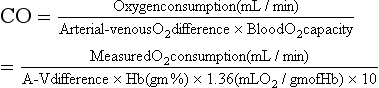Chapter 12
Bedside Hemodynamic Monitoring
1. What is a Swan-Ganz catheter?
2. How is a Swan-Ganz catheter constructed?
3. What information can be gained from a Swan-Ganz catheter?
4. How is a Swan-Ganz catheter inserted?
5. Describe the normal pressure waveforms along the path of an advancing Swan-Ganz catheter.
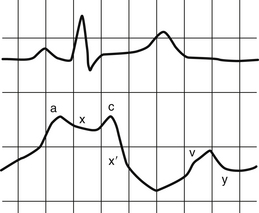
The a wave is produced by atrial contraction and follows the electrical P wave of an electrocardiogram (ECG). The x descent reflects atrial relaxation. The c wave is produced at the beginning of ventricular systole as the closed tricuspid valve bulges into the right atrium. The x′ descent is thought to be the result of the descent of the atrioventricular ring during ventricular contraction as well as continued atrial relaxation. The v wave is caused by venous filling of the atrium during ventricular systole, when the tricuspid valve is closed. This should correspond with the electrical T wave. However, at the bedside, due to a lag in pressure transmission, the a wave will align with the QRS complex and the v wave will follow the T wave. Finally, the y descent is produced by rapid atrial emptying, when the tricuspid valve opens at the onset of diastole (Fig. 12-1).
6. How is the location of the catheter determined?
7. How do we know that the catheter is in the true wedge position?
There are three ways to confirm that the catheter is in the wedge position (Figures 12-1 and 12-2). At the bedside, an atrial tracing (reflecting left atrial pressure) will be seen when the catheter is in the wedge position. Secondly, if the catheter is withdrawn from the wedge position, the mean arterial pressure should be observed to rise from the wedge pressure (reflecting a physiologic gradient between the mean pulmonary artery and mean wedge pressure). Gentle aspiration of blood from the distal port should reveal high-oxygenated blood if the catheter is truly wedged. Additionally, in the catheterization lab, fluoroscopy can be used to determine that the catheter is in a distal pulmonary arteriole, immobile in the wedge position.
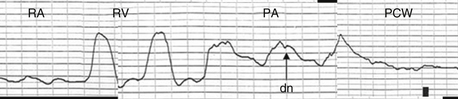
Figure 12-2 Pressure tracings as a catheter is advanced through the right-sided chambers. As the catheter moves from the right atrium to the right ventricle, a ventricular wave form is seen representing isovolumic contraction, ejection, and diastole. When the catheter passes into the pulmonary artery, the diastolic pressure rises. The dicrotic notch (dn), is produced by the closure of the pulmonic valve. If the catheter is advanced further, it attains the wedge position. PA, Pulmonary artery; PCW, pulmonary capillary wedge; RA, right atrium; RV, right ventricle.
8. What does the PAWP signify?
When the catheter is in the wedge position (Fig. 12-3, B), proximal blood flow is occluded and a static column of blood is created between the catheter tip and the distal cardiac chambers. With the balloon shielding the catheter tip from the pressure in the pulmonary artery proximally, the pressure transducer measures pressure distally in the pulmonary arterioles. This pressure closely approximates left atrial pressure. When the mitral valve is open at end diastole, left ventricular (LV) end diastolic pressure is measured (Fig. 12-3, C), assuming that there is no obstruction between the catheter tip and the LV (i.e., mitral stenosis). The PAWP can be used to approximate LV preload.
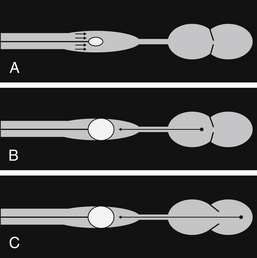
Figure 12-3 A, With the balloon tip deflated, the pressure transducer at the catheter tip sees blood flow from the proximal pulmonary artery. B, With the balloon tip inflated, proximal blood flow is occluded and a static column of blood is created between the catheter tip and the distal cardiac chamber. With the mitral valve closed, left atrial pressure is approximated. C, With the mitral valve open at end diastole, left ventricular end diastolic pressure can be measured, assuming there is no significant mitral stenosis.
9. How is cardiac output determined?
With thermodilution, 5 to 10 mL of normal saline is injected rapidly via the proximal port into the right atrium. The injectate mixes completely with blood and causes a drop in temperature that is measured continuously by a thermocouple near the catheter tip. The area under the curve is calculated and is inversely related to cardiac output (Fig. 12-4). This method of measurement is not reliable in patients with low cardiac output or significant tricuspid regurgitation. In a low cardiac output state, blood is rewarmed by the walls of the cardiac chambers and surrounding tissue, resulting in an overestimation of cardiac output.
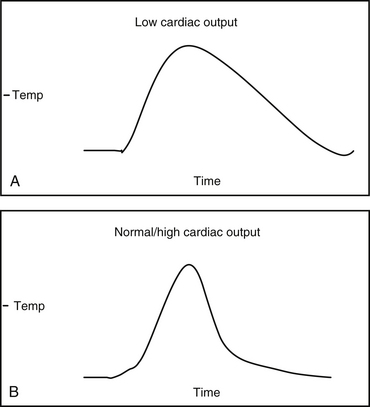
Figure 12-4 Area under the curve (AUC) is inversely proportional to cardiac output. The illustration depicts a larger AUC in a patient with low cardiac output (A). B, Temperature equilibrates faster in a patient with a higher cardiac output, resulting in a smaller AUC.
Alternatively, the Fick method can be used to calculate cardiac output.
This method is based on the principle that the consumption of a substance (oxygen) by any organ is determined by the arterial-venous (A-V) difference of the substance and the blood flow (CO) to that organ. The consumption of oxygen by a patient can be measured using a covered hood in the cardiac catheterization lab and the arterial-venous difference can be measured by obtaining blood samples from the right atrium and pulmonary artery. This method is more accurate in patients with atrial fibrillation, tricuspid regurgitation, and low cardiac output. Common sources of error include improper collection of blood samples.
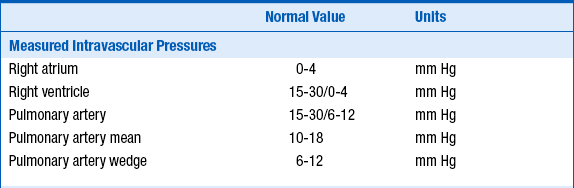
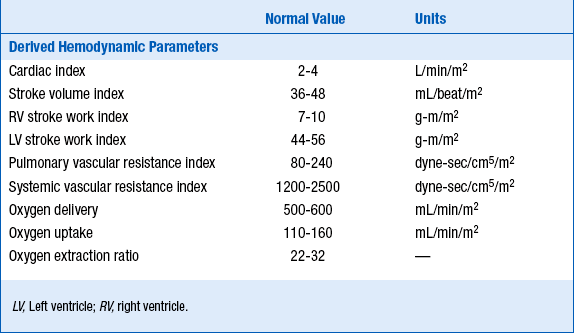
10. What are normal values for intravascular pressures and hemodynamic parameters?
The normal values for intravascular pressures and hemodynamic measurements are given in Table 12-1.
11. Why are cardiac output and LV preload important?
In certain clinical situations, the knowledge of cardiac output and PAWP (surrogate of LV preload, see Question 8) can help to make diagnoses and/or guide management (see Question 12). PAWP can be applied to the Starling curve and help to predict whether cardiac output may improve if filling pressures are altered.
12. When is placing a Swan-Ganz catheter clinically indicated and do all patients derive clinical benefit?
Heart Failure/Shock
 To differentiate between cardiogenic and noncardiogenic pulmonary edema when a trial of diuretic and/or vasodilator therapy has failed
To differentiate between cardiogenic and noncardiogenic pulmonary edema when a trial of diuretic and/or vasodilator therapy has failed
 To differentiate causes of shock and to guide management when a trial of intravascular volume expansion has failed (see Question 15)
To differentiate causes of shock and to guide management when a trial of intravascular volume expansion has failed (see Question 15)
 Determination of whether pericardial tamponade is present when clinical assessment is inconclusive and echocardiography is unavailable
Determination of whether pericardial tamponade is present when clinical assessment is inconclusive and echocardiography is unavailable
 Assessment of valvular heart disease
Assessment of valvular heart disease
 Determination of reversibility of pulmonary vasoconstriction in patients being considered for heart transplantation
Determination of reversibility of pulmonary vasoconstriction in patients being considered for heart transplantation
 Management of congestive heart failure refractory to standard medical therapy, especially in the setting of acute myocardial infarction (MI)
Management of congestive heart failure refractory to standard medical therapy, especially in the setting of acute myocardial infarction (MI)
Acute Myocardial Infarction
American College of Cardiology/American Heart Association (ACC/AHA) guidelines state that Swan-Ganz catheters should be used in those patients who have progressive hypotension unresponsive to fluids and in patients with suspected mechanical complications of ST elevation MI if an echocardiogram has not been performed (class I). However, mortality benefit has not been demonstrated in a randomized trial.
The use of Swan-Ganz catheters can also be considered in the following situations:
 Diagnosis of mechanical complications of MI (i.e., mitral regurgitation, ventricular septal defect)
Diagnosis of mechanical complications of MI (i.e., mitral regurgitation, ventricular septal defect)
 Diagnosis of intracardiac shunts and to establish their severity before surgical correction (see Question 16)
Diagnosis of intracardiac shunts and to establish their severity before surgical correction (see Question 16)
 Guidance of management of cardiogenic shock with pharmacologic and/or mechanical support
Guidance of management of cardiogenic shock with pharmacologic and/or mechanical support
 Guidance of management of right ventricular infarction with hypotension and/or signs of low cardiac output not responding to intravascular volume expansion and/or low doses of inotropic drugs
Guidance of management of right ventricular infarction with hypotension and/or signs of low cardiac output not responding to intravascular volume expansion and/or low doses of inotropic drugs
 Short-term guidance of pharmacologic and/or mechanical management of acute mitral regurgitation before surgical correction
Short-term guidance of pharmacologic and/or mechanical management of acute mitral regurgitation before surgical correction
Perioperative Use
 In patients undergoing cardiac surgery, to differentiate between causes of low cardiac output or to differentiate between right and left ventricular dysfunction, when clinical assessment and echocardiography is inadequate
In patients undergoing cardiac surgery, to differentiate between causes of low cardiac output or to differentiate between right and left ventricular dysfunction, when clinical assessment and echocardiography is inadequate
 Guidance of perioperative management in selected patients with decompensated heart failure, undergoing intermediate- or high-risk noncardiac surgery
Guidance of perioperative management in selected patients with decompensated heart failure, undergoing intermediate- or high-risk noncardiac surgery
Use in Intensive Care Units
 Many studies have shown that clinical data predict PAWP and cardiac output poorly, and that insertion of the catheter frequently changes patient management. However, despite widespread use of these devices in intensive care units, only a few observational studies have shown their use to decrease mortality. A 2005 meta-analysis of several randomized trials showed that the use of Swan-Ganz catheters was associated with neither benefit nor increased mortality. Current thinking is reflected in a 1997 pulmonary artery consensus statement, recommending that the decision to insert a Swan-Ganz catheter be made on an individual basis, such that the potential risks and benefits are considered in each case.
Many studies have shown that clinical data predict PAWP and cardiac output poorly, and that insertion of the catheter frequently changes patient management. However, despite widespread use of these devices in intensive care units, only a few observational studies have shown their use to decrease mortality. A 2005 meta-analysis of several randomized trials showed that the use of Swan-Ganz catheters was associated with neither benefit nor increased mortality. Current thinking is reflected in a 1997 pulmonary artery consensus statement, recommending that the decision to insert a Swan-Ganz catheter be made on an individual basis, such that the potential risks and benefits are considered in each case.
14. What diagnoses can the catheter help make?
The characteristic waveform of the Swan-Ganz catheter is altered in several disease states.
 In pericardial tamponade, equalization of diastolic pressures across all chambers is seen (Fig. 12-5).
In pericardial tamponade, equalization of diastolic pressures across all chambers is seen (Fig. 12-5).
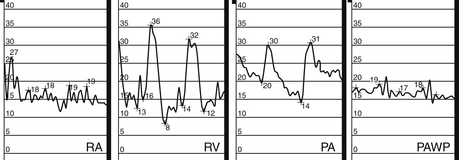
Figure 12-5 Pressure tracings in cardiac tamponade. PA, Pulmonary artery; PAWP, pulmonary artery wedge pressure; RA, right atrium; RV, right ventricle. There is equalization of all diastolic pressures across all chambers.
 In atrial fibrillation, the a wave disappears from the right atrial pressure tracing, while in atrial flutter, mechanical flutter waves occur at a rate of 300 per minute.
In atrial fibrillation, the a wave disappears from the right atrial pressure tracing, while in atrial flutter, mechanical flutter waves occur at a rate of 300 per minute.
 “Cannon” a waves occur when the atria contract against closed valves due to atrioventricular dissociation. Irregular cannon a waves during a wide-complex tachycardia strongly suggest ventricular tachycardia.
“Cannon” a waves occur when the atria contract against closed valves due to atrioventricular dissociation. Irregular cannon a waves during a wide-complex tachycardia strongly suggest ventricular tachycardia.
 Complications of MI can be detected on the PAWP tracing, such as giant v waves seen with acute mitral insufficiency, and the “dip and plateau” pattern of the RV pressure tracing seen with RV infarction.
Complications of MI can be detected on the PAWP tracing, such as giant v waves seen with acute mitral insufficiency, and the “dip and plateau” pattern of the RV pressure tracing seen with RV infarction.
15. How can etiologies of shock be differentiated by Swan-Ganz catheterization?
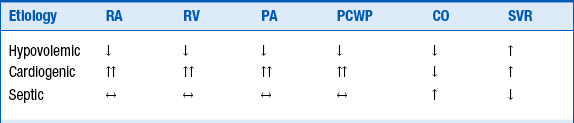
Table 12-2 presents the hemodynamic parameters in different etiologies of shock.
16. How can left-to-right intracardiac shunts be diagnosed by Swan-Ganz catheterization?
17. What complications are associated with use of a Swan-Ganz catheter?
 All complications of central venous cannulation including bleeding and infection.
All complications of central venous cannulation including bleeding and infection.
 Local infection rates range from 18% to 63% in patients with catheter in place for an average of 3 days.
Local infection rates range from 18% to 63% in patients with catheter in place for an average of 3 days.
 Bloodstream infections have been reported in up to 5% of patients.
Bloodstream infections have been reported in up to 5% of patients.
 Transient right bundle branch block
Transient right bundle branch block
 Complete heart block (especially in patients with preexisting left bundle branch block)
Complete heart block (especially in patients with preexisting left bundle branch block)
 Clinically insignificant ventricular arrhythmias can occur in up to 30% to 60% of patients.
Clinically insignificant ventricular arrhythmias can occur in up to 30% to 60% of patients.
 Sustained arrhythmias usually occur in patients with myocardial ischemia or infarction.
Sustained arrhythmias usually occur in patients with myocardial ischemia or infarction.
 Pulmonary infarction (incidence 0-1.3%)
Pulmonary infarction (incidence 0-1.3%)
18. How can complications be minimized?
 The use of fluoroscopy should be considered for placement of the catheter, particularly if access is obtained from a nontraditional site, or if the patient has a dilated right ventricle.
The use of fluoroscopy should be considered for placement of the catheter, particularly if access is obtained from a nontraditional site, or if the patient has a dilated right ventricle.
 Consideration should be given to removing the catheter after the first set of data is obtained.
Consideration should be given to removing the catheter after the first set of data is obtained.
 The duration the catheter is kept in place should be minimized, as infectious and thrombotic complications increase significantly after 3 to 4 days.
The duration the catheter is kept in place should be minimized, as infectious and thrombotic complications increase significantly after 3 to 4 days.
 Use of the introducer side arm for infusion of medications should be minimized.
Use of the introducer side arm for infusion of medications should be minimized.
 Manipulation of the catheter should only be performed by trained personnel.
Manipulation of the catheter should only be performed by trained personnel.
19. The wedge tracing is abnormal. What do I do?
 Check a chest radiogram for proper catheter position. The tip should lie in lung zone 3, below the level of the left atrium.
Check a chest radiogram for proper catheter position. The tip should lie in lung zone 3, below the level of the left atrium.
 Aspirate and flush the catheter to remove clots and bubbles.
Aspirate and flush the catheter to remove clots and bubbles.
 Check all connecting lines and stopcocks.
Check all connecting lines and stopcocks.
 Confirm that the pressure transducers are zeroed to the level of the right atrium.
Confirm that the pressure transducers are zeroed to the level of the right atrium.
 Check that the balloon is not overinflated; try letting out the air and refilling it slowly.
Check that the balloon is not overinflated; try letting out the air and refilling it slowly.
 Consider the possibility that the tracing really is a wedge tracing with a giant v wave, as is seen in acute mitral insufficiency and several other conditions.
Consider the possibility that the tracing really is a wedge tracing with a giant v wave, as is seen in acute mitral insufficiency and several other conditions.
20. The cardiac output doesn’t make sense. What is wrong?
 Check that at least three values were averaged and that the range of these values is no greater than 20% of the mean.
Check that at least three values were averaged and that the range of these values is no greater than 20% of the mean.
 Check the chest radiogram: is the distal tip of the catheter in the pulmonary artery and the proximal port in the right atrium?
Check the chest radiogram: is the distal tip of the catheter in the pulmonary artery and the proximal port in the right atrium?
 Check to see if the computer is calibrated to the proper temperatures.
Check to see if the computer is calibrated to the proper temperatures.
 If the computer can display the time versus temperature curve, check that the curve is shaped properly.
If the computer can display the time versus temperature curve, check that the curve is shaped properly.
Bibliography, Suggested Readings, and Websites
1. Baim D.S., ed. Grossman’s Cardiac Catheterization, Angiography, and Intervention. Philadelphia: Lippincott Williams & Wilkins, 2006.
2. Binanay, C., Califf, R.M., Hasselblad, V., et al. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA. 2005;294:1625–1633.
3. Leatherman, J.W., Marini, J.J., Clinical use of the pulmonary artery catheter. Hall J.B., Schmidt G.A., Wood L.D.H., eds. Principles of Critical Care ed 2 New York: McGraw-Hill; 1998:155–177.
4. Mueller, H.S., Chatterjee, K., Davis, K.B., et al. American College of Cardiology consensus statement. Present use of bedside right heart catheterization in patients with cardiac disease. J Am Coll Cardiol. 1998;32:840–864.
5. Pulmonary Artery Catheter Consensus Conference Participants. Pulmonary artery catheter consensus conference: Consensus statement. Crit Care Med. 1997;25(6):910–925.
6. Robin, E.D. The cult of the Swan-Ganz catheter. Ann Intern Med. 1985;103:445–449.
7. Sandham, J.D., Hull, R.D., Brant, R.F., et al. A randomized, controlled trial of the use of pulmonary-artery catheters in high-risk surgical patients. N Engl J Med. 2003;348:5–14.
8. Shah, M.R., Hasselblad, V., Stevenson, L.W., et al. Impact of the pulmonary artery catheter in critically ill patients: meta-analysis of randomized clinical trials. JAMA. 2005;294:1664–1670.
9. Sharkey, S.W. Beyond the wedge: Clinical physiology and the Swan Ganz catheter. Am J Med. 1987;83:111–122.
10. Sise, M.J., Hollingsworth, P., Brimm, J.E., et al. Complications of the flow-directed pulmonary-artery catheter: A prospective analysis of 219 patients. Crit Care Med. 1981;9:315–318.
11. Walston, A., Kendall, M.E. Comparison of pulmonary wedge and left atrial pressure in man. Am Heart J. 1973;86:159–164.
12. Zipes D.P., Libby P., Bonow R.O., Braunwald E., eds. Braunwald’s Heart Disease. Philadelphia: Elsevier Saunders, 2005.