CHAPTER 99 Benign and Malignant Tumors of the Nasopharynx
The nasopharynx (NP) is an embryologic confluence of the end of the nasal structures and the beginning of the pharynx. It is also bounded superiorly by the ectodermal origins of the cranial base and upper cervical spines posteriorly. For this reason, a large range of pathologies occur in this small confine (Table 99-1). Because of the NP capacity for tumor growth and expansion, the symptoms in general arise late. Thus they can reach significant sizes before presentation because of the diagnostic inaccessibility of the NP area.
Table 99-1 Benign and Malignant Tumors of the Nasopharynx
Diagnostic Approach for Nasopharyngeal Masses
Squamous Papilloma
This benign epithelial tumor is common in the anterior and posterior nasal space. The epithelial changes of such a tumor may be inverting at the basal membrane, whence they are termed “Schneiderian” or “inverting” papilloma. The presentation is unusually incidental for lesions of the nasopharynx (which is not common), and diagnosis should be done with a biopsy. The base of the lesion should be completely removed with a margin of normal tissue. The use of powered instrumentation is helpful in difficult recesses of the nasopharynx. Further detail on the management of inverting papilloma of the nose and paranasal sinuses is available in Chapters 49 and 99.
Craniopharyngioma
Craniopharyngiomas are histologically benign tumors that arise developmentally from Rathke’s pouch. They usually present with symptoms similar to pituitary tumors (endocrine and visual disturbances), but occasionally they may present with nasal symptoms such as nasal obstruction due to extension into the nasopharynx. In children, these lesions have been known to mimic presentation similar to adenoidal hypertrophy.14 Treatment is surgical excision with or without radiation therapy. The gamma knife has been used as the primary treatment.
Angiofibroma
Growth Patterns
The tumor mass is locally infiltrative and usually has a wide-based attachment to the NP and to its surrounding related anatomy. JNA is a slow-growing vascular tumor arising in the area of the sphenopalatine foramen at the root of the pterygoid process on the lateral nasal wall. The tumor expands laterally via the pterygopalatine fossa to the infratemporal fossa but has no resistance to growth into the NP and choanal space and thereby may expand into the anterior nasal cavity and all the related sinuses. It can have intracranial extensions into the middle and anterior cranial fossae, although this is uncommon. It can also extend bilaterally to the maxillary sinuses and superiorly to the cranial skull base. Because JNA commonly occurs in young males, this has prompted studies related to the androgen receptor (AR) and estrogen receptor (ER) in the tumor tissue. A recent study suggests a stronger relationship to ER positivity, which may provide a platform for ER modulators in the management of JNA.1
Pathologic Features
The gross pathology usually shows a sessile, lobulated, rubbery dark red to tan gray mass that can be large in size. Mucosal ulceration is uncommonly seen and the tumor is unencapsulated and composed of an admixture of vascular tissue and fibrous stroma. The vessel walls lack elastic fibers and have incomplete or absent smooth muscle that will account for their tendency to bleed.2
Clinical Features
Although JNA is typically diagnosed between the ages of 10 and 25 in males, many case reports exist for female patients and also in patients who are in the later decades of life. JNA patients present with nasal obstruction (usually unilateral), epistaxis, blood-stained sputum, and serous otitis media. Less common symptoms include nasal discharge, facial swelling, proptosis, sinusitis, and anosmia. The duration of symptoms is usually long and often mild and innocuous. JNA cases occurring in sites other than the NP have been reported. The next commonest site, the maxillary sinuses, is associated with a greater proportion of females.3
Diagnosis
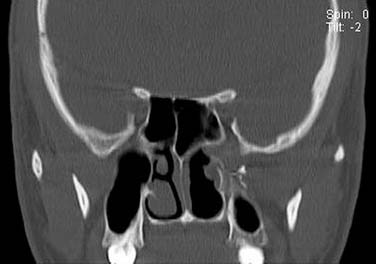
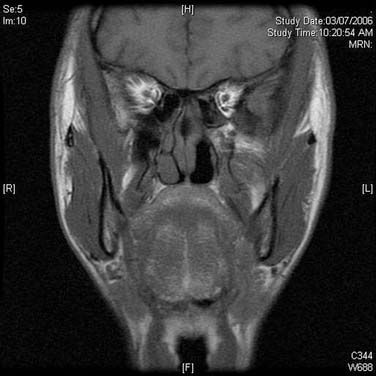
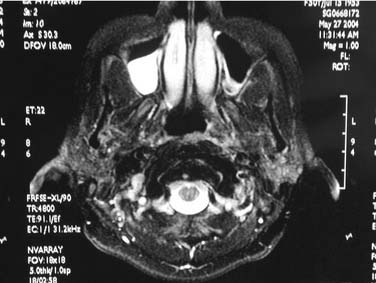
On endoscopic examination, JNA presents as a rubbery vascular mass protruding into the anterior nasal space and may have excessive bleeding on contact. A CT scan with contrast will show an enhancing soft tissue mass arising from the NP or lateral wall of the nose. Although the pterygopalatine fossa may be widened by the tumor (Fig. 99-1), bone erosion is not present in general. MRI will show a vascular tumor with flow voids within the mass and enhances on gadolinium. MR angiography will be the least invasive form of vascular imaging that will show the feeding vessels arising from the branches of the external carotid system. The authors have found the MRI with contrast to be more useful for follow-up evaluation (Fig. 99-2). The use of 99mTc-RBC single photon emission computed tomography has been suggested to be accurate in the diagnosis of JNA.4 However, final proof of diagnosis is histologic.
Staging
In 1984 Chandler and colleagues5 proposed a staging system (Table 99-2) on the basis of clinical and CT assessment. This staging system is logical and easy to understand and it is useful for reporting. Due to the large anatomic region of stage 3, Sessions and Radkowshi have suggested refining stage 3. However, none of the systems has been used extensively for comparing outcomes because published case series have usually been of small numbers.
Table 99-2 The 2002 UICC TNM Staging of Nasopharyngeal Carcinoma
| T Classification | |
| Tx | Primary tumor unable to be assessed |
| T0 | No evidence of tumor |
| T1 | Confined to nasopharynx |
| T2a | Extends to nasal cavity, oropharynx |
| T2b | Tumor extends to parapharyngeal space |
| T3 | Tumor involves sinuses, orbit, skull base, hypopharynx |
| T4 | Intracranial or infratemporal involvement |
| N Classification | |
| N0 | No nodal involvement |
| N1 | Ipsilateral lymph nodes < 6 cm |
| N2 | Bilateral lymph nodes < 6 cm |
| N3 | Lymph node > 6 cm; supraclavicular node |
| M Classification | |
| M0 | No distant metastasis |
| M1 | Distant metastasis (includes mediastinal nodes) |
| Stage Classification | |
| Stage I | T1N0M0 |
| Stage II | T1N1M0, T2N0M0, T2N1M0 |
| Stage III | T3N0-2M0, T1-2N2M0 |
| Stage IVa | T4, any NM0 |
| Stage IVb | any TN3M0 |
| Stage IVc | any T, any N, M1 |
TNM, tumor node metastasis; UICC, Union Internationale Contre Cancre.
Management
For extension outside the NP into the paranasal sinuses, a Le Fort type 1 osteotomy will allow an inferior approach to the maxillary and ethmoid sinuses as well as the pterygopalatine canal. For lesions involving the infratemporal fossa or large lesions involving the majority of the sinuses, a facial translocation approach affords a large window of access. The facial translocation involves the rotation of the facial cheek flap as a single osteoplastic unit with the underlying maxilla rotated laterally after osteotomies to the maxillary nasal, orbital maxillary, and zygomatic arches. The senior author has also incorporated the entire nose and cheek as a single rotation based laterally. This allows for excellent access to the infratemporal fossa and hemostatic options in the event of bleeding. A lateral infratemporal fossa approach has been recently used as reported by Tyagi and colleagues.6
Endoscopic sinus surgery techniques have advanced significantly over the past 2 decades and have allowed the intranasal access and nasal anatomy to be approached in an extremely competent fashion. Several authors have published their experience of successful endoscopic resection of such tumors. Several reports of endoscopic resection have reasonable follow-up durations (13 patients in 23 months) and low recurrence rates (8%).7 Combination approaches of type 3 JNA with open and endoscopic approaches have also reported good success but of shorter-term results.8 For larger lesions such as stage 3A disease, Hofmann and colleagues have reported successful endoscopic resection with long-term results (52 months) in a series of 21 patients and have reported that extension to the medial aspect of infratemporal fossa and retromaxillary space was not a contraindication to an endonasal endoscopic approach.
Preoperative embolization of the tumor mass is used by some to reduce intraoperative bleeding. However, the risk of recurrence has been highlighted with the use of preoperative embolization.9 In extensive tumors where the feeding vessels come from the internal carotid system, a laser-assisted extracranial-intracranial bypass has been used before the definite surgery in order to prevent neurologic deficits.10
Recurrence rates have been reported to be as high as 35% depending on the length of follow-up. For patients with recurrent disease, the options remain for observation alone, revision surgery, or radiation. Radiation has been used for treatment of JNA, especially those that recur after surgery and those with intracranial extension where clearance of margins may be difficult. Cummings and colleagues11 reported a series of 56 patients treated with 3000 cGY. However, issues of impact on growth centers, cataract formation, and osteoradionecrosis have been reported, as have cases of malignant transformation.12 For these various reasons, observation, sometimes followed by involution with aging or repeat surgery, is an option in the presence of recurrence. In patients who cannot undergo surgery, recalcitrant cases of tumor recurrence despite several repeat resections and inaccessible recurrences may be considered for radiation therapy with full disclosure of possible long-term, late effects (e.g., radiation-induced malignancy, carotid artery damage). Park13 recently reported using the gamma knife with good success for a case of recurrent intraorbital JNA.
Adenoid Cystic Carcinoma of the Nasopharynx
Adenocystic carcinoma of the sinonasal tract constitutes 20% of all adenoid cystic carcinomas of the head and neck. They, along with other tumors of salivary gland origin, arise from the minor salivary glands in this region. Those of the NP are not common but can be insidious and slow growing.14 The lesion can extend from the nasopharynx into the anterior skull base and can present as bilateral nasal lesions. In the latter, there is a need to differentiate this from simple bilateral nasal polyps. Patients with either condition may present with simple nasal obstruction with hyposmia. Other accompanying symptoms do not usually occur, unless palpable neck metastasis is detectable at presentation. Otitis media with effusion may exist if the eustachian tube is involved. These tumors have the propensity for perineural spread and thus MR imaging is important in the pretreatment evaluation. The primary treatment of such lesions is surgical resection. However, proximity of the nasopharynx to the sphenoid, and, hence, the internal carotid arteries and intracranial structures, plus their propensity for centrifugal spread along nerves, may preclude complete removal. Postoperative radiotherapy is important in local control of such lesions.
Nasopharyngeal Carcinoma
Nasopharyngeal carcinoma (NPC) is unique in its epidemiologic pattern. It is common in certain ethnic groups, with the highest incidence in southern China, Hong Kong, and southeast Asia. The majority of patients with NPC are diagnosed with advanced disease. However, over the past 20 years, the survivals of these patients have improved.15 This has been attributed to the combined use of chemotherapy and radiation to treat the patients with advanced disease.16 Furthermore, with the advent of improved techniques in radiation, the side effects, which plagued long-term survivors in the past, may be reduced.17,18 The strategy in the management of NPC is to find novel methods of early detection and develop improved techniques of effective primary treatment with the focus on reducing morbidity from the treatment. In this chapter we describe the epidemiology, etiology, pathology, clinical features, staging, treatment, and prognosis of NPC.
Epidemiology
The highest incidence of NPC is recorded in Guangzhou, China, where the age-adjusted incidence rate is reported to be more than 30 per 100,000.19 Hong Kong has a rate of 20.2 per 100,000 in males and 7.8 per 100,000 in females.15 Patients who are affected by NPC are ethnic Chinese with an ancestry from the southern part of China. In southern China the two main ethnic subgroups are the Cantonese and the Fujians. In Hong Kong the vast majority of the population is Cantonese. In Singapore the overall incidence in males from 1998 to 2002 was 10.8 per 100,000.20 This lower rate may be in part accounted for by the demographics of Singapore, which unlike China, Hong Kong, and Taiwan, has a more heterogeneous population. The population in Singapore is approximately 70% Chinese, with the remaining major ethnic groups being Malays and Indians. The majority of the NPC patients in Singapore are Chinese (Fujians and Cantonese).21 The incidence of NPC in Singaporean male Chinese is 12.5 per 100,000. These incidence rates in southern China, Hong Kong, and southeast Asia are among the highest in the world and have remained stable for many years. However, in the most recent review of the latest trend in the incidence of NPC, both Singapore and Hong Kong have reported a significant decrease for the first time after many years of stable rates.15 Why there was a fall in incidence is unclear, but one theory advanced to account for this is the change in dietary habits.22,23 Other ethnic groups, with rates that are reported to be intermediate to high, include the Eskimos,24,25 Polynesians, and indigenous Mediterranean population.26 NPC is not common in whites. In North America the highest incidences are seen in the ethnic Chinese who are first-generation immigrants.23 Subsequent-generation immigrants have a lower incidence, although it is still higher than the other ethnic groups. Overall, these incidence rates are lower than those in southern China and southeast Asia. This pattern of decreasing incidence rates seen in emigrant Chinese is a well-known phenomenon. It is likely that with migration, there is over time a greater variation in the gene pool arising from factors such as intermarriage, although the adoption of different dietary habits cannot be discounted. The epidemiologic pattern indicates that NPC occurs commonly in Chinese. This will suggest the possibility of a genetic link or a shared habit such as dietary intake.
Etiology
Three main factors are believed to be associated with the development of NPC.27 These are genetic and environmental factors, as well as EBV. The knowledge relating to these factors that are associated with NPC has been advanced over the past 20 years. However, we are still unable to assimilate the information in an orderly fashion to translate the knowledge into prevention, early detection, or markedly improved survivals. Nevertheless, the current school of thought among those involved in NPC research believes that this cancer is probably a result of interaction among the three main factors.
Genetic Factors and Nasopharyngeal Carcinoma
A large body of evidence indicates that genetic factors play a significant role in NPC. Family clusters with NPC are not uncommon. Ung and colleagues28 had reported that the risk of NPC in a first-degree family member could be as high as eight times. Reports from Hong Kong and Guangzhou have also estimated this risk to be as high as 20 times when compared with the general population. We had reported that 15.5% of all NPC patients have had a first-degree relative with NPC.29 The majority of first-degree family members who are affected are usually siblings (70%) rather than parents and children (30%).29 The mean duration between two siblings who are affected within a family is 5.3 years, whereas that between a parent and child is 25 years.29 Experts also believe that the risk for a first-degree relative also developing NPC is higher if the index NPC patient is diagnosed at age younger than 40 years.30 The presence of such family clusters may indeed be due to common genetic factors, but it can also be argued that shared environmental factors may play a role.
The association of HLA alleles with NPC was established in 1975.31 These alleles are the HLA class I alleles belonging to the HLA A, B, and D groups in chromosome 6. HLA A2, Bw46, B 17, Bw58, DR3, and DR9 have been consistently found to be significantly more prevalent in NPC patients in contrast to the general population.31–35 These haplotypes are associated with increased risks for NPC. Further refinements in establishing these haplotypes have been reported with the highest risks established consistently for patients with the haplotypes A2 and B46.34,36 It has also been found that multiple alleles confer protective effects. The evidence suggests it is likely that the tumor susceptibility gene has a recessive mode of inheritance because an NPC-linked gene with a dominant mode will not be associated with such wide allelic variations in protective effects.37 It has also been established that most Chinese patients with NPC have the HLA-A0207 haplotype in contrast to the HLA-A0201 found in whites.38 Microsatellite instability in the nearby region of the HLA loci has been described, suggesting the possibility of tumor susceptibility factors.39 NPC patients may have alleles in these loci that may contain genes that contribute to the oncogenesis of normal nasopharynx cells. However, linkage analysis has shown that the tumor susceptibility gene is not likely to be at the position of the high-risk A2 B46 haplotypes.
Chromosome changes that have been described include deletions in chromosomes 3, 9, and 11.40–43 The significance of these chromosomal changes is the implied loss of function of known specific factors: for example, chromosome 9p21 region codes for known cell cycle regulators such as p15 and p16. The protein p16 has been reported to be inactivated in NPC patients.44,45 Other chromosomal changes have been sporadically reported. Each of these genetic deletions has also been associated with an implied tumor suppressor gene. Novel markers have also been reported to suggest the possibility of other tumor suppressor gene candidates.46 Despite this wealth of information, it is difficult to determine if these changes are downstream events rather than early changes. What will be helpful will be genome-wide scans of NPC tumor samples and normal tissues, as well as using techniques such as comparative genomic hybridization to narrow in on the true early changes.
Environmental Factors and Nasopharyngeal Carcinoma
The most common environmental factor associated with NPC is diet. It has been suggested that certain diets high in preservatives are associated with NPC. These include salted fish, eggs, and vegetables.47–51 The origin of these associations is traced to the epidemiologic studies performed on a group of high-incidence NPC patients, the “boat people” of Hong Kong. These people had originated from a common region in China many years ago and had settled into a life based largely on living in boats. The diets are necessarily based on preserving the food from the sea (e.g., salted fish). Subsequently large epidemiologic studies have suggested similar findings, too.52 It is believed that the risk is associated with early exposure or during weaning period.50,53 The carcinogen believed to be the inciting factor is nitrosamines. Other environmental causes that have been thought to be associated include chemical fumes and wood dust.54 Smoking is not an associated factor unlike squamous cell carcinoma of the aerodigestive tract. However, it is always difficult to attribute food alone as the factor that leads to oncogenesis because many confounding factors are also often present.
Epstein-Barr Virus and Nasopharyngeal Carcinoma
The IgM and IgG class of antibodies to antigens on the EBV are raised in acute and convalescent phases of infection. The antigens on the EBV are either the nuclear core early antigen (Ea) or the viral capsid antigen (VCA). Hence in the majority of any population, the IgG VCA and Ea will be raised. In NPC, however, the IgA VCA and Ea are raised.55 This is used as a marker, with laboratories using immunofluorescence to detect these antibodies. The sensitivity and specificity of these two antibodies are high.
EBV antigens are also found on NPC cells. EBV expresses both lytic and nuclear antigens. Lytic antigens include latent membrane proteins 1, 2, and 3 (LMP-1, -2, -3). Nuclear antigens include the Epstein-Barr nuclear antigens 1 to 6 (EBNA-1 to 6). In NPC, EBNA-1 is always expressed, whereas LMP-1 is consistently expressed in significant levels. Both of these are latent proteins. EBNA-1 is believed to be responsible for maintaining the viral episomes in the tumor cells. LMP-1 has been shown to induce cellular growth by inducing epithelial hyperplasia and altered keratin gene expression.56,57 It reduces cytokeratin expression and inhibits squamous cell differentiation. It has been shown to up-regulate epidermal growth factor receptors. NPC also expresses Epstein-Barr encoded ribonucleic acids (EBERs) within the cytoplasm. These EBV antigens and EBERs are not present in normal nasopharynx cells.
Clinical Presentation
Virtually all NPC patients are symptomatic at the time of diagnosis. Less than 1% of NPC patients are asymptomatic and diagnosed incidentally. This may occur because of imaging performed for some other indications or an abnormal EBV serology is detected because of health screening. Three quarters of NPC patients are males. More than 80% are diagnosed between 30 and 60 years of age with more than 50% diagnosed between 30 and 50 years. The proportion of NPC patients diagnosed at a relatively young age has a significant socioeconomic impact on these patients and their families. Approximately 15% to 20% of NPC patients will also report a first-degree relative who was similarly affected.29
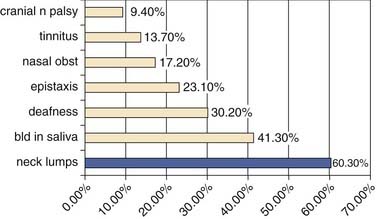
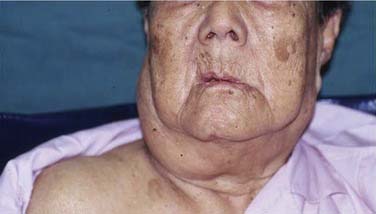
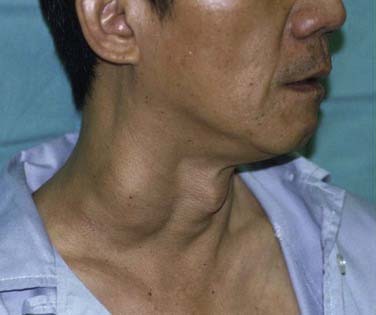
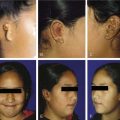
The most common presentation in NPC is the presence of a neck lump (Fig. 99-3). About 60% of our patients presented with a neck lump. These neck lumps are due to metastatic disease in the cervical lymph nodes. It is not uncommon for the enlarged nodes to be matted. The nodal metastasis is usually located in the superior aspect of the neck (Fig. 99-4), corresponding to high level V and level II. The first echelon of nodal metastasis is in the retropharyngeal nodes. These retropharyngeal nodes may be so bulky that they can be visualized on physical examination as nodular swellings in the posterior pharyngeal wall. Occasionally NPC patients may present with enlarged level III nodes (Fig. 99-5) and less commonly level IV. Enlarged submental lymph nodes at the time of diagnosis are rare and not encountered in our series. Some patients may present with a parotid swelling due to metastatic parotid lymph nodes, although these are also uncommon. Metastatic NPC nodes are usually firm. If left for a significant duration of time, a node may enlarge to such an extent that central necrosis occurs, followed by abscess formation. In endemic regions such as China, Hong Kong, and Singapore, a Chinese adult male presenting with large bulky bilateral nodes is usually either NPC or lymphoma. Although 60% of NPC patients present with a neck lump due to nodal metastasis, at least 80% of NPC will be classified as N1. This is because even in the NPC patients without palpable nodes, imaging will show metastatic nodes in at least another 20%.
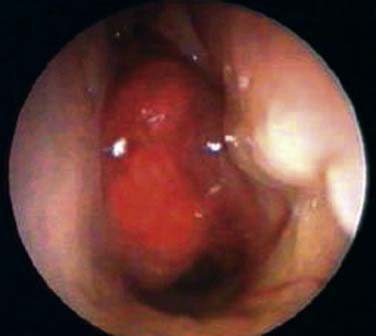
Examination of the nasopharynx in NPC patients usually reveals an exophytic mass that may occupy the whole postnasal space (Fig. 99-6). This may be ulcerated and contact bleeding is usually encountered. However, in about 10% of NPC patients the lesion is submucosal. In these cases the mucosal surface of the nasopharynx is normal in appearance. Occasionally, only a slight irregular mucosa is detected. This may consist of a small area of heaped-up mucosa or just an erythematous change, both of which could be passed off as normal. The significance of submucosal NPC in a patient is unclear, although a review of our patients suggested that this group might have more aggressive disease.
Diagnosis of Nasopharyngeal Carcinoma
The classification of NPC58,59 is shown in Table 99-3. The vast majority of patients with NPC—at least 90%—in the endemic region have a histologic pattern termed as nonkeratinizing undifferentiated carcinoma (type 2b or type III). Squamous cell carcinoma is uncommon in endemic regions.
Table 99-3 World Health Organization (WHO) Classification of Nasopharyngeal Carcinoma
| Type 1 (I) | Squamous cell carcinoma |
| Type 2a (II) | Keratinizing undifferentiated carcinoma |
| Type 2b (III) | Nonkeratinizing undifferentiated carcinoma |
Types 1, 2a, and 2b correspond to WHO types I, II, and III, respectively, and are used interchangeably.
Staging of Nasopharyngeal Carcinoma
The TNM staging system is shown in Table 99-2. This staging system is based on clinical and radiologic examination. In general, two thirds of NPC patients are classified as stage III or IV at the time of diagnosis. Only about 10% of newly diagnosed NPC patients have stage I disease.
Computed Tomography Scan/Magnetic Resonance Imaging
CT scan had been for many years the essential staging investigation for assessing the primary tumor, as well as regional disease. The soft tissue of the nasopharynx is shown well and CT is particularly useful in delineating clival and skull base erosion.60 It has been the cornerstone of radiologic staging of NPC for many years. However, MRI has been used increasingly in many centers. This is because of the superior definition afforded by MRI in detecting soft tissue changes and intracranial involvement. It is not uncommon for MRI to show features consistent with disease that is not shown on a CT scan.61
Other Staging Investigations
These include chest radiograph, ultrasound of liver, and bone scans. Alternatively, CT scans of the lungs and liver may be performed. The main purpose is to locate distant metastasis. Of these the highest pick-up rate appears to be the bone scan. This is consistent with the skeleton being the most common distant site of metastasis in NPC. In the past 5 years, PET scans have come into more prominent use. Much of the information in the literature on the use of PET CT in NPC is with regard to detecting local and regional recurrences or residual disease, as well as in the detection of distant metastasis.62 It is a useful imaging tool for patients with indeterminate CT or MRI abnormalities that may represent local, regional, or distant recurrences. Enhancement will suggest a higher possibility of recurrences in such indeterminate imaging abnormalities. However, caution has to be advised in the interpretation of current data on PET CT, especially as a tool for staging newly diagnosed NPC patients. The main criticisms of these reports have been the lack of a standardized methodology to determine the histopathology of the tissues that have high uptake of agents like 18-fluoro-2-deoxyglucose (FDG). Oftentimes these reports will include any form of enhancement on PET CT to represent metastasis without confirmation of the pathology. Inflammatory reactions and radiation necrosis, without malignancy, can lead to high uptake.63 Literature on the efficacy and cost-effectiveness of using PET CT as a staging tool in newly diagnosed NPC patients to assess the primary tumor and regional metastasis is scant.
Treatment of Nasopharyngeal Carcinoma
Pretreatment Planning
The treatment of a newly diagnosed NPC patient is best served by a multidisciplinary team of head and neck surgeon, radiation oncologist, medical oncologist, pathologist, and radiologist. This includes audiological investigations and dental clearance. In addition, if chemotherapy is part of the treatment, hematologic and biochemical investigations are performed including creatinine clearance. Dental clearance involves ensuring that oral hygiene is maintained because the oral cavity will inevitably be in the radiation field. The decaying, unhealthy tooth is extracted to prevent osteomyelitis from setting in during the postradiation period. However, there is no evidence that prophylactic extraction will reduce osteoradionecrosis in patients undergoing radiation of the head and neck.64,65 Allowing such prophylactic extractions will potentially delay treatment and increase costs for the patient.
Treatment
NPC is treated by radiation. In general, patients with stage I and II NPC are treated by radiation only, whereas stage III and stage IV patients are treated by concurrent chemotherapy and radiation. In addition, there is evidence that patients diagnosed with stage IV NPC with locally advanced disease may be better controlled by neoadjuvant cisplatin followed by chemoradiation.66 Radiation is administered by linear accelerator, and the dose reaches 60 to 70 Gy in the nasopharynx and both necks. This is given daily at up to 2 Gy in 35 to 40 fractions. The main side effects include mucositis and xerostomia. The former may take up to 3 months after the last day of treatment to heal. Xerostomia affects every patient and can be permanent, although the younger patients show much better improvement with time. Other side effects include sinusitis, crusting, and blood-stained nasal discharge. Otitis media with effusion can develop or be worsened with radiation. Sensorineural hearing loss has been attributed to radiation, although the cochlea is resistant and it has been shown that this may be due to other causes such as infiltration by tumor cells.67 Trismus is particularly more severe in those treated for skull base involvement. It may take several years for trismus to develop. Late side effects include cranial nerve palsies, which may give rise to difficulties in distinguishing trismus from recurrent disease. The most common cranial nerve to be affected postradiation, other than the cochlear-vestibular nerve, is the hypoglossal nerve. In the past 5 to 10 years, data have emerged to confirm the efficacy of using intensity-modulated radiation therapy (IMRT) to treat NPC patients. This has resulted in control rates similar to conventional radiation.68 It has also resulted in better treatment outcomes, especially xerostomia17 and skin burns.
The use of concurrent chemotherapy and radiation to effectively treat local and regionally advanced NPC patients was reported by Al-Sarraf.69 There is compelling evidence for using concurrent chemotherapy and radiation in advanced stage NPC.70 The concurrent chemotherapy regimen includes cisplatin and 5-fluorouracil (5-FU). We had found this to give good local and regional control, but it increases both acute and late toxicities. Two of the significant toxicities associated with cisplatin are idiosyncratic sensorineural hearing loss and peripheral neuropathy. To avoid these toxicities, other agents such as Taxol combined with 5-FU and hydroxyurea are alternatives that can be considered.71
Surgical Treatment of Nasopharyngeal Carcinoma
The indications for surgical treatment of NPC are currently for local and regional recurrences.
Surgical Treatment of Local Recurrences
Approximately 5% to 10% of all newly diagnosed NPC patients will develop local recurrences. Up to 50% of these patients will be amenable to salvage by surgery. The remaining 50% are usually too far advanced for surgical intervention. This may be due to intracranial invasion or carotid artery encasement. In some patients this may be due to concomitant distant metastasis. The surgical treatment of locally recurrent or residual NPC is nasopharyngectomy. In those who can be salvaged surgically, the recurrent T (rT) classification is often rT1 to rT3.72 The success of surgical intervention in recurrent NPC is strongly correlated with T classification. The best results are obtained with rT1 patients. Patients with rT3 recurrences are considered by some to be not salvageable. In particular if there is clival erosion, some surgeons consider this to be a situation in which complete tumor extirpation is not possible. Hence careful and diligent follow-up coupled with biopsies of any suspicious areas of the nasopharynx are crucial factors that allow for early detection of recurrences or residual tumor.
Endoscopic Approach
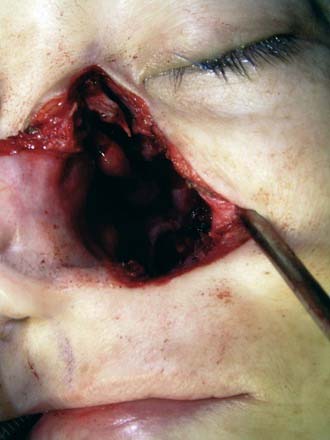
This approach is clearly possible for small recurrences.73 It is best limited to recurrences that are centrally placed on the posterior wall of the nasopharynx (Fig. 99-7). Recurrences that extend out to the pterygopalatine fossa, the soft palate, and beyond may be too large for endoscopic nasopharyngectomy, although experienced endoscopic skull base surgeons may not necessarily consider this an absolute contraindication. The success of this approach lies in being able to choose the patients well. Besides proper instruments, the key to being able to have excellent visualization and control of the surgical approach lies in resection of the posterior nasal septum. Depending on the site and size of the recurrences, the medial maxillary wall may have to be resected. It is also important to remember to resect adequately the roof of the nasopharynx and drilling down the vomer. The depth of resection should include at least the prevertebral muscles. Once the resection and frozen sections are completed, the wound in the resected nasopharynx is packed with antiseptic gauze and left for at least 1 week.
Lateral Rhinotomy and Medial Maxillectomy Approach
This approach can be used for tumors slightly larger than those indicated for endoscopic approach. It may be used for recurrent or residual tumors limited to the nasopharynx or with extension out to the pterygopalatine fossa. It may also be combined with a transoral approach to resect tumors with oropharyngeal extension. Alternatively it may be extended for tumors that invade the orbit. After completion of the lateral rhinotomy, the medial wall of the maxilla is removed as far out laterally as possible while preserving the infraorbital nerve. The nasolacrimal duct is marsupialized. Resecting the inferior half of the middle turbinate will also help with surgical access. The key to visualizing the entire nasopharynx well is in resecting the posterior nasal septum (Fig. 99-8). The surgical access is adequate but not as wide as the view afforded by the maxillary swing approach. It does not require any palatal split. The wound almost always heals well and cosmetically it is not an issue with the patient. Trismus is uncommon and palatal fistula does not exist. However, postoperatively these patients often complain of headaches, which usually take about a month to settle. The problem is the prolonged period of nasal clearance required to rid the crusts that develop. Exposed bone may lead to localized osteoradionecrosis.
Maxillary Swing
This is an elegant operation described by Wei in 1991.74 It provides a wide access for resecting nasopharyngeal tumors. The procedure involves a Weber-Ferguson incision. The maxilla is exposed, and osteotomies are made such that the maxilla will be rotated laterally but the skin and subcutaneous tissues continue to provide the blood supply to the maxilla because it is not dissected off the anterior wall of the bone (Fig. 99-9). The medial maxillary wall is removed. This is an approach that provides excellent access to remove tumors that have infiltrated the pterygopalatine space. It is also argued that the internal carotid artery may be controlled should there be tumor extension laterally. It does, however, require a palatal split and this can lead to palatal fistulas.
Other Surgical Approaches
Surgical Treatment of Regional Recurrences
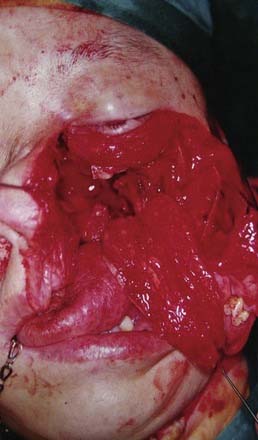
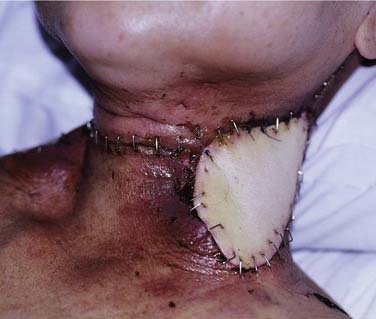
The treatment of regional recurrence and residual regional disease is by neck dissection (Fig. 99-10). The evidence clearly indicates that a radical neck dissection should be performed.75 This is because regional disease is often along the chain of nodes of the upper internal jugular vein, as well as the accessory nerve. Furthermore, the metastatic node, unlike similar nodes in squamous cell carcinoma of the head and neck, tends to have a propensity for extracapsular spread. Hence the internal jugular vein, accessory nerve, and sternocleidomastoid muscle should be sacrificed. In fact, it is not uncommon to perform an extended radical neck dissection involving resection of the skin. Levels I to V nodes are removed. Although retropharyngeal nodes are said to be the first echelon of nodes in NPC, these do not need to be removed because oftentimes the radiation will have encompassed the retropharyngeal nodes. The main problems with performing a radical neck dissection for recurrent NPC are related to bleeding and wound infection, because a good proportion of these patients will have had radiation with or without chemotherapy. The risk of carotid blowout is higher if the wound breaks down and the carotids are exposed. This may be prevented by the use of a pedicled flap such as the pectoralis major (see Fig. 99-10) or latissimus dorsi flap. The effectiveness of the treatment is good, with overall survival after radical neck dissection reaching above 50%.76 Regional control is often achieved and further recurrences are often at distant sites.
Prognosis
The poor prognostic factor in NPC is stage IV disease. In particular, N3 disease is associated with poorer survival and higher rates of distant metastasis. Patients with stage III or IV disease who have had concurrent chemoradiation have a 5-year overall survival rate of about 70%.77 Stage I and II NPC patients treated by radiation alone have 5-year overall survival rates of 80% and higher. The use of chemoradiation for stage III and IV patients has seen improvements in local and regional control, but distant metastasis remains the main failure leading to death in NPC patients.78
Al-Sarraf M, LeBlanc M, Giri PG, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomised Intergroup study 0099. J Clin Oncol. 1998;16(Apr):1310-1317.
Makek MS, Andrews JC, Fisch U. Malignant transformation of a nasopharyngeal angiofibroma. Laryngoscope. 1989;99:1088-1092.
Chan AT, Leung SF, Ngan RK, et al. Overall survival after concurrent cisplatin-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma. J Natl Cancer Inst. 2005;97:536-539.
Chan AT, Ma BB, Lo YM, et al. Phase II study of neoadjuvant carboplatin and paclitaxel followed by radiotherapy and concurrent cisplatin in patients with locoregionally advanced nasopharyngeal carcinoma: therapeutic monitoring with plasma Epstein-Barr virus DNA. J Clin Oncol. 2004;22:3053-3060.
Chan SH, Day NE, Kunaratnam N, et al. HLA and nasopharyngeal carcinoma in Chinese—a further study. Int J Cancer. 1983;32:171-176.
Chandler JR, Goulding R, Moskowitz L, et al. Nasopharyngeal angiofibromas: staging and management. Ann Otol Rhinol Laryngol. 1984;93(4 Pt 1):322-329.
Chong VF, Fan YF. Skull base erosion in nasopharyngeal carcinoma: detection by CT and MRI. Clin Radiol. 1996;51:625-631.
Fam FM, Tsai WL, Chen HC, et al. Intensity modulated or conformal radiotherapy improves the quality of life of patients with nasopharyngeal carcinoma: comparisons of four radiotherapy techniques. Cancer. 2007;109:313-321.
Ho JHC. An epidemiologic and clinical study of nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 1978;4:181-198.
Lanier A, Bender T, Talbot M, et al. Nasopharyngeal carcinoma in Alaskan Eskimo Indians, and Aleuts: a review of cases and study of Epstein-Barr virus, HLA and environmental risk factors. Cancer. 1980;46:2100-2106.
Lee AW, Foo W, Mang O, et al. Changing epidemiology of nasopharyngeal carcinoma in Hong Kong over a 20-year period (1980-1999): an encouraging reduction in both incidence and mortality. Int J Cancer. 2003;103:680-685.
Leung TW, Tung SY, Sze WK, et al. Treatment results of 1070 patients with nasopharyngeal carcinoma: an analysis of survival and failure patterns. Head Neck. 2005;27:555-565.
Loh KS, Goh BC, Hsieh WS, et al. Familial nasopharyngeal carcinoma in a cohort of 200 patients. Arch Otolaryngol Head Neck Surg. 2006;132:82-85.
Luo J, Chia KS, Chia SE, et al. Secular trends of nasopharyngeal carcinoma in Singapore, Hong Kong and Los Angeles Chinese populations, 1973-1997. Eur J Epidemiol. 2007;22:513-521.
McCombe A, Lund VJ, Howard DJ. Recurrence in juvenile angiofibroma. Rhinology. 1990;28:97-102.
O’Meara WP, Lee N. Advances in nasopharyngeal carcinoma. Curr Opin Oncol. 2005;17:225-230.
Sciarretta V, Pasquini E, Farneti G, et al. Endsocopic sinus surgery for the treatment of vascular tumours. Am J Rhinol. 2006;20:426-431.
Shanmugaratnam K. Nasopharyngeal carcinoma: epidemiology, histopathology and aetiology. Ann Acad Med (Singapore). 1980;9:289-295.
Sun LM, Epplein M, Li CI, et al. Trends in the incidence rates of nasopharyngeal carcinoma in Chinese Americans living in the Los Angeles County and the San Francisco metropolitan area, 1992-2002. Am J Epidemiol. 2005;162:1174-1178.
Tyagi I, Syal R, Goyal A. Recurrent and residual juvenile angiofibromas. JLO. 2007;121:460-467.
Ung A, Chen CJ, Levine PH, et al. Familial and sporadic cases of nasopharyngeal carcinoma in Taiwan. Anticancer Res. 1999;19:661-665.
Wei WI, Lam KH, Sham JS. New approach to the nasopharynx: the maxillary swing approach. Head Neck. 1991;13:200-207.
Wenig BM. Atlas of Head and Neck Pathology. Philadelphia: WB Saunders; 1993.
Wong SCA, Soo RA, Lu JJ, et al. Paclitaxel, 5-fluorouracil and hydroxyurea concurrent with radiation in locally advanced nasopharyngeal carcinoma. Ann Oncol. 2006;17:1152-1157.
Yu MC, Huang TB, Henderson BE. Diet and nasopharyngeal carcinoma: a case-control study in Guangzhou, China. Int J Cancer. 1989;43:1077-1082.
1. Montag AG, Tretiakova M, Ricardson M. Steroid hormone receptor expression in nasopharyngeal angiofibromas. Consistent expression of estrogen receptor beta. Am J Clin Pathol. 2006;125:832-837.
2. Wenig BM. Atlas of Head and Neck Pathology. Philadelphia: WB Saunders; 1993.
3. Windfuhr JP, Remmert S. Extranasopharyngeal angiofibroma: etiology, incidence and management. Act Otolaryngol. 2004;124:880-889.
4. Uslu C, Yildirim M, Uslu H, et al. 99m Tc-labelled red blood cell single-photon emission computed tomography for the diagnosis and follow-up of juvenile nasopharyngeal angiofibroma. Nucl Med Commun. 2006;27:489-494.
5. Chandler JR, Goulding R, Moskowitz L, et al. Nasopharyngeal angiofibromas: staging and management. Ann Otol Rhinol Laryngol. 1984;93(4 Pt 1):322-329.
6. Tyagi I, Syal R, Goyal A. Recurrent and residual juvenile angiofibromas. JLO. 2007;121:460-467.
7. Sciarretta V, Pasquini E, FarnetiG, et al. Endsocopic sinus surgery for the treatment of vascular tumours. Am J Rhinol. 2006;20:426-431.
8. El-Banhawy OA, Ragab A, El-Sharnoby MM. Surgical resection of type 3 juvenile angiofibroma without preoperative embolization. Int J Paediatric Otorhinolaryngol. 2006;70:1715-1723.
9. McCombe A, Lund VJ, Howard DJ. Recurrence in juvenile angiofibroma. Rhinology. 1990;28:97-102.
10. Graamans K, Tulleken CA. Laser assisted bypass of the internal carotid artery prior to the treatment of an extensive angiofibroma. Skull Base Surgery. 1998;8:205-210.
11. Cummings BJ, Blend R, Keane T, et al. Primary radiation therapy for juvenile nasopharyngeal angiofibroma. Laryngoscope. 1984;94:1599-1605.
12. Makek MS, Andrews JC, Fisch U. Malignant transformation of a nasopharyngeal angiofibroma. Laryngoscope. 1989;99:1088-1092.
13. Park CK, Kim DG, Paek SH, et al. Recurrent juvenile nasopharyngeal angiofibroma treated with gamma knife surgery. J Korean Med Sci. 2006;21:773-777.
14. Mohanty S, Balakrishnan S. Management of extra sellar craniopharyngioma masquerading as hypertrophied adenoid tissue in a 6-year-old boy. Int J Pediatr Otorhinolaryngol. 2008;72:1441-1444.
15. Lee AW, Foo W, Mang O, et al. Changing epidemiology of nasopharyngeal carcinoma in Hong Kong over a 20-year period (1980-1999): an encouraging reduction in both incidence and mortality. Int J Cancer. 2003;103:680-685.
16. O’Meara WP, Lee N. Advances in nasopharygeal carcinoma. Curr Opin Oncol. 2005;17:225-230.
17. Kam MK, Leung SF, Zee B, et al. Prospective randomized study of intensity-modulated radiotherapy on salivary gland function in early-stage nasopharyngeal carcinoma patients. J Clin Oncol. 2007;25:4873-4879.
18. Fam FM, Tsai WL, Chen HC, et al. Intensity modulated or conformal radiotherapy improves the quality of life of patients with nasopharyngeal carcinoma: comparisons of four radiotherapy techniques. Cancer. 2007;109:313-321.
19. Simons MJ, Shanumugaratnam K, eds. The Biology of Nasopharyngeal Carcinoma. Epidemiology of Nasopharyngeal Carcinoma. UICC Technical Report Series Geneva International Union Against Cancer. 1982;71:10-16.
20. Seow A, Koh WP, Chia KS, et al. Trends and incidences of cancer in Singapore 1968-2002. Singapore Cancer Registry Report No 6:88-89.
21. Shanmugaratnam K. Variations in nasopharyngeal carcinoma incidence among specific Chinese communities (dialect groups) in Singapore. IARC Sci Publ. 1978;20:191-198.
22. Luo J, Chia KS, Chia SE, et al. Secular trends of nasopharyngeal carcinoma in Singapore, Hong Kong and Los Angeles Chinese populations, 1973-1997. Eur J Epidemiol. 2007;22:513-521.
23. Sun LM, Epplein M, Li CI, et al. Trends in the incidence rates of nasopharyngeal carcinoma in Chinese Americans living in the Los Angeles County and the San Francisco metropolitan area, 1992-2002. Am J Epidemiol. 2005;162:1174-1178.
24. Lanier A, Bender T, Talbot M, et al. Nasopharyngeal carcinoma in Alaskan Eskimo Indians, and Aleuts: a review of cases and study of Epstein-Barr virus, HLA and environmental risk factors. Cancer. 1980;46:2100-2106.
25. Ireland B, Lanier AP, Knutson L, et al. Increased risk of cancer in siblings of Alaskan native patients with nasopharyngeal carcinoma. Int J Epimediol. 1988;17:509-511.
26. Shanmugaratnam K. Nasopharyngeal carcinoma: epidemiology, histopathology and aetiology. Ann Acad Med (Singapore). 1980;9:289-295.
27. Hirayama T. Descriptive and analytical epidemiology of nasopharyngeal cancer. IARC Sci Publ. 1978;20:167-189.
28. Ung A, Chen CJ, Levine PH, et al. Familial and sporadic cases of nasopharyngeal carcinoma in Taiwan. Anticancer Res. 1999;19:661-665.
29. Loh KS, Goh BC, Hsieh WS, et al. Familial nasopharyngeal carcinoma in a cohort of 200 patients. Arch Otolaryngol Head Neck Surg. 2006;132:82-85.
30. Jia WH, Feng BJ, Xu ZL, et al. Familial risk and clustering of nasopharyngeal carcinoma in Guangdong, China. Cancer. 2004;101:363-369.
31. Simons MJ, Wee GB, Chan SH, et al. Probable identification of an HL-A second-locus antigen associated with a high risk of nasopharyngeal carcinoma. Lancet. 1975;1:142-143.
32. Chan SH. HLA and nasopharyngeal carcinoma—a position in 1980. Ann Acad Med (Singapore). 1980;9:296-299.
33. Chan SH, Chew CT, Prasad U, et al. HLA and nasopharyngeal carcinoma in Malays. Br J Cancer. 1985;51:389-392.
34. Chan SH, Day NE, Kunaratnam N, et al. HLA and nasopharyngeal carcinoma in Chinese—a further study. Int J Cancer. 1983;32:171-176.
35. Simons MJ, Wee GB, Goh EH, et al. Immunogenetic aspects of nasopharyngeal carcinoma. IV. Increased risk in Chinese of nasopharyngeal carcinoma associated with a Chinese-related HLA profile (A2, Singapore 2). J Natl Cancer Inst. 1976;57:977-980.
36. Lu CC, Chen JC, Jin YT, et al. Genetic susceptibility to nasopharyngeal carcinoma within the HLA-A locus in Taiwanese. Int J Cancer. 2003;103:745-751.
37. Hu SP, Day NE, Li DR, et al. Further evidence for an HLA-related recessive mutation in nasopharyngeal carcinoma among the Chinese. Br J Cancer. 2005;92:967-970.
38. Hildesheim A, Apple RJ, Chen CJ, et al. Association of HLA class I and II alleles and extended haplotypes with nasopharyngeal carcinoma in Taiwan. J Natl Cancer Inst. 2002;94:1780-1789.
39. Ooi EE, Ren EC, Chan SH. Association between microsatellites within the human MHC and nasopharyngeal carcinoma. Int J Cancer. 1997;74:229-232.
40. Huang DP, Lo KW, Choi PH, et al. Loss of heterozygosity on the short arm of chromosome 3 in nasopharyngeal carcinoma. Cancer Genet Cytogenet. 1991;54:91-99.
41. Lo KW, Tsao SW, Leung SF, et al. Detailed deletion mapping on the short arm of chromosome 3 in nasopharyngeal carcinoma. Int J Oncol. 1994;4:1359-1364.
42. Huang DP, Lo KW, van Hasselt A, et al. A region of homozygous deletion on chromosome 9p21-22 in primary nasopharyngeal carcinoma. Cancer Res. 1995;55:2039-2043.
43. Hui AB, Lo KW, Leung SF, et al. Loss of heterozygosity on the long arm of chromosome 11 in nasopharyngeal carcinoma. Cancer Res. 1996;56:3225-3229.
44. Lo KW, Huang DP, Lau KM. P16 gene alterations in nasopharyngeal carcinoma. Cancer Res. 1995;55:2039-2043.
45. Lo KW, Cheung ST, Leung SF, et al. Hypermethylation of the p16 gene in nasopharyngeal carcinoma. Cancer Res. 1996;56:2721-2725.
46. Qiu GH, Tan LK, Loh KS, et al. The candidate tumor suppressor gene BLU, located at the commonly deleted region 3p21.3, is an E2F-regulated, stress-responsive gene and inactivated by both epigenetic and genetic mechanisms in nasopharyngeal carcinoma. Oncogene. 2004;23:4793-4806.
47. Ho JHC. An epidemiologic and clinical study of nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 1978;4:181-198.
48. Ning JP, Yu MC, Wang QS, et al. Consumption of salted fish and other risk factors for nasopharyngeal carcinoma (NPC) in Tianjin, a low-risk region for NPC in the People’s Republic of China. J Natl Cancer Inst. 1990;82:291-296.
49. Yu MC, Ho JHC, Lai SH, et al. Cantonese-style salted fish as a cause of nasopharyngeal carcinoma: report of a case-controlled study in Hong Kong. Cancer Res. 1986;46:956-961.
50. Yu MC, Huang TB, Henderson BE. Diet and nasopharyngeal carcinoma: a case-control study in Guangzhou, China. Int J Cancer. 1989;43:1077-1082.
51. Yu MC, Mo CC, Chong WX, et al. Preserved foods and nasopharyngeal carcinoma: a case-control study in Guangxi, China. Cancer Res. 1988;48:1954-1959.
52. Yang XR, Diehl S, Pfeiffer R, et al. Evaluation of risk factors for nasopharyngeal carcinoma in high-risk nasopharyngeal carcinoma families in Taiwan. Cancer Epidemiol Biomarkers Prev. 2005;14:90-905.
53. Zheng YM, Tuppin P, Hubert A, et al. Environmental and dietary risk factors for nasopharyngeal carcinoma: a case-control study in Zangwu County, Guangxi, China. Br J Cancer. 1994;69:508-514.
54. Hildesheim A, Dosemeci M, Chan CC, et al. Occupational exposure to wood, formaldehyde, and solvents and risk of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2001;10:1145-1153.
55. Henle G, Henle W. Epstein-Barr virus-specific IgA serum antibodies as an outstanding feature of nasopharyngeal carcinoma. Int J Cancer. 1976 Jan 15;17(1):1-7.
56. Miller WE, Earp HS, Raab-Traub N. The Epstein-Barr virus latent membrane protein 1 induces expression of the epidermal growth factor receptor. J Virol. 1995;69:4390-4398.
57. Paine E, Scheinman RI, Baldwin ASJr, et al. Expression of LMP1 in epithelial cells leads to the activation of a select subset of NF-kappa B/Rel family proteins. J Virol. 1995;69:4572-4576.
58. Shanmugaratnam K, Sobin L. Histological Typing of Upper Respiratory Tract Tumors: International Typing of Tumors. Geneva: World Health Organization; 1978.
59. Sobin LH, Shanmugaratnam K. WHO Classification of Upper Aerodigestive Tract Diseases. Berlin: Springer-Verlag; 1991.
60. Chong VF, Fan YF. Skull base erosion in nasopharyngeal carcinoma: detection by CT and MRI. Clin Radiol. 1996;51:625-631.
61. Chong VF, Mukherji SK, Ng SH, et al. Nasopharyngeal carcinoma: review of how imaging affects staging. J Comput Assist Tomogr. 1999;23:984-993.
62. Liu FY, Lin CY, Chang JT, et al. 18F-FDG PET can replace conventional work-up in primary M staging of nonkeratinizing nasopharyngeal carcinoma. J Nucl Med. 2007;48:1614-1619.
63. Hung GU, Tsai SC, Lin WY. Extraordinarily high F-18 FDG uptake caused by radiation necrosis in a patient with nasopharyngeal carcinoma. Clin Nucl Med. 2005;30:558-559.
64. Oh HK, Chambers MS, Garden AS, et al. Risk of osteoradionecrosis after extraction of impacted third molars in irradiated head and neck cancer patients. J Oral Maxillofac Surg. 2004;62:125-126.
65. Chang DT, Sandow PR, Morris CG, et al. Do pre-irradiation dental extractions reduce the risk of osteoradionecrosis of the mandible? Head Neck. 2007;29:528-536.
66. Chan AT, Ma BB, Lo YM, et al. Phase II study of neoadjuvant carboplatin and paclitaxel followed by radiotherapy and concurrent cisplatin in patients with locoregionally advanced nasopharyngeal carcinoma: therapeutic monitoring with plasma Epstein-Barr virus DNA. J Clin Oncol. 2004;22:3053-3060.
67. Gibb AG, Loh KS. The role of radiation in delayed hearing loss in nasopharyngeal carcinoma. J Laryngol Otol. 2000;114:139-144.
68. Kam MK, Teo PM, Chau RM, et al. Treatment of nasopharyngeal carcinoma with intensity modulated radiotherapy: the Hong Kong experience. Int J Radiat Oncol Biol Phys. 2004;60:1440-1450.
69. Al-Sarraf M, LeBlanc M, Giri PG, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomised Intergroup study 0099. J Clin Oncol. 1998;16(Apr):1310-1317.
70. Chan AT, Teo PM, Ngan RK, et al. Concurrent chemotherapy-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: progression-free survival analysis of a phase III randomized trial. J Clin Oncol. 2002 Apr 15;20(8):2038-2044.
71. Wong SCA, Soo RA, Lu JJ, et al. Paclitaxel, 5-fluorouracil and hydroxyurea concurrent with radiation in locally advanced nasopharyngeal carcinoma. Ann Oncol. 2006;17:1152-1157.
72. Hsu MM, Hong RL, Ting LL, et al. Factors affecting the overall survival after salvage surgery in patients with recurrent nasopharyngeal carcinoma at the primary site: experience with 60 cases. Arch Otolaryngol Head Neck Surg. 2001;127:798-802.
73. Chen MK, Lai JC, Chang CC, et al. Minimally invasive endoscopic nasopharyngectomy in the treatment of recurrent T1-2a nasopharyngeal carcinoma. Laryngoscope. 2007;117:894-896.
74. Wei WI, Lam KH, Sham JS. New approach to the nasopharynx: the maxillary swing approach. Head Neck. 1991;13:200-207.
75. Wei WI, Ho WK, Cheng AC, et al. Management of extensive cervical nodal metastasis in nasopharyngeal carcinoma after radiotherapy: a clinicopathological study. Arch Otolaryngol Head Neck Surg. 2001;127:1457-1462.
76. Yen KL, Hsu LP, Sheen TS, et al. Salvage neck dissection for cervical recurrence of nasopharyngeal carcinoma. Arch Otolaryngol Head Neck Surg. 1997;123:725-729.
77. Chan AT, Leung SF, Ngan RK, et al. Overall survival after concurrent cisplatin-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma. J Natl Cancer Inst. 2005;97:536-539.
78. Leung TW, Tung SY, Sze WK, et al. Treatment results of 1070 patients with nasopharyngeal carcinoma: an analysis of survival and failure patterns. Head Neck. 2005;27:555-565.