12. Becker’s Dystrophy
Definition
Becker’s dystrophy is a genetic variation of Duchenne’s muscular dystrophy. This form is very similar to pseudohypertrophic muscular dystrophy (Duchenne’s), but the onset of the disease occurs later in life and progresses more slowly. However, the ultimate debilitation is the same as with Duchenne’s.
Incidence
The Becker’s dystrophy phenotype occurs at a rate of approximately 24:1,000,000 population. The onset may occur any time from 3 years of age through adulthood, even late adulthood.
Etiology
The genotype for Becker’s dystrophy is the result of a sex-linked transmission on the X chromosome in the Xp 21 stripe. The Becker phenotype correlates with point mutations that preserve reading frame or genetic deletions that cause less structural compromise.
Signs and Symptoms
• Age, usually 10 to 20 years
• Calf enlargement
• Gait disturbances
• Kyphoscoliosis
• Progressive development of muscle weakness
• Progressive diminishment of deep tendon reflexes
• Waning forced vital capacity and other lung volumes
Medical Management
Prednisone is currently the only medication that has demonstrated any benefit in the treatment of Becker’s dystrophy. The dose is 0.75 to 1.5 mg/kg/day in divided doses. The muscle wasting produced by this disease is retarded or delayed by the administration of prednisone. The benefit of prednisone administration may become evident as soon as 1 month after initiation of treatment, but those benefits generally last only about 3 years. These benefits may also be somewhat diminished by sequelae resulting from chronic steroid administration.
The remainder of medical management of Becker’s dystrophy concentrates on measures and strategies to maximize functional status, maintain muscle tone, and delay reliance on a wheelchair for as long as possible. Exercising joints and stretching muscles daily can delay the onset of debilitating contractures. These exercises work synergistically with the application of various supportive braces, such as ankle-foot or knee-ankle-foot orthoses, to help maintain the ability to stand, whether mobile or not, and further contribute to the delay of debilitating contractures and scoliosis.
Becker’s dystrophy is a progressive disease that ultimately culminates in muscle-joint contractures, profound muscle weakness, and disability. When the patient becomes wheelchair dependent, the possibility of developing pressure sores increases. This possibility is further exacerbated by the chronic administration of corticosteroids. With the development of pressure sores comes the greater potential for infection and sepsis. Muscle wasting or atrophy and weakness contribute to alteration in pulmonary function, particularly forced vital capacity (FVC). Diminished pulmonary function can lead to the development of atelectasis and pneumonia. Frequent use of incentive spirometry can help prevent atelectasis and pneumonia. The decline in pulmonary function may necessitate some manner of ventilatory support, ranging from noninvasive, such as continuous positive airway pressure (CPAP), to minimally invasive, such as bilevel positive airway pressure (BiPAP), to invasive, such as tracheal intubation or tracheostomy, with or without mechanical ventilation. Tracheal intubation should be a short-term measure that should be supplanted by tracheostomy, whether or not it is combined with mechanical ventilation.
The sequelae of chronic steroid administration can contribute to the development of complications. The patient who depends on steroids may be more susceptible to skin breakdown and infection, whether via pressure sore or the pulmonary system, as well as weight gain and cushingoid habitus. The weight gain and cushingoid habitus may further exacerbate the potential for pressure sore development, infection, and diminution of pulmonary function.
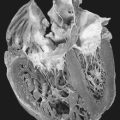
Dystrophin is found not only in the skeletal muscle, but also in significant quantity in the cardiac and brain tissues. Pseudohypertrophy may also occur in the heart muscle tissue, leading to cardiac fibrosis, which results in reduced cardiac output with ensuing pulmonary congestion, spiraling downward to fulminate congestive heart failure. Cardiac fibrosis also affects the cardiac conduction system. Fibrotic changes in cardiac conduction may be evidenced by tall R waves in the V 1 lead, deep Q waves in the AVL, AVR, and AVF leads, shortened P-R interval, and sinus tachycardia. As a result, lethal dysrhythmias may occur. Loss of dystrophin from cerebral tissue leads to developmental delays and diminished intellectual development. There is a shift to the left with regard to intelligence quotient (IQ) distribution; the mean IQ has been reported at 83.
Complications
• Atelectasis
• Cardiac conduction abnormalities
• Cardiac fibrosis
• Congestive heart failure
• Cushingoid habitus
• Decline in pulmonary function
• Developmental delays, diminished intellectual development
• Infection
• Muscle wasting or atrophy
• Pneumonia
• Pressure sores
• Pseudohypertrophy (skeletal and/or cardiac muscle)
• Sepsis
• Weight gain
Anesthesia Implications
Preoperative pulmonary function testing is appropriate to assess the degree of incapacity the disease has produced, and it contributes to the decision as to which anesthetic technique to use and whether the patient will require ventilatory support postoperatively. For the patient with severely diminished pulmonary function, regional anesthesia—specifically subarachnoid or epidural—may not be appropriate. A severe decline in pulmonary function implies severe muscle weakness, especially of the accessory muscles of breathing (intercostal and sternocleidomastoid muscles), which may be further exacerbated by instillation of such a regional technique. The work of breathing may quickly overwhelm the patient’s already limited energy reserves. Periods of postoperative respiratory embarrassment or dysfunction may be drawn out by as much as 36 hours, even though the patient’s muscle strength may appear to recover to the same level as observed preoperatively.
Heart failure is a real possibility during anesthesia, particularly for major surgical procedures, despite normal results on preoperative electrocardiogram and echocardiogram. Stress echocardiography using angiotensin has been advocated to detect latent heart failure and to identify any contraction abnormalities that may be induced.
Administration of anesthesia medications requires much consideration. Malignant hyperthermia (MH)–like symptoms may occur in the patient with Becker’s dystrophy, as well as in those with other muscular dystrophies. The potent inhalational agents are not recommended—nor is succinylcholine—in the patient with Becker’s dystrophy because of the potential production of MH-like symptoms, including rhabdomyolysis. Inhalation agents may also exacerbate any cardiac dysfunction that may be present or induce latent dysfunction. Hypnotics must also be selected judiciously. Thiopental may be used, but the doses must be significantly reduced. Propofol may be preferable to all others, but the induction dose may be larger than anticipated; moreover, administration of propofol must be closely scrutinized relative to the patient’s functional myocardial status because of the high degree of myocardial depression propofol produces. Parenteral narcotic analgesics may be used to supplant the need for potent inhalational agents. However, the choice of opiate must take into account the need for postoperative ventilation. Therefore shorter-acting opiates, such as alfentanil or remifentanil, may be more appropriate. Nondepolarizing muscle relaxants are likely to demonstrate increases in both effect as well as duration of action. Recovery from nondepolarizing muscle relaxants has been reported to be as much as three to six times longer than usually expected. The patient with Becker’s dystrophy—as well as those with all muscular dystrophies—experience abnormalities of smooth muscle. The combination of these abnormalities, inactivity, and general anesthesia may delay gastric emptying, thus increasing the potential for regurgitation and aspiration during induction/intubation and emergence/extubation.