1 Basics of Percutaneous Coronary Interventions
This chapter is the extension of the PCI chapter from The Cardiac Catheterization Handbook, fifth edition, and presents the basic method and mechanisms of balloon angioplasty and stenting as an introduction to the practice of interventional cardiology. The various techniques of PCI can be placed into niche applications for specific devices (Table 1-1).
Overview of the Basic PCI Method
Steps in the PCI Procedure
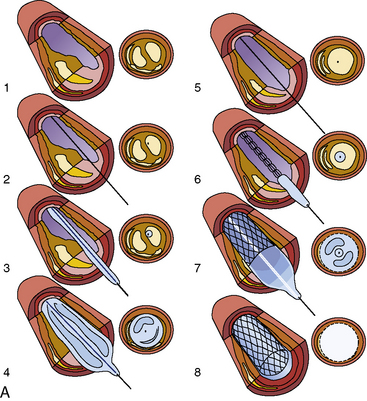
The steps in the PCI procedure are shown in Figure 1-1. First, a guiding catheter is seated in the coronary ostium. A thin, steerable guidewire is introduced into the guide catheter and then into the coronary artery and positioned across the stenosis into the distal aspect of the artery. A very small angioplasty balloon catheter is placed on the guidewire, inserted through the guiding catheter, and is positioned in the artery across the stenotic area by tracking it over the guidewire. Once correctly placed within the narrowed area to be treated, the balloon on the PCI catheter is inflated several times for brief periods (10–60 seconds). The inflation and deflation of the balloon expand the stenosis and restore blood flow to an area of the heart previously deprived by the stenosed artery.
After the stent struts have been expanded and implanted into the artery wall, the balloon is deflated, and the delivery catheter and guidewire are removed. Intravascular ultrasound (IVUS) imaging is often used to confirm appropriate vessel-stent matching and full stent strut apposition (contact without space against the wall). After IVUS and final angiography have been performed, the guide catheter is removed. The femoral or radial arterial sheath is removed, and hemostasis is obtained in the laboratory. The patient is then transferred to a recovery area and then to the patient’s room. If no complications occur, the patient is discharged the next morning. The patient usually returns to work shortly (<2 days) thereafter. The definitions of a successful PCI procedure are summarized in Table 1-2.
| Percutaneous coronary intervention (PCI) success may be defined by angiographic, procedural, and clinical criteria. |
| Angiographic Success |
• A successful PCI should achieve angiographic success without in-hospital major clinical complications (e.g., death, myocardial infarction [MI], emergency coronary artery bypass surgery) during hospitalization. MI is often defined as the development of Q waves in addition to elevation of troponins three times the upper limit of the laboratory’s normal value. Cardiac troponin T and I as measurements of myocardial necrosis are more sensitive and specific than CK-MB. Enzyme elevation in the absence of new Q waves is counted as MI, peri-procedural. There is no consensus on what level of troponin alone is clinically important enough to change major management following the interventional procedure.
• A clinically successful PCI is an anatomical and procedural success with relief of signs and/or symptoms of myocardial ischemia after recovery from the procedure. The long-term clinical success requires that the patient have persistent relief of signs and symptoms of myocardial ischemia for more than 6 months. Restenosis is the principal cause of lack of long-term clinical success when short-term clinical success has been achieved.
Mechanisms of Angioplasty and Stenting
Indications for PCI
In general, PCI is indicted for patients with the following:
• Stable angina pectoris unrelieved by optimal medical therapy with objective evidence of ischemia (abnormal stress test or abnormal stress thallium) and a coronary lesion in a vessel supplying a large area of myocardium
• Angina pectoris after CABG surgery
• Symptomatic restenosis after previous PCI
• Relative contraindications to PCI
• Unsuitable coronary anatomy (e.g., multiple severe complex lesions or diffuse distal disease)
• High-risk coronary anatomy in which closure of vessel would result in death
Contraindications to PCI
PCI Equipment
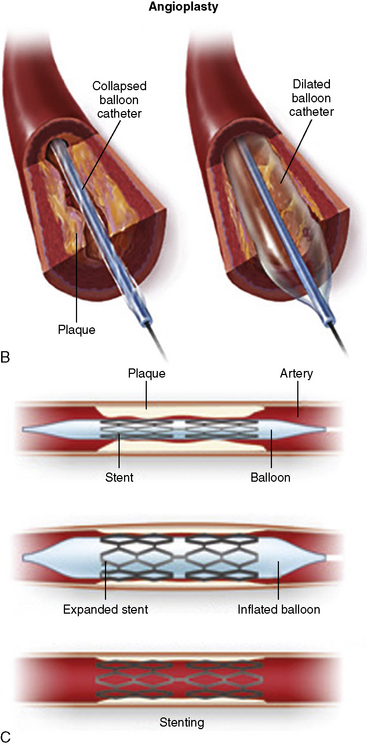
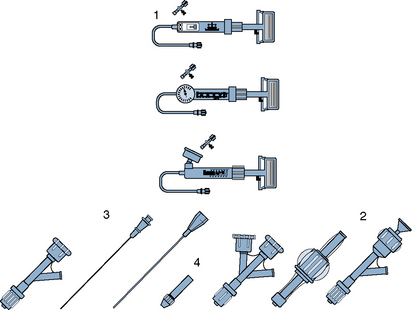
The most commonly used PCI equipment consists of four basic elements: a guiding catheter, a balloon catheter, a coronary guidewire, and a stent (Fig. 1-2 and Table 1-3 list approximate costs of such equipment).
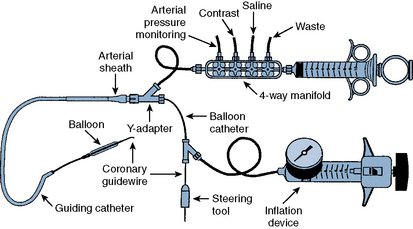
Figure 1-2 Diagram of components of percutaneous coronary intervention equipment.
(Adapted from Safian RD, Freed MS, eds. The manual of interventional cardiology, 3rd ed. Birmingham, MI: Physicians’ Press, 2001.)
Table 1-3 Approximate Costs of Coronary Angioplasty Equipment
| Equipment | Cost ($US) |
|---|---|
| Balloon dilatation catheter | 300 OTW/250 Rx |
| Guiding catheter | 65 |
| Guidewire | 80 |
| Exchange guidewire (300 cm) | 120 |
| Indeflator | 55 |
| Y connector | 31 |
| Sheath introducer | 8 |
| Torque tool | 18 |
| Nonballoon devices | |
| Stent (noncoated) | 1300 |
| Stent (drug-eluting) | 2300 |
| Rotablator | 1330 |
| IABP | 840 |
IABP, intra-aortic balloon pump; OTW, over-the-wire; Rx, rapid exchange.
Guiding Catheter
A special large-lumen catheter is used to deliver the coronary balloon catheter and other interventional devices to the vessel that contains the lesion to be dilated. The features of the guide catheter noted in Figure 1-3 differentiate it from diagnostic catheters.
Functions
A guiding catheter serves three major functions during angioplasty:
Backup Support for Balloon Catheter and Stent Advancement
The improved quality and size of currently used stents have reduced the need for robust backup support in most situations. For more complex and technically difficult lesions, the choice of an appropriate guiding catheter for extra support and lesion visualization remains essential (see Chapter 3). When there is insufficient backup in crossing a very tight stenosis, the guiding catheter will disengage from the coronary ostium and back out into the aortic root. When pressure is applied to the stent catheter during attempts to cross the lesion, repositioning the guide catheter in a stepwise fashion while the stent is advanced may overcome this loss of support. However, aggressive intubation of the coronary ostium may damage the vessel, stopping the procedure prematurely, or may require additional stenting for an ostial dissection.
Characteristics
Compared with the diagnostic catheters, the guiding catheters have thinner walls, larger lumens, and stiffer shafts (Fig. 1-3). A large catheter lumen is achieved at the expense of catheter wall thickness and thus may result in decreased catheter wall strength, less torque control, or catheter kinking. The guiding catheters are generally stiffer to provide backup support during the PCI catheter advancement into the coronary artery and, therefore, respond differently to manipulation than diagnostic catheters. The guiding catheter tip is not tapered. Pressure-wave damping upon engaging the coronary ostium is seen more often than with similar-sized diagnostic angiographic catheters. Some guide catheters have relatively shorter and more flexible tips to decrease catheter-induced trauma.
Balloon Dilatation Catheter Systems
Types
There are three types of PCI balloon catheters (Fig. 1-4):
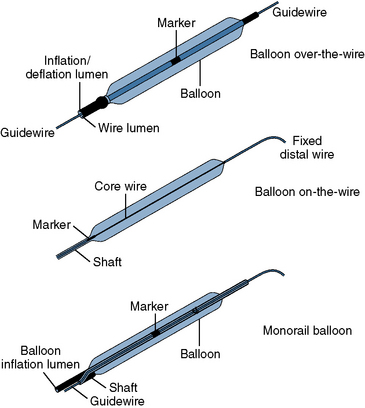
Figure 1-4 Three common types of coronary balloon angioplasty catheter design.
(Adapted from Freed MS, Grines C, eds. New manual of interventional cardiology. Birmingham, MI: Physicians’ Press, 1992: 29.)
The OTW and monorail balloons, but not fixed-wire balloons, are also used to deliver stents that are mounted by the manufacturer on a specific balloon. The advantages and limitations are summarized in Table 1-4.
Table 1-4 Advantages and Limitations of Angioplasty Balloon Types
| Advantages | Limitations |
|---|---|
| Over the wire | |
| Distal wire position | Two experienced personnel required |
| Distal port available for pressure measurement or contrast media injection | Larger profile |
| Accepts multiple guidewires | |
| Rapid exchange | |
| Distal wire position Enhanced visualization Low-profile balloons Single-operator system |
Exchanging balloons at hemostatic valve may be technically demanding |
| Fixed wire | |
| Enhanced visualization | Lack of through lumen |
| Single-operator system | Inability to recross lesion without removing |
| Use with small guiding catheters system | |
| Low-profile balloons | |
Modified from Kern MJ, ed. The cardiac catheterization handbook, 2nd ed. St Louis, MO: Mosby, 1995.
OTW Angioplasty Balloon Catheters
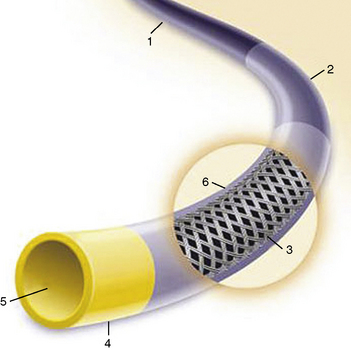
Historically, the OTW balloon was the first introduced and has remained popular in a few centers. A standard OTW angioplasty balloon catheter has a central lumen throughout the length of the catheter for the guidewire and another, separate lumen for balloon inflation (Fig. 1-5). These balloons are approximately 145 to 155 cm long and are designed to be used with guidewires of various dimensions (0.010–0.014 inches). The major OTW advantage is the ability to maintain distal artery access with the balloon beyond the lesion while one guidewire is exchanged for another. The OTW system tracks very well because the whole balloon length has a wire lumen. It permits long guidewire exchanges, and because of the through lumen, it allows for delivery contrast and drugs distally in an artery. To exchange PCI catheters, the balloon is advanced over the wire to a distal position. The standard short (145-cm) wire is then removed from the balloon. A longer guidewire (300 cm) is then inserted to maintain distal wire position while the balloon catheter is completely withdrawn over the guidewire and another balloon catheter is introduced over the same long guidewire for additional dilatations. OTW catheters can accept multiple guidewires, which allows for exchanging additional devices that may require stronger, stiffer, or specialized guidewires.
Rapid-Exchange (Monorail) Balloon Catheters
“Rapid-exchange” or monorail catheters were developed to permit the exchange of angioplasty balloon catheters by a single operator. Rapid-exchange catheters have only a short (30–40 cm) length of the catheter shaft containing two lumens (Fig. 1-6). One lumen, in the distal 30-cm portion of the catheter shaft, houses the guidewire. The remaining lumen runs the entire length of the catheter and is used for balloon inflation. Because only a limited portion of the balloon requires dual lumens, rapid-exchange catheters are smaller in diameter than are OTW balloon catheters.
Characteristics
The mechanical aspects of balloon inflations apply the following fundamental principles:
PCI Guidewires
Characteristics
Malleability is the ability to shape the spring tip and maintain a desired tip shape. Repeated attempts with different wire tip configurations may be required to cross distal stenoses. The manufacturer preforms some guidewire tip shapes. Guidewire tip shaping is accomplished by bending the wire between the thumb and index finger, rolling the guidewire tip over a needle, or bending the wire tip at the end of an introducer tool. In general, the length of the distal bend in a large vessel should approximate half the usual diameter of the vessel (about 2 mm). A larger bend may be needed to reach a takeoff. When the wire is steered into an abruptly angled branch, a double 45-degree bend is often helpful (Table 1-5).
Accessory Equipment (Fig. 1-8)
Stents
Stents reduce abrupt vessel closure from dissections and coronary restenosis. The implantation of coronary stents has superseded traditional balloon angioplasty and is the most widely practiced procedure of PCI. The multitude of stent designs has arisen because of patent design issues, improved scaffolding mechanisms, and unique coatings. Most of the advances in stent design have been to increase deliverability. Stent delivery depends on both stent flexibility and profile, which must be designed without sacrificing radial strength and scaffolding length. Characteristics of an ideal stent are listed in Table 1-6.
Types
Dimensions and Designs
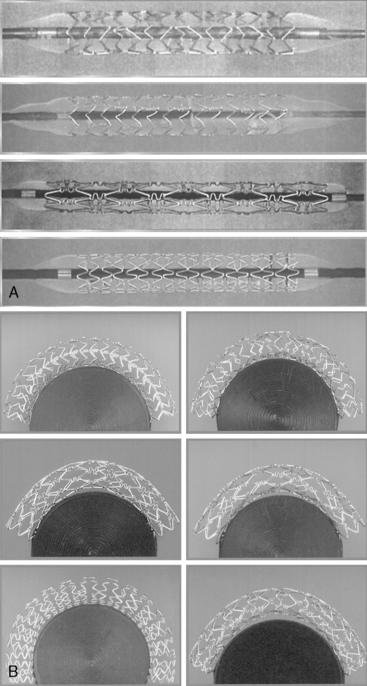
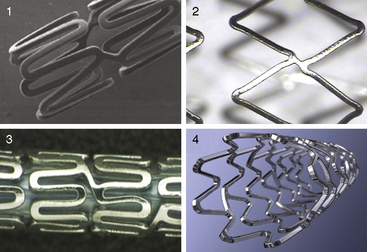
Each stent has specific characteristics that differentiate their selection for clinical use. Figure 1-9 shows several currently available stents. Table 1-7 lists stent types, characteristics, and specialized features of material and cell configuration. Figure 1-10, A, depicts different polymer metallic stents. Figure 1-10, B, is an example of stent placement in right coronary angioplasty (RCA).
| Stent Type | Description | Examples |
|---|---|---|
| Drug-eluting stent | A stent that slowly releases a drug to block cell proliferation and/or restenosis | Cypher (J&J/Cordis) Taxus (Boston Scientific) Xience V (Abbott Vascular) Endeavor (Medtronic) |
| Bare metal stent, stainless steel | A vascular thin metal wire or mesh stent without a coating, typically first-generation technology | Bx Velocity (J&J/Cordis) Express2 (Medtronic) Millennium Matrix (Sahajanand Medical Technologies) |
| Bare metal stent, CoCr | A vascular thin metal wire or mesh without a coating, typically next-generation technology | Driver (Medtronic) Multi-Link Vision (Abbott Vascular) Corronnium (Sahajanand Medical Technologies) |
| Absorbable stent | Completely biodegradable, bioabsorbable stent, typically polymer or magnesium, sometimes coated with antirestenotic agent | AMS (Biotronik) ABSORB trial (Abbott Vascular) REVA/RESORB trial (REVA medical) |
| Bioactive stent | A stent that reacts with the body’s natural processes to achieve an antirestenotic effect | Genous (OrbusNeich) Titan2 BAS (Hexacath) |
| Radioactive stent | Stent with a radiation-emitting coating | (Name undisclosed) (MoBeta, Inc.) |
| Drug-eluting balloon | Angioplasty balloon that, after deflation, leaves behind an antirestenotic drug | SeQuent Please (B. Braun Melsungen) DIOR (EuroCor) Elutax (Aachen Resonance) |
Contraindications
Relative contraindications to stenting can be determined based on patient and anatomical factors.
Patient Factors
• Gastrointestinal bleeding that prevents 4 to 5 hours of anticoagulation during or following the stent procedure
• Inability to take dual antiplatelet therapy (e.g., ASA and a thiopyridine-like clopidogrel)
• Conditions prone to hemorrhage; intracranial hemorrhage, recent surgery, or bleeding diathesis
Complex Stenting
Stenting for patients with complex anatomy should be carefully considered. Any of the following characteristics are considered at higher risk for complications and are discussed in detail in Chapters 8 and 9:
• Long lesions requiring more than one stent per lesion
• Small coronary artery reference vessel diameters (<2.5 mm)
• Significant thrombus at the lesion site
• Lesions in saphenous vein grafts, the left main coronary artery, ostial locations, or bifurcation lesions
• Diffuse disease or poor outflow distal to the identified lesion
• Very tortuous vessels in the region of the obstruction or proximal to the lesion
• Unprotected left main stenosis
• Complex coronary artery disease (CAD) with significant impairment of left ventricular function
General Notes for Stent Deployment
1. When multiple lesions are stented, the distal lesion should be treated initially, followed by the proximal lesion. Stenting in this order obviates the need to recross the proximal stent with the distal stent and reduces the chances of stent delivery failure or loss of stent when an undeployed stent is pulled back for whatever reason.
2. When recrossing a recently implanted stent, ensure that the guidewire traverses the stent and does not go between the stent and the vessel wall, which may result in inadvertent dislodgement of the stent during further balloon/stent passage.
3. If there is stent inflow or outflow obstruction or residual distal vessel narrowing, a freshly prepared balloon catheter can be advanced into and through the stented area for further dilatations. Re-wrapping previously used balloons should also be considered.
4. Eliminate any inflow or outflow narrowing by additional balloon inflations or stent implantation (especially if the stent margin has a dissection).
5. An acceptable angiographic result is a residual narrowing of less than 10% by visual estimate, but a truly optimal result must be confirmed by IVUS.
6. Vasospasm may occur during the procedure when high inflation pressures are used for stent optimization. Vasospasm is self-limiting, nearly always resolves with time or intracoronary nitroglycerin, and has not been associated with any unfavorable clinical events. Extraordinarily high-pressure inflations (>16 atm) are generally unnecessary and have been associated with stent overexpansion and higher in-stent restenosis rates.
IVUS Optimization Based on the Reference Vessel Area
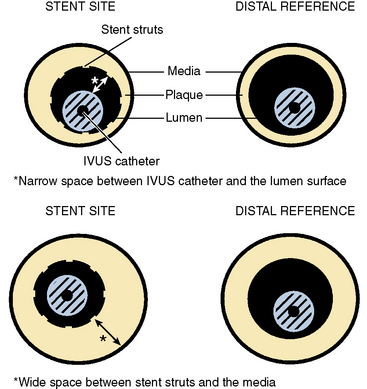
Using criteria based only on IVUS vessel area has the inherent flaw of not incorporating stent expansion relative to the reference lumen CSA. The use of a criterion of 50% of the average vessel area would leave a significant number of patients with a stent that was underexpanded compared with the distal reference lumen. The use of a criterion of 60% of the average would position the final stent lumen between the CSAs of the proximal and distal reference lumens (Fig. 1-11). The use of reference vessel criteria has the disadvantage of requiring multiple additional measurements, in contrast to using the reference lumen criterion, which requires only a few.
Stent Expansion Strategies
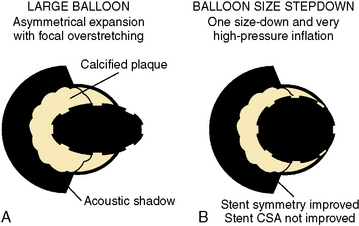
Asymmetrical Stent Expansion
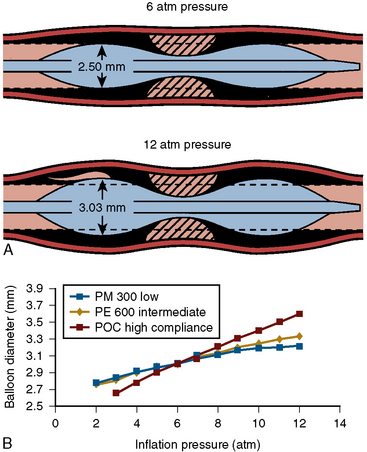
Stent expansion should be symmetrical in soft plaques. Very hard plaques (fibrotic or calcified), seen in approximately 20% to 30% of lesions, are not easily compressed by the balloon/stent, resulting in asymmetrical stent expansion into the normal arc of the vessel. In lesions with a significant arc (= 270 degrees) of dense or hard fibrocalcific disease, asymmetrical stent expansion occurs with a minimum to maximum lumen diameter ratio (symmetry index) of less than 0.7. In such lesions, further inflation leads to focal overstretching in the less diseased arc of the vessel. The symmetry index can worsen after further dilation, especially if an oversized balloon is used (Fig. 1-12). Using a balloon that is 0.25 to 0.5 mm smaller than the size of the vessel, and very high pressures (>18 ATM), may improve the symmetry index but will not necessarily increase the CSA of the lumen at the stent site.
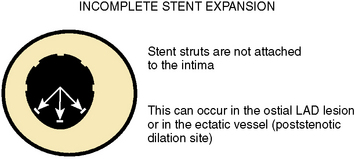
Incomplete Stent Expansion
Adequate stent expansion is dependent on the plaque burden. Optimal stent expansion in lesions with 50% to 70% diameter stenosis or lesions with a spiral dissection can be easily accomplished because there is not much atheroma. In lesions with more than 90% diameter stenosis, optimal stenting is more difficult to achieve and is associated with a higher percentage of asymmetrical stent expansion. Incomplete stent expansion (i.e., when the stent struts do not contact the intimal surface) can occur, particularly in ectatic vessels (at poststenotic dilation or aneurysm sites) and in the ostial left anterior descending artery (LAD), where the operator is cautious about performing a high-pressure balloon inflation in the left main trunk (Fig. 1-13). In the latter case, dilation of the ostial lesion with only the shoulder of the balloon does not provide sufficient expansion force to implant the stent fully. Table 1-6 suggests ways to redeploy stent after initial failure to expand.
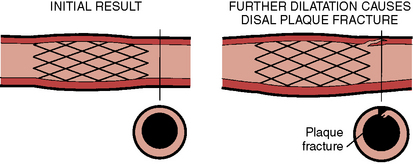
Dissection at the Stent Margin
Stent dilations sometimes cause a plaque fracture or dissection at the edge of the stent and vessel, which requires additional stents to stabilize the newly produced dissection (Fig. 1-14). Plaque fracture may result from misplacement of the balloon post dilation, especially if the balloon is clearly oversized relative to the angiographic vessel size. Plaque fracture can also occur even when the balloon is positioned within the stented segment, especially in calcific lesions or vessels. In more elastic or soft lesions, this is less likely to occur, but it can be seen at the stent margins when the stents are deployed on bend lesions.
Managing Complications During Stent Delivery and Implantation
Delivery Failure
Failure to deliver the stent is most often due to:
1. Suboptimal guide catheter support
2. Failure to predilate a significant coronary lesion
3. Unsuspected proximal tortuosity or calcification of the vessel with unanticipated vessel rigidity and acute angulation
In arteries that are highly tortuous and have multiple bends and folds, guidewire selection is an important factor in stent delivery success. Extra-support guidewires may not be ideal for initially crossing lesions, producing folds and pseudo stenosis, and conventional guidewires that are softer may permit delivery of the stent system without encountering the pseudo stenosis (Table 1-8).
Table 1-8 Technical Manipulations When a Stent Fails to Advance
• Advance a second stiffer wire to straighten the artery (the buddy wire technique). This stiff wire can cause wire bias.
• Advance the stent on the second stiffer buddy wire. Occasionally stents may advance more easily over a softer wire.
• Shape the wire along the curve of the artery to lessen wire bias so there is less friction or resistance at the outer curve of the vessel and the path of the wire is more coaxial with the path of the vessel.
• If the problem is due to tortuosity of the proximal segment, change the stent to a shorter one.
• Select a different type of stent with better flexibility.
• Gently bend the stent to conform it along the curve of the artery.
• Change to a guide with a different curve, to achieve better backup, and more coaxial to allow less friction at the ostium.
• Best technical manipulation: Steer the wire into a different direction, or to a different branch to lessen wire bias and increase more wire centering.
• Rotate the balloon catheter while advancing it and let the catheter enter the stent by itself through its rotational energy (like torquing the Judkins right catheter).
• Use a shorter balloon or stent.
• Use a more flexible balloon or stent.
• Use a fixed-wire balloon to cross the stent.
• Use a fixed-wire balloon to track alongside a buddy wire.
• Mount a stent on a balloon with the tip partially inflated.
• If only the balloon needs to enter the stented segment, inflate the balloon with 1 to 2 atm so the balloon centers the wire in the lumen and facilitates the crossing of the wire and balloon.
Modified from Nguyen T, Douglas JS Jr, Hieu NL, et al. Basic stenting. J Interv Cardiol 2002;15:237–241.
Expansion Failure or “Persistent” Stent Narrowing
Loss of Access to the Stent
1. Loss of guidewire access to a stent may result in a complication, especially if the stent has been inadequately expanded or when new lesions have been produced distal or proximal to the implanted stent. Recrossing a recently deployed stent is facilitated by using a soft guidewire with an exaggerated tip loop to prolapse through the stent. Care should always be taken so that the wire does not enter under a stent strut between the strut and the arterial wall. Once the guidewire has crossed the stent, a second problem may be encountered of inability to advance a balloon for high-pressure post-stent deployment.
2. Recrossing stents with balloons may be difficult when the proximal border of the stent is on a tortuous vessel segment, forcing the tip of the dilatation balloon into the vessel wall where it is blocked by the stent struts. Several approaches can be used to overcome this problem. The guide catheter can be repositioned in a more coaxial manner. A stiffer guidewire can be advanced to reshape the curve of the artery. The balloon can be withdrawn slightly, rotated, and readvanced during inspiration or coughing (the balloon’s profile should be as low as possible). Several operators have recommended putting a curve onto a stiff part of the guidewire and using it to advance across a tortuous segment proximal to a stent and placing a curve on the balloon by forming it with the finger and using a technique similar to that of putting a gentle curve on a guidewire. Table 1-8 summarizes several technical manipulations that may be used to recross a deployed stent. Table 1-5 lists unique guidewire tip shapes that will help in difficult PCI situations.
Stent-Related Dissection, Thrombosis, and Ischemia
The following factors are associated with an increased risk of stent thrombosis and ischemia:
Stent Implantation Before Noncardiac Surgery
Pre-PCI Preparation—Holding Area
• Patient preparation (intravenous access, meds, consent)
• Patient and family teaching (procedure, results, complications)
• Cardiothoracic surgeon consultation, particularly for high-risk, multivessel disease, or decreased LV function
• Appropriate laboratory data (type and cross-match, complete blood cell and platelet counts, prothrombin time [PT], partial thromboplastin time [PTT], electrolytes, blood urea nitrogen [BUN], creatinine)
Patient Preparation in Catheterization Suite
• Electrocardiogram ([ECG]; inferior and anterior wall leads): 12-lead (radiolucent) ECG.
• Skin-prepare both inguinal areas or wrist for radial artery (venous access for temporary pacing no longer routine; consider for high-risk patient, acute myocardial infarction, left bundle branch block requiring RCA PCI; Rotablator or thrombus aspiration device).
• Aspirin (325 mg PO); failure to administer aspirin before PCI is associated with a two to three times higher acute complication rate.
• Plavix (600 mg PO, best 24 hours beforehand); best outcomes are associated with Plavix preloading.
• Continue patient’s routine antihypertensive medications.
• Heparin 40 to 70 U/kg bolus (or 40 U/kg bolus if GPIIb/IIIa blocker used). Target activated clotting time (ACT) more than 200 seconds. Heparin is critical for PCI, despite controversies regarding dosing and unpredictable therapeutic responses. Higher levels of anticoagulation are roughly correlated with fewer complications during coronary angioplasty, albeit at the expense of increased bleeding complications at higher heparin doses. Weight-adjusted heparin provides a clinically superior anticoagulation method over fixed heparin dosing.
• Consider glycoprotein IIb/IIIa blockers.
• Premedication (e.g., fentanyl [25–50 mg IV] and Versed [1-2 mg IV]).
Postprocedure Angiograms and Hemostasis
• Remove guidewire for final images after additional intracoronary nitroglycerin. Angiography with guidewire in place may disguise intimal flap or dissection.
• Femoral angiography before vascular closure device selection (perform right anterior oblique view for right femoral artery and left anterior oblique for left femoral artery). Alternatively, if not suitable for closure device, secure arterial and venous sheaths in place. Remove in 4 hours when ACT less than 150 seconds. These considerations do not apply to radial artery access.
• No postprocedure heparin infusions unless there are highly unusual circumstances.
Post-PCI Care—Medications
• Order aspirin (325 mg orally daily).
• Give clopidogrel (600 mg loading dose and 75 mg/day orally, at least 4 weeks after stenting with a bare metal stent and 12 months with a drug-eluting stent).
• Initiate statin drugs if not already prescribed.
• Restart or initiate antihypertensive or antianginal medications depending on the patient’s clinical needs.
• Resume prior medications for other conditions (e.g., GERD).
Follow-up Schedule and Stress Testing
PCI Program Without Surgical Backup
1. The operators must be experienced interventionalists who regularly perform elective intervention at a surgical center (75 cases/year). The institution must perform a minimum of 36 primary PCI procedures per year.
2. The nursing and technical catheterization laboratory staff must be experienced in handling acutely ill patients and must be comfortable with interventional equipment. They must have acquired experience in dedicated interventional laboratories at a surgical center. They participate in a 24-hour, 365-day call schedule.
3. The catheterization laboratory itself must be well equipped, with optimal imaging systems, resuscitative equipment, and intra-aortic balloon pump (IABP) support, and must be well stocked with a broad array of interventional equipment.
4. The cardiac care unit nurses must be adept in hemodynamic monitoring and IABP management.
5. The hospital administration must fully support the program and enable the fulfillment of the institutional requirements listed previously.
6. There must be formalized written protocols in place for immediate (within 1 hour) and efficient transfer of patients to the nearest cardiac surgical facility that is reviewed or tested on a regular (quarterly) basis.
7. Primary intervention must be performed routinely as the treatment of choice around the clock for a large proportion of patients with acute myocardial infarction to ensure streamlined care paths and increased case volumes.
8. Case selection for the performance of primary angioplasty must be rigorous. Criteria for the types of lesion appropriate for primary angioplasty and for the selection for transfer for emergency aortocoronary bypass surgery are shown in Table 1-9.
9. There must be an ongoing program of outcomes analysis and formalized periodic case review.
10. Institutions should participate in a 3- to 6-month period of implementation, during which time development of a formalized primary PCI program is instituted that includes establishing standards, training staff, detailed logistic development, and creation of a quality assessment and error management system. (Smith et al. 1993) (See Smith SC Jr, Dove JT, Jacobs AK, et al. ACC/AHA guidelines for percutaneous coronary intervention (revision of the 1993 PTCA guidelines)—executive summary: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (Committee to revise the 1993 guidelines for percutaneous transluminal coronary angioplasty) endorsed by the Society for Cardiac Angiography and Interventions. Circulation 2001;103:3019–3041.)
Table 1-9 Patient Selection for PCI at Hospitals Without On-Site Cardiac Surgery
PCI, percutaneous coronary intervention; TIMI, thrombolysis in myocardial infarction.
Adapted from Wharton TJ Jr, McNamara NS, Fedele FA, et al. Primary angioplasty for the treatment of acute myocardial infarction: experience at two community hospitals without cardiac surgery. J Am Coll Cardiol 1999;33:1257–1265.
Training for Coronary Angioplasty
Advances in interventional procedures have maintained high and durable success rates despite increasingly complex procedures. The need for appropriate training and guidelines for the procedure is obvious. Recent guidelines for the assessment and proficiencies of coronary interventional procedures have been summarized in a report from the joint task force from the AHA/ACC (Table 1-10). ABIM board certification in interventional cardiology requires documentation of training in an accredited fellowship program during which a minimum of 125 coronary angioplasty procedures must be performed, including 75 performed with the trainee as primary operator (Table 1-11).
Table 1-10 Considerations for the Assessment and Maintenance of Proficiency in Coronary Interventional Procedures
• Procedural volume of 75 per year
• Continuation of privileges based on outcome benchmark rates with consideration of not granting privileges to operators who exceed adjusted case-mix benchmark complication rates for a 2-year period
• Ongoing quality assessment comparing results with current benchmarks, with risk stratification of complication rates
From Hirshfeld JW, Elllis SG, Faxon DP. Recommendations for the assessment and maintenance of proficiency in coronary interventional procedures: statement of the American College of Cardiology. J Am Coll Cardiol 1998;31:722–743.
Table 1-11 Recommendations for Clinical Competence in Percutaneous Transluminal Coronary Angiography: Minimum Recommended Number of Cases per Year
| Total number of cases | 125 |
| Cases as primary operator | 75 |
| Practicing, number of cases per year | 50-75 to maintain competency |
Anderson H.V., Shaw R.E., Brindis R.G., et al. Relationship between procedure indications and outcomes of percutaneous coronary interventions by American College of Cardiology/American Heart Association Task Force Guidelines. Circulation. Nov 2005;112:2786–2791.
Baim D.S. Approaches to mechanical coronary thrombectomy. J Invasive Cardiol. 2006;18(1–10 suppl C):28C–31C.
Baim D.S. Grossman’s cardiac catheterization, angiography, and intervention, 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2006.
Berger A., Botman K.J., MacCarthy P.A., et al. Long-term clinical outcome after fractional flow reserve-guided percutaneous coronary intervention in patients with multivessel disease. J Am Coll Cardiol. 2005;46:438–442.
Boden W.E. Optimal medical therapy with or without PCI for stable coronary artery disease. N Engl J Med. 2007;356:1503–1516.
Brilakis E.S., Banerjee S., Berger P.B. Perioperative management of patients with coronary stents. J Am Coll Cardiol. 2007;49:2145–2150.
Eagle K.A., Berger P.B., Calkins H., et al. ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery executive summary: a report of the American College of Cardiology/American Heart Association Task force on Practice Guidelines (Committee to Update the 1996 Guidelines on Perioperative Cardiovascular Evaluation for Non cardiac Surgery). Circulation. 2002;105:1257–1267.
Garg S., Serruys P.W. Coronary stents: current status. J Am Coll Cardiol. Aug 31 2010;56:S1–S42.
Garg S., Serruys P.W. Coronary stents: looking forward. J Am Coll Cardiol. Aug 31 2010;56:S43–S78.
Grüntzig A., Sennig A., Siegenthaler W.E. Nonoperative dilatation of coronary artery stenosis: percutaneous transluminal coronary angioplasty. N Engl J Med. 1979;301:61–68.
Harper R.W., Mottram P.M., McGaw D.J. Closure of secundum atrial septal defects with the Amplatzer septal occluder device: techniques and problems. Catheter Cardiovasc Interv. 2002;57(4):508–524.
Holmes D.R.Jr, Kereiakes D., Garg S., et al. Stent thrombosis. J Am Coll Cardiol. 2010;56:1357–1365.
Inglessis I., Landzberg M.J. Interventional catheterization in adult congenital heart disease. Circ J. 2007;115:1622–1633.
Kaluza G.L., Joseph J., Lee J.R., et al. Catastrophic outcomes of noncardiac surgery soon after coronary stenting. J Am Coll Cardiol. 2000;35:1288–1294.
Katritsis D.G., Ioannidis J.P.A. Percutaneous coronary intervention versus conservative therapy in nonacute coronary artery disease: a meta-analysis. Circulation. 2005;111:2906–2912.
King S.B., Chair for the Writing Committee Members ACCF/AHA/SCAI. 2007 Update of the Clinical Competence Statement on Cardiac Interventional Procedures, 2010.
McFadden E.P., Stabile E., Regar E., et al. Late thrombosis in drug-eluting coronary stents after discontinuation of antiplatelet therapy. Lancet. 2004;364:1519–1521.
McFalls E.O., Ward H.B., Moritz T.E., et al. Coronary-artery revascularization before elective major vascular surgery. N Engl J Med. 2004;351:2795–2804.
Moussa I., Oetgen M., Roubin G., et al. Effectiveness of clopidogrel and aspirin versus ticlopidine and aspirin in preventing stent thrombosis after coronary stent implantation. Circulation. 1999;99:2364–2366.
Nasser M., Kapeliovich M., Markiewicz W. Late thrombosis of sirolimus-eluting stents following noncardiac surgery. Catheter Cardiovasc Interv. 2005;65:516–519.
Patel M.R., Dehme G.Jr, Hirshfeld J.W., et al. ACCF/SCAI/STS/AATS/AHA/ASNC 2009 Appropriateness criteria for coronary revascularization: a report by the American College of Cardiology Foundation Appropriateness Criteria Task Force, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, American Association for Thoracic Surgery, American Heart Association, and the American Society of Nuclear Cardiology Endorsed by the American Society of Echocardiography, the Heart Failure Society of America and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2009;53:530–553.
Poldermans D., Schouten O., Vidakovic R., et al. A clinical randomized trial to evaluate the safety of a noninvasive approach in high-risk patients undergoing major vascular surgery: the DECREASE-V pilot study. J Am Coll Cardiol. 2007;49:1763–1769.
Sant’Anna F.M., Silva E.E., Batista L.A., et al. Influence of routine assessment of fractional flow reserve on decision making during coronary interventions. Am J Cardiol. 2007;99:504–508.
Varma C., Benson L.N., Silversides C., et al. Outcomes and alternative techniques for device closure of the large secundum atrial septal defect. Catheter Cardiovasc Interv. 2004;61:131–139.
Wilson S.H., Fasseas P., Orford J.L., et al. Clinical outcome of patients undergoing noncardiac surgery in the two months following coronary stenting. J Am Coll Cardiol. 2003;42:234–240.
Zanchetta M., Onorato E., Rigatelli G., et al. Intracardiac echocardiology-guided transcatheter closure of secundum atrial septal defect. A new efficient device selection method. J Am Coll Cardiol. 2003;42:1677–1682.