooks DC, et al. Management of bile leaks following laparoscopic cholecystectomy. Surg Endosc. 1993;7:292-295.
Bujanda L, et al. MRCP in the diagnosis of iatrogenic bile duct injury. NMR Biomed. 2003;16:475-478.
Burhenne HJ. Garland lecture: percutaneous extraction of retained biliary tract stones—661 patients. Am J Roentgenol. 1980;134:889-898.
Calne RY. A new technique for biliary drainage in orthotopic liver transplantation utilizing the gallbladder as a pedicle graft conduit between the donor and recipient common bile ducts. Ann Surg. 1976;184:605-609.
Cantwell CP, et al. Thirty years’ experience with balloon dilation of benign postoperative biliary strictures: long-term outcomes. Radiology. 2008;249(3):1050-1057.
Carroll BJ, et al. Routine cholangiography reduces sequelae of common bile duct injuries. Surg Endosc. 1996;10:1194-1197.
Casillas RA, Yegiyants S, Collins JC. Early laparoscopic cholecystectomy is the preferred management of acute cholecystitis. Arch Surg. 2008;143:533-537.
Cattell RB, Braasch JW. Two-stage repairs of benign strictures of the bile duct. Surg Gynecol Obstet. 1959;109:691-696.
Cetta F, et al. Clip migration within the common duct after laparoscopic cholecystectomy: a case of transient acute pancreatitis in the absence of associated stones. Endoscopy. 1997;29:S59-S60.
Chamberlain RS, Sakpal SV. A comprehensive review of single-incision laparoscopic surgery (SILS) and natural orifice transluminal endoscopic surgery (NOTES) techniques for cholecystectomy. J Gastrointest Surg. 2009;13:1733-1740.
Chapman WC, et al. Postcholecystectomy bile duct strictures: management and outcome in 130 patients. Arch Surg. 1995;130:597-602.
Chen HH, et al. Twenty-two year experience with the diagnosis and treatment of intrahepatic calculi. Surg Gynecol Obstet. 1984;159:519-524.
Chijiiwa K, et al. Current management and long-term prognosis of hepatolithiasis. Arch Surg. 1995;130:194-197.
Choi TK, Wong J, Ong GB. The surgical management of primary intrahepatic stones. Br J Surg. 1982;69:86-90.
Chow A, Purkayastha S, Paraskeva P. Appendicectomy and cholecystectomy using single-incision laparoscopic surgery (SILS): the first UK experience. Surg Innov. 2009;16:211-217.
Chu KM, et al. Malignancy associated with hepatolithiasis. Hepatogastroenterology. 1997;44:352-357.
Coakley FV, et al. Complex postcholecystectomy biliary disorders: preliminary experience with evaluation by means of breath-hold MR cholangiography. Radiology. 1998;209:141-146.
Colonna JO, et al. Biliary strictures complicating liver transplantation: incidence, pathogenesis, management, and outcome. Ann Surg. 1992;216:344-350.
Colovic RB. Isolated segmental, sectoral and right hepatic bile duct injuries. World J Gastroenterol. 2009;15(12):1415-1419.
Couinaud C. Les hepato-cholangiostomies digestives. Presse Med. 1953;61:468-470.
Couinaud C. Recherches sur la chirugie du confluent biliare superieur et des canaux hepatiques. Presse Med. 1955;63:669-674.
Crist DW, Kadir S, Cameron JL. The value of preoperatively placed percutaneous biliary catheters in reconstruction of the proximal part of the biliary tract. Surg Gynecol Obstet. 1987;165:421-424.
Csendes A, et al. Late results of immediate primary end-to-end repair in accidental section of the common bile duct. Surg Gynecol Obstet. 1989;168:125-130.
Csikesz NG, et al. Surgeon volume metrics in laparoscopic cholecystectomy. Dig Dis Sci. 2009;55(8):2398-2405.
Czerniak A, et al. Liver atrophy complicating benign bile duct strictures: surgical and interventional radiologic approaches. Am J Surg. 1986;152:294-300.
Davidoff AM, et al. Mechanisms of major biliary injury during laparoscopic cholecystectomy. Ann Surg. 1992;215:196-202.
Degiannis E, et al. Gunshot injuries of the extrahepatic biliary ducts. Eur J Surg. 2001;167:618-621.
de Reuver PR, et al. Long-term results of a primary end-to-end anastomosis in peroperative detected bile duct injury. J Gastrointest Surg. 2007;11:296-302.
de Reuver PR, et al. Impact of bile duct injury after laparoscopic cholecystectomy on quality of life: a longitudinal study after multidisciplinary treatment. Endoscopy. 2008;40:637-643.
Deziel DJ, et al. Complications of laparoscopic cholecystectomy: a national survey of 4,292 hospitals and an analysis of 77,604 cases. Am J Surg. 1993;165:9-14.
Dolay K, et al. Migrated endoclip and stone formation after cholecystectomy: a new danger of acute pancreatitis. World J Gastroenterol. 2007;13:6446-6448.
Duffield JS, et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56-65.
Dusunceli E, Erden A, Erden I. Anatomic variations of the bile ducts: MRCP findings [in Turkish]. Tani Girisim Radyol. 2004;10:296-303.
Ell C, et al. Endoscopic treatment of the “sump syndrome” after choledochoduodenostomy: a new technique using an amplatzer septal occluder. Z Gastroenterol. 2006;44:1231-1235.
Ellis H, Hoile RW. Vein patch repair of the common bile duct. J R Soc Med. 1980;73:635-637.
Escudero-Fabre A, et al. Choledochoduodenostomy: analysis of 71 cases followed for 5 to 15 years. Ann Surg. 1991;213:635-642.
Felekouras E, et al. Emergency liver resection for combined biliary and vascular injury following laparoscopic cholecystectomy: case report and review of the literature. South Med J. 2007;100:317-320.
Fiddian-Green RG, et al. Median hepatotomy using ultrasonic dissection for complex hepatobiliary problems. Arch Surg. 1988;123:901-907.
Fischer CP, et al. Timing of referral impacts surgical outcomes in patients undergoing repair of bile duct injuries. HPB (Oxford). 2009;11:32-37.
Fletcher DR, et al. Complications of cholecystectomy: risks of the laparoscopic approach and protective effects of operative cholangiography: a population-based study. Ann Surg. 1999;229:449-457.
Florence MG, Hart MJ, White TT. Ampullary disconnection during the course of biliary and duodenal surgery. Am J Surg. 1981;142:100-105.
Friedman SL. Molecular mechanisms of hepatic fibrosis and principles of therapy. J Gastroenterol. 1997;32:424-430.
Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655-1669.
Frilling A, et al. Major bile duct injuries after laparoscopic cholecystectomy: a tertiary center experience. J Gastrointest Surg. 2004;8(6):679-685.
Fujii T, et al. Efficacy of endoscopic diagnosis and treatment for postoperative biliary leak. Hepatogastroenterology. 1998;45:656-661.
Gigot J, et al. The dramatic reality of biliary tract injury during laparoscopic cholecystectomy: an anonymous multicenter Belgian survey of 65 patients. Surg Endosc. 1997;11(12):1171-1178.
Goldman LD, Steer ML, Silen W. Recurrent cholangitis after biliary surgery. Am J Surg. 1983;145:450-454.
Gupta N, et al. Management and outcome of patients with combined bile duct and hepatic artery injuries. Arch Surg. 1998;133:176-181.
Gurusamy K, et al. Meta-analysis of randomized controlled trials on the safety and effectiveness of early versus delayed laparoscopic cholecystectomy for acute cholecystitis. Br J Surg. 2009;97:141-150.
Hadjis NS, Collier NA, Blumgart LH. Malignant masquerade at the hilum of the liver. Br J Surg. 1985;72:659-661.
Heinrich S, et al. Right hemihepatectomy for bile duct injury following laparoscopic cholecystectomy. Surg Endosc. 2003;17:1494-1495.
Hepp J, Couinaud C. L’abord et l’utilisation du canal hepatique gauche dans les reparations de la voie biliare principale. Presse Med. 1956;64:947-948.
Hirano Y, et al. Efficacy of multi-slice computed tomography cholangiography before laparoscopic cholecystectomy. ANZ J Surg. 2006;76:693-695.
Hochwald SN, et al. Association of preoperative biliary stenting with increased postoperative infectious complications in proximal cholangiocarcinoma. Arch Surg. 1999;134:261-266.
Hogan AM, et al. Quality of life after iatrogenic bile duct injury: a case control study. Ann Surg. 2009;249:292-295.
Horiguchi J, et al. Traumatic biliary stricture successfully treated by percutaneous transhepatic bile duct dilatation: a case report. Hepatogastroenterology. 1998;45:2038-2041.
House MG, et al. Incidence and outcome of biliary strictures after pancreaticoduodenectomy. Ann Surg. 2006;243:571-576.
Hublet A, et al. Laparoscopic ultrasonography as a good alternative to intraoperative cholangiography (IOC) during laparoscopic cholecystectomy: results of prospective study. Acta Chir Belg. 2009;109:312-316.
Hunter JG. Avoidance of bile duct injury during laparoscopic cholecystectomy. Am J Surg. 1991;162:71-76.
Inui H, Kwon AH, Kamiyama Y. Managing bile duct injury during and after laparoscopic cholecystectomy. J Hepatobiliary Pancreat Surg. 1998;5:445-449.
Ishizuka D, Shirai Y, Hatakeyama K. Ischemic biliary stricture due to lymph node dissection in the hepatoduodenal ligament. Hepatogastroenterology. 1998;45:2048-2050.
Itani KM, Taylor TV. The challenge of therapy for pancreatitis-related common bile duct stricture. Am J Surg. 1995;170:543-546.
Jalan R, et al. Transjugular intrahepatic portosystemic stent-shunt (TIPSS) occlusion and the role of biliary venous fistulae. J Hepatol. 1996;24:169-176.
Jarnagin WR, et al. Expression of variant fibronectins in wound healing: cellular source and biological activity of the EIIIA segment in rat hepatic fibrogenesis. J Cell Biol. 1994;127:2037-2048.
Jeng KS, Ohta I, Yang FS. Reappraisal of the systematic management of complicated hepatolithiasis with bilateral intrahepatic biliary strictures. Arch Surg. 1996;131:141-147.
Johnson AG, Lyon IM, Blumgart LH. Stricture of common hepatic duct after right hepatic lobectomy treated by Longmire’s operation. J R Soc Med. 1979;72:136-139.
Johnson AG, Stevens AE. Importance of the size of the stoma in choledochoduodenostomy. Gut. 1969;10:68-70.
Kalvaria I, et al. The spectrum and natural history of common bile duct stenosis in chronic alcohol-induced pancreatitis. Ann Surg. 1989;210:608-613.
Kaman L, et al. Comparison of major bile duct injuries following laparoscopic cholecystectomy and open cholecystectomy. ANZ J Surg. 2006;76:788-791.
Kassab C, et al. Endoscopic management of post-laparoscopic cholecystectomy biliary strictures. Long-term outcome in a multicenter study. Gastroenterol Clin Biol. 2006;30(1):124-129.
Kholdebarin R, et al. Risk factors for bile duct injury during laparoscopic cholecystectomy: a case-control study. Surg Innov. 2008;15:114-119.
Kim KH, et al. Clinical significance of intrahepatic biliary stricture in efficacy of hepatic resection for intrahepatic stones. J Hepatobiliary Pancreat Surg. 1998;5:303-308.
Kirkland JG, et al. Reversible surgical model of biliary inflammation and obstructive jaundice in mice. J Surg Res. 2009;164(2):221-227.
Kocabiyik N, et al. Anatomical assessment of bile ducts of Luschka in human fetuses. Surg Radiol Anat. 2009;31:517-521.
Kohn GP, Martinie JB. Laparoscopic robot-assisted completion cholecystectomy: a report of three cases. Int J Med Robot. 2009;5:406-409.
Kullman E, et al. Value of routine intraoperative cholangiography in detecting aberrant bile ducts and bile duct injuries during laparoscopic cholecystectomy. Br J Surg. 1996;83:171-175.
Kum CK, et al. Laparoscopic cholecystectomy for acute cholecystitis: is it really safe? World J Surg. 1996;20:43-48.
Kune GA, Sali A. Benign biliary strictures. In: Kune, GA, Sali, A. The Practice of Biliary Surgery. 2nd ed. Oxford: Blackwell; 1981:192-220.
Kuzela L, et al. Prospective follow-up of patients with bile duct strictures secondary to laparoscopic cholecystectomy, treated endoscopically with multiple stents. Hepatogastroenterology. 2005;52:1357-1361.
Laurent A, et al. Major hepatectomy for the treatment of complex bile duct injury. Ann Surg. 2008;248(1):77-83.
Lemmer ER, et al. The management of biliary complications following orthotopic liver transplantation. S Afr J Surg. 1997;35:77-81.
Li J, et al. Management of concomitant hepatic artery injury in patients with iatrogenic major bile duct injury after laparoscopic cholecystectomy. Br J Surg. 2008;95:460-465.
Liguory C, et al. Endoscopic treatment of postoperative biliary fistulae. Surgery. 1991;110:779-783.
Lillemoe KD. Benign post-operative bile duct strictures. Baillieres Clin Gastroenterol. 1997;11:749-779.
Lillemoe KD, et al. Major bile duct injuries during laparoscopic cholecystectomy: follow-up after combined surgical and radiologic management. Ann Surg. 1997;225:459-468.
Lillemoe KD, et al. Isolated right segmental hepatic duct injury: a diagnostic and therapeutic challenge. J Gastrointest Surg. 2000;4:168-177.
Liu CL, et al. Safety of donor right hepatectomy without abdominal drainage: a prospective evaluation in 100 consecutive liver donors. Liver Transpl. 2005;11:314-319.
Lo CM. Complications and long-term outcome of living liver donors: a survey of 1,508 cases in five Asian centers. Transplantation. 2003;75:S12-S15.
Longmire WP, Sandford MC. Intrahepatic cholangiojejunostomy with partial hepatectomy for biliary obstruction. Surgery. 1948;128:330-347.
Longmire WP, Sandford MC. Intrahepatic cholangiojejunostomy for biliary obstuction—further studies: report of 4 cases. Ann Surg. 1949;130:455-460.
Lopez-Santamaria M, et al. Late biliary complications in pediatric liver transplantation. J Pediatr Surg. 1999;34:316-320.
Ludwig K, et al. Surgical strategies in the laparoscopic therapy of cholecystolithiasis and common duct stones. ANZ J Surg. 2002;72(8):547-552.
Luman W. Distal biliary stricture as a complication of sclerosant injection for bleeding duodenal ulcer. Gut. 1994;35:1665-1667.
MacFadyen BVJr, et al. Bile duct injury after laparoscopic cholecystectomy: the United States experience. Surg Endosc. 1998;12:315-321.
Macfarlane B, et al. Endoscopic retrograde cholangiography in the diagnosis and endoscopic management of biliary complications after liver transplantation. Eur J Gastroenterol Hepatol. 1996;8:1003-1006.
Machi J, et al. The routine use of laparoscopic ultrasound decreases bile duct injury: a multicenter study. Surg Endosc. 2009;23:384-388.
Maher JJ, McGuire RF. Extracellular matrix gene expression increases preferentially in rat lipocytes and sinusoidal endothelial cells during hepatic fibrosis in vivo. J Clin Invest. 1990;86:1641-1648.
Maki T, Sato T, Matsushiro T. A reappraisal of surgical treatment for intrahepatic gallstones. Ann Surg. 1972;175:155-165.
Matthews JB, et al. Recurrent cholangitis with and without anastomotic stricture after biliary-enteric bypass. Arch Surg. 1993;128:269-272.
Matthews JB, et al. Biliary stricture following hepatic resection. HPB Surg. 1991;3:181-190.
McArthur MS, Longmire WPJr. Peptic ulcer disease after choledochojejunostomy. Am J Surg. 1971;122:155-158.
McDonald ML, et al. Benign biliary strictures: repair and outcome with a contemporary approach. Surgery. 1995;118:582-590.
McMahon AJ, et al. Bile duct injury and bile leakage in laparoscopic cholecystectomy. Br J Surg. 1995;82:307-313.
McPherson GA, et al. Percutaneous transhepatic drainage in obstructive jaundice: advantages and problems. Br J Surg. 1982;69:261-264.
McPherson SJ, et al. Percutaneous transjejunal biliary intervention: 10-year experience with access via Roux-en-Y loops. Radiology. 1998;206:665-672.
Melton GB, et al. Major bile duct injuries associated with laparoscopic cholecystectomy: effect of surgical repair on quality of life. Ann Surg. 2002;235:888-895.
Mercado MA, et al. An unusual cause of bilioenteric anastomotic dysfunction after iatrogenic bile duct injury. Ann Hepatol. 2006;5:120-122.
Michalowski K, et al. Laparoscopic subtotal cholecystectomy in patients with complicated acute cholecystitis or fibrosis. Br J Surg. 1998;85:904-906.
Millis JM, et al. Management of bile duct strictures: an evolving strategy. Arch Surg. 1992;127:1077-1082.
Mirizzi PL. Sindrome del conducto hepatico (sindrome hepaticiano). J. Int Surg. 1948;8:731-734.
Misra S, et al. Percutaneous management of bile duct strictures and injuries associated with laparoscopic cholecystectomy: a decade of experience. J Am Coll Surg. 2004;198:218-226.
Molnar W, Stockum AE. Transhepatic dilatation of choledochoenterostomy strictures. Radiology. 1978;129:59-64.
Moore AVJr, et al. Percutaneous dilation of benign biliary strictures. Radiology. 1987;163:625-628.
Moore DE, et al. Long-term detrimental effect of bile duct injury on health-related quality of life. Arch Surg. 2004;139:476-481.
Moossa AR, et al. Laparoscopic injuries to the bile duct: a cause for concern. Ann Surg. 1992;215:203-208.
Mueller PR, et al. Biliary stricture dilatation: multicenter review of clinical management in 73 patients. Radiology. 1986;160:17-22.
Nakajima J, et al. Laparoscopic subtotal cholecystectomy for severe cholecystitis. Surg Today. 2009;39:870-875.
Nealon WH, Urrutia F. Long-term follow-up after bilioenteric anastomosis for benign bile duct stricture. Ann Surg. 1996;223:639-645.
Nelson MT, Nakashima M, Mulvihill SJ. How secure are laparoscopically placed clips? An in vitro and in vivo study. Arch Surg. 1992;127:718-720.
Neri V, et al. Difficult cholecystectomies: validity of the laparoscopic approach. JSLS. 2003;7:329-333.
Nicholson T, et al. Hepatic artery angiography and embolization for hemobilia following laparoscopic cholecystectomy. Cardiovasc Intervent Radiol. 1999;22:20-24.
Northover JM, Terblanche J. A new look at the arterial supply of the bile duct in man and its surgical implications. Br J Surg. 1979;66:379-384.
Nuzzo G, et al. Bile duct injury during laparoscopic cholecystectomy: results of an Italian national survey on 56,591 cholecystectomies. Arch Surg. 2005;140:986-992.
Okamura T, et al. Surgical technique for repair of benign strictures of the bile ducts, preserving the papilla of Vater. World J Surg. 1985;9:619-625.
Olsen D. Bile duct injuries during laparoscopic cholecystectomy. Surg Endosc. 1997;11:133-138.
Ong GB. A study of recurrent pyogenic cholangitis. Arch Surg. 1962;84:199-225.
Orons PD, Sheng R, Zajko AB. Hepatic artery stenosis in liver transplant recipients: prevalence and cholangiographic appearance of associated biliary complications. Am J Roentgenol. 1995;165:1145-1149.
Palanivelu C, et al. Transumbilical flexible endoscopic cholecystectomy in humans: first feasibility study using a hybrid technique. Endoscopy. 2008;40:428-431.
Pellegrini CA, Thomas MJ, Way LW. Recurrent biliary stricture: patterns of recurrence and outcome of surgical therapy. Am J Surg. 1984;147:175-180.
Perakath B, et al. Post-cholecystectomy benign biliary stricture with portal hypertension: is a portosystemic shunt before hepaticojejunostomy necessary? Ann R Coll Surg Engl. 2003;85:317-320.
Pitt HA, et al. Factors influencing outcome in patients with postoperative biliary strictures. Am J Surg. 1982;144:14-21.
Pitt HA, et al. Benign postoperative biliary strictures: operate or dilate? Ann Surg. 1989;210:417-425.
Pottakkat B, et al. Recurrent bile duct stricture: causes and long-term results of surgical management. J Hepatobiliary Pancreat Surg. 2007;14:171-176.
Pottakkat B, et al. Surgical management of patients with post-cholecystectomy benign biliary stricture complicated by atrophy–hypertrophy complex of the liver. HPB (Oxford). 2009;11:125-129.
Ragozzino A, et al. Value of MR cholangiography in patients with iatrogenic bile duct injury after cholecystectomy. Am J Roentgenol. 2004;183:1567-1572.
Raoul JL, et al. Cystic duct clip migration into the common bile duct: a complication of laparoscopic cholecystectomy treated by endoscopic biliary sphincterotomy. Gastrointest Endosc. 1992;38:608-611.
Richardson MC, Bell G, Fullarton GM. Incidence and nature of bile duct injuries following laparoscopic cholecystectomy: an audit of 5913 cases. West of Scotland Laparoscopic Cholecystectomy Audit Group. Br J Surg. 1996;83:1356-1360.
Roslyn JJ, et al. Open cholecystectomy: a contemporary analysis of 42,474 patients. Ann Surg. 1993;218:129-137.
Rossi RL, Tsao JI. Biliary reconstruction. Surg Clin North Am. 1994;74:825-841.
Russell JC, et al. Bile duct injuries, 1989-1993: a statewide experience. Connecticut Laparoscopic Cholecystectomy Registry. Arch Surg. 1996;131:382-388.
Salinas G, et al. Early experience in human hybrid transgastric and transvaginal endoscopic cholecystectomy. Surg Endosc. 2010;24(5):1092-1098.
Sanchez-Urdazpal L, et al. Diagnostic features and clinical outcome of ischemic-type biliary complications after liver transplantation. Hepatology. 1993;17:605-609.
Sarles H, Sahel J. Cholestasis and lesions of the biliary tract in chronic pancreatitis. Gut. 1978;19:851-857.
Sarmiento JM, et al. Quality-of-life assessment of surgical reconstruction after laparoscopic cholecystectomy-induced bile duct injuries: what happens at 5 years and beyond? Arch Surg. 2004;139:483-488.
Sato T, et al. Effect of biliary reconstruction procedures on gastric acid secretion. Am J Surg. 1982;144:549-553.
Sato M, et al. Long-term results of hepatic resection for hepatolithiasis. HPB Surg. 1995;9:37-41.
Sato M, et al. Intrahepatic cholangiocarcinoma associated with hepatolithiasis. Hepatogastroenterology. 1998;45:137-144.
Savader SJ, et al. Laparoscopic cholecystectomy-related bile duct injuries: a health and financial disaster. Ann Surg. 1997;225:268-273.
Saxon RS, et al. Transjugular intrahepatic portosystemic shunt patency and the importance of stenosis location in the development of recurrent symptoms. Radiology. 1998;207:683-693.
Schaffner F, et al. Mechanism of cholestasis. 4. Structural and biochemical changes in the liver and serum in rats after bile duct ligation. Gastroenterology. 1971;60:888-897.
Schein CJ, Gliedman ML. Choledochoduodenostomy as an adjunct to choledocholithotomy. Surg Gynecol Obstet. 1981;152:797-804.
Schmets L, et al. Postradiotherapy benign biliary stricture: successful treatment by self-expandable metallic stent. Gastrointest Endosc. 1996;43:149-152.
Schmidt SC, et al. Long-term results and risk factors influencing outcome of major bile duct injuries following cholecystectomy. Br J Surg. 2005;92:76-82.
Schol FP, Go PM, Gouma DJ. Outcome of 49 repairs of bile duct injuries after laparoscopic cholecystectomy. World J Surg. 1995;19:753-756.
Schwarz W, et al. Percutaneous transhepatic drainage preoperatively for benign biliary strictures. Surg Gynecol Obstet. 1981;152:466-468.
Schweizer WP, et al. Combined surgical and interventional radiological approach for complex benign biliary tract obstruction. Br J Surg. 1991;78:559-563.
Sharma AK, et al. Pitfalls in the management of Mirizzi’s syndrome. Trop Gastroenterol. 1998;19:72-74.
Sheen-Chen SM, Chou FF. Choledochotomy for biliary lithiasis: is routine T-tube drainage necessary? A prospective controlled trial. Acta Chir Scand. 1990;156:387-390.
Sheen-Chen SM, et al. Ductal dilatation and stenting for residual hepatolithiasis: a promising treatment strategy. Gut. 1998;42:708-710.
Sicklick JK, et al. Surgical management of bile duct injuries sustained during laparoscopic cholecystectomy: perioperative results in 200 patients. Ann Surg. 2005;241:786-792.
Sikora SS, et al. Liver histology in benign biliary stricture: fibrosis to cirrhosis . . . and reversal? J Gastroenterol Hepatol. 2008;23:1879-1884.
Sirichindakul B, et al. Partial segment-IV/V liver resection facilitates the repair of complicated bile duct injury. Hepatogastroenterology. 2009;56:956-959.
Smith EE, et al. The management of post-traumatic intrahepatic cutaneous biliary fistulas. Br J Surg. 1982;69:317-318.
Smith R. Hepaticojejunostomy with transhepatic intubation: a technique for very high strictures of the hepatic ducts. Br J Surg. 1964;51:186-194.
Smith R. Strictures of the bile ducts. Proc R Soc Med. 1969;62:131-137.
Smits ME, et al. Long-term results of endoscopic stenting and surgical drainage for biliary stricture due to chronic pancreatitis. Br J Surg. 1996;83:764-768.
Soderlund C, Frozanpor F, Linder S. Bile duct injuries at laparoscopic cholecystectomy: a single-institution prospective study—acute cholecystitis indicates an increased risk. World J Surg. 2005;29:987-993.
Soleimani M, et al. Partial cholecystectomy as a safe and viable option in the emergency treatment of complex acute cholecystitis: a case series and review of the literature. Am Surg. 2007;73:498-507.
Sorensen VJ, et al. Primary common bile duct closure following exploration: an effective alternative to routine biliary drainage. Am Surg. 1994;60:451-454.
Soupault R, Couinaud C. Sur un procede nouveau de derivation biliare intra-hepatique. Presse Med. 1957;65:1157-1159.
Southern Surgeons Club. A prospective analysis of 1518 laparoscopic cholecystectomies. N Engl J Med. 1991;325(21):1517-1518.
Spanos CP, Syrakos T. Bile leaks from the duct of Luschka (subvesical duct): a review. Langenbecks Arch Surg. 2006;391:441-447.
Stabile BE, et al. Stricture of the common bile duct from chronic pancreatitis. Surg Gynecol Obstet. 1987;165:121-126.
Standfield NJ, Salisbury JR, Howard ER. Benign non-traumatic inflammatory strictures of the extrahepatic biliary system. Br J Surg. 1989;76:849-852.
Stewart L, Way LW. Bile duct injuries during laparoscopic cholecystectomy: factors that influence the results of treatment. Arch Surg. 2003;130:1123-1128.
Stewart L, Way LW. Laparoscopic bile duct injuries: timing of surgical repair does not influence success rate. A multivariate analysis of factors influencing surgical outcomes. HPB (Oxford). 2009;11(6):516-522.
Strasberg SM, Hertl M, Soper NJ. An analysis of the problem of biliary injury during laparoscopic cholecystectomy. J Am Coll Surg. 1995;180:101-125.
Tacchino R, Greco F, Matera D. Single-incision laparoscopic cholecystectomy: surgery without a visible scar. Surg Endosc. 2009;23:896-899.
Tantia O, et al. Iatrogenic biliary injury: 13,305 cholecystectomies experienced by a single surgical team over more than 13 years. Surg Endosc. 2008;22:1077-1086.
Teplick SK, et al. Percutaneous transhepatic choledochoplasty and dilatation of choledochoenterostomy strictures. JAMA. 1980;244:1240-1242.
Thompson JEJr, et al. Broad-spectrum penicillin as an adequate therapy for acute cholangitis. Surg Gynecol Obstet. 1990;171:275-282.
Thomson BN, et al. Liver resection and transplantation in the management of iatrogenic biliary injury. World J Surg. 2007;31:2363-2369.
Tocchi A, et al. The long-term outcome of hepaticojejunostomy in the treatment of benign bile duct strictures. Ann Surg. 1996;224:162-167.
Tomonaga T, et al. Laparoscopic intracorporeal ultrasound cystic duct length measurement: a new technique to prevent common bile duct injuries. Surg Endosc. 1999;13:183-185.
Torkington J, Chalmers RT, Horner J. Laparoscopic cholecystectomy, bile duct injury and the British and Irish surgeon. Ann R Coll Surg Engl. 1999;81:71-72.
Tornqvist B, et al. Long-term effects of iatrogenic bile duct injury during cholecystectomy. Clin Gastroenterol Hepatol. 2009;7(9):1013-1018.
Toufanian A, Carey LC, Martin ETJr. Transhepatic biliary dilatation: an alternative to surgical reconstruction. Curr Surg. 1978;35:70-73.
Tu Z, et al. Primary choledochorrhaphy after common bile duct exploration. Dig Surg. 1999;16:137-139.
Uenishi T, et al. Right hepatic lobectomy for recurrent cholangitis after bile duct and hepatic artery injury during laparoscopic cholecystectomy: report of a case. Hepatogastroenterology. 1999;46:2296-2298.
van Boeckel PG, Vleggaar FP, Siersema PD. Plastic or metal stents for benign extrahepatic biliary strictures: a systematic review. BMC Gastroenterol. 2009;9:96.
vanSonnenberg E, et al. Complications of laparoscopic cholecystectomy: coordinated radiologic and surgical management in 21 patients. Radiology. 1993;188:399-404.
Venerito M, et al. Cholangitis as a late complication of choledochoduodenostomy: the sump syndrome. Endoscopy. 2009;41(Suppl 2):E142-E143.
Vogel SB, et al. Evaluation of percutaneous transhepatic balloon dilatation of benign biliary strictures in high-risk. patients. Am J Surg. 1985;149:73-79.
Vougas V, et al. A prospective randomised trial of bile duct reconstruction at liver transplantation: T tube or no T tube? Transpl Int. 1996;9:392-395.
Voyles CR, Blumgart LH. A technique for the construction of high biliary-enteric anastomoses. Surg Gynecol Obstet. 1982;154:885-887.
Waage A, Nilsson M. Iatrogenic bile duct injury: a population-based study of 152,776 cholecystectomies in the Swedish Inpatient Registry. Arch Surg. 2006;141:1207-1213.
Walsh RM, et al. Trends in bile duct injuries from laparoscopic cholecystectomy. J Gastrointest Surg. 1998;2:458-462.
Walsh RM, et al, 2004: Management of failed biliary repairs for major bile duct injuries after laparoscopic cholecystectomy. 199;2:192-197
Way LW. Bile duct injury during laparoscopic cholecystectomy. Ann Surg. 1992;215:195.
Way LW, Dunphy JE. Biliary stricture. Am J Surg. 1972;124:287-295.
Weber A, et al. Long-term outcome of endoscopic therapy in patients with bile duct injury after cholecystectomy. J Gastroenterol Hepatol. 2009;24:762-769.
Weinbren K, Hadjis NS, Blumgart LH. Structural aspects of the liver in patients with biliary disease and portal hypertension. J Clin Pathol. 1985;38:1013-1020.
Wetter LA, et al. Differential diagnosis of sclerosing cholangiocarcinomas of the common hepatic duct (Klatskin tumors). Am J Surg. 1991;161:57-62.
Wherry DC, et al. An external audit of laparoscopic cholecystectomy in the steady state performed in medical treatment facilities of the Department of Defense. Ann Surg. 1996;224:145-154.
Williams HJJr, Bender CE, May GR. Benign postoperative biliary strictures: dilation with fluoroscopic guidance. Radiology. 1987;163:629-634.
Wojcicki M, Milkiewicz P, Silva M. Biliary tract complications after liver transplantation: a review. Dig Surg. 2008;25:245-257.
Woods MS, et al. Characteristics of biliary tract complications during laparoscopic cholecystectomy: a multi-institutional study. Am J Surg. 1994;167:27-33.
Yoon KH, et al. Biliary stricture caused by blunt abdominal trauma: clinical and radiologic features in five patients. Radiology. 1998;207:737-741.
Yoshida J, et al. Hepatolithiasis: outcome of cholangioscopic lithotomy and dilation of bile duct stricture. Surgery. 1998;123:421-426.
Zaydfudim V, Wright JK, Pinson CW. Liver transplantation for iatrogenic porta hepatis transection. Am Surg. 2009;75:313-316.
Zornig C, et al. Transvaginal NOTES hybrid cholecystectomy: feasibility results in 68 cases with mid-term follow-up. Endoscopy. 2009;41:391-394.